Multifractal Heart Rate Value Analysis: A Novel Approach for Diabetic Neuropathy Diagnosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Photoplethysmography Recording
2.3. HRV Analysis
2.4. Statistical Analysis
3. Results
3.1. Linear Analysis Is Not Able to Discriminate Asymptomatic Neuropathy Patients to Healthy Patients
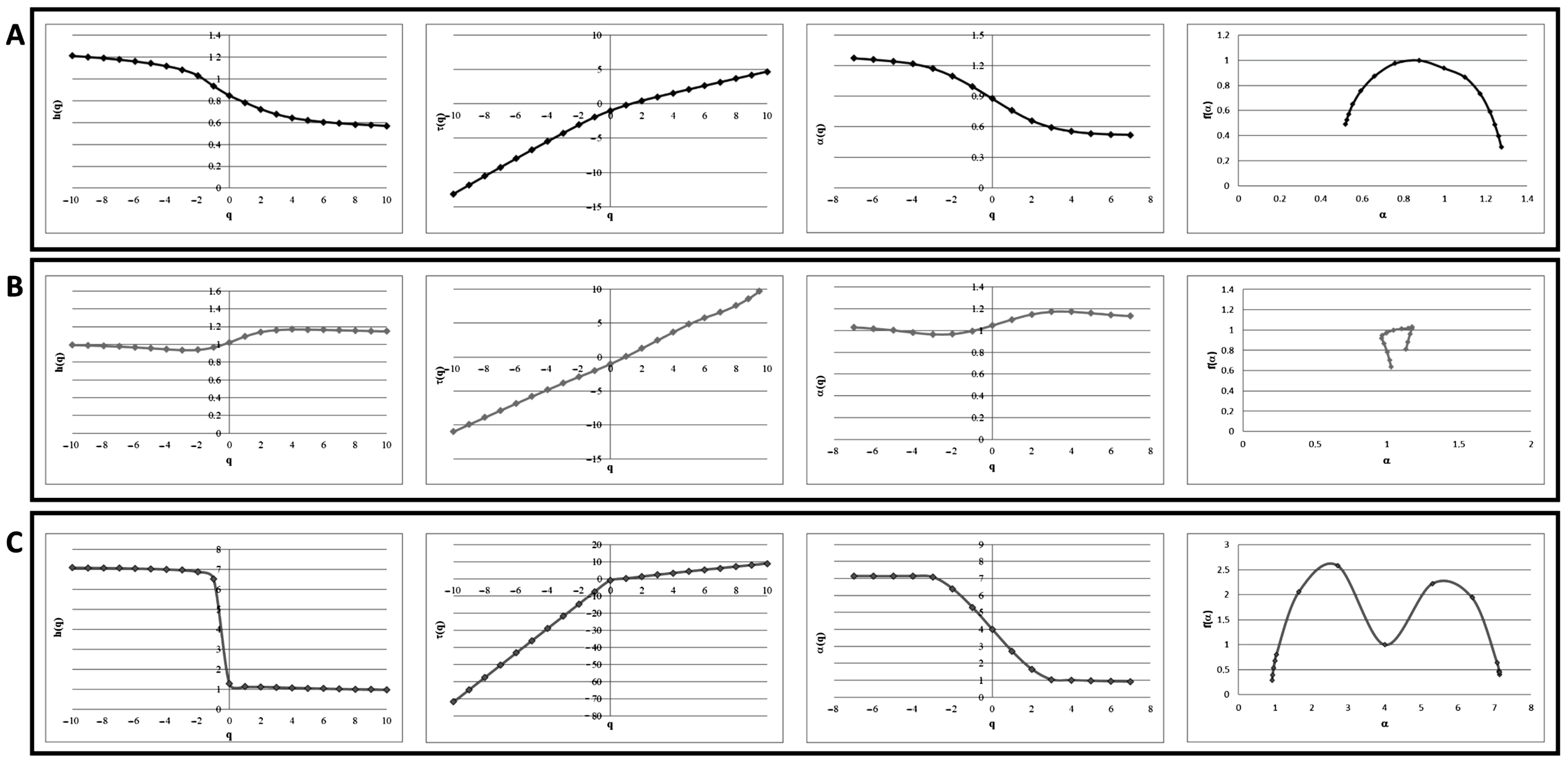
3.2. Multifractal Analysis Is Suitable to Distinguish Not-Full-Blown Neuropathy Patients to Full-Blown Neuropathy Patient
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stolar, M. Glycemic control and complications in type 2 diabetes mellitus. Am. J. Med. 2010, 123, S3–S11. [Google Scholar] [CrossRef] [PubMed]
- Vinik, A.I.; Park, T.S.; Stansberry, K.B.; Pittenger, G.L. Diabetic neuropathies. Diabetologia 2000, 43, 957–973. [Google Scholar] [CrossRef]
- Lai, Y.R.; Chiu, W.C.; Huang, C.C.; Tsai, N.W.; Wang, H.C.; Lin, W.C.; Cheng, B.C.; Su, Y.J.; Su, C.M.; Hsiao, S.Y.; et al. HbA1C Variability Is Strongly Associated With the Severity of Peripheral Neuropathy in Patients With Type 2 Diabetes. Front. Neurosci. 2019, 13, 90. [Google Scholar] [CrossRef] [PubMed]
- Bansal, V.; Kalita, J.; Misra, U.K. Diabetic neuropathy. Postgrad. Med. J. 2006, 82, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Dyck, P.J.; Kratz, K.M.; Karnes, J.L.; Litchy, W.J.; Klein, R.; Pach, J.M.; Wilson, D.M.; O’Brien, P.C.; Melton, L.J., 3rd; Service, F.J. The prevalence by staged severity of various types of diabetic neuropathy, retinopathy, and nephropathy in a population-based cohort: The Rochester Diabetic Neuropathy Study. Neurology 1993, 43, 817–824. [Google Scholar] [CrossRef]
- Brock, C.; Brock, B.; Pedersen, A.G.; Drewes, A.M.; Jessen, N.; Farmer, A.D. Assessment of the cardiovascular and gastrointestinal autonomic complications of diabetes. World J. Diabetes 2016, 7, 321–332. [Google Scholar] [CrossRef]
- The Diabetes Control and Complications Trial Research Group. The effect of intensive diabetes therapy on the development and progression of neuropathy. Ann. Intern. Med. 1995, 122, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Spallone, V.; Ziegler, D.; Freeman, R.; Bernardi, L.; Frontoni, S.; Pop-Busui, R.; Stevens, M.; Kempler, P.; Hilsted, J.; Tesfaye, S.; et al. Cardiovascular autonomic neuropathy in diabetes: Clinical impact, assessment, diagnosis, and management. Diabetes Metab. Res. Rev. 2011, 27, 639–653. [Google Scholar] [CrossRef] [PubMed]
- Pop-Busui, R.; Evans, G.W.; Gerstein, H.C.; Fonseca, V.; Fleg, J.L.; Hoogwerf, B.J.; Genuth, S.; Grimm, R.H.; Corson, M.A.; Prineas, R.; et al. Effects of cardiac autonomic dysfunction on mortality risk in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Diabetes Care 2010, 33, 1578–1584. [Google Scholar] [CrossRef] [PubMed]
- Pastore, D.; Deja-Simoni, A.; De Stefano, A.; Pacifici, F.; Cela, E.; Infante, M.; Coppola, A.; Di Daniele, N.; Lauro, D.; Della-Morte, D.; et al. Risk factors for diabetic foot ulcers: An Albanian retrospective study of inpatients with type 2 diabetes. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 558–572. [Google Scholar] [CrossRef]
- Chun, M.Y.; Park, H.K.; Hwang, H.S.; Han, J.I.; Chee, Y.J.; Lee, J.S. The Association between Symptoms of Autonomic Neuropathy and the Heart Rate Variability in Diabetics. Korean J. Fam. Med. 2011, 32, 292–298. [Google Scholar] [CrossRef]
- Chessa, M.; Butera, G.; Lanza, G.A.; Bossone, E.; Delogu, A.; De Rosa, G.; Marietti, G.; Rosti, L.; Carminati, M. Role of heart rate variability in the early diagnosis of diabetic autonomic neuropathy in children. Herz 2002, 27, 785–790. [Google Scholar] [CrossRef]
- Ewing, D.J.; Clarke, B.F. Diabetic autonomic neuropathy: Present insights and future prospects. Diabetes Care 1986, 9, 648–665. [Google Scholar] [CrossRef] [PubMed]
- Pagani, M. Heart rate variability and autonomic diabetic neuropathy. Diabetes Nutr. Metab. 2000, 13, 341–346. [Google Scholar]
- Peng, Y.; Liu, Y.S.; Wu, M.Y.; Chen, C.N.; Li, C.Q.; Jiang, A.Q.; Li, C.X.; Wang, Y.; Tian, G.; Pan, S.Y.; et al. Evaluation of the Degree of Agreement of Four Methods for Diagnosing Diabetic Autonomic Neuropathy. Front. Neurol. 2021, 12, 637099. [Google Scholar] [CrossRef] [PubMed]
- Dube, S.; Hulke, S.M.; Wakode, S.L.; Khadanga, S.; Thakare, A.E.; Bharshankar, R.N.; Pakhare, A. Effectiveness of Semmes Weinstein 10 gm monofilament in diabetic peripheral neuropathy taking nerve conduction and autonomic function study as reference tests. J. Fam. Med. Prim. Care 2022, 11, 6204–6208. [Google Scholar] [CrossRef]
- Ewing, D.J.; Campbell, I.W.; Clarke, B.F. Heart rate changes in diabetes mellitus. Lancet 1981, 1, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Sassi, R.; Signorini, M.G.; Cerutti, S. Multifractality and heart rate variability. Chaos 2009, 19, 028507. [Google Scholar] [CrossRef] [PubMed]
- Mani, A.R.; Montagnese, S.; Jackson, C.D.; Jenkins, C.W.; Head, I.M.; Stephens, R.C.; Moore, K.P.; Morgan, M.Y. Decreased heart rate variability in patients with cirrhosis relates to the presence and degree of hepatic encephalopathy. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G330–G338. [Google Scholar] [CrossRef] [PubMed]
- Gholami, M.; Mazaheri, P.; Mohamadi, A.; Dehpour, T.; Safari, F.; Hajizadeh, S.; Moore, K.P.; Mani, A.R. Endotoxemia is associated with partial uncoupling of cardiac pacemaker from cholinergic neural control in rats. Shock 2012, 37, 219–227. [Google Scholar] [CrossRef]
- Karimi Moridani, M.; Setarehdan, S.K.; Motie Nasrabadi, A.; Hajinasrollah, E. Non-linear feature extraction from HRV signal for mortality prediction of ICU cardiovascular patient. J. Med. Eng. Technol. 2016, 40, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, P.C.; Amaral, L.A.; Goldberger, A.L.; Havlin, S.; Rosenblum, M.G.; Struzik, Z.R.; Stanley, H.E. Multifractality in human heartbeat dynamics. Nature 1999, 399, 461–465. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, G.; Della-Morte, D.; Pastore, D.; Donadel, G.; De Stefano, A.; Palmieri, F. Identifying patterns in multiple biomarkers to diagnose diabetic foot using an explainable genetic programming-based approach. Future Gener. Comput. Syst. 2023, 140, 138–150. [Google Scholar] [CrossRef]
- Islam, S.; Kim, D.; Lee, Y.S.; Moon, S.S. Association between diabetic peripheral neuropathy and heart rate variability in subjects with type 2 diabetes. Diabetes Res. Clin. Pract. 2018, 140, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Stauss, H.M. Heart rate variability. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 285, R927–R931. [Google Scholar] [CrossRef] [PubMed]
- Akselrod, S.; Gordon, D.; Madwed, J.B.; Snidman, N.C.; Shannon, D.C.; Cohen, R.J. Hemodynamic regulation: Investigation by spectral analysis. Am. J. Physiol. 1985, 249, H867–H875. [Google Scholar] [CrossRef]
- Berger, R.D.; Akselrod, S.; Gordon, D.; Cohen, R.J. An efficient algorithm for spectral analysis of heart rate variability. IEEE Trans. Biomed. Eng. 1986, 33, 900–904. [Google Scholar] [CrossRef] [PubMed]
- Bassingthwaighte, J.B.; Raymond, G.M. Evaluation of the dispersional analysis method for fractal time series. Ann. Biomed. Eng. 1995, 23, 491–505. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liao, D.W.; Li, J.W.; Liao, G.P. Two-dimensional multifractal detrended fluctuation analysis for plant identification. Plant Methods 2015, 11, 12. [Google Scholar] [CrossRef]
- Agashe, S.; Petak, S. Cardiac Autonomic Neuropathy in Diabetes Mellitus. Methodist. Debakey Cardiovasc. J. 2018, 14, 251–256. [Google Scholar] [CrossRef]
- O’Brien, I.A.; O’Hare, P.; Corrall, R.J. Heart rate variability in healthy subjects: Effect of age and the derivation of normal ranges for tests of autonomic function. Br. Heart J. 1986, 55, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Ewing, D.J.; Campbell, I.W.; Clarke, B.F. Assessment of cardiovascular effects in diabetic autonomic neuropathy and prognostic implications. Ann. Intern. Med. 1980, 92, 308–311. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, I.A.; McFadden, J.P.; Corrall, R.J. The influence of autonomic neuropathy on mortality in insulin-dependent diabetes. Q. J. Med. 1991, 79, 495–502. [Google Scholar] [PubMed]
| ID | Age | Sex | Heart Rate (p/m) | Tachogram Standard Deviation (ms) | Total PSD (ms2/Hz) | VLF (ms2/Hz) | LF (Symph) (ms2/Hz) | HF (Parasymph) (ms2/Hz) | LF (u. n.) | HF (u. n.) | LF/HF |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 49 | M | 85.3 | 45.8 | 2097 | 388 | 898 | 811 | 0.53 | 0.47 | 1.11 |
| 2 | 27 | F | 73.6 | 52.6 | 2768 | 838 | 999 | 875 | 0.53 | 0.47 | 1.14 |
| 3 | 32 | F | 68.4 | 54.2 | 2937 | 1251 | 935 | 645 | 0.59 | 0.41 | 1.45 |
| 4 | 43 | M | 73.9 | 45.3 | 2056 | 835 | 653 | 519 | 0.56 | 0.44 | 1.26 |
| 5 | 29 | F | 72.7 | 58.9 | 3478 | 1659 | 625 | 1118 | 0.36 | 0.64 | 0.56 |
| 6 | 20 | M | 66.9 | 51.9 | 2700 | 1518 | 732 | 402 | 0.65 | 0.35 | 1.82 |
| 7 | 31 | F | 83.5 | 49.7 | 2473 | 395 | 999 | 939 | 0.52 | 0.48 | 1.06 |
| 8 | 37 | M | 71.6 | 43.2 | 1874 | 501 | 817 | 478 | 0.63 | 0.37 | 1.71 |
| 9 | 39 | F | 70.4 | 55.3 | 3060 | 1513 | 758 | 722 | 0.51 | 0.49 | 1.05 |
| 10 | 44 | M | 82.3 | 44.2 | 1960 | 648 | 764 | 480 | 0.61 | 0.39 | 1.59 |
| Average | 35.10 | 74.86 | 50.11 | 2540.30 | 954.60 | 818.00 | 698.90 | 0.55 | 0.45 | 1.28 | |
| St. Dev. | 8.89 | 6.51 | 537.52 | 491.61 | 134.99 | 235.12 | 0.08 | 0.08 | 0.38 |
| ID | Age | Sex | Heart Rate (p/m) | Tachogram Standard Deviation (ms) | Total PSD (ms2/Hz) | VLF (ms2/Hz) | LF (Symph) (ms2/Hz) | HF (Parasymph) (ms2/Hz) | LF (u. n.) | HF (u. n.) | LF/HF |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 44 | M | 90.3 | 42.7 | 1833.00 | 356.00 | 832 | 645 | 0.56 | 0.44 | 1.29 |
| 2 | 40 | F | 87.5 | 43.2 | 1869.00 | 158.00 | 900 | 811 | 0.53 | 0.47 | 1.11 |
| 3 | 47 | M | 83.5 | 40.9 | 1686.80 | 123.80 | 832 | 731 | 0.53 | 0.47 | 1.14 |
| 4 | 39 | F | 73.6 | 42.5 | 1816.25 | 387.25 | 800 | 629 | 0.56 | 0.44 | 1.27 |
| 5 | 49 | M | 88.5 | 44.0 | 1957.23 | 437.23 | 830 | 690 | 0.55 | 0.45 | 1.20 |
| 6 | 35 | F | 68.3 | 65.3 | 4276.00 | 1385.00 | 1805 | 1086 | 0.62 | 0.38 | 1.66 |
| 7 | 52 | M | 94.4 | 44.1 | 1948.00 | 571.00 | 816 | 561 | 0.59 | 0.41 | 1.45 |
| 8 | 46 | F | 74.6 | 52.1 | 2714.41 | 1301.41 | 797 | 616 | 0.56 | 0.44 | 1.29 |
| 9 | 47 | M | 68.0 | 50.8 | 2581.00 | 590.00 | 1655 | 336 | 0.83 | 0.17 | 4.93 |
| 10 | 36 | F | 64.2 | 39.6 | 1574.00 | 450.00 | 722 | 322 | 0.69 | 0.31 | 2.24 |
| Average | 43.50 | 79.29 | 46.52 | 2225.57 | 575.97 | 998.90 | 642.70 | 0.60 | 0.40 | 1.76 | |
| St. Dev. | 5.72 | 10.80 | 808.44 | 431.96 | 389.41 | 220.95 | 0.09 | 0.09 | 1.16 |
| ID | Age | Sex | Heart Rate (p/m) | Tachogram Standard Deviation (ms) | Total PSD (ms2/Hz) | VLF (ms2/Hz) | LF (Symph) (ms2/Hz) | HF (Parasymph) (ms2/Hz) | LF (u. n.) | HF (u. n.) | LF/HF |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 55 | M | 70.5 | 19.3 | 373 | 246 | 56 | 39 | 0.59 | 0.41 | 1.44 |
| 2. | 50 | F | 72.3 | 24.8 | 617 | 306 | 157 | 98 | 0.62 | 0.38 | 1.60 |
| 3. | 44 | M | 77.9 | 21.0 | 445 | 62 | 279 | 75 | 0.79 | 0.21 | 3.72 |
| 4 | 40 | F | 68.6 | 32.2 | 1040 | 360 | 364 | 280 | 0.57 | 0.43 | 1.30 |
| 5 | 52 | M | 100.0 | 30.3 | 922 | 326 | 390 | 147 | 0.73 | 0.27 | 2.65 |
| 6 | 41 | F | 79.6 | 24.0 | 578 | 193 | 289 | 62 | 0.82 | 0.18 | 4.66 |
| 7 | 43 | M | 88.2 | 30.9 | 959 | 296 | 476 | 148 | 0.76 | 0.24 | 3.22 |
| 8 | 52 | F | 72.0 | 24.8 | 618 | 373 | 113 | 95 | 0.54 | 0.46 | 1.19 |
| 9 | 54 | M | 91.8 | 16.5 | 274 | 118 | 58 | 55 | 0.51 | 0.49 | 1.05 |
| 10 | 46 | F | 73.8 | 25.9 | 671 | 437 | 123 | 80 | 0.61 | 0.39 | 1.54 |
| Average | 47.70 | 79.47 | 24.97 | 649.70 | 271.70 | 230.50 | 107.90 | 0.65 | 0.35 | 2.24 | |
| St. Dev. | 5.56 | 10.49 | 255.91 | 117.64 | 149.17 | 70.34 | 0.11 | 0.11 | 1.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coppola, A.; Conte, S.; Pastore, D.; Chiereghin, F.; Donadel, G. Multifractal Heart Rate Value Analysis: A Novel Approach for Diabetic Neuropathy Diagnosis. Healthcare 2024, 12, 234. https://doi.org/10.3390/healthcare12020234
Coppola A, Conte S, Pastore D, Chiereghin F, Donadel G. Multifractal Heart Rate Value Analysis: A Novel Approach for Diabetic Neuropathy Diagnosis. Healthcare. 2024; 12(2):234. https://doi.org/10.3390/healthcare12020234
Chicago/Turabian StyleCoppola, Andrea, Sergio Conte, Donatella Pastore, Francesca Chiereghin, and Giulia Donadel. 2024. "Multifractal Heart Rate Value Analysis: A Novel Approach for Diabetic Neuropathy Diagnosis" Healthcare 12, no. 2: 234. https://doi.org/10.3390/healthcare12020234
APA StyleCoppola, A., Conte, S., Pastore, D., Chiereghin, F., & Donadel, G. (2024). Multifractal Heart Rate Value Analysis: A Novel Approach for Diabetic Neuropathy Diagnosis. Healthcare, 12(2), 234. https://doi.org/10.3390/healthcare12020234









