Short-Term Impact of Low-Intensity Exercise with Blood Flow Restriction on Mild Knee Osteoarthritis in Older Adults: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Intervention
2.3. Assessments
2.3.1. Clinical Symptoms
2.3.2. Lower Extremity Function
2.3.3. Muscle Volume
2.3.4. Isokinetic Knee Strength
2.3.5. General Muscle Condition
2.3.6. Safety
2.4. Sample Size Determination and Statistical Analysis
3. Results
3.1. Participants
3.2. Clinical Symptoms
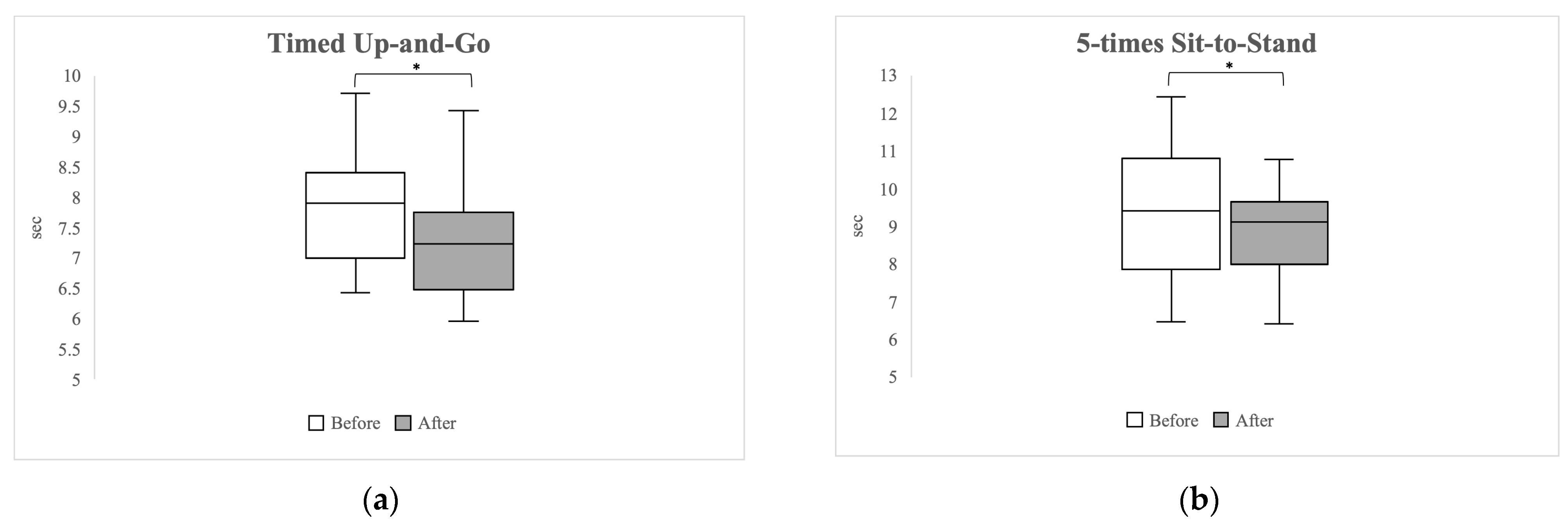
3.3. Lower Extremity Function
3.4. Muscle Volume
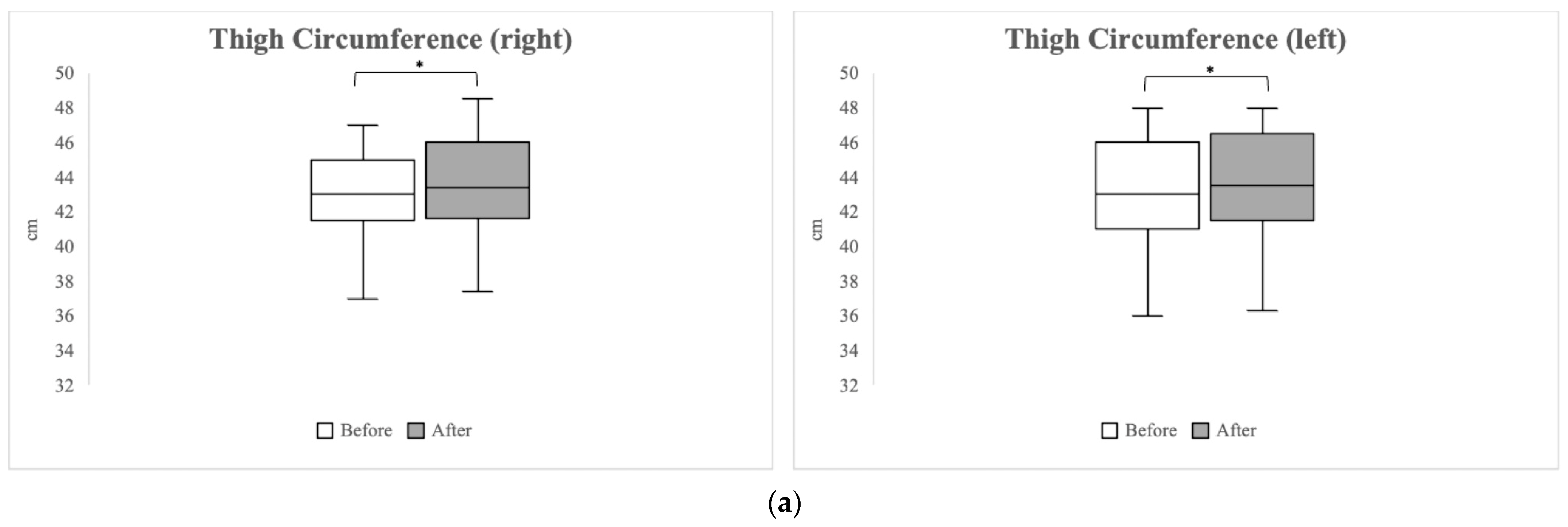
3.4.1. Anthropometric Measurements
3.4.2. Muscle Thickness
3.5. Isokinetic Knee Strength
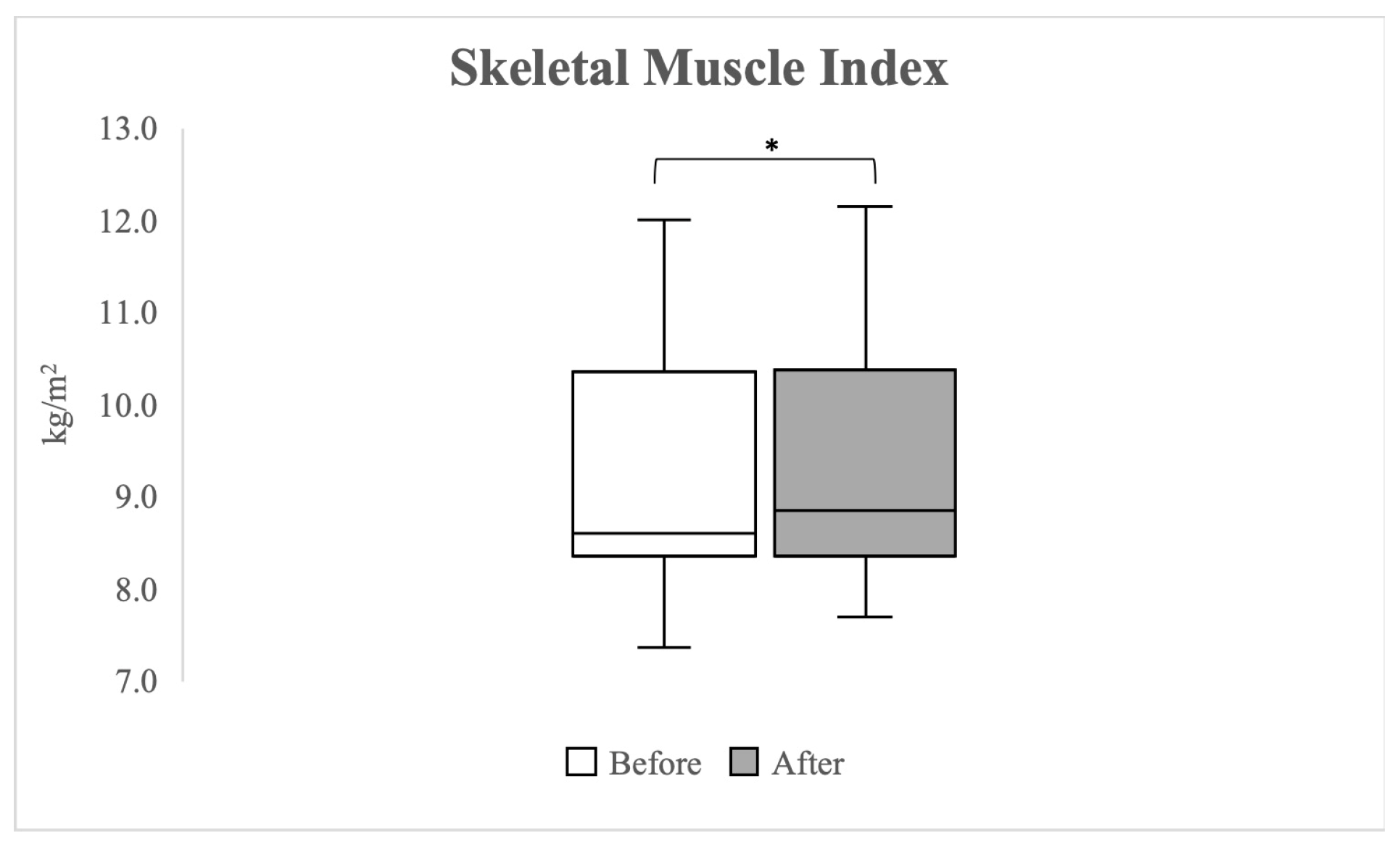
3.6. General Muscle Condition
3.7. Safety
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Madry, H.; Kon, E.; Condello, V.; Peretti, G.M.; Steinwachs, M.; Seil, R.; Berruto, M.; Engebretsen, L.; Filardo, G.; Angele, P. Early osteoarthritis of the knee. Knee Surg. Sports Traumatol. Arthrosc. 2016, 24, 1753–1762. [Google Scholar] [CrossRef]
- Zeng, C.Y.; Zhang, Z.R.; Tang, Z.M.; Hua, F.Z. Benefits and mechanisms of exercise training for knee osteoarthritis. Front. Physiol. 2021, 12, 794062. [Google Scholar] [CrossRef]
- Mesa-Castrillon, C.I.; Simic, M.; Ferreira, M.L.; Hatswell, K.; Luscombe, G.; de Gregorio, A.M.; Davis, P.R.; Bauman, A.; Bunker, S.; Clavisi, O. EHealth to empower patients with musculoskeletal pain in rural Australia (EMPoweR) a randomised clinical trial: Study protocol. BMC Musculoskelet. Disord. 2021, 22, 11. [Google Scholar] [CrossRef] [PubMed]
- Sharma, L. Osteoarthritis of the knee. N. Engl. J. Med. 2021, 384, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Takagi, S.; Omori, G.; Koga, H.; Endo, K.; Koga, Y.; Nawata, A.; Endo, N. Quadriceps muscle weakness is related to increased risk of radiographic knee OA but not its progression in both women and men: The Matsudai Knee Osteoarthritis Survey. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 2607–2614. [Google Scholar] [CrossRef] [PubMed]
- Nur, H.; Sertkaya, B.S.; Tuncer, T. Determinants of physical functioning in women with knee osteoarthritis. Aging Clin. Exp. Res. 2018, 30, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Sturnieks, D.L.; Tiedemann, A.; Chapman, K.; Munro, B.; Murray, S.M.; Lord, S.R. Physiological risk factors for falls in older people with lower limb arthritis. J. Rheumatol. 2004, 31, 2272–2279. [Google Scholar] [PubMed]
- Kim, H.T.; Kim, H.J.; Ahn, H.Y.; Hong, Y.H. An analysis of age-related loss of skeletal muscle mass and its significance on osteoarthritis in a Korean population. Korean J. Intern. Med. 2016, 31, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Ro, H.J.; Chung, S.G.; Kang, S.H.; Seo, K.M.; Kim, D.K. Low Skeletal muscle mass in the lower limbs is independently associated to knee osteoarthritis. PLoS ONE 2016, 11, e0166385. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Han, K.; McAlindon, T.E.; Park, Y.G.; Park, S.H. Lower leg muscle mass relates to knee pain in patients with knee osteoarthritis. Int. J. Rheum. Dis. 2018, 21, 126–133. [Google Scholar] [CrossRef]
- Fransen, M.; McConnell, S.; Harmer, A.R.; Van der Esch, M.; Simic, M.; Bennell, K.L. Exercise for osteoarthritis of the knee. Cochrane Database Syst. Rev. 2015, 1, CD004376. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zheng, X.; Huang, H.; Liu, C.; Wan, Q.; Shang, S. The effects of a home-based exercise intervention on elderly patients with knee osteoarthritis: A quasi-experimental study. BMC Musculoskelet. Disord. 2019, 20, 160. [Google Scholar] [CrossRef]
- DeVita, P.; Aaboe, J.; Bartholdy, C.; Leonardis, J.M.; Bliddal, H.; Henriksen, M. Quadriceps-strengthening exercise and quadriceps and knee biomechanics during walking in knee osteoarthritis: A two-centre randomized controlled trial. Clin. Biomech. 2018, 59, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Su, Y.; Chen, S.; Zhang, Y.; Zhang, Z.; Liu, C.; Lu, M.; Liu, F.; Li, S.; He, Z.; et al. The effects of resistance exercise in patients with knee osteoarthritis: A systematic review and meta-analysis. Clin. Rehabil. 2016, 30, 947–959. [Google Scholar] [CrossRef] [PubMed]
- Messier, S.P.; Callahan, L.F.; Beavers, D.P.; Queen, K.; Mihalko, S.L.; Miller, G.D.; Losina, E.; Katz, J.N.; Loeser, R.F.; Quandt, S.A.; et al. Weight-loss and exercise for communities with arthritis in North Carolina (we-can): Design and rationale of a pragmatic, assessor-blinded, randomized controlled trial. BMC Musculoskelet. Disord. 2017, 18, 91. [Google Scholar] [CrossRef]
- American College of Sports Medicine. American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med. Sci. Sports Exerc. 2009, 41, 687–708. [Google Scholar] [CrossRef]
- Lixandrao, M.E.; Ugrinowitsch, C.; Berton, R.; Vechin, F.C.; Conceicao, M.S.; Damas, F.; Libardi, C.A.; Roschel, H. Magnitude of muscle strength and mass adaptations between high-load resistance training versus low-load resistance training associated with blood-flow restriction: A systematic review and meta-analysis. Sports Med. 2018, 48, 361–378. [Google Scholar] [CrossRef]
- Jan, M.H.; Lin, J.J.; Liau, J.J.; Lin, Y.F.; Lin, D.H. Investigation of clinical effects of high- and low-resistance training for patients with knee osteoarthritis: A randomized controlled trial. Phys. Ther. 2008, 88, 427–436. [Google Scholar] [CrossRef]
- Bennell, K.L.; Dobson, F.; Hinman, R.S. Exercise in osteoarthritis: Moving from prescription to adherence. Best Pract. Res. Clin. Rheumatol. 2014, 28, 93–117. [Google Scholar] [CrossRef] [PubMed]
- Grantham, B.; Korakakis, V.; O’Sullivan, K. Does blood flow restriction training enhance clinical outcomes in knee osteoarthritis: A systematic review and meta-analysis. Phys. Ther. Sport 2021, 49, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Hendry, M.; Williams, N.H.; Markland, D.; Wilkinson, C.; Maddison, P. Why should we exercise when our knees hurt? A qualitative study of primary care patients with osteoarthritis of the knee. Fam. Pract. 2006, 23, 558–567. [Google Scholar] [CrossRef]
- Patterson, S.D.; Hughes, L.; Warmington, S.; Burr, J.; Scott, B.R.; Owens, J.; Abe, T.; Nielsen, J.L.; Libardi, C.A.; Laurentino, G. Blood flow restriction exercise: Considerations of methodology, application, and safety. Front. Physiol. 2019, 10, 533. [Google Scholar] [CrossRef] [PubMed]
- Gronfeldt, B.M.; Lindberg Nielsen, J.; Mieritz, R.M.; Lund, H.; Aagaard, P. Effect of blood-flow restricted vs heavy-load strength training on muscle strength: Systematic review and meta-analysis. Scand. J. Med. Sci. Sports 2020, 30, 837–848. [Google Scholar] [CrossRef]
- Pearson, S.J.; Hussain, S.R. A review on the mechanisms of blood-flow restriction resistance training-induced muscle hypertrophy. Sports Med. 2015, 45, 187–200. [Google Scholar] [CrossRef]
- Miller, B.C.; Tirko, A.W.; Shipe, J.M.; Sumeriski, O.R.; Moran, K. The systemic effects of blood flow restriction training: A systematic review. Int. J. Sports Phys. Ther. 2021, 16, 978–990. [Google Scholar] [CrossRef]
- Hwang, P.S.; Willoughby, D.S. Mechanisms behind blood flow–restricted training and its effect toward muscle growth. J. Strength Cond. Res. 2019, 33, S167–S179. [Google Scholar] [CrossRef]
- Bobes Álvarez, C.; Issa-Khozouz Santamaria, P.; Fernández-Matías, R.; Pecos-Martín, D.; Achalandabaso-Ochoa, A.; Fernández-Carnero, S.; Martínez-Amat, A.; Gallego-Izquierdo, T. Comparison of blood flow restriction training versus non-occlusive training in patients with anterior cruciate ligament reconstruction or knee osteoarthritis: A systematic review. J. Clin. Med. 2020, 10, 68. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Patel, B.H.; Kym, C.; Nwachukwu, B.U.; Beletksy, A.; Forsythe, B.; Chahla, J. Perioperative blood flow restriction rehabilitation in patients undergoing ACL reconstruction: A systematic review. Orthop. J. Sports Med. 2020, 8, 2325967120906822. [Google Scholar] [CrossRef]
- Takarada, Y.; Takazawa, H.; Ishii, N. Applications of vascular occlusion diminish disuse atrophy of knee extensor muscles. Med. Sci. Sports Exerc. 2000, 32, 2035–2039. [Google Scholar] [CrossRef]
- Barber-Westin, S.; Noyes, F. Blood flow-restricted training for lower extremity muscle weakness due to knee pathology: A systematic review. Sports Health 2019, 11, 69–83. [Google Scholar] [CrossRef]
- Hughes, L.; Paton, B.; Rosenblatt, B.; Gissane, C.; Patterson, S.D. Blood flow restriction training in clinical musculoskeletal rehabilitation: A systematic review and meta-analysis. Br. J. Sports Med. 2017, 51, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, R.B.; Gualano, B.; Rodrigues, R.; Kurimori, C.O.; Fuller, R.; Lima, F.R.; Ana Lucia, D.E.S.-P.; Roschel, H. Benefits of Resistance training with blood flow restriction in knee osteoarthritis. Med. Sci. Sports Exerc. 2018, 50, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Harper, S.A.; Roberts, L.M.; Layne, A.S.; Jaeger, B.C.; Gardner, A.K.; Sibille, K.T.; Wu, S.S.; Vincent, K.R.; Fillingim, R.B.; Manini, T.M.; et al. Blood-flow restriction resistance exercise for older adults with knee osteoarthritis: A pilot randomized clinical trial. J. Clin. Med. 2019, 8, 265. [Google Scholar] [CrossRef] [PubMed]
- Guex, K.; Daucourt, C.; Borloz, S. Validity and reliability of maximal-strength assessment of knee flexors and extensors using elastic bands. J. Sport Rehabil. 2015, 24, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Page, P. Clinical force production of Thera-Band^(! R) elastic bands. J. Orthop. Sports Phys. Ther. 2000, 30, A47–A48. [Google Scholar]
- Brzycki, M. Strength testing—Predicting a one-rep max from reps-to-fatigue. J. Phys. Educ. Recreat. Danc. 1993, 64, 88–90. [Google Scholar] [CrossRef]
- Alghadir, A.; Anwer, S.; Brismée, J.-M. The reliability and minimal detectable change of Timed Up and Go test in individuals with grade 1–3 knee osteoarthritis. BMC Musculoskelet. Disord. 2015, 16, 174. [Google Scholar] [CrossRef]
- Lord, S.R.; Murray, S.M.; Chapman, K.; Munro, B.; Tiedemann, A. Sit-to-stand performance depends on sensation, speed, balance, and psychological status in addition to strength in older people. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2002, 57, M539–M543. [Google Scholar] [CrossRef]
- Santos, L.P.; Gonzalez, M.C.; Orlandi, S.P.; Bielemann, R.M.; Barbosa-Silva, T.G.; Heymsfield, S.B.; Group, C.S. New prediction equations to estimate appendicular skeletal muscle mass using calf circumference: Results from NHANES 1999–2006. J. Parenter. Enter. Nutr. 2019, 43, 998–1007. [Google Scholar] [CrossRef] [PubMed]
- Fukuoka, Y.; Narita, T.; Fujita, H.; Morii, T.; Sato, T.; Sassa, M.H.; Yamada, Y. Importance of physical evaluation using skeletal muscle mass index and body fat percentage to prevent sarcopenia in elderly Japanese diabetes patients. J. Diabetes Investig. 2019, 10, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Yoo, T.K.; Rhim, H.C.; Lee, Y.-T.; Yoon, K.J.; Park, C.-H. Relationship between hyperhomocysteinemia and coexisting obesity with low skeletal muscle mass in asymptomatic adult population. Sci. Rep. 2022, 12, 12439. [Google Scholar] [CrossRef] [PubMed]
- Bontemps, B.; Gruet, M.; Louis, J.; Owens, D.J.; Miric, S.; Erskine, R.M.; Vercruyssen, F. The time course of different neuromuscular adaptations to short-term downhill running training and their specific relationships with strength gains. Eur. J. Appl. Physiol. 2022, 122, 1071–1084. [Google Scholar] [CrossRef]
- Tidball, J.G.; Villalta, S.A. Regulatory interactions between muscle and the immune system during muscle regeneration. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2010, 298, R1173–R1187. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.E.; De Freitas, M.C.; Zanchi, N.E.; Lira, F.S.; Cholewa, J.M. The role of inflammation and immune cells in blood flow restriction training adaptation: A review. Front. Physiol. 2018, 9, 1376. [Google Scholar] [CrossRef]
- Takarada, Y.; Nakamura, Y.; Aruga, S.; Onda, T.; Miyazaki, S.; Ishii, N. Rapid increase in plasma growth hormone after low-intensity resistance exercise with vascular occlusion. J. Appl. Physiol. 2000, 88, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e302. [Google Scholar] [CrossRef]
- McGrath, R.P.; Kraemer, W.J.; Snih, S.A.; Peterson, M.D. Handgrip strength and health in aging adults. Sports Med. 2018, 48, 1993–2000. [Google Scholar] [CrossRef]
- Morimoto, A.; Suga, T.; Tottori, N.; Wachi, M.; Misaki, J.; Tsuchikane, R.; Isaka, T. Association between hand muscle thickness and whole-body skeletal muscle mass in healthy adults: A pilot study. J. Phys. Ther. Sci. 2017, 29, 1644–1648. [Google Scholar] [CrossRef][Green Version]
- Song, J.; Chang, A.H.; Chang, R.W.; Lee, J.; Pinto, D.; Hawker, G.; Nevitt, M.; Dunlop, D.D. Relationship of knee pain to time in moderate and light physical activities: Data from Osteoarthritis Initiative. Semin. Arthritis Rheum. 2018, 47, 683–688. [Google Scholar] [CrossRef]
- Lees, F.D.; Clark, P.G.; Nigg, C.R.; Newman, P. Barriers to exercise behavior among older adults: A focus-group study. J. Aging Phys. Act. 2005, 13, 23–33. [Google Scholar] [CrossRef]


| Gender (male%) | 40% |
| Age (years) | 59.20 ± 4.97 |
| Height (cm) | 159.80 ± 6.30 |
| Weight (kg) | 64.05 ± 9.70 |
| BMI | 24.99 ± 2.63 |
| KL grade I (n) | 8 |
| KL graded II (n) | 7 |
| Pre-Exercise | Post-Exercise | p-Value | |
|---|---|---|---|
| Knee pain (NRS) | 2.00 (1.00–4.00) | 1.00 (0.00–2.00) | 0.004 |
| WOMAC-Pain | 2.00 (1.00–5.00) | 1.00 (0.00–3.00) | 0.013 |
| WOMAC-Stiffness | 1.00 (0.00–3.00) | 0.00 (0.00–2.00) | 0.039 |
| WOMAC-Physical function | 4.00 (2.00–8.00) | 2.00 (0.00–10.00) | 0.008 |
| WOMAC-Total | 7.00 (3.00–17.00) | 3.00 (0.00–16.00) | 0.003 |
| Muscles | Pre-Exercise (cm) | Post-Exercise (cm) | p-Value |
|---|---|---|---|
| Right RF | 1.81 (1.57–1.97) | 1.87 (1.69–2.09) | 0.061 |
| Left RF | 1.82 (1.42–2.29) | 1.92 (1.54–2.15) | 0.041 |
| Right VM | 2.95 (2.65–3.62) | 3.19 (2.72–3.94) | 0.015 |
| Left VM | 2.86 (2.53–3.89) | 3.19 (2.69–4.15) | 0.011 |
| Right VL | 2.22 (1.86–2.60) | 2.51 (2.30–2.84) | 0.002 |
| Left VL | 2.20 (1.70–2.50) | 2.50 (2.24–2.81) | 0.014 |
| Right BF | 2.88 (2.53–3.33) | 2.98 (2.63–3.53) | 0.088 |
| Left BF | 2.68 (2.49–2.94) | 3.28 (2.81–3.41) | 0.001 |
| Pre-Exercise (Nm) | Post-Exercise (Nm) | p-Value | ||
|---|---|---|---|---|
| 60°/s | Right KE | 75.00 (64.00–113.00) | 77.00 (62.00–96.00) | 0.887 |
| Left KE | 81.00 (66.00–123.00) | 79.00 (71.00–107.00) | 0.319 | |
| Right KF | 37.00 (24.00–58.00) | 39.00 (34.00–56.00) | 0.073 | |
| Left KF | 39.00 (27.00–66.00) | 45.00 (31.00–58.00) | 0.379 | |
| 180°/s | Right KE | 48.00 (41.00–73.00) | 52.00 (39.00–72.00) | 0.197 |
| Left KE | 50.00 (41.00–77.00) | 50.00 (45.00–85.00) | 0.018 | |
| Right KF | 24.00 (18.00–39.00) | 27.00 (20.00–45.00) | 0.148 | |
| Left KF | 24.00 (18.00–41.00) | 27.00 (20.00–37.00) | 0.674 | |
| Pre-Exercise | Post-Exercise | p-Value | |
|---|---|---|---|
| CRP (mg/L) | 0.66 (0.17–0.86) | 0.47 (0.32–1.07) | 0.530 |
| ESR (mm/h) | 16.00 (5.00–21.00) | 16.00 (4.00–21.00) | 0.752 |
| D-dimer (μg/mL) | 0.27 (0.27–0.32) | 0.27 (0.27–0.34) | 0.088 |
| CPK (IU/L) | 87.00 (65.00–160.00) | 89.00 (76.00–161.00) | 0.139 |
| LDH (IU/L) | 360.00 (311.00–402.00) | 363.00 (284.00–451.00) | 0.955 |
| Myoglobin (ng/mL) | 27.00 (21.00–30.40) | 27.60 (21.00–31.20) | 0.878 |
| Lactic acid (mmol/L) | 1.50 (1.30–1.90) | 2.20 (1.30–2.70) | 0.047 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.-H.; Kang, S.-H.; Kim, N.; Choi, J.; Kang, S. Short-Term Impact of Low-Intensity Exercise with Blood Flow Restriction on Mild Knee Osteoarthritis in Older Adults: A Pilot Study. Healthcare 2024, 12, 308. https://doi.org/10.3390/healthcare12030308
Kim K-H, Kang S-H, Kim N, Choi J, Kang S. Short-Term Impact of Low-Intensity Exercise with Blood Flow Restriction on Mild Knee Osteoarthritis in Older Adults: A Pilot Study. Healthcare. 2024; 12(3):308. https://doi.org/10.3390/healthcare12030308
Chicago/Turabian StyleKim, Kang-Ho, Seung-Ho Kang, Nackhwan Kim, Jaehyeong Choi, and Seok Kang. 2024. "Short-Term Impact of Low-Intensity Exercise with Blood Flow Restriction on Mild Knee Osteoarthritis in Older Adults: A Pilot Study" Healthcare 12, no. 3: 308. https://doi.org/10.3390/healthcare12030308
APA StyleKim, K.-H., Kang, S.-H., Kim, N., Choi, J., & Kang, S. (2024). Short-Term Impact of Low-Intensity Exercise with Blood Flow Restriction on Mild Knee Osteoarthritis in Older Adults: A Pilot Study. Healthcare, 12(3), 308. https://doi.org/10.3390/healthcare12030308





