Abstract
Early diagnosis of a Human Immunodeficiency Virus (HIV)-infected person represents a cornerstone of HIV prevention, treatment, and care. Numerous publications have developed recommendations where HIV serology is indicated to reduce missed diagnostic opportunities (MDOs). This retrospective study analyses new HIV infection diagnoses and the relationship between late diagnosis (LD)/advanced HIV disease (AHD), baseline characteristics, and MDOs. Sociodemographic data and data related to contact with the health system in the 5 years before diagnosis were collected. Most of the 273 diagnoses were made in primary care (48.5%). Approximately 50.5% and 34.4% had LD and AHD criteria, respectively. Female sex was associated with a higher incidence of LD. Persons infected through the heterosexual route and those at an older age had a higher risk for LD and AHD. People with previous HIV serology presented a lower percentage of LD and AHD. In total, 10% of the health contact instances were classified as MDOs, mostly occurring in primary care. A significant increase in the median of MDOs was observed in patients with LD/AHD. Female sex and hepatitis C virus co-infection were associated with an increase in the number of MDOs. The high percentage of LD and AHD and the significant number of MDOs show that the current screening system should be improved.
1. Introduction
Early diagnosis and treatment of people living with Human Immunodeficiency Virus (HIV) is essential to prevent disease progression. Otherwise, it may increase morbidity and mortality, possible virus transmission, and delay immunity recovery [1,2]. In addition, late diagnosis (LD) (CD4 lymphocyte count at diagnosis < 350 cells/μL) [3] is associated with increased costs, especially those related to hospital care [4]. However, early diagnosis of HIV is difficult, as people may not have symptoms for years.
In 2019, 2698 new HIV cases were reported in Spain. This was the first year in the last 10 years in which the number of new HIV cases decreased from the usual 3000+ reported [5]. The percentage of advanced HIV disease (AHD) cases (CD4 lymphocyte count at diagnosis < 200 cells/μL) was 25.1% in that year, and LD cases were 45.9%, with fewer cases in men who have sex with men (MSM) than in heterosexuals and in intravenous drug users (IVDUs) [3,6]. The data available for 2020 and 2021 regarding new HIV cases in Spain (1925 and 2786 cases, respectively) are likely to be influenced by the evolution of the SARS-CoV-2 pandemic.
It has been estimated that 14% of HIV-infected people in Spain are undiagnosed [7], and between 54% and 65% of new infections are caused by people unaware of their HIV status [8].
Promoting early diagnosis is one of the priority strategies of HIV prevention and care programmes in developed countries [9]. Different national and international organizations stress that more efforts are needed to achieve this. In addition, simple and inexpensive HIV diagnostic tests are now available.
In 2014, the Spanish Ministry of Health published the “Guide of recommendations for the early diagnosis of HIV in healthcare settings”, which considered routine testing as a viable option [10]. The Spanish guideline aligns with the World Health Organization and with the European guidelines [11,12,13]. These guidelines emphasize the importance of reducing missed diagnostic opportunities (MDOs) and make recommendations about in which situations the HIV serology would be indicated. These cases include conditions or diseases with an HIV prevalence greater than 0.1%, which are cost-effective and have the potential to enable earlier HIV diagnosis [14].
As reported in numerous articles, HIV-positive people who have not yet been diagnosed consult for many episodes at different levels of the healthcare system before diagnosis, generating MDOs [15,16].
This study aims to analyse new HIV diagnoses in our health area over the last 7 years and the relationship between LD/AHD, baseline sociodemographic characteristics, and the MDOs detected in these patients.
2. Materials and Methods
This was a retrospective, observational, and descriptive study of patients ≥ 18 years of age diagnosed and treated for HIV infection from a health catchment area of 550,000 inhabitants between 1 January 2013 and 31 December 2019.
Patients who transferred to our health area but had already been diagnosed and those who did not sign the corresponding informed consent form were excluded from the study.
Sociodemographic data and data related to HIV diagnosis (date of diagnosis, the level of care requesting the diagnostic test, previous serological determinations, route of infection, and immunovirological status of the patient), as well as the presence of other sexually transmitted infections (STIs) and exitus at the end of the study (31 December 2021), were collected.
The patient data were extracted from the Electronic Health Record, where there is information about contact with the health system at the three levels of care (primary care, specialised care, and emergency department) and analytical and other tests carried out in our health area and other health areas of the Autonomous Community of Galicia, in the northwest of Spain.
All contact with the health system at different levels of care in 5 years before the diagnosis of HIV infection (health contact (HC)) was analysed. A single HC instance was considered if the patient consulted several times for the same reason in <2 weeks in primary care. The recorded HC instances were categorised according to whether they corresponded to MDOs. They were considered HIV MDOs if the reason for the consultation met the criteria for “offer of HIV testing” in the guidelines for recommendations for the early diagnosis of HIV in healthcare settings [10] and no HIV diagnostic test was requested.
The statistical analyses were carried out with SPSS 24.0, except for the trend analyses, performed with the Joinpoint Regression Program, version 5.0.2. Numerical variables were presented as median and interquartile range for discrete variables and mean and standard deviation for continuous variables, and categorical variables were presented as frequencies using percentages and confidence intervals.
HC instances and MDOs were classified, and MDO prevalence by the level of care and other sociodemographic and immunovirological variables was analysed using the chi-square or Fisher’s exact test and the non-parametric Mann–Whitney U test, Kruskal–Wallis, or Spearman’s correlation coefficient for categorical and continuous variables, respectively. Repeated measurements were compared using the Wilcoxon test. This study investigated the impact of various factors on LD and AHD, including sociodemographic variables, MDOs, and the number of HC instances. The relationship between HIV test presence before diagnosis, the number of HC instances, and the immunological status at diagnosis (LD, AHD) was assessed using multivariate logistic regression.
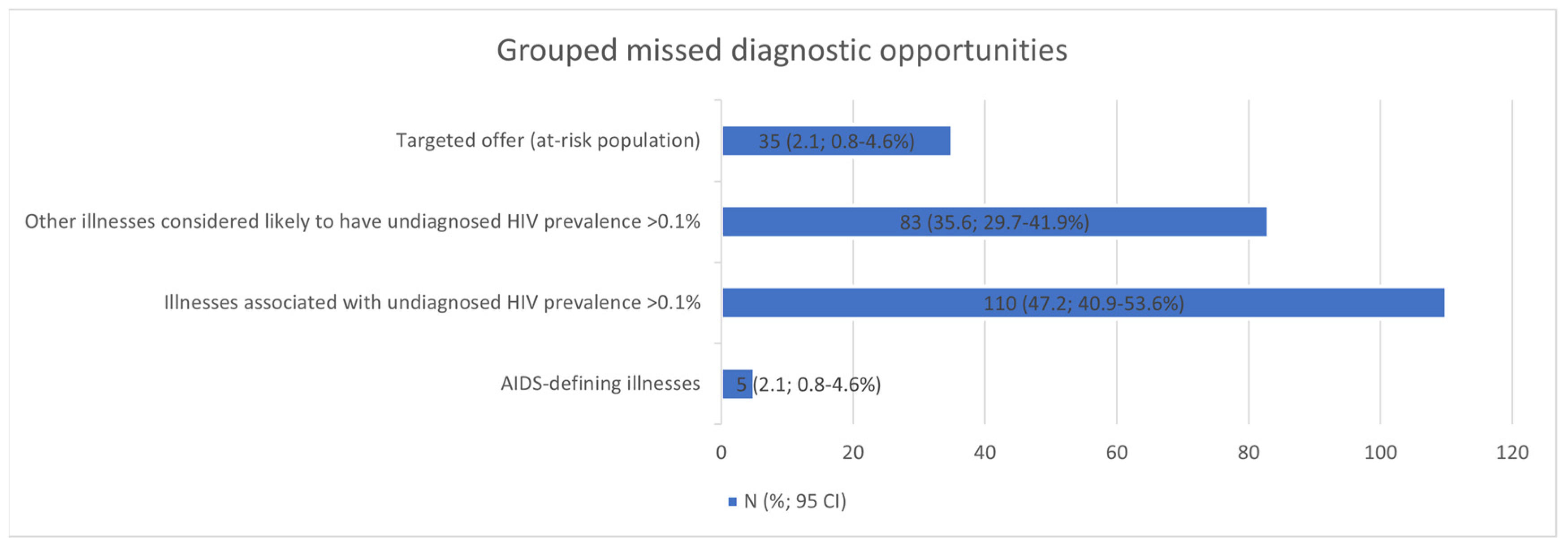
All detected MDOs were grouped into four clusters by diagnostic test indicator status (AIDS-defining illnesses; illnesses associated with undiagnosed HIV prevalence > 0.1%; other illnesses considered likely to have undiagnosed HIV prevalence > 0.1%; and targeted offer (at-risk population)) [10,13], and their prevalences were analysed.
Finally, the effect of the study variables (odd ratio (OR) estimation) was determined using logistic regression models on the risk of LD or AHD (multivariate regression).
The p-values ≤ 0.05 were considered statistically significant.
This study was approved by the corresponding Research Ethics Committee (CEI A Coruña-Ferrol, 2014/564, date of approval 24 November 2014). This study was conducted in accordance with the Helsinki Declaration of Good Clinical Practice.
3. Results
A total of 273 patients diagnosed and treated for HIV infection were included. The mean age at diagnosis was 39.6 ± 11.0 years, with 61.2% of the patients in the 31–50 age group. Of the newly diagnosed patients, 86.1% were men and most were Caucasian (76.9%), although there was a high percentage of Latinos (people from or with origins that trace back to Latin America, including Central America, South America, and the Caribbean) (19.8%). The main mode of HIV transmission was sexual (86.1%), mainly MSM (57.9%). Viral hepatitis B or C co-infection was present in 7.7% of patients (Table 1).

Table 1.
Sociodemographic and immunovirological variables and level of diagnostic care.
At diagnosis, the median CD4 cell count was 345.0 (123–510) cells/μL, and the mean viral load was 5.09 ± 0.89 log copies/mL. Clinical classification is detailed in Table 1. AIDS criteria were present in 35.5% of the patients at diagnosis. Of the study population, 50.5% and 34.4% had LD and AHD criteria, respectively. No differences were observed when analysing the evolution of LD and AHD cases over the study period, and the trend remained stable (Table 2).

Table 2.
Late diagnosis/advanced HIV disease time evolution.
At the end of this study, five patients died, and only one death was related to HIV infection (pneumonia).
Most new diagnoses were made in primary care (48.5%) and in specialised care (38.1%). Of the patients diagnosed in specialised care, 17.6% were diagnosed during hospitalisation.
Regarding the influence of sociodemographic variables on LD and AHD (Table 3), women presented a higher number of LD and AHD compared with men, although it was only significant in the case of LD (p = 0.043). Furthermore, the mean age of patients with LD and AHD was higher than patients without LD and AHD (p < 0.001). Regarding race, the percentage of patients with AHD at diagnosis was higher in the Latino group compared with the Black group (p = 0.02).

Table 3.
Late diagnosis and advanced HIV disease relationship with other variables studied.
A higher viral load at diagnosis was observed in those patients with LD and AHD (p < 0.001), and hepatitis C virus (HCV) co-infection was significantly related to AHD at diagnosis (p = 0.032).
A lower percentage of LD and AHD at diagnosis was found among MSM patients compared to the other modes of HIV transmission.
The number of patients with LD is independent of the level of care. However, a higher percentage of patients with AHD was observed among those diagnosed in an inpatient episode than those diagnosed in primary care (p = 0.004).
In total, 30.4% of patients had at least one negative HIV serology 5 years before diagnosis. The population with prior serology had a significantly lower percentage of LD, AHD, and Acquired Immunodeficiency Syndrome (AIDS) than those without (Table 4).

Table 4.
Late diagnosis, advanced HIV disease, and AIDS relationship with previous HIV serology.
In the logistic regression analysis, the variables implicated in an increased risk of LD were age (each year increase in age increases the risk of AHD by 3%), viral load, and route of transmission (heterosexual vs. MSM multiplies the risk by 2.2 and unknown route multiplies the risk by 4.1) (Table 5).

Table 5.
Late diagnosis/advanced HIV disease adjusted logistic regression model vs. other variables studied.
The variables that increased the risk of AHD were age (each year increase in age increases the risk of AHD by 3%), transmission route (heterosexual vs. MSM increases the risk of AHD by 2.3 times compared to MSM, and unknown route increases the risk by 4.7 times), and HCV co-infection (HCV co-infection increases the risk of AHD by 10 times) (Table 5).
In the study population, a total of 1,987 HC instances were analysed. Of these consultations, 63.7% were performed in primary care, 21.7% in the emergency department and, 14.2% in specialised care consultations. The number of HC instances performed by level of care, as well as the mean per patient, is detailed in Table 6.

Table 6.
Health contact/missed diagnostic opportunities relationship with other variables studied.
The relationship between the number of HC instances, LD, and AHD was analysed. Patients who met AHD criteria at diagnosis had more HC instances (p = 0.039). This association was not detected in the case of LD (p = 0.386).
Of all the HC instances analysed, 233 were considered MDOs (11.7%). A total of 57.9% of the MDOs occurred in primary care, while 23.6% and 18.5% were found in emergency department and specialised care consultations, respectively. When analysing the MDOs per patient, one MDO was detected in 83 patients, two to three MDOs in 36 patients, and only 2 patients had five and six MDOs. No MDOs were detected in the remaining patients diagnosed in the study period (152; 55.7%).
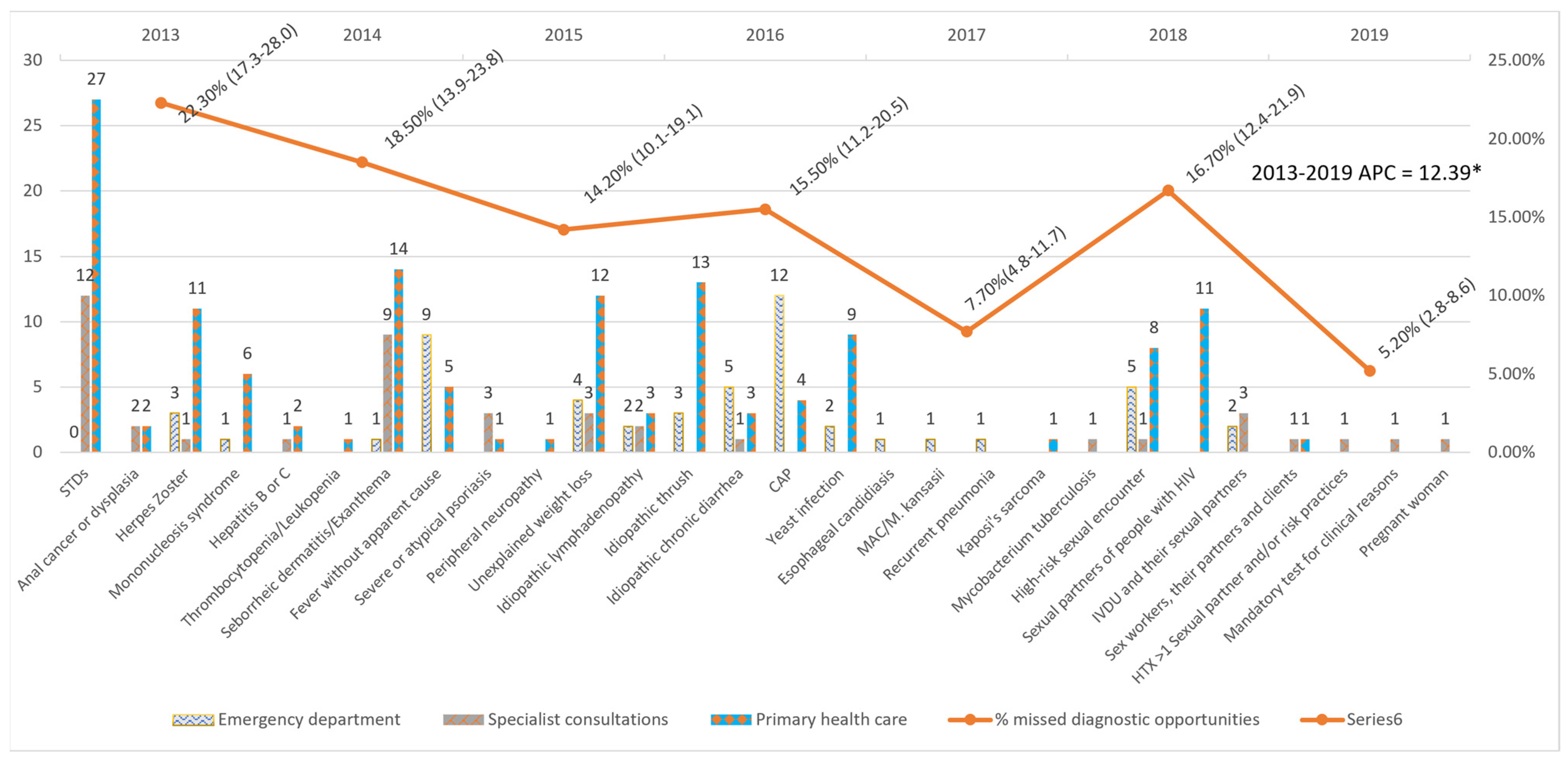
An average annual decrease of 12.39% (24.39–1.53%) was observed in the number of MDOs detected yearly, which was significant despite the increase in 2018. The trend remained constant over the entire period (Figure 1).
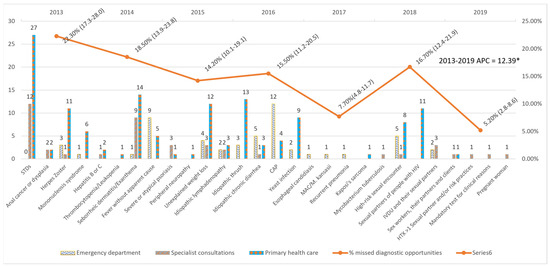
Figure 1.
Missed diagnostic opportunities by indicator condition and evolution of the percentage of Missed diagnostic opportunities per year. STDs: Sexually transmitted diseases; CAP: Community-acquired pneumonia; MAC: Mycobacterium avium complex; IVDU: Intravenous drug users; HTX: heterosexual; APC: Annual Percent Change. * APC is significantly different from zero at the alfa = 0.05 level.
The five most frequent MDOs were STIs (42), candidiasis (28), seborrhoeic dermatitis/exanthema (24), unjustified weight loss (19), and pneumonia (17). Breaking down the MDOs by the level of care, the two most frequent MDOs in the emergency department were pneumonia (12) and fever without apparent cause (9); in specialised care outpatient clinics, STIs (12) and seborrhoeic dermatitis/exanthema (9); and in primary care, STIs (27) and seborrhoeic dermatitis/exanthema (14) (Table 6). The MDOs detected were grouped into four groups by indicator condition, and their prevalence was analysed, which is detailed in Figure 2.
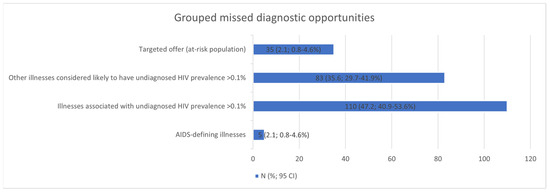
Figure 2.
Missed diagnostic opportunities grouped into four clusters. CI: confidence interval.
The only baseline characteristics associated with an increased risk of MDOs were sex (higher number of MDOs in women) (p = 0.012) and HCV co-infection (p = 0.032).
A significant increase in median MDOs was observed in patients with LD (p < 0.001) or AHD (p < 0.001) at diagnosis (Table 6).
4. Discussion
Our study showed that half of the new HIV diagnoses in our health area were LDs, and one-third were AHD cases. Women had a higher incidence of LD, and older people were more at risk of having LD or AHD. Also, heterosexual patients had a higher risk of LD or AHD, in contrast to MSM. Contrary to expectations, almost 12% of HC instances were considered MDOs and were more frequent in women and HCV-co-infected individuals. Probably most importantly, patients with LD or AHD had more MDOs, a critical point to try to improve HIV screening.
The baseline characteristics of new HIV diagnoses in our health area resembled those described in other studies [17] and the published national data [6], with a similar mean age at diagnosis (39.6 vs. 36.0), a higher prevalence of new diagnoses among the population aged 31–50 years, a predominance of new diagnoses among men (86.1% vs. 84.3%), and sexual intercourse as the main factor of transmission (86.1% vs. 82.7%), predominating in MSM (57.9% vs. 55.2%). Our findings align with previous research, indicating that the patient profile has remained consistent in recent years. As in our study, other authors, such as Mínguez-Gallego et al., observed an increase in foreign patients among the new diagnoses, with the population of Latino origin standing out (19.8% vs. 22.4%) [6]. It might be necessary to update the current guide, as there has been a shift in the profile of the diagnosed population. Conducting a serology test for this population during their first interaction with the healthcare system could be beneficial. Most new diagnoses were detected in primary care (48.5%). This contrasts with other populations, such as the USA, where most diagnoses occur in infectious disease clinics or during hospital admission [18,19,20]. However, we observed similar results in populations with similar healthcare systems [21,22], where the cornerstone of the system is in primary care. In our study, the low percentage of patients diagnosed in hospitalisation processes (17.6%) is noteworthy when compared with reports of other studies [20]. However, Mínguez-Gallego et al., in their study, detected an upward trend in diagnoses during hospitalisation [17]. On the other hand, Levy et al. highlight how AHD patients accounted for more than 50% of inpatient diagnoses [23]. It is important to note that the emergency department did not diagnose any patients with HIV. Serology tests were only conducted on those who were hospitalised or referred to specialist care consultations. This could be because serology tests are voluntary and require informed consent from the person being tested, along with a brief pre-test information session, as per our country’s regulations. Furthermore, the limited time available to care for patients in emergency departments may also contribute to the lack of HIV diagnoses.
One effective approach to improve diagnoses in emergency departments is to emphasize and disseminate guidelines for normalizing their use and reducing the number of procedures needed to request serology.
The results of the median CD4 counts obtained in our study are comparable to those reported in the epidemiological surveillance report on HIV and AIDS in Spain in 2020 (345.0 vs. 371.0) [6]. Similar data were reported in other MDO studies, such as those of Gullón et al. and Hopkins et al. (352.0 and 404.0, respectively) [24,25]. Similar to those of most national studies [24,26], CD4 counts differed according to the transmission route, being lower among MSM patients with LD and AHD. In our study, we observed an increase in LD and AHD with increasing patient age and HIV viral load at diagnosis, as well as a higher number of LD in women. According to the available data, the percentage of new diagnoses in the female sex decreases yearly in Spain [6]. However, according to national data, they are more likely to have LD, confirmed in our population. Possible explanations for this finding could be related to low risk awareness or lack of disease awareness compared to more exposed populations. Furthermore, our results showed a greater number of AHD in HCV-co-infected patients, similar to that reported by Gullón et al. [24]. The poor immunological condition of these patients may be a result of comorbidities and the progression of the disease worsened by this factor. However, it could also indicate a failure to follow the recommended guidelines, especially since most of the co-infected patients are IVDUs.
Despite the recommended early diagnostic measures, the percentage of patients with LD and AHD at the time of diagnosis remains high. Gargallo-Bernad et al. and Gullón et al. have published LD percentages similar to our population [21,24], although the national average has been lower [27], and neighbouring countries have reported higher values [28]. We observed a higher percentage of patients with AHD or AIDS at the time of diagnosis than in other national and international studies [24,27,28]. However, there are studies in other regions of Spain with similar results to ours [29]. In contrast to other North American studies [30], in our setting, no association of LDs with the place of diagnosis was seen. However, an increase in AHD was observed in patients diagnosed with HIV during hospitalisation, which is logical, as patients are usually admitted for advanced clinical symptoms of immunosuppression. This again underscores the importance of primary care diagnosis in reducing AHD incidence [31].
The presence of prior serology significantly decreased the number of patients with LD, AHD, and AIDS. These results agreed with those obtained in other studies [26,29]. Both studies were carried out in similar health systems, where free access to this type of test is guaranteed. This ease of access may be the key factor that explains the relationship between the presence of a previous serology and the lower number of LD, AHD, and AIDS cases. This highlights the importance of establishing tools for more agile and population-wide screening. An example that is easy to implement and is currently being used to achieve HCV “microelimination” is to perform serology in the entire population in a specific age range and make a consultation in primary care, regardless of the reason for the consultation [32,33]. Despite early diagnosis guidelines, improved diagnostic circuits, and new tools available (rapid tests and others), the percentage of patients with LD and AHD remained constant throughout our study period. These results are in agreement with those reported nationally [6]. According to WHO guidelines, it is important to tailor guidelines based on the specific characteristics of the target population. An update to the guidelines may be necessary based on changes in the diagnostic profile, lack of screening identified in emergency departments, and stagnation in improving CD4 counts.
Although there was a diversity of results, the high percentage of LDs and ADs showed that the current diagnostic method [10], mainly based on testing patients with pathologies most likely linked to HIV infection, is not effective enough. It relies primarily on the person having a health contact instance, and secondly, the physician must recognise the current pathology as a signal for HIV testing. Neilan et al. conclude that routine screening at age 25 is more cost-effective than the usual recommendations [34]. Given the costs of current tests, establishing age ranges among the sexually active and carrying out systematic screening can be an interesting tool to reduce the number of late diagnoses. Another tool implemented with promising results is automating diagnostic screening recommendations using algorithms in the Electronic Health Record [35]. The automation of these systems, feasible in any health system with electronic medical records, can contribute to reducing the stigma associated with these tests and normalizing them, reducing the time until diagnosis. The emergence of rapid tests and self-testing may explain why the percentage of patients with previous serology was similar to other national and international articles (30.4% vs. 29.9% vs. 27.3% vs. 27.4%) [25,26,29] and lower than reported before the advent of these tests [36]. However, standardisation of these tests remains essential. According to the CDC (Centers for Disease Control and Prevention), patients consult an average of 2.5 times in primary care before a diagnostic test is ordered [37]. Rapid tests could decrease the time to diagnosis, but there is a need to raise awareness in society for more widespread use.
As in our population, patient age at diagnosis and mode of transmission are common risk factors for LD and AHD in most studies [17,21,24,38]. Furthermore, we observed in our population that HCV co-infection increased the likelihood of being diagnosed with a CD4 count < 200 cells/μL by 10-fold. As mentioned before, this might be attributed to the poor immunological condition of these patients. The results of national studies such as the one by Mínguez-Gallego et al. [17], which are in line with our findings, show that geographical origin is not found to be a risk factor for LD, AHD, or both, which could be due to the universal access to healthcare in our country. This is in contrast to studies conducted outside Spain [39]. However, Gullón et al. and Gargallo-Bernad et al. also found an association between LD and the immigrant population in Spain [21,24]. These differences within the same healthcare system may be attributed to the regions where the study was conducted, with a larger impact in areas where there is a higher number of immigrants.
Comparing the identified HC instances in this study with those of other studies is difficult, as there are many ways to classify them (including only HC instances produced in the emergency department, in a single centre, etc.), or they directly reflect the number of MDOs without indicating the analysed HC instances [21,24,30]. Most of the HC instances in our study came from primary care, which is expected considering the structure of our health system [21]. When studying the relationship between the number of HC instances with LD and AHD, we detected that the population with a higher number of HC instances had a lower CD4 count at the time of diagnosis, in line with other published studies [16,19] and reaching statistical significance in AHD. Of all the analysed HC instances, 11.7% were classified according to established criteria as MDOs. Powell et al. state that 25% of new diagnoses with previous HC instances had at least one indicator condition for HIV serology [16]. When breaking down MDOs by healthcare level at diagnosis, we found that the majority occur in primary care, as was the case for the number of diagnoses and HC instances. Studies with similar health systems show identical results [21,40]. Overall, the number of MDOs remained stable with slight fluctuations over the years, highlighting the few improvements that have been made in the screening process. As in most published studies [21,23,24,40,41], the most frequent MDOs are STIs (18.0%), followed by candidiasis and seborrhoeic dermatitis/exanthema. When MDOs were broken down by healthcare level, STIs were the main cause of MDOs in primary care and specialised care. However, in the emergency department, the main cause was pneumonia, identified by both Powell et al. and Nanditha et al. as the leading cause of MDOs [16,42]. The high percentage of MDOs in patients consulting for STIs again highlights how current screening systems are not fully effective, as the diagnosis of an STI does not determine HIV screening. The opposite is true for diagnosing Pneumocystis jirovecii infection, where no MDOs were detected.
The main limitations of our study were the small sample size compared with other populations studied and the single-centre retrospective observational design, which could have introduced uncontrolled bias. In addition, when extracting information from computerised medical records, information that was not transcribed or not correctly coded (reason for consultation, diagnosis, and others) could have been lost. All contact instances analysed were captured from a single public centre; therefore, any other contact in other private or public centres may lead to underestimating the MDOs. The articles reviewed showed high variability in study populations and ease of access to diagnostic tests across different health systems. In addition, there was no consensus on the definition of LD and AHD, further complicating the comparison of different studies. There were also differences in the classification of indicator conditions considered MDOs, making comparing different studies difficult. Furthermore, the fact that the WHO guidelines suggest that each country must adapt its guidelines to the target population makes the comparison between studies more difficult.
Further studies comparing the current HIV serology system by indicator pathology with screening algorithms based on information available in the medical record or with population-based screening based on rapid tests would be desirable.
5. Conclusions
Despite recommendations to perform an HIV diagnostic test upon detection of one of the indicator conditions, this study shows that the number of MDOs remains considerable, resulting in a higher percentage of patients with LD and AHD among the population with detected MDOs. The number of LDs and AHD decreases among the population with previous serology. Implementing other strategies for better population screening is essential in areas such as emergency departments and especially among subgroups with a higher risk of experiencing MDOs and being diagnosed in more advanced stages of immunosuppression.
Author Contributions
Conceptualization, V.G.-A. and P.C.-S.; methodology, V.G.-A., P.C.-S. and Á.M.-d.-C.; software, J.S.-H.; validation, L.M.-F. and M.I.M.H.; formal analysis J.S.-H.; investigation, V.G.-A., S.R.-S. and A.M.-P.; resources, Á.M.-d.-C. and M.I.M.H.; writing—original draft, V.G.-A.; writing—review and editing, L.M.-F., P.C.-S., S.R.-S. and Á.M.-d.-C.; visualization, V.G.-A., S.R.-S. and A.M.-P.; project administration, P.C.-S. All authors have read and agreed to the published version of the manuscript.
Funding
The APC was funded by Fundación Profesor Novoa Santos, A Coruña, Spain (ESG15335219).
Institutional Review Board Statement
This study was approved by the corresponding Research Ethics Committee (CEI A Coruña-Ferrol, 2014/564, date of approval 24 November 2014). The study was conducted in accordance with the Helsinki Declaration of Good Clinical Practice.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lundgren, J.D.; Babiker, A.G.; Gordin, F.; Emery, S.; Grund, B.; Sharma, S.; Avihingsanon, A.; Cooper, D.A.; Fätkenheuer, G.; Llibre, J.M.; et al. Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. N. Engl. J. Med. 2015, 373, 795–807. [Google Scholar] [CrossRef]
- Rodger, A.J.; Cambiano, V.; Bruun, T.; Vernazza, P.; Collins, S.; Degen, O.; Corbelli, G.M.; Estrada, V.; Geretti, A.M.; Beloukas, A.; et al. Risk of HIV Transmission through Condomless Sex in Serodifferent Gay Couples with the HIV-Positive Partner Taking Suppressive Antiretroviral Therapy (PARTNER): Final Results of a Multicentre, Prospective, Observational Study. Lancet 2019, 393, 2428–2438. [Google Scholar] [CrossRef]
- Antinori, A.; Coenen, T.; Costagiola, D.; Dedes, N.; Ellefson, M.; Gatell, J.; Girardi, E.; Johnson, M.; Kirk, O.; Lundgren, J.; et al. Late Presentation of HIV Infection: A Consensus Definition. HIV Med. 2011, 12, 61–64. [Google Scholar] [CrossRef]
- Krentz, H.B.; Auld, M.C.; Gill, M.J. The High Cost of Medical Care for Patients Who Present Late (CD4 <200 Cells/MicroL) with HIV Infection. HIV Med. 2004, 5, 93–98. [Google Scholar] [CrossRef]
- Ministerio de Sanidad—Ciudadanos—Informes Previos Vigilancia. Available online: https://www.sanidad.gob.es/ciudadanos/enfLesiones/enfTransmisibles/sida/vigilancia/informesprevios.htm (accessed on 21 March 2023).
- Vigilancia Epidemiológica Del Vih y Sida en España 2020. Available online: https://www.sanidad.gob.es/ciudadanos/enfLesiones/enfTransmisibles/sida/vigilancia/docs/Informe_VIH_SIDA_WEB.pdf (accessed on 21 March 2023).
- Estimación Del Continuo de Atención Del Vih en España. 2016. Available online: https://www.isciii.es/QueHacemos/Servicios/VigilanciaSaludPublicaRENAVE/EnfermedadesTransmisibles/Documents/VIH/INFORMES%20ESPECIALES/ESTIMACION_DEL_CONTINUO_DE_ATENCION_DEL_VIH_EN_ESPANA_2019.pdf (accessed on 21 March 2023).
- Marks, G.; Crepaz, N.; Janssen, R.S. Estimating Sexual Transmission of HIV from Persons Aware and Unaware That They Are Infected with the Virus in the USA. AIDS 2006, 20, 1447–1450. [Google Scholar] [CrossRef]
- World Health Organization. Clinical Guidelines: HIV Diagnosis. In Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach, 2nd ed.; World Health Organization: Geneva, Switzerland, 2016; Chapter 2; pp. 17–50. Available online: https://www.who.int/publications/i/item/9789241549684 (accessed on 21 March 2023).
- Ministerio de Sanidad, Servicios Sociales e Igualdad. Guía de Recomendaciones Para el Diagnóstico Precoz del VIH en el Ámbito Sanitario. 2014. Available online: https://www.sanidad.gob.es/ciudadanos/enfLesiones/enfTransmisibles/sida/docs/GuiaRecomendacionesDiagnosticoPrecozVIH.pdf (accessed on 21 March 2023).
- World Health Organization. Regional Office for Europe. Scaling Up HIV Testing and Counseling in the WHO European Region as an Essential Component of Efforts to Achieve Universal Access to HIV Prevention, Treatment, Care and Support: Policy Framework; World Health Organization, Regional Office for Europe: Geneva, Switzerland, 2010; Available online: https://iris.who.int/handle/10665/353068 (accessed on 26 December 2023).
- European Centre for Disease Prevention and Control, HIV Testing—Increasing Uptake and Effectiveness in the European Union, Publications Office. 2010. Available online: https://data.europa.eu/doi/10.2900/35061 (accessed on 26 December 2023).
- Eurotest. HIV Indicator Conditions: Guidance for Implementing HIV Testing in Adults in Health Care Settings. Available online: https://www.eurotest.org/media/0ymdzdvu/guidancepdf.pdf (accessed on 26 December 2023).
- Sullivan, A.K.; Raben, D.; Reekie, J.; Rayment, M.; Mocroft, A.; Esser, S.; Leon, A.; Begovac, J.; Brinkman, K.; Zangerle, R.; et al. Feasibility and Effectiveness of Indicator Condition-Guided Testing for HIV: Results from HIDES I (HIV Indicator Diseases across Europe Study). PLoS ONE 2013, 8, e52845. [Google Scholar] [CrossRef]
- Champenois, K.; Cousien, A.; Cuzin, L.; Le Vu, S.; Deuffic-Burban, S.; Lanoy, E.; Lacombe, K.; Patey, O.; Béchu, P.; Calvez, M.; et al. Missed Opportunities for HIV Testing in Newly-HIV-Diagnosed Patients, a Cross Sectional Study. BMC Infect. Dis. 2013, 13, 200. [Google Scholar] [CrossRef] [PubMed]
- Powell, M.; Krentz, H.B.; Eagles, M.E.; Gill, M.J. Missed Opportunities within Healthcare for an Earlier Diagnosis of HIV. Int. J. STD AIDS. 2020, 31, 1169–1177. [Google Scholar] [CrossRef]
- Mínguez-Gallego, C.; Vera-Remartinez, E.J.; Albert-Coll, M.; Roldán-Puchalt, M.C.; Aguilar-Climent, M.; Rovira-Ferrando, R.E.; Andrés-Soler, J.; Roig-Espert, B.; Penadés-Vidal, M.; Usó-Blasco, J. Cambios en las características clínico-epidemiológicas de los nuevos casos de infección por el VIH-1 en Castellón (España) y su repercusión en la presentación tardía (1987–2011). Enfermedades Infecc. Microbiol. Clínica 2015, 33, 173–180. [Google Scholar] [CrossRef]
- Rüütel, K.; Lemsalu, L.; Lätt, S.; Epštein, J.; on behalf of OptTEST by HiE. Missed Opportunities for HIV Testing in People Diagnosed with HIV, Estonia, 2014 to 2015. Eurosurveillance 2019, 24, 1800382. [Google Scholar] [CrossRef] [PubMed]
- DeRose, J.; Zucker, J.; Cennimo, D.; Swaminathan, S. Missed Testing Opportunities for HIV Screening and Early Diagnosis in an Urban Tertiary Care Center. AIDS Res. Treat. 2017, 2017, 5708620. [Google Scholar] [CrossRef]
- Downing, A.; Garcia-Diaz, J.B. Missed Opportunities for HIV Diagnosis. J. Int. Assoc. Provid. AIDS Care JIAPAC 2017, 16, 14–17. [Google Scholar] [CrossRef]
- Gargallo-Bernad, C.; Sangrós-González, F.J.; Arazo-Garcés, P.; Martínez-Álvarez, R.; Malo-Aznar, C.; Gargallo-Bernad, A.; Ballester-Luna, A.; Cabrero-Pascual, L.E.; Gil-Orna, P.; Abadía-Gallego, V.J.; et al. Oportunidades perdidas en el diagnóstico de la infección por el virus de inmunodeficiencia humana en la Comunidad de Aragón. Importancia del diagnóstico tardío. Enfermedades Infecc. Microbiol. Clínica 2019, 37, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Lhopitallier, L.; Moulin, E.; Hugli, O.; Cavassini, M.; Darling, K.E.A. Missed Opportunities for HIV Testing among Patients Newly Presenting for HIV Care at a Swiss University Hospital: A Retrospective Analysis. BMJ Open 2018, 8, e019806. [Google Scholar] [CrossRef]
- Levy, I.; Maor, Y.; Mahroum, N.; Olmer, L.; Wieder, A.; Litchevski, V.; Mor, O.; Rahav, G. Missed Opportunities for Earlier Diagnosis of HIV in Patients Who Presented with Advanced HIV Disease: A Retrospective Cohort Study. BMJ Open 2016, 6, e012721. [Google Scholar] [CrossRef] [PubMed]
- Gullón, A.; Verdejo, J.; de Miguel, R.; Gómez, A.; Sanz, J. Factors Associated with Late Diagnosis of HIV Infection and Missed Opportunities for Earlier Testing. AIDS Care 2016, 28, 1296–1300. [Google Scholar] [CrossRef]
- Hopkins, C.; Reid, M.; Gilmour, J.; Werder, S.; Briggs, S. Missed Opportunities for Earlier Diagnosis of Human Immunodefi-ciency Virus Infection among Adults Presenting to Auckland District Health Board Hospital Services. Intern. Med. J. 2019, 49, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Sanz, J.; Rodríguez Albarrán, J.; Torralba, M. Late Diagnosis of HIV Infection: Missed Opportunities. Med. Clínica Engl. Ed. 2019, 152, 466–467. [Google Scholar] [CrossRef]
- Unidad de Vigilancia de VIH, ITS y Hepatitis. Vigilancia Epidemiológica del VIH y Sida en España 2019: Sistema de Información Sobre Nuevos Diagnósticos de VIH y Registro Nacional de Casos de Sida; Plan Nacional Sobre el Sida—D.G. de Salud Pública/Centro Nacional de Epidemiología—ISCIII: Madrid, Spain, 2020; Available online: https://www.sanidad.gob.es/ciudadanos/enfLesiones/enfTransmisibles/sida/vigilancia/docs/Informe_VIH_SIDA_20201130.pdf (accessed on 21 March 2023).
- Van den Bogaart, L.; Ranzani, A.; Oreni, L.; Giacomelli, A.; Corbellino, M.; Rusconi, S.; Galli, M.; Antinori, S.; Ridolfo, A.L. Overlooked Cases of HIV Infection: An Italian Tale of Missed Diagnostic Opportunities. Eur. J. Intern. Med. 2020, 73, 30–35. [Google Scholar] [CrossRef]
- Rivero Marcotegui, M.; Layana Echezuri, E.; Repáraz Padrós, J.; Irigoyen Olaiz, C.; Arraiza Cruchaga, M.; Úriz Ayestarán, J. Late diagnosis of HIV infection: Missed diagnostic opportunities. An. Sist. Sanit. Navar. 2014, 37, 329–338. [Google Scholar] [CrossRef]
- Lyons, M.S.; Lindsell, C.J.; Wayne, D.B.; Ruffner, A.H.; Hart, K.W.; Fichtenbaum, C.J.; Trott, A.T.; Sullivan, P.S. Comparison of Missed Opportunities for Earlier HIV Diagnosis in 3 Geographically Proximate Emergency Departments. Ann. Emerg. Med. 2011, 58, S17–S22.e1. [Google Scholar] [CrossRef]
- semFYC—Medicina Familiar y Comunitaria. Medicina Resolutiva. semFYC. Available online: https://www.semfyc.es/grupos/la-semfyc-alerta-de-que-cerca-del-50-de-nuevos-diagnosticos-de-vih-son-tardios-por-lo-que-es-necesario-promover-el-diagnostico-precoz-en-ap/ (accessed on 21 March 2023).
- Servicio Galego de Saude (Sergas)—Salud Pública—Hepatitis C. Estrategia Para la Eliminación de la Hepatitis C Como Problema de Salud Pública en Galicia. Available online: https://www.sergas.es/Saude-publica/Documents/6927/Estrategia_eliminacion_hepatitis_C_en%20Galicia_cast.pdf (accessed on 21 March 2023).
- Ministerio de Sanidad—Áreas—VIH y SIDA—Hepatitis Víricas. Guía de Cribado de la Infección por el VHC. Available online: https://www.sanidad.gob.es/ciudadanos/enfLesiones/enfTransmisibles/sida/docs/GUIA_DE_CRIBADO_DE_LA_INFECCION_POR_EL_VHC_2020.pdf (accessed on 21 March 2023).
- Neilan, A.M.; Dunville, R.; Ocfemia, M.C.B.; Salomon, J.A.; Francke, J.A.; Bulteel, A.J.B.; Wang, L.Y.; Hsu, K.K.; DiNenno, E.A.; Walensky, R.P.; et al. The Optimal Age for Screening Adolescents and Young Adults Without Identified Risk Factors for HIV. J. Adolesc. Health 2018, 62, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Tapp, H.; Ludden, T.; Shade, L.; Thomas, J.; Mohanan, S.; Leonard, M. Electronic Medical Record Alert Activation Increase Hepatitis C and HIV Screening Rates in Primary Care Practices within a Large Healthcare System. Prev. Med. Rep. 2020, 17, 101036. [Google Scholar] [CrossRef]
- Deblonde, J.; Hamers, F.F.; Callens, S.; Lucas, R.; Barros, H.; Rüütel, K.; Hemminki, E.; Temmerman, M. HIV Testing Practices as Reported by HIV-Infected Patients in Four European Countries. AIDS Care 2014, 26, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC) Missed Opportunities for Earlier Diagnosis of HIV Infection—South Caro-lina, 1997–2005. MMWR Morb. Mortal. Wkly. Rep. 2006, 55, 1269–1272. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5547a2.htm (accessed on 29 March 2023).
- Sobrino-Vegas, P.; García-San Miguel, L.; Caro-Murillo, A.M.; Miró, J.M.; Viciana, P.; Tural, C.; Saumoy, M.; Santos, I.; Sola, J.; del Amo, J.; et al. Delayed Diagnosis of HIV Infection in a Multicenter Cohort: Prevalence, Risk Factors, Response to HAART and Impact on Mortality. Curr. HIV Res. 2009, 7, 224–230. [Google Scholar] [CrossRef]
- Zoufaly, A.; An der Heiden, M.; Marcus, U.; Hoffmann, C.; Stellbrink, H.J.; Voss, L.; van Lunzen, J.; Hamouda, O.; The ClinSurv Study Group. Late Presentation for HIV Diagnosis and Care in Germany. HIV Med. 2012, 13, 172–181. [Google Scholar] [CrossRef]
- Espinel, M.; Belza, M.J.; Cabeza-de-Vaca, C.; Arranz, B.; Guerras, J.M.; Garcia-Soltero, J.; Hoyos, J. Indicator Condition Based HIV Testing: Missed Opportunities for Earlier Diagnosis in Men Who Have Sex with Men. Enfermedades Infecc. Microbiol. Clin. Engl. Ed. 2018, 36, 465–471. [Google Scholar] [CrossRef]
- Muelas Fernandez, M.; Rojas Lievano, J.F.; Perez Vidal, R.; Flor Perez, A.; Tapiz Reula, A.; Mallolas Masferrer, J. Prevalencia de diagnóstico tardío en infección por VIH. Med. Clínica 2020, 155, 388–391. [Google Scholar] [CrossRef]
- Nanditha, N.G.A.; St-Jean, M.; Tafessu, H.; Guillemi, S.A.; Hull, M.W.; Lu, M.; Henry, B.; Barrios, R.; Montaner, J.S.G.; Lima, V.D. Missed Opportunities for Earlier Diagnosis of HIV in British Columbia, Canada: A Retrospective Cohort Study. PLoS ONE 2019, 14, e0214012. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).