Predictive Validity of the Johns Hopkins Fall Risk Assessment Tool for Older Patients in Stroke Rehabilitation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. JHFRAT
2.3. Monitoring of Falls
2.4. Measurement of HGS
2.5. Data Collection
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- W.H.O. Falls. Available online: https://www.who.int/news-room/fact-sheets/detail/falls (accessed on 20 December 2023).
- Hauer, K.; Lamb, S.E.; Jorstad, E.C.; Todd, C.; Becker, C.; PROFANE-Group. Systematic review of definitions and methods of measuring falls in randomised controlled fall prevention trials. Age Ageing 2006, 35, 5–10. [Google Scholar] [CrossRef]
- Nyberg, L.; Gustafson, Y. Patient falls in stroke rehabilitation: A challenge to rehabilitation strategies. Stroke 1995, 26, 838–842. [Google Scholar] [CrossRef] [PubMed]
- Vaishya, R.; Vaish, A. Falls in older adults are serious. Indian J. Orthop. 2020, 54, 69–74. [Google Scholar] [CrossRef]
- Tinetti, M.E.; Speechley, M.; Ginter, S.F. Risk factors for falls among elderly persons living in the community. N. Engl. J. Med. 1988, 319, 1701–1707. [Google Scholar] [CrossRef]
- Graafmans, W.C.; Ooms, M.E.; Hofstee, H.M.; Bezemer, P.D.; Bouter, L.M.; Lips, P. Falls in the elderly: A prospective study of risk factors and risk profiles. Am. J. Epidemiol. 1996, 143, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Jalayondeja, C.; Sullivan, P.E.; Pichaiyongwongdee, S. Six-month prospective study of fall risk factors identification in patients post-stroke. Geriatr. Gerontol. Int. 2014, 14, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Hou, Y.; Li, H.; Lin, J. Influencing factors of weak grip strength and fall: A study based on the China Health and Retirement Longitudinal Study (CHARLS). BMC Public Health 2022, 22, 2337. [Google Scholar] [CrossRef]
- Neri, S.G.R.; Lima, R.M.; Ribeiro, H.S.; Vainshelboim, B. Poor handgrip strength determined clinically is associated with falls in older women. J. Frailty Sarcopenia Falls 2021, 6, 43–49. [Google Scholar] [CrossRef]
- Moreland, J.D.; Richardson, J.A.; Goldsmith, C.H.; Clase, C.M. Muscle weakness and falls in older adults: A systematic review and meta-analysis. J. Am. Geriatr. Soc. 2004, 52, 1121–1129. [Google Scholar] [CrossRef]
- Bobowik, P.; Wiszomirska, I. Diagnostic dependence of muscle strength measurements and the risk of falls in the elderly. Int. J. Rehabil. Res. 2020, 43, 330–336. [Google Scholar] [CrossRef]
- Maulden, S.A.; Gassaway, J.; Horn, S.D.; Smout, R.J.; DeJong, G. Timing of initiation of rehabilitation after stroke. Arch. Phys. Med. Rehabil. 2005, 86 (Suppl. 2), S34–S40. [Google Scholar] [CrossRef] [PubMed]
- Cumbler, E.U.; Simpson, J.R.; Rosenthal, L.D.; Likosky, D.J. Inpatient falls: Defining the problem and identifying possible solutions. Part I: An evidence-based review. Neurohospitalist 2013, 3, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Joint Commission International, International Patient Safety Goals [Online]. Joint Commission International. 2017. Available online: https://www.jointcommissioninternational.org/improve/international-patient-safety-goals/ (accessed on 21 December 2023).
- Chen, Y.; Lv, L.; Wu, C.; Wen, H.; Cai, H.; Xiao, Y.; Zhu, H. Assessment of the predictive ability of the Johns Hopkins Fall Risk Assessment Tool (Chinese Version) in inpatient settings. J. Adv. Nurs. 2022, 78, 4054–4061. [Google Scholar] [CrossRef] [PubMed]
- Poe, S.S.; Dawson, P.B.; Cvach, M.; Burnett, M.; Kumble, S.; Lewis, M.; Thompson, C.B.; Hill, E.E. The johns Hopkins fall risk assessment tool: A Study of Reliability and Validity. J. Nurs. Care Qual. 2018, 33, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Kim, J.A.; Choi, Y.K.; Kim, Y.J.; Park, M.H.; Kim, H.Y.; Song, M.S. A comparative study on the validity of fall risk assessment scales in Korean hospitals. Asian Nurs. Res. (Korean Soc. Nurs. Sci.) 2011, 5, 28–37. [Google Scholar] [CrossRef]
- Cox, R.; Buckholtz, B.; Bradas, C.; Bowden, V.; Kerber, K.; McNett, M.M. Risk factors for falls among hospitalized acute post–ischemic stroke patients. J. Neurosci. Nurs. 2017, 49, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Sonoda, S.; Misawa, K.; Saitoh, E.; Shimizu, Y.; Kotake, T. Incidence and consequence of falls in inpatient rehabilitation of stroke patients. Exp. Aging Res. 2005, 31, 457–469. [Google Scholar] [CrossRef] [PubMed]
- Teasell, R.; McRae, M.; Foley, N.; Bhardwaj, A. The incidence and consequences of falls in stroke patients during inpatient rehabilitation: Factors associated with high risk. Arch. Phys. Med. Rehabil. 2002, 83, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Persson, C.U.; Kjellberg, S.; Lernfelt, B.; Westerlind, E.; Cruce, M.; Hansson, P.O. Risk of falling in a stroke unit after acute stroke: The Fall Study of Gothenburg (FallsGOT). Clin. Rehabil. 2018, 32, 398–409. [Google Scholar] [CrossRef]
- Czernuszenko, A.; Członkowska, A. Risk factors for falls in stroke patients during inpatient rehabilitation. Clin. Rehabil. 2009, 23, 176–188. [Google Scholar] [CrossRef]
- Dudzińska-Griszek, J.; Szuster, K.; Szewieczek, J. Grip strength as a frailty diagnostic component in geriatric inpatients. Clin. Interv. Aging 2017, 12, 1151–1157. [Google Scholar] [CrossRef]
- Hur, E.Y.; Jin, Y.; Jin, T.; Lee, S.M. Longitudinal evaluation of Johns Hopkins fall risk assessment tool and nurses’ experience. J. Nurs. Care Qual. 2017, 32, 242–251. [Google Scholar] [CrossRef]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e2. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, L.; Gustafson, Y. Fall prediction index for patients in stroke rehabilitation. Stroke 1997, 28, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Yeung, S.S.Y.; Reijnierse, E.M.; Pham, V.K.; Trappenburg, M.C.; Lim, W.K.; Meskers, C.G.M.; Maier, A.B. Sarcopenia and its association with falls and fractures in older adults: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2019, 10, 485–500. [Google Scholar] [CrossRef] [PubMed]
- Hajian-Tilaki, K. Receiver operating characteristic (ROC) curve analysis for medical diagnostic test evaluation. Caspian J. Intern. Med. 2013, 4, 627–635. [Google Scholar]
- Centers for Disease Control and Prevention. Preventing Falls: A Guide to Implementing Effective Community-Based Fall Prevention Programs. Available online: https://www.cdc.gov/falls/programs/community_prevention.html (accessed on 21 December 2023).
- Beaudart, C.; Rolland, Y.; Cruz-Jentoft, A.J.; Bauer, J.M.; Sieber, C.; Cooper, C.; Al-Daghri, N.; Araujo de Carvalho, I.; Bautmans, I.; Bernabei, R.; et al. Assessment of muscle function and physical performance in daily clinical practice: A position paper endorsed by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO). Calcif. Tissue. Int. 2019, 105, 1–14. [Google Scholar] [CrossRef]
- Alonso, A.C.; Ribeiro, S.M.; Luna, N.M.S.; Peterson, M.D.; Bocalini, D.S.; Serra, M.M.; Brech, G.C.; Greve, J.M.D.A.; Garcez-Leme, L.E. Association between handgrip strength, balance, and knee flexion/extension strength in older adults. PLoS ONE 2018, 13, e0198185. [Google Scholar] [CrossRef]
- Vassallo, M.; Sharma, J.C.; Briggs, R.S.; Allen, S.C. Characteristics of early fallers on elderly patient rehabilitation wards. Age Ageing 2003, 32, 338–342. [Google Scholar] [CrossRef]
- Campbell, G.B.; Matthews, J.T. An integrative review of factors associated with falls during post-stroke rehabilitation. J. Nurs. Scholarsh. 2010, 42, 395–404. [Google Scholar] [CrossRef]
- Damoiseaux-Volman, B.A.; van Schoor, N.M.; Medlock, S.; Romijn, J.A.; van der Velde, N.; Abu-Hanna, A. External validation of the Johns Hopkins Fall Risk Assessment Tool in older Dutch hospitalized patients. Eur. Geriatr. Med. 2023, 14, 69–77. [Google Scholar] [CrossRef] [PubMed]
- John, G.; Bardini, C.; Combescure, C.; Dällenbach, P. Urinary incontinence as a predictor of death: A systematic review and meta-analysis. PLoS ONE 2016, 11, e0158992. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.; Chung, H.S.; Kim, Y.J.; Kim, S.J.; Kwon, O.; Lee, Y.G.; Yu, J.M.; Cho, S.T. The impact of urinary incontinence on falls: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0251711. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Medication Safety in Polypharmacy [Technical Report]. Available online: https://iris.who.int/handle/10665/325454 (accessed on 21 December 2023).
- Woolcott, J.C.; Richardson, K.J.; Wiens, M.O.; Patel, B.; Marin, J.; Khan, K.M.; Marra, C.A. Meta-analysis of the impact of 9 medication classes on falls in elderly persons. Arch. Intern. Med. 2009, 169, 1952–1960. [Google Scholar] [CrossRef] [PubMed]

| Variables | All Patients (N = 175) | Patients with Low HGS (n = 135) |
|---|---|---|
| Age, years | 76.0 [70.0–82.0] | 78.0 [72.0–83.0] |
| Sex | ||
| Male | 71 (40.6%) | 52 (38.5%) |
| Female | 104 (59.4%) | 83 (61.5%) |
| BMI, kg/m2 | 23.5 [21.4–26.8] | 23.4 [20.9–26.8] |
| Stroke history | ||
| First | 128 (73.1%) | 94 (69.6%) |
| Recurrent (2nd) | 34 (19.4%) | 29 (21.5%) |
| Recurrent (3rd) | 13 (7.4%) | 12 (8.9%) |
| Time from onset of stroke to transfer, days | 12.0 [8.0–17.0] | 14.0 [8.0–18.5] |
| Lesion | ||
| Ischemic | 146 (83.4%) | 109 (80.7%) |
| Hemorrhagic | 29 (16.6%) | 26 (19.3%) |
| NIHSS score | 4.0 [2.0–8.0] | 4.0 [2.0–9.0] |
| LOS, days | 41.0 [32.0–52.0] | 42.0 [33.0–53.0] |
| HGS of non-hemiplegic hand, kg | 14.0 [8.0–22.0] | 11.0 [4.0–16.0] |
| BBS score | 8.0 [2.0–29.0] | 6.0 [1.0–22.0] |
| FMA score of hemiplegic lower extremity | 24.0 [8.5–29.0] | 22.0 [6.0–28.5] |
| MBI | 24.0 [7.0–45.5] | 13.0 [5.0–39.0] |
| MMSE score | 17.0 [10.5–23.5] | 15.0 [8.0–21.0] |
| BDI score | 13.0 [7.0–25.0] | 15.0 [7.0–26.0] |
| Aphasia | ||
| Absence | 114 (65.1%) | 82 (60.7%) |
| Presence | 61 (34.9%) | 53 (39.3%) |
| Spatial neglect | ||
| Absence | 159 (90.9%) | 122 (90.4%) |
| Presence | 16 (9.1%) | 13 (9.6%) |
| Visual field problems | ||
| Absence | 168 (96.0%) | 129 (95.6%) |
| Presence | 7 (4.0%) | 6 (4.4%) |
| Visual acuity problems | ||
| Absence | 168 (96.0%) | 132 (97.8%) |
| Presence | 7 (4.0%) | 3 (2.2%) |
| Hearing loss | ||
| Absence | 162 (92.6%) | 127 (94.1%) |
| Presence | 13 (7.4%) | 8 (5.9%) |
| Total number of medications used | 10.2 ± 3.1 | 10.4 ± 3.1 |
| Falls | ||
| No | 163 (93.1%) | 127 (94.1%) |
| Yes | 12 (6.9%) | 8 (5.9%) |
| Variable | All Patients | Patients with Low HGS | ||||||
|---|---|---|---|---|---|---|---|---|
| Non-Fallers (n = 163) | Fallers (n = 12) | Total (N = 175) | p | Non-Fallers (n = 127) | Fallers (n = 8) | Total (N = 135) | p | |
| Age, years | 76.0 [70.0–82.0] | 79.0 [72.0–81.5] | 76.0 [70.0–82.0] | 0.552 | 77.0 [72.0–83.0] | 79.5 [78.0–82.0] | 78.0 [72.0–83.0] | 0.508 |
| Sex | 0.701 | >0.999 | ||||||
| Male | 65 (39.9%) | 6 (50.0%) | 71 (40.6%) | 49 (38.6%) | 3 (37.5%) | 52 (38.5%) | ||
| Female | 98 (60.1%) | 6 (50.0%) | 104 (59.4%) | 78 (61.4%) | 5 (62.5%) | 83 (61.5%) | ||
| BMI, kg/m2 | 23.5 [21.5–26.8] | 22.8 [20.6–28.1] | 23.5 [21.4–26.8] | 0.969 | 23.5 [21.2–26.8] | 21.3 [19.6–25.8] | 23.4 [20.9–26.8] | 0.278 |
| Stroke history | 0.557 | 0.657 | ||||||
| First | 119 (73.0%) | 9 (75.0%) | 128 (73.1%) | 88 (69.3%) | 6 (75.0%) | 94 (69.6%) | ||
| Recurrent (2nd) | 31 (19.0%) | 3 (25.0%) | 34 (19.4%) | 27 (21.3%) | 2 (25.0%) | 29 (21.5%) | ||
| Recurrent (3rd) | 13 (8.0%) | 0 (0.0%) | 13 (7.4%) | 12 (9.4%) | 0 (0.0%) | 12 (8.9%) | ||
| Time from onset of stroke to transfer, days | 12.0 [8.0–17.0] | 15.0 [9.0–24.5] | 12.0 [8.0–17.0] | 0.252 | 13.0 [8.0–18.0] | 20.5 [15.0–30.0] | 14.0 [8.0–18.5] | 0.030 * |
| Lesion | 0.681 | 0.375 | ||||||
| Ischemic | 137 (84.0%) | 9 (75.0%) | 146 (83.4%) | 104 (81.9%) | 5 (62.5%) | 109 (80.7%) | ||
| Hemorrhagic | 26 (16.0%) | 3 (25.0%) | 29 (16.6%) | 23 (18.1%) | 3 (37.5%) | 26 (19.3%) | ||
| NIHSS score | 4.0 [2.0– 8.0] | 4.5 [0.5– 5.0] | 4.0 [2.0– 8.0] | 0.248 | 4.0 [2.0– 9.0] | 3.5 [0.5– 7.0] | 4.0 [2.0– 9.0] | 0.230 |
| LOS, days | 40.0 [32.0–51.5] | 45.5 [34.0–52.0] | 41.0 [32.0–52.0] | 0.425 | 42.0 [33.0–53.0] | 48.5 [34.5–57.0] | 42.0 [33.0–53.0] | 0.439 |
| HGS of non-hemiplegic hand, kg | 14.0 [6.8–20.0] | 20.0 [10.0–27.0] | 14.0 [8.0–22.0] | 0.171 | 11.0 [4.0–16.0] | 11.0 [9.0–20.0] | 11.0 [4.0–16.0] | 0.559 |
| BBS score | 7.0 [2.0–28.5] | 16.0 [7.0–47.5] | 8.0 [2.0–29.0] | 0.061 | 6.0 [1.0–22.0] | 11.0 [6.5–33.0] | 6.0 [1.0–22.0] | 0.237 |
| FMA score of hemiplegic lower extremity | 22.0 [8.0–29.0] | 26.5 [24.0–30.5] | 24.0 [8.5–29.0] | 0.090 | 21.0 [6.0–28.0] | 26.5 [24.0–31.5] | 22.0 [6.0–28.5] | 0.087 |
| MBI | 22.0 [6.5–45.5] | 37.0 [16.5–56.0] | 24.0 [7.0–45.5] | 0.212 | 13.0 [4.5–39.0] | 28.5 [10.0–56.0] | 13.0 [5.0–39.0] | 0.277 |
| MMSE score | 17.0 [10.0–23.0] | 21.0 [12.5–27.5] | 17.0 [10.5–23.5] | 0.242 | 15.0 [8.0–21.0] | 14.5 [9.0–21.5] | 15.0 [8.0–21.0] | 0.948 |
| BDI score | 13.0 [7.0–25.0] | 12.0 [6.5–24.0] | 13.0 [7.0–25.0] | 0.887 | 15.0 [7.0–26.0] | 14.0 [6.0–34.5] | 15.0 [7.0–26.0] | 0.830 |
| Aphasia | >0.999 | >0.999 | ||||||
| Absence | 106 (65.0%) | 8 (66.7%) | 114 (65.1%) | 77 (60.6%) | 5 (62.5%) | 82 (60.7%) | ||
| Presence | 57 (35.0%) | 4 (33.3%) | 61 (34.9%) | 50 (39.4%) | 3 (37.5%) | 53 (39.3%) | ||
| Spatial neglect | 0.535 | 0.738 | ||||||
| Absence | 147 (90.2%) | 12 (100.0%) | 159 (90.9%) | 114 (89.8%) | 8 (100.0%) | 122 (90.4%) | ||
| Presence | 16 (9.8%) | 0 (0.0%) | 16 (9.1%) | 13 (10.2%) | 0 (0.0%) | 13 (9.6%) | ||
| Visual field problems | >0.999 | >0.999 | ||||||
| Absence | 156 (95.7%) | 12 (100.0%) | 168 (96.0%) | 121 (95.3%) | 8 (100.0%) | 129 (95.6%) | ||
| Presence | 7 (4.3%) | 0 (0.0%) | 7 (4.0%) | 6 (4.7%) | 0 (0.0%) | 6 (4.4%) | ||
| Visual acuity problems | 0.976 | 0.426 | ||||||
| Absence | 157 (96.3%) | 11 (91.7%) | 168 (96.0%) | 125 (98.4%) | 7 (87.5%) | 132 (97.8%) | ||
| Presence | 6 (3.7%) | 1 (8.3%) | 7 (4.0%) | 2 (1.6%) | 1 (12.5%) | 3 (2.2%) | ||
| Hearing loss | >0.999 | >0.999 | ||||||
| Absence | 151 (92.6%) | 11 (91.7%) | 162 (92.6%) | 119 (93.7%) | 8 (100.0%) | 127 (94.1%) | ||
| Presence | 12 (7.4%) | 1 (8.3%) | 13 (7.4%) | 8 (6.3%) | 0 (0.0%) | 8 (5.9%) | ||
| Total number of medications used | 10.2 ± 3.0 | 11.1 ± 4.5 | 10.2 ± 3.1 | 0.500 | 10.3 ± 3.0 | 11.6 ± 4.9 | 10.4 ± 3.1 | 0.474 |
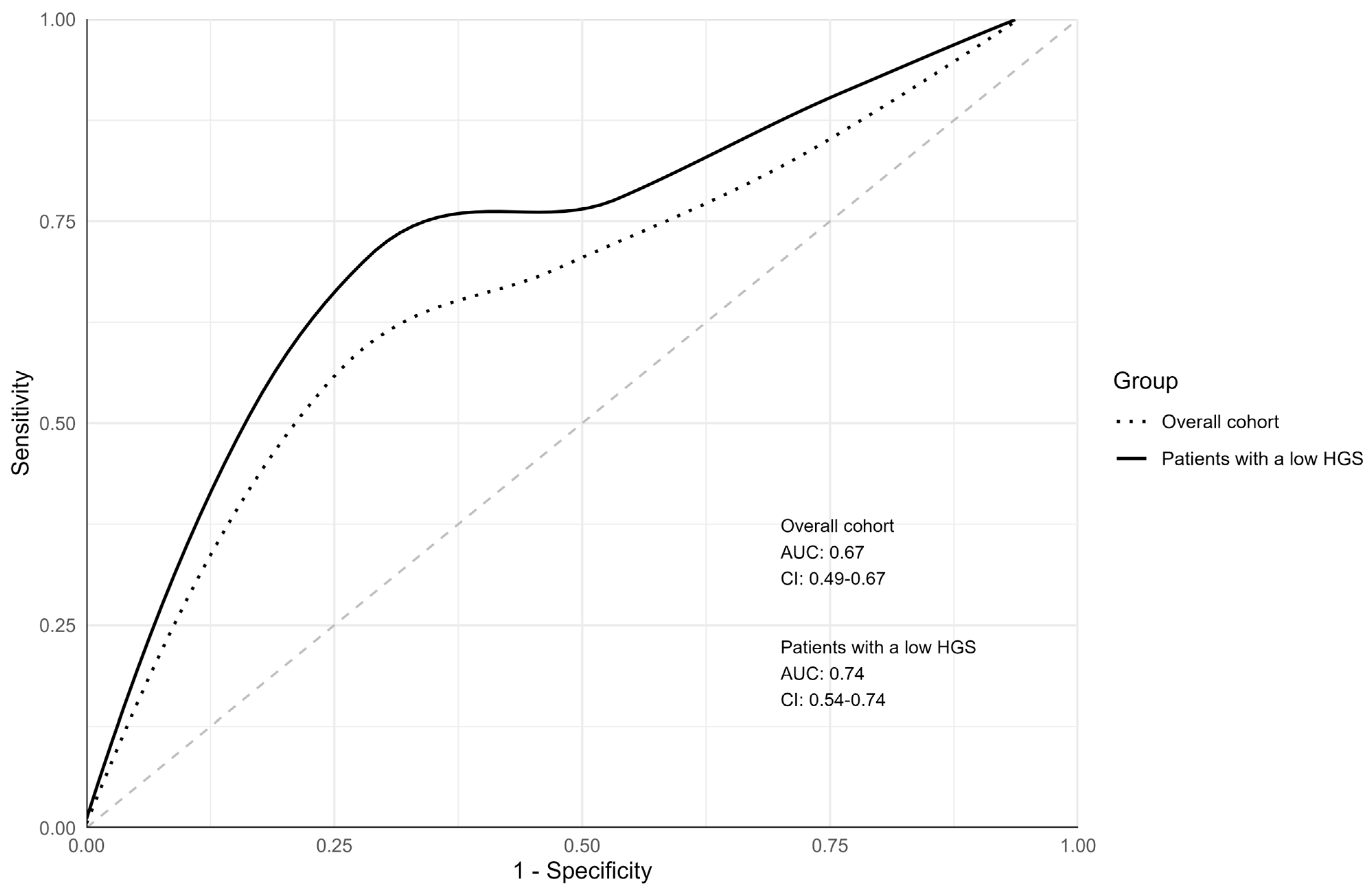
| Group | Cutoff Score | Sensitivity | Specificity | PPV (95% CI) | NPV (95% CI) | Youden Index |
|---|---|---|---|---|---|---|
| Total cohort | 6 | 0.83 | 0.26 | 0.08 (0.04–0.14) | 0.95 (0.85–0.99) | 0.09 |
| 7 | 0.75 | 0.35 | 0.08 (0.04–0.14) | 0.95 (0.86–0.99) | 0.10 | |
| 8 | 0.67 | 0.44 | 0.08 (0.04–0.15) | 0.95 (0.87–0.99) | 0.11 | |
| 9 | 0.67 | 0.53 | 0.09 (0.04–0.18) | 0.96 (0.89–0.99) | 0.19 | |
| 10 | 0.67 | 0.60 | 0.11 (0.05–0.20) | 0.96 (0.90–0.99) | 0.27 | |
| 11 | 0.67 | 0.68 | 0.13 (0.06–0.25) | 0.97 (0.91–0.99) | 0.35 | |
| 12 | 0.58 | 0.75 | 0.15 (0.06–0.28) | 0.96 (0.91–0.99) | 0.33 | |
| 13 | 0.50 | 0.80 | 0.15 (0.06–0.31) | 0.96 (0.91–0.98) | 0.30 | |
| Patients with low HGS | 6 | 1.00 | 0.24 | 0.08 (0.03–0.14) | 1.00 (0.88–1.00) | 0.24 |
| 7 | 0.88 | 0.31 | 0.07 (0.03–0.15) | 0.97 (0.87–1.00) | 0.18 | |
| 8 | 0.75 | 0.38 | 0.07 (0.03–0.15) | 0.96 (0.86–1.00) | 0.13 | |
| 9 | 0.75 | 0.46 | 0.08 (0.03–0.17) | 0.97 (0.89–1.00) | 0.21 | |
| 10 | 0.75 | 0.54 | 0.09 (0.03–0.19) | 0.97 (0.90–1.00) | 0.29 | |
| 11 | 0.75 | 0.63 | 0.11 (0.04–0.23) | 0.98 (0.91–1.00) | 0.38 | |
| 12 | 0.75 | 0.72 | 0.14 (0.05–0.29) | 0.98 (0.92–1.00) | 0.47 | |
| 13 | 0.62 | 0.77 | 0.15 (0.05–0.31) | 0.97 (0.92–0.99) | 0.40 |
| Total Cohort | Patients with Low HGS | |||||||
|---|---|---|---|---|---|---|---|---|
| Non-Fallers (n = 163) | Fallers (n = 12) | Total (N = 175) | p | Non-Fallers (n = 127) | Fallers (n = 8) | Total (N = 135) | p | |
| Age (years) | 0.462 | 0.779 | ||||||
| 60–69 | 40 (24.5%) | 2 (16.7%) | 42 (24.0%) | 23 (18.1%) | 1 (12.5%) | 24 (17.8%) | ||
| 70–79 | 70 (42.9%) | 4 (33.3%) | 74 (42.3%) | 56 (44.1%) | 3 (37.5%) | 59 (43.7%) | ||
| ≥80 | 53 (32.5%) | 6 (50.0%) | 59 (33.7%) | 48 (37.8%) | 4 (50.0%) | 52 (38.5%) | ||
| History of one fall within 6 months before admission | 0.850 | 0.534 | ||||||
| No | 146 (89.6%) | 10 (83.3%) | 156 (89.1%) | 113 (89.0%) | 6 (75.0%) | 119 (88.1%) | ||
| Yes | 17 (10.4%) | 2 (16.7%) | 19 (10.9%) | 14 (11.0%) | 2 (25.0%) | 16 (11.9%) | ||
| Intestinal and urinary elimination | 0.002 * | 0.033 * | ||||||
| Normal | 157 (96.3%) | 9 (75.0%) | 166 (94.8%) | 121 (95.3%) | 6 (75.0%) | 127 (94.1%) | ||
| Incontinence or urgency or frequency | 5 (3.1%) | 3 (25.0%) | 8 (4.6%) | 5 (3.9%) | 2 (25.0%) | 7 (5.2%) | ||
| Urgency/frequency and incontinence | 1 (0.6%) | 0 (0.0%) | 1 (0.6%) | 1 (0.8%) | 0 (0.0%) | 1 (0.7%) | ||
| Use of drugs entailing high risk of falls | 0.255 | 0.024 * | ||||||
| None | 132 (81.0%) | 8 (66.7%) | 140 (80.0%) | 103 (81.1%) | 5 (62.5%) | 108 (80.0%) | ||
| 1 high fall-risk drug | 28 (17.2%) | 3 (25.0%) | 31 (17.7%) | 23 (18.1%) | 2 (25.0%) | 25 (18.5%) | ||
| 2 or more high fall-risk drug | 3 (1.8%) | 1 (8.3%) | 4 (2.3%) | 1 (0.8%) | 1 (12.5%) | 2 (1.5%) | ||
| Equipment that may compromise mobility | 0.427 | 0.242 | ||||||
| No | 102 (62.6%) | 7 (58.3%) | 109 (62.3%) | 73 (57.5%) | 3 (37.5%) | 76 (56.3%) | ||
| 1 | 34 (20.9%) | 3 (25.0%) | 37 (21.1%) | 29 (22.8%) | 3 (37.5%) | 32 (23.7%) | ||
| 2 | 16 (9.8%) | 0 (0.0%) | 16 (9.1%) | 14 (11.0%) | 0 (0.0%) | 14 (10.4%) | ||
| ≥3 | 11 (6.7%) | 2 (16.7%) | 13 (7.4%) | 11 (8.7%) | 2 (25.0%) | 13 (9.6%) | ||
| Mobility impairment | 2.4 ± 1.6 | 3.2 ± 1.3 | 2.5 ± 1.6 | 0.121 | 2.3 ± 1.5 | 3.0 ± 1.5 | 2.4 ± 1.5 | 0.237 |
| Cognition impairment | 1.0 [0.0–5.0] | 2.5 [0.0–5.5] | 1.0 [0.0–5.0] | 0.456 | 2.0 [0.0–5.0] | 4.5 [0.5–6.0] | 2.0 [0.0–5.0] | 0.389 |
| Total score | 8.0 [5.0–1.5] | 12.5 [6.5–15.0] | 8.0 [5.5–12.0] | 0.053 | 9.2 ± 4.4 | 13.1 ± 4.7 | 9.4 ± 4.5 | 0.016 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, S.; Kim, J.-S.; Choi, Y.-A. Predictive Validity of the Johns Hopkins Fall Risk Assessment Tool for Older Patients in Stroke Rehabilitation. Healthcare 2024, 12, 791. https://doi.org/10.3390/healthcare12070791
Hong S, Kim J-S, Choi Y-A. Predictive Validity of the Johns Hopkins Fall Risk Assessment Tool for Older Patients in Stroke Rehabilitation. Healthcare. 2024; 12(7):791. https://doi.org/10.3390/healthcare12070791
Chicago/Turabian StyleHong, Seungho, Ji-Sook Kim, and Young-Ah Choi. 2024. "Predictive Validity of the Johns Hopkins Fall Risk Assessment Tool for Older Patients in Stroke Rehabilitation" Healthcare 12, no. 7: 791. https://doi.org/10.3390/healthcare12070791
APA StyleHong, S., Kim, J.-S., & Choi, Y.-A. (2024). Predictive Validity of the Johns Hopkins Fall Risk Assessment Tool for Older Patients in Stroke Rehabilitation. Healthcare, 12(7), 791. https://doi.org/10.3390/healthcare12070791








