Correlation Between Metabolic Score for Visceral Fat and Cardiovascular-Kidney-Metabolic Syndrome: Analysis of NHANES 2011–2020
Abstract
:1. Introduction
2. Materials and Methods
2.1. Research Design and Population Selection
2.2. Observed Variable
2.2.1. Definition of the METS-VF Index and CKM Syndrome
2.2.2. Covariate Selection
2.3. Statistical Analysis
3. Results
3.1. Characteristics of the Baseline Population
3.2. The Correlation Between the METS-VF and CKM Syndrome
3.3. Collinearity Diagnostics Analysis
3.4. Subgroup and Interaction Analysis
3.5. RCS Analysis
3.6. ROC Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CKM | Cardiovascular-kidney-metabolic syndrome |
| METS-VF | Metabolic Score for Visceral Fat |
| NHANES | National Health and Nutrition Examination Survey |
| RCS | Restricted cubic spline |
| ROC | Receiver operating characteristic |
| CVD | Cardiovascular disease |
| CKD | Chronic kidney disease |
| DM | Diabetes mellitus |
| BMI | Body mass index |
| VAI | Visceral adiposity index |
| HR | Hazard ratio |
| 95% CI | 95% confidence interval |
| VIF | Variance inflation factor |
References
- Ndumele, C.E.; Rangaswami, J.; Chow, S.L.; Neeland, I.J.; Tuttle, K.R.; Khan, S.S.; Coresh, J.; Mathew, R.O.; Baker-Smith, C.M.; Carnethon, M.R. Cardiovascular-Kidney-Metabolic Health: A Presidential Advisory From the American Heart Association. Circulation 2023, 148, 1606–1635. [Google Scholar]
- Aggarwal, R.; Ostrominski, J.W.; Vaduganathan, M. Prevalence of Cardiovascular-Kidney-Metabolic Syndrome Stages in US Adults, 2011–2020. JAMA 2024, 331, 1858–1860. [Google Scholar]
- Mehta, R.; Buzkova, P.; Patel, H.; Cheng, J.; Kizer, J.R.; Gottdiener, J.S.; Psaty, B.; Khan, S.S.; Ix, J.H.; Isakova, T.; et al. Cardiac Mechanics and Kidney Function Decline in the Cardiovascular Health Study. Kidney360 2023, 4, 622–630. [Google Scholar]
- Zannad, F.; Rossignol, P. Cardiorenal Syndrome Revisited. Circulation 2018, 138, 929–944. [Google Scholar]
- Patel, K.P.; Katsurada, K.; Zheng, H. Cardiorenal Syndrome: The Role of Neural Connections Between the Heart and the Kidneys. Circ. Res. 2022, 130, 1601–1617. [Google Scholar] [CrossRef]
- Kjaergaard, A.D.; Teumer, A.; Witte, D.R.; Stanzick, K.J.; Winkler, T.W.; Burgess, S.; Ellervik, C. Obesity and Kidney Function: A Two-Sample Mendelian Randomization Study. Clin. Chem. 2022, 68, 461–472. [Google Scholar]
- García-Carrasco, A.; Izquierdo-Lahuerta, A.; Medina-Gómez, G. The Kidney-Heart Connection in Obesity. Nephron 2021, 145, 604–608. [Google Scholar]
- Ostrominski, J.W.; Arnold, S.V.; Butler, J.; Fonarow, G.C.; Hirsch, J.S.; Palli, S.R.; Donato, B.M.K.; Parrinello, C.M.; O’Connell, T.; Collins, E.B.; et al. Prevalence and Overlap of Cardiac, Renal, and Metabolic Conditions in US Adults, 1999–2020. JAMA Cardiol. 2023, 8, 1050–1060. [Google Scholar]
- Huang, Y.; Liu, Y.; Ma, Y.; Tu, T.; Liu, N.; Bai, F.; Xiao, Y.; Liu, C.; Hu, Z.; Lin, Q.; et al. Associations of Visceral Adipose Tissue, Circulating Protein Biomarkers, and Risk of Cardiovascular Diseases: A Mendelian Randomization Analysis. Front. Cell. Dev. Biol. 2022, 10, 840866. [Google Scholar]
- Chen, Q.; Wu, Y.; Gao, Y.; Zhang, Z.; Shi, T.; Yan, B. Effect of visceral adipose tissue mass on coronary artery disease and heart failure: A Mendelian randomization study. Int. J. Obes. 2022, 46, 2102–2106. [Google Scholar] [CrossRef]
- Dalah, E.; Hasan, H.; Madkour, M.; Obaideen, A.; Faris, M.A. Assessing visceral and subcutaneous adiposity using segmented T2-MRI and multi-frequency segmental bioelectrical impedance: A sex-based comparative study. Acta Biomed. 2021, 92, e2021078. [Google Scholar]
- Mayoral, L.P.; Andrade, G.M.; Mayoral, E.P.; Huerta, T.H.; Canseco, S.P.; Rodal Canales, F.J.; Cabrera-Fuentes, H.A.; Cruz, M.M.; Pérez Santiago, A.D.; Alpuche, J.J.; et al. Obesity subtypes, related biomarkers & heterogeneity. Indian J. Med. Res. 2020, 151, 11–21. [Google Scholar]
- Perdomo, C.M.; Cohen, R.V.; Sumithran, P.; Clément, K.; Frühbeck, G. Contemporary medical, device, and surgical therapies for obesity in adults. Lancet 2023, 401, 1116–1130. [Google Scholar]
- Caballero, B. Humans against Obesity: Who Will Win? Adv. Nutr. 2019, 10, S4–S9. [Google Scholar]
- Gutin, I. Body mass index is just a number: Conflating riskiness and unhealthiness in discourse on body size. Sociol. Health. Illn. 2021, 43, 1437–1453. [Google Scholar]
- Godziuk, K.; Hawker, G.A. Obesity and body mass index: Past and future considerations in osteoarthritis research. Osteoarthritis. Cartil. 2024, 32, 452–459. [Google Scholar]
- Dierkes, J.; Dahl, H.; Lervaag Welland, N.; Sandnes, K.; Sæle, K.; Sekse, I.; Marti, H.P. High rates of central obesity and sarcopenia in CKD irrespective of renal replacement therapy—An observational cross-sectional study. BMC Nephrol. 2018, 19, 259. [Google Scholar]
- Lin, T.Y.; Lim, P.S.; Hung, S.C. Impact of Misclassification of Obesity by Body Mass Index on Mortality in Patients With CKD. Kidney Int. Rep. 2018, 3, 447–455. [Google Scholar] [PubMed]
- Ogden, C.L.; Carroll, M.D.; Fakhouri, T.H.; Hales, C.M.; Fryar, C.D.; Li, X.; Freedman, D.S. Prevalence of Obesity Among Youths by Household Income and Education Level of Head of Household—United States 2011-2014. MMWR. Morb. Mortal. Wkly. Rep. 2018, 67, 186–189. [Google Scholar] [CrossRef]
- Bello-Chavolla, O.Y.; Antonio-Villa, N.E.; Vargas-Vázquez, A.; Viveros-Ruiz, T.L.; Almeda-Valdes, P.; Gomez-Velasco, D.; Mehta, R.; Elias-López, D.; Cruz-Bautista, I.; Roldán-Valadez, E.; et al. Metabolic Score for Visceral Fat (METS-VF), a novel estimator of intra-abdominal fat content and cardio-metabolic health. Clin. Nutr. 2020, 39, 1613–1621. [Google Scholar]
- Kapoor, N.; Jiwanmall, S.A.; Nandyal, M.B.; Kattula, D.; Paravathareddy, S.; Paul, T.V.; Furler, J.; Oldenburg, B.; Thomas, N. Metabolic Score for Visceral Fat (METS-VF) Estimation—A Novel Cost-Effective Obesity Indicator for Visceral Adipose Tissue Estimation. Diabetes. Metab. Syndr. Obes. 2020, 13, 3261–3267. [Google Scholar] [PubMed]
- Feng, Y.; Yang, X.; Li, Y.; Wu, Y.; Han, M.; Qie, R.; Huang, S.; Wu, X.; Zhang, Y.; Zhang, J.; et al. Metabolic Score for Visceral Fat: A novel predictor for the risk of type 2 diabetes mellitus. Br. J. Nutr. 2022, 128, 1029–1036. [Google Scholar] [PubMed]
- Liu, H.; Dong, H.; Zhou, Y.; Jin, M.; Hao, H.; Yuan, Y.; Jia, H. The association between Metabolic Score for Visceral Fat and depression in overweight or obese individuals: Evidence from NHANES. Front. Endocrinol. 2024, 15, 1482003. [Google Scholar]
- Zhu, Y.; Zou, H.; Guo, Y.; Luo, P.; Meng, X.; Li, D.; Xiang, Y.; Mao, B.; Pan, L.; Kan, R.; et al. Associations between metabolic score for visceral fat and the risk of cardiovascular disease and all-cause mortality among populations with different glucose tolerance statuses. Diabetes. Res. Clin. Pract. 2023, 203, 110842. [Google Scholar]
- Feng, L.; Chen, T.; Wang, X.; Xiong, C.; Chen, J.; Wu, S.; Ning, J.; Zou, H. Metabolism Score for Visceral Fat (METS-VF): A New Predictive Surrogate for CKD Risk. Diabetes. Metab. Syndr. Obes. 2022, 15, 2249–2258. [Google Scholar]
- KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases. Kidney Int. 2021, 100, S1–S276.
- Sebastian, S.A.; Padda, I.; Johal, G. Cardiovascular-Kidney-Metabolic (CKM) syndrome: A state-of-the-art review. Curr. Probl. Cardiol. 2024, 49, 102344. [Google Scholar]
- Borghi, C.; Agabiti-Rosei, E.; Johnson, R.J.; Kielstein, J.T.; Lurbe, E.; Mancia, G.; Redon, J.; Stack, A.G.; Tsioufis, K.P. Hyperuricaemia and gout in cardiovascular, metabolic and kidney disease. Eur. J. Intern. Med. 2020, 80, 1–11. [Google Scholar]
- Tain, Y.L.; Hsu, C.N. The Renin-Angiotensin System and Cardiovascular-Kidney-Metabolic Syndrome: Focus on Early-Life Programming. Int. J. Mol. Sci. 2024, 25, 3298. [Google Scholar] [CrossRef]
- Tain, Y.L.; Hsu, C.N. Maternal Polyphenols and Offspring Cardiovascular-Kidney-Metabolic Health. Nutrients 2024, 16, 3168. [Google Scholar] [CrossRef]
- de Oliveira, A.A.; Vergara, A.; Wang, X.; Vederas, J.C.; Oudit, G.Y. Apelin pathway in cardiovascular, kidney, and metabolic diseases: Therapeutic role of apelin analogs and apelin receptor agonists. Peptides 2022, 147, 170697. [Google Scholar] [CrossRef] [PubMed]
- Nosalski, R.; Guzik, T.J. Perivascular adipose tissue inflammation in vascular disease. Br. J. Pharmacol. 2017, 174, 3496–3513. [Google Scholar] [CrossRef] [PubMed]
- Rana, M.N.; Neeland, I.J. Adipose Tissue Inflammation and Cardiovascular Disease: An Update. Curr. Diab. Rep. 2022, 22, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am. J. Physiol. Cell. Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef]
- Neeland, I.J.; Ross, R.; Després, J.P.; Matsuzawa, Y.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; Santos, R.D.; Arsenault, B.; et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: A position statement. Lancet. Diabetes. Endocrinol. 2019, 7, 715–725. [Google Scholar] [CrossRef]
- Rosito, G.A.; Massaro, J.M.; Hoffmann, U.; Ruberg, F.L.; Mahabadi, A.A.; Vasan, R.S.; O’Donnell, C.J.; Fox, C.S. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: The Framingham Heart Study. Circulation 2008, 117, 605–613. [Google Scholar] [CrossRef]
- Soltanieh, S.; Salavatizadeh, M.; Poustchi, H.; Yari, Z.; Mansour, A.; Khamseh, M.E.; Malek, M.; Alaei-Shahmiri, F.; Hekmatdoost, A. The association of dietary inflammatory index (DII) and central obesity with non-alcoholic fatty liver disease (NAFLD) in people with diabetes (T2DM). Heliyon 2023, 9, e13983. [Google Scholar] [CrossRef]
- Kuang, M.; Qiu, J.; Li, D.; Hu, C.; Zhang, S.; Sheng, G.; Zou, Y. The newly proposed Metabolic Score for Visceral Fat is a reliable tool for identifying non-alcoholic fatty liver disease, requiring attention to age-specific effects in both sexes. Front. Endocrinol. 2023, 14, 1281524. [Google Scholar] [CrossRef]
- Elisha, B.; Messier, V.; Karelis, A.; Coderre, L.; Bernard, S.; Prud’homme, D.; Rabasa-Lhoret, R. The Visceral Adiposity Index: Relationship with cardiometabolic risk factors in obese and overweight postmenopausal women—A MONET group study. Appl. Physiol. Nutr. Metab. 2013, 38, 892–899. [Google Scholar] [CrossRef]
- Tan, A.; Yang, S.; Pan, Y.; Lin, Q. Metabolism score for visceral fat (METS-VF): An innovative and powerful predictor of stroke. Arch. Med. Sci. 2024, 20, 1710–1714. [Google Scholar] [CrossRef]
- Huang, J.C.; Huang, Y.C.; Lu, C.H.; Chuang, Y.S.; Chien, H.H.; Lin, C.I.; Chao, M.F.; Chuang, H.Y.; Ho, C.K.; Wang, C.L.; et al. Exploring the Relationship Between Visceral Fat and Coronary Artery Calcification Risk Using Metabolic Score for Visceral Fat (METS-VF). Life 2024, 14, 1399. [Google Scholar] [CrossRef] [PubMed]
- Antonio-Villa, N.E.; Juárez-Rojas, J.G.; Posadas-Sánchez, R.; Reyes-Barrera, J.; Medina-Urrutia, A. Visceral adipose tissue is an independent predictor and mediator of the progression of coronary calcification: A prospective sub-analysis of the GEA study. Cardiovasc. Diabetol. 2023, 22, 81. [Google Scholar] [PubMed]
- Wander, P.L.; Hayashi, T.; Sato, K.K.; Uehara, S.; Hikita, Y.; Leonetti, D.L.; Kahn, S.E.; Fujimoto, W.Y.; Boyko, E.J. Design and validation of a novel estimator of visceral adipose tissue area and comparison to existing adiposity surrogates. J. Diabetes. Complicat. 2018, 32, 1062–1067. [Google Scholar]
- Feng, Y.; Yang, X.; Li, Y.; Wu, Y.; Han, M.; Qie, R.; Huang, S.; Wu, X.; Zhang, Y.; Liu, D.; et al. Metabolic Score for Visceral Fat: A reliable indicator of visceral obesity for predicting risk for hypertension. Nutrition 2022, 93, 111443. [Google Scholar]
- Yang, R.; Kuang, M.; Qiu, J.; Yu, C.; Sheng, G.; Zou, Y. Assessing the usefulness of a newly proposed metabolic score for visceral fat in predicting future diabetes: Results from the NAGALA cohort study. Front. Endocrinol. 2023, 14, 1172323. [Google Scholar]
- Tripathi, H.; Singh, A.; Farheen; Prakash, B.; Dubey, D.K.; Sethi, P.; Jadon, R.S.; Ranjan, P.; Vikram, N.K. The Metabolic Score for Visceral Fat (METS-VF) as a predictor of diabetes mellitus: Evidence from the 2011–2018 NHANES study. PLoS ONE 2025, 20, e0317913. [Google Scholar]
- Rask-Andersen, M.; Johansson, Å. Illuminating the ’healthy obese’ phenotype. Nat. Metab. 2023, 5, 193–194. [Google Scholar]
- Torun, C.; Ankaralı, H.; Caştur, L.; Uzunlulu, M.; Erbakan, A.N.; Akbaş, M.M.; Gündüz, N.; Doğan, M.B.; Oğuz, A. Is Metabolic Score for Visceral Fat (METS-VF) a Better Index Than Other Adiposity Indices for the Prediction of Visceral Adiposity. Diabetes Metab. Syndr. Obes. 2023, 16, 2605–2615. [Google Scholar] [CrossRef]
- Liu, Q.; Cui, H.; Si, F.; Wu, Y.; Yu, J. Association of Cumulative Exposure to Metabolic Score for Visceral Fat With the Risk of Cardiovascular Disease and All-Cause Mortality: A Prospective Cohort Study. J. Cachexia. Sarcopenia Muscle 2025, 16, e13702. [Google Scholar] [CrossRef]
- Cao, Y.; Wen, W.; Zhang, H.; Li, W.; Huang, G.; Huang, Y. The association between visceral fat metabolic score and stroke: Mediation by declining kidney function. Diabetol. Metab. Syndr. 2025, 17, 50. [Google Scholar]
- Yu, P.; Meng, X.; Kan, R.; Wang, Z.; Yu, X. Association between metabolic scores for visceral fat and chronic kidney disease: A cross-sectional study. Front. Endocrinol. 2022, 13, 1052736. [Google Scholar]
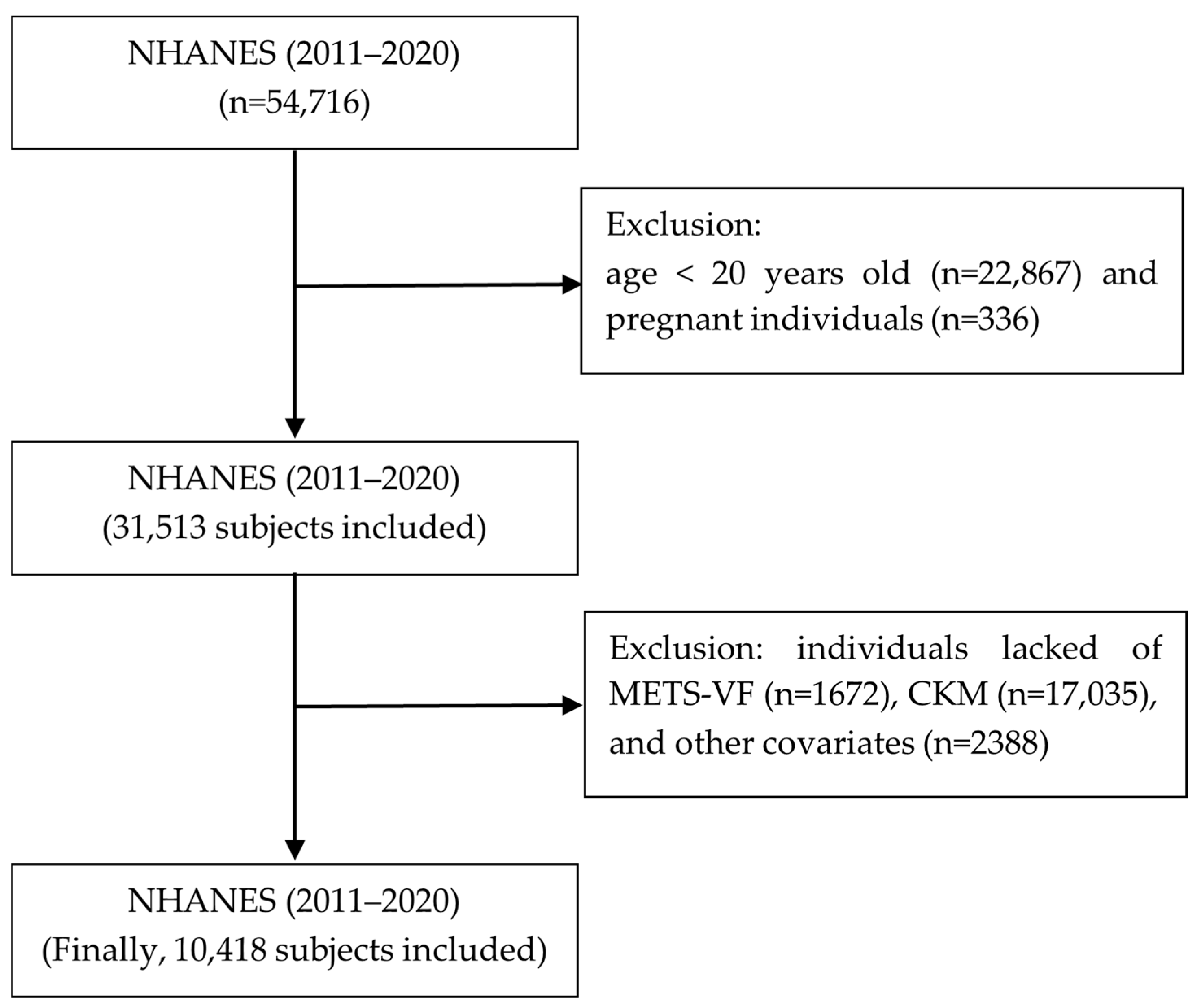


| Characteristics | CKM 0 | CKM 1 | CKM 2 | CKM 3 | CKM 4 | p-Value |
|---|---|---|---|---|---|---|
| (n = 838) | (n = 2138) | (n = 5797) | (n = 538) | (n = 1107) | ||
| Age | 34.40 (0.61) | 40.38 (0.62) | 48.73 (0.34) | 74.93 (0.46) | 64.52 (0.56) | <0.0001 |
| Sex | <0.0001 | |||||
| Female | 62.06 (2.22) | 50.96 (1.60) | 48.12 (0.86) | 36.99 (3.12) | 42.81 (2.28) | |
| Male | 37.94 (2.22) | 49.04 (1.60) | 51.88 (0.86) | 63.01 (3.12) | 57.19 (2.28) | |
| Race | <0.0001 | |||||
| Mexican American | 4.72 (0.74) | 10.72 (1.40) | 9.35 (0.80) | 4.78 (0.90) | 3.99 (0.69) | |
| Non-Hispanic Black | 7.11 (0.80) | 9.46 (0.86) | 10.50 (0.94) | 12.15 (1.55) | 10.53 (1.08) | |
| Non-Hispanic White | 73.62 (2.03) | 63.44 (2.04) | 65.87 (1.58) | 68.93 (2.79) | 74.05 (1.94) | |
| Other Hispanic | 5.87 (1.13) | 7.48 (0.74) | 5.92 (0.61) | 6.42 (1.24) | 4.10 (0.65) | |
| Other Race | 8.69 (1.07) | 8.90 (0.85) | 8.37 (0.50) | 7.72 (1.35) | 7.32 (1.17) | |
| Drinking | <0.0001 | |||||
| Never | 9.57 (1.50) | 8.48 (0.97) | 9.65 (0.58) | 17.62 (2.39) | 11.18 (1.13) | |
| Former | 4.12 (0.91) | 5.17 (0.53) | 9.00 (0.62) | 17.78 (2.30) | 20.42 (1.83) | |
| Now | 86.32 (1.85) | 86.35 (1.20) | 81.35 (0.88) | 64.59 (2.81) | 68.40 (1.99) | |
| Smoking | <0.0001 | |||||
| Never | 68.68 (2.17) | 60.21 (1.43) | 55.30 (1.02) | 45.29 (3.37) | 38.49 (2.11) | |
| Former | 15.08 (1.66) | 24.20 (1.61) | 25.49 (0.92) | 39.46 (3.60) | 39.15 (2.16) | |
| Now | 16.24 (1.49) | 15.59 (1.22) | 19.20 (0.78) | 15.26 (1.94) | 22.36 (2.33) | |
| SBP | 108.12 (0.42) | 112.25 (0.29) | 125.53 (0.28) | 144.39 (1.52) | 128.62 (0.78) | <0.0001 |
| Hypertension | <0.0001 | |||||
| No | 100.00 (0.00) | 100.00 (0.00) | 46.23 (1.00) | 19.70 (2.55) | 27.66 (2.12) | |
| Yes | 0.00 (0.00) | 0.00 (0.00) | 53.77 (1.00) | 80.30 (2.55) | 72.34 (2.12) | |
| BMI | 21.71 (0.10) | 28.30 (0.17) | 30.79 (0.17) | 28.96 (0.30) | 30.74 (0.33) | 0.0001 |
| Fat mass | 17.42 (0.25) | 27.91 (0.32) | 32.46 (0.29) | 29.41 (0.56) | 32.42 (0.56) | < 0.0001 |
| Lean mass | 43.15 (0.39) | 51.33 (0.32) | 54.25 (0.26) | 50.20 (0.68) | 52.81 (0.60) | <0.0001 |
| VAI | 0.77 (0.02) | 1.01 (0.02) | 2.35 (0.05) | 2.33 (0.26) | 2.26 (0.10) | <0.0001 |
| METS-VF | 5.62 (0.03) | 6.63 (0.02) | 7.01 (0.01) | 7.41 (0.02) | 7.31 (0.03) | <0.0001 |
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| HR (95%CI) | p-Value | HR (95%CI) | p-Value | HR (95%CI) | p-Value | |
| METS-VF | ||||||
| METS-VF as continuous variable | 1.377 (1.310, 1.448) | <0.0001 | 1.363 (1.266, 1.468) | <0.0001 | 1.665 (1.457, 1.903) | <0.0001 |
| Tertile 1 | Reference | Reference | Reference | |||
| Tertile 2 | 1.438 (1.318, 1.570) | <0.0001 | 1.340 (1.186, 1.514) | <0.0001 | 1.153 (1.031, 1.289) | 0.009 |
| Tertile 3 | 1.477 (1.373, 1.589) | <0.0001 | 1.359 (1.228, 1.503) | <0.0001 | 1.004 (0.847, 1.189) | 0.967 |
| p for trend | <0.0001 | <0.0001 | 0.943 | |||
| Covariates | VIF | Tolerance |
|---|---|---|
| BMI | 1.92 | 0.52 |
| VAI | 1.43 | 0.70 |
| Fat mass | 1.07 | 0.93 |
| Lean mass | 1.10 | 0.91 |
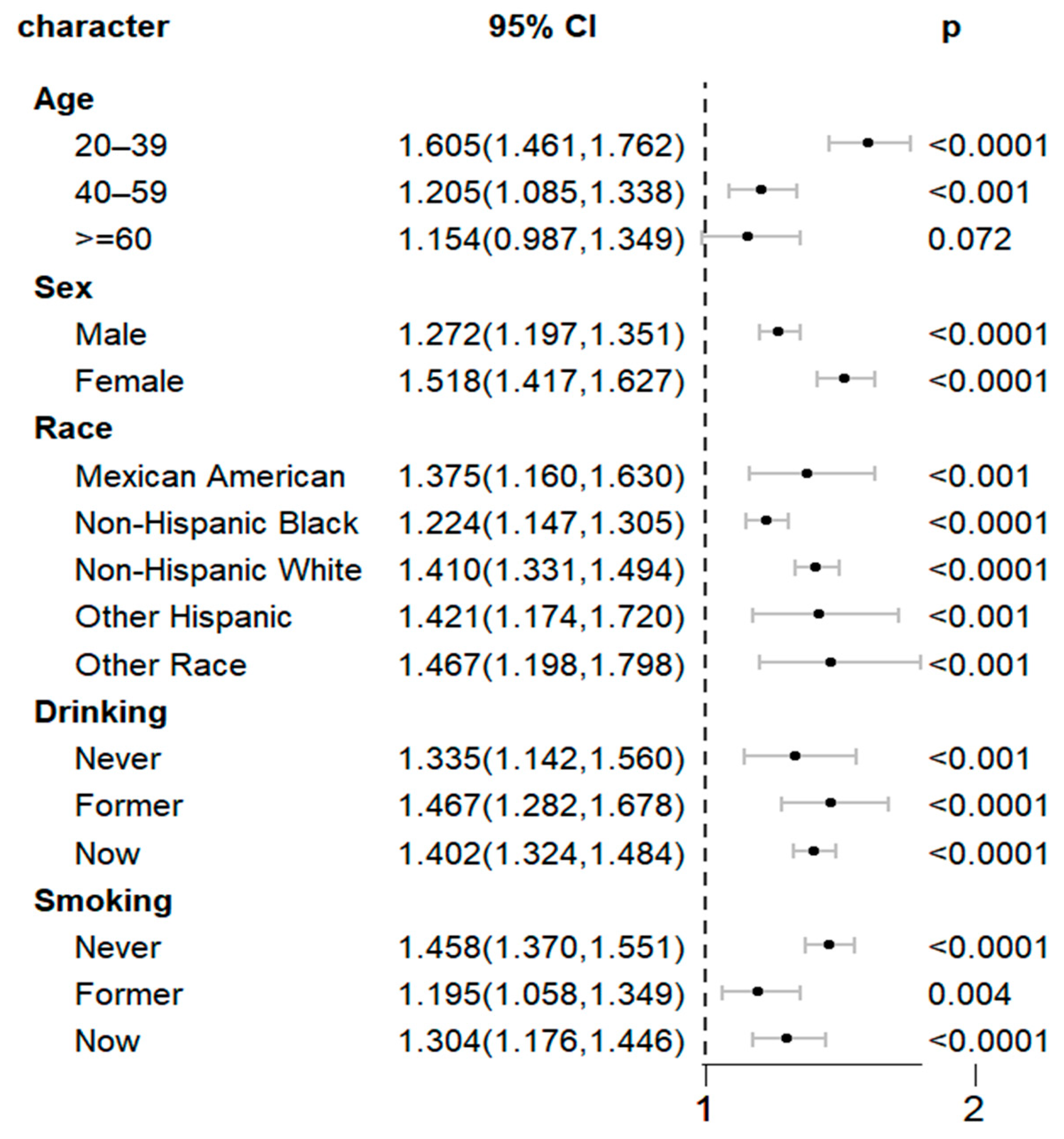
| Character | Q1 | Q2 | Q3 | p for Trend | p for Interaction |
|---|---|---|---|---|---|
| Age | 0.076 | ||||
| 20–39 | 1 | 1.630 (1.410, 1.885) | 1.478 (1.198, 1.825) | <0.0001 | |
| 40–59 | 1 | 1.262 (1.105, 1.440) | 1.271 (1.109, 1.457) | 0.001 | |
| ≥60 | 1 | 1.158 (0.850, 1.578) | 1.248 (0.966, 1.612) | 0.037 | |
| Sex | 0.07 | ||||
| Male | 1 | 1.322 (1.179, 1.483) | 1.403 (1.277, 1.542) | <0.0001 | |
| Female | 1 | 1.550 (1.400, 1.717) | 1.543 (1.373, 1.735) | <0.0001 | |
| Race | 0.261 | ||||
| Mexican American | 1 | 1.330 (1.079, 1.640) | 1.520 (1.222, 1.891) | <0.001 | |
| Non-Hispanic Black | 1 | 1.214 (1.069, 1.378) | 1.209 (1.086, 1.346) | <0.001 | |
| Non-Hispanic White | 1 | 1.466 (1.307, 1.644) | 1.540 (1.403, 1.690) | <0.0001 | |
| Other Hispanic | 1 | 1.515 (1.222, 1.879) | 1.547 (1.183, 2.022) | 0.001 | |
| Other Race | 1 | 1.689 (1.389, 2.055) | 1.401 (0.967, 2.029) | 0.016 | |
| Drinking | 0.484 | ||||
| Never | 1 | 1.301 (0.977, 1.733) | 1.261 (0.973, 1.636) | 0.095 | |
| Former | 1 | 1.589 (1.263, 1.998) | 1.769 (1.519,2.061) | <0.0001 | |
| Now | 1 | 1.452 (1.309, 1.611) | 1.521 (1.382, 1.674) | <0.0001 | |
| Smoking | 0.449 | ||||
| Never | 1 | 1.493 (1.332, 1.673) | 1.500 (1.362, 1.652) | <0.0001 | |
| Former | 1 | 1.277 (1.084, 1.503) | 1.375 (1.183, 1.600) | <0.0001 | |
| Now | 1 | 1.402 (1.193, 1.647) | 1.348 (1.120, 1.623) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, X.; Yin, X.; Liu, Q.; Liu, J.; Li, Y. Correlation Between Metabolic Score for Visceral Fat and Cardiovascular-Kidney-Metabolic Syndrome: Analysis of NHANES 2011–2020. Healthcare 2025, 13, 694. https://doi.org/10.3390/healthcare13070694
Fang X, Yin X, Liu Q, Liu J, Li Y. Correlation Between Metabolic Score for Visceral Fat and Cardiovascular-Kidney-Metabolic Syndrome: Analysis of NHANES 2011–2020. Healthcare. 2025; 13(7):694. https://doi.org/10.3390/healthcare13070694
Chicago/Turabian StyleFang, Xi, Xuemin Yin, Qianfang Liu, Jing Liu, and Ying Li. 2025. "Correlation Between Metabolic Score for Visceral Fat and Cardiovascular-Kidney-Metabolic Syndrome: Analysis of NHANES 2011–2020" Healthcare 13, no. 7: 694. https://doi.org/10.3390/healthcare13070694
APA StyleFang, X., Yin, X., Liu, Q., Liu, J., & Li, Y. (2025). Correlation Between Metabolic Score for Visceral Fat and Cardiovascular-Kidney-Metabolic Syndrome: Analysis of NHANES 2011–2020. Healthcare, 13(7), 694. https://doi.org/10.3390/healthcare13070694







