Bayesian Analysis of Length of Stay Determinants in ERAS-Guided Hip Arthroplasty
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Study Setting and Sampling
2.3. Inclusion and Exclusion Criteria
2.4. Data Source
2.5. Data Collection and Data Analysis
2.6. Ethical Approval
3. Results
3.1. Characteristics of Patients
3.2. Univariate Analysis of LOS for Primary THA Recipients
3.3. Multifactorial Analysis of LOS for Primary THA Recipients
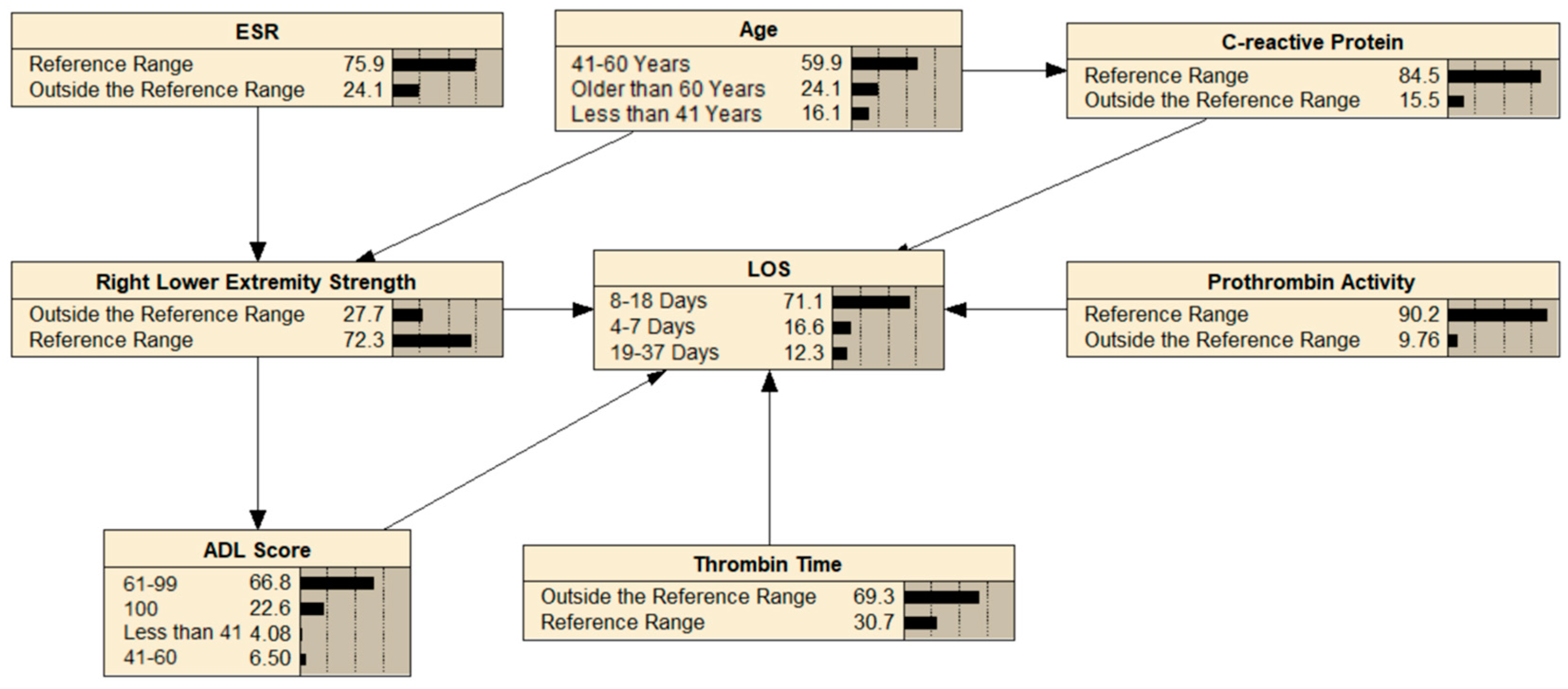
3.4. Building a BN
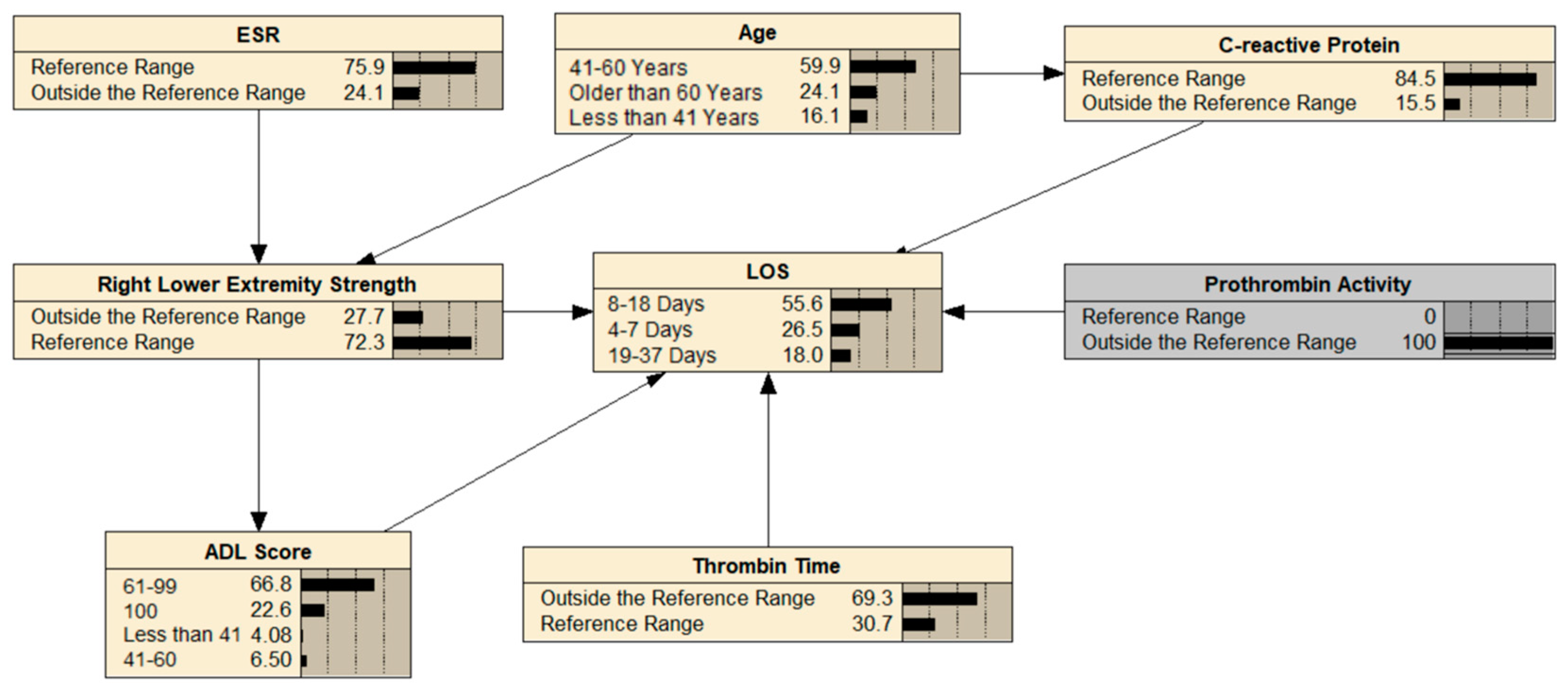
3.5. BN Reasoning
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bian, Y.; Cheng, K.; Chang, X.; Weng, X. Reports and Analysis of Amount of Hip and Knee Arthroplasty in China from 2011 to 2019. Chin. J. Orthop. 2020, 1453–1460. [Google Scholar] [CrossRef]
- Tsuchiya, M.; Fukushima, K.; Ohashi, Y.; Mamorita, N.; Saito, H.; Uchida, K.; Uchiyama, K.; Takahira, N.; Takaso, M. Is the Increase in the Number of Total Hip Arthroplasties in Japan Due to an Aging Society? J. Orthop. Sci. 2024. [Google Scholar] [CrossRef]
- Katano, H.; Ozeki, N.; Kohno, Y.; Nakagawa, Y.; Koga, H.; Watanabe, T.; Jinno, T.; Sekiya, I. Trends in Arthroplasty in Japan by a Complete Survey, 2014–2017. J. Orthop. Sci. 2021, 26, 812–822. [Google Scholar] [CrossRef] [PubMed]
- Tham, K.W.; Abdul Ghani, R.; Cua, S.C.; Deerochanawong, C.; Fojas, M.; Hocking, S.; Lee, J.; Nam, T.Q.; Pathan, F.; Saboo, B.; et al. Obesity in South and Southeast Asia—A New Consensus on Care and Management. Obes. Rev. 2023, 24, e13520. [Google Scholar] [CrossRef]
- Steinmetz, J.D.; Culbreth, G.T.; Haile, L.M.; Rafferty, Q.; Lo, J.; Fukutaki, K.G.; Cruz, J.A.; Smith, A.E.; Vollset, S.E.; Brooks, P.M.; et al. Global, Regional, and National Burden of Osteoarthritis, 1990–2020 and Projections to 2050: A Systematic Analysis for the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023, 5, e508–e522. [Google Scholar] [CrossRef]
- Ferguson, R.J.; Palmer, A.J.; Taylor, A.; Porter, M.L.; Malchau, H.; Glyn-Jones, S. Hip Replacement. Lancet 2018, 392, 1662–1671. [Google Scholar] [CrossRef]
- Zhou, S.; Bender, A.; Kutzner, I.; Dymke, J.; Maleitzke, T.; Perka, C.; Duda, G.N.; Winkler, T.; Damm, P. Loading of the Hip and Knee During Swimming: An in Vivo Load Study. J. Bone Jt. Surg. Am. 2023, 105, 1962–1971. [Google Scholar] [CrossRef]
- Small, S.R.; Khalid, S.; Price, A.J.; Doherty, A. Device-Measured Physical Activity in 3506 Individuals with Knee or Hip Arthroplasty. Med. Sci. Sports Exerc. 2024, 56, 805–812. [Google Scholar] [CrossRef]
- Tanzer, M.; Pedneault, C.; Yakobov, E.; Hart, A.; Sullivan, M. Marital Relationship and Quality of Life in Couples Following Hip Replacement Surgery. Life 2021, 11, 401. [Google Scholar] [CrossRef]
- Xiao, M.; Kristensen, S.R.; Marti, J.; Mossialos, E. The Impact of Patient Safety Incidents during Hip and Knee Replacements on Patients’ Health Related Quality of Life: A before and after Study Using Longitudinal Data Linked to Patient-Reported Outcome Measures. Int. J. Surg. 2023, 109, 1085–1093. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, Y.; Li, Y.; Liu, R.; Rai, S.; Li, J.; Hong, P. Enhanced Recovery after Surgery in Patients after Hip and Knee Arthroplasty: A Systematic Review and Meta-Analysis. Postgrad. Med. J. 2024, 100, 159–173. [Google Scholar] [CrossRef]
- Taylor, A.J.; Kay, R.D.; Bryman, J.A.; Tye, E.Y.; Longjohn, D.B.; Najibi, S.; Runner, R.P. Outcomes of an Institutional Rapid Recovery Protocol for Total Joint Arthroplasty at a Safety Net Hospital. J. Am. Acad. Orthop. Surg. Glob. Res. Rev. 2022, 6, e21.00173. [Google Scholar] [CrossRef]
- Mateshaytis, J.; Trudeau, P.; Bisch, S.; Pin, S.; Chong, M.; Nelson, G. Improving the Rate of Same-Day Discharge in Gynecologic Oncology Patients Undergoing Minimally Invasive Surgery-An Enhanced Recovery After Surgery Quality Improvement Initiative. J. Minim. Invasive Gynecol. 2024, 31, 309–320. [Google Scholar] [CrossRef]
- Vanni, F.; Foglia, E.; Pennestrì, F.; Ferrario, L.; Banfi, G. Introducing Enhanced Recovery after Surgery in a High-Volume Orthopaedic Hospital: A Health Technology Assessment. BMC Health Serv. Res. 2020, 20, 773. [Google Scholar] [CrossRef]
- Crossley, N.; Sweeney, B. Patient and Service-Level Factors Affecting Length of Inpatient Stay in an Acute Mental Health Service: A Retrospective Case Cohort Study. BMC Psychiatry 2020, 20, 438. [Google Scholar] [CrossRef]
- Digitale, J.C.; Martin, J.N.; Glymour, M.M. Tutorial on Directed Acyclic Graphs. J. Clin. Epidemiol. 2022, 142, 264–267. [Google Scholar] [CrossRef]
- Sener, E.; Demir, I. Gaussian Bayesian Network Model of Healthcare, Food and Energy Sectors in the Pandemic: Türkiye Case. Heliyon 2024, 10, e23798. [Google Scholar] [CrossRef] [PubMed]
- Pearl, J. Fusion, Propagation, and Structuring in Belief Networks. In Probabilistic and Causal Inference: The Works of Judea Pearl; Association for Computing Machinery: New York, NY, USA, 2022; Volume 36, pp. 139–188. ISBN 978-1-4503-9586-1. [Google Scholar]
- Campos, J.P.A.F.; Machado, I.G.; Bessani, M. Multi-Agent Genetic Algorithm for Bayesian Networks Structural Learning. Knowl. Based Syst. 2025, 310, 113025. [Google Scholar] [CrossRef]
- Song, W.; Gong, H.; Wang, Q.; Zhang, L.; Qiu, L.; Hu, X.; Han, H.; Li, Y.; Li, R.; Li, Y. Using Bayesian Networks with Max-Min Hill-Climbing Algorithm to Detect Factors Related to Multimorbidity. Front. Cardiovasc. Med. 2022, 9, 984883. [Google Scholar] [CrossRef]
- Martinkovich, S.C.; Trott, G.L.; Garay, M.; Sewecke, J.J.; Sauber, T.J.; Sotereanos, N.G. Patient Characteristics and Surgical Start Time Affect Length of Stay Following Anterior Total Hip Arthroplasty. J. Arthroplast. 2020, 35, 2114–2118. [Google Scholar] [CrossRef]
- Girbino, K.L.; Klika, A.K.; Barsoum, W.K.; Bloomfield, M.R.; Briskin, I.N.; Brooks, P.J.; Higuera, C.A.; Kamath, A.F.; Krebs, V.E.; McLaughlin, J.P.; et al. Understanding the Main Predictors of Length of Stay After Total Hip Arthroplasty: Patient-Related or Procedure-Related Risk Factors? J. Arthroplast. 2021, 36, 1663–1670.e4. [Google Scholar] [CrossRef]
- Missmann, M.; Grenier, J.-P.; Raas, C. Modifiable Factors Influencing Length of Stay after Total Knee Arthroplasty. Eur. J. Orthop. Surg. Traumatol. 2023, 33, 1565–1572. [Google Scholar] [CrossRef]
- Tornese, D.; Robustelli, A.; Ricci, G.; Rancoita, P.M.V.; Maffulli, N.; Peretti, G.M. Predictors of Postoperative Hospital Length of Stay after Total Knee Arthroplasty. Singap. Med. J. 2024, 65, 68. [Google Scholar] [CrossRef]
- Golinelli, D.; Grassi, A.; Tedesco, D.; Sanmarchi, F.; Rosa, S.; Rucci, P.; Amabile, M.; Cosentino, M.; Bordini, B.; Fantini, M.P.; et al. Patient Reported Outcomes Measures (PROMs) Trajectories after Elective Hip Arthroplasty: A Latent Class and Growth Mixture Analysis. J. Patient Rep. Outcomes 2022, 6, 95. [Google Scholar] [CrossRef] [PubMed]
- Ripollés-Melchor, J.; Abad-Motos, A.; Díez-Remesal, Y.; Aseguinolaza-Pagola, M.; Padin-Barreiro, L.; Sánchez-Martín, R.; Logroño-Egea, M.; Catalá-Bauset, J.C.; García-Orallo, S.; Bisbe, E.; et al. Association Between Use of Enhanced Recovery After Surgery Protocol and Postoperative Complications in Total Hip and Knee Arthroplasty in the Postoperative Outcomes Within Enhanced Recovery After Surgery Protocol in Elective Total Hip and Knee Arthroplasty Study (POWER2). JAMA Surg. 2020, 155, e196024. [Google Scholar] [CrossRef] [PubMed]
- Kelly, M.E.; Turcotte, J.J.; Aja, J.M.; MacDonald, J.H.; King, P.J. General vs Neuraxial Anesthesia in Direct Anterior Approach Total Hip Arthroplasty: Effect on Length of Stay and Early Pain Control. J. Arthroplast. 2021, 36, 1013–1017. [Google Scholar] [CrossRef]
- Tirumala, V.; Bounajem, G.; Klemt, C.; Maier, S.P.; Padmanabha, A.; Kwon, Y.-M. Outcome of Spinal Versus General Anesthesia in Revision Total Hip Arthroplasty: A Propensity Score-Matched Cohort Analysis. JAAOS J. Am. Acad. Orthop. Surg. 2021, 29, e656. [Google Scholar] [CrossRef]
- Kishawi, D.; Schwarzman, G.; Mejia, A.; Hussain, A.K.; Gonzalez, M.H. Low Preoperative Albumin Levels Predict Adverse Outcomes After Total Joint Arthroplasty. JBJS 2020, 102, 889. [Google Scholar] [CrossRef]
- Yiğit, Ş.; Akar, M.S.; Şahin, M.A.; Arslan, H. Periprosthetic Infection Risks and Predictive Value of C-Reactive Protein/Albumin Ratio for Total Joint Arthroplasty. Acta Biomed. 2021, 92, e2021324a. [Google Scholar] [CrossRef]
- Fu, W.; Kan, Q.; Li, B.; Zhang, X. Prognosis Model of Advanced Non-Small-Cell Lung Cancer Based on Max-Min Hill-Climbing Algorithm. Comput. Math. Methods Med. 2022, 2022, 9173913. [Google Scholar] [CrossRef]
- LeBrun, D.G.; Nguyen, J.T.; Fisher, C.; Tuohy, S.; Lyman, S.; Gonzalez Della Valle, A.; Ast, M.P.; Carli, A.V. The Risk Assessment and Prediction Tool (RAPT) Score Predicts Discharge Destination, Length of Stay, and Postoperative Mobility After Total Joint Arthroplasty. J. Arthroplast. 2023, 38, S121–S129. [Google Scholar] [CrossRef] [PubMed]
- Vasta, S.; Papalia, R.; Torre, G.; Vorini, F.; Papalia, G.; Zampogna, B.; Fossati, C.; Bravi, M.; Campi, S.; Denaro, V. The Influence of Preoperative Physical Activity on Postoperative Outcomes of Knee and Hip Arthroplasty Surgery in the Elderly: A Systematic Review. J. Clin. Med. 2020, 9, 969. [Google Scholar] [CrossRef] [PubMed]
- Hewlett-Smith, N.; Pope, R.; Furness, J.; Simas, V.; Hing, W. Prognostic Factors for Inpatient Functional Recovery Following Total Hip and Knee Arthroplasty: A Systematic Review. Acta Orthop. 2020, 91, 313–318. [Google Scholar] [CrossRef]
- Stone, K.; Zwiggelaar, R.; Jones, P.; Parthaláin, N.M. A Systematic Review of the Prediction of Hospital Length of Stay: Towards a Unified Framework. PLoS Digit. Health 2022, 1, e0000017. [Google Scholar] [CrossRef]
- Li, G.; Weng, J.; Xu, C.; Wang, D.; Xiong, A.; Zeng, H. Factors Associated with the Length of Stay in Total Knee Arthroplasty Patients with the Enhanced Recovery after Surgery Model. J. Orthop. Surg. Res. 2019, 14, 343. [Google Scholar] [CrossRef]
- Sanchez-Ramirez, D.C.; van der Leeden, M.; van der Esch, M.; Gerritsen, M.; Roorda, L.D.; Verschueren, S.; van Dieën, J.; Dekker, J.; Lems, W.F. Association of Serum C-Reactive Protein and Erythrocyte Sedimentation Rate with Muscle Strength in Patients with Knee Osteoarthritis. Rheumatology 2013, 52, 727–732. [Google Scholar] [CrossRef]
- Mathiessen, A.; Conaghan, P.G. Synovitis in Osteoarthritis: Current Understanding with Therapeutic Implications. Arthritis Res. Ther. 2017, 19, 18. [Google Scholar] [CrossRef]
- Cannataro, R.; Carbone, L.; Petro, J.L.; Cione, E.; Vargas, S.; Angulo, H.; Forero, D.A.; Odriozola-Martínez, A.; Kreider, R.B.; Bonilla, D.A. Sarcopenia: Etiology, Nutritional Approaches, and miRNAs. Int. J. Mol. Sci. 2021, 22, 9724. [Google Scholar] [CrossRef]
- Hirsch, K.R.; Wolfe, R.R.; Ferrando, A.A. Pre- and Post-Surgical Nutrition for Preservation of Muscle Mass, Strength, and Functionality Following Orthopedic Surgery. Nutrients 2021, 13, 1675. [Google Scholar] [CrossRef]
- Holstege, M.S.; Lindeboom, R.; Lucas, C. Preoperative Quadriceps Strength as a Predictor for Short-Term Functional Outcome After Total Hip Replacement. Arch. Phys. Med. Rehabil. 2011, 92, 236–241. [Google Scholar] [CrossRef]
- Pfitzner, T.; Krocker, D.; Perka, C.; Matziolis, G. Das C-Reaktive Protein. Orthopäde 2008, 37, 1116–1120. [Google Scholar] [CrossRef]
- Shetty, S.; Ethiraj, P.; Shanthappa, A.H. C-Reactive Protein Is a Diagnostic Tool for Postoperative Infection in Orthopaedics. Cureus 2022, 14, e22270. [Google Scholar] [CrossRef]
- Liang, Y.; Xu, K.; Liu, W.; Liu, X.; Yuan, P.; Xu, P.; Li, H. Monomeric C-reactive Protein Level Is Associated with Osteoarthritis. Exp. Ther. Med. 2022, 23, 277. [Google Scholar] [CrossRef]
- Si, H.; Yang, T.; Zeng, Y.; Zhou, Z.; Pei, F.; Lu, Y.; Cheng, J.; Shen, B. Correlations between Inflammatory Cytokines, Muscle Damage Markers and Acute Postoperative Pain Following Primary Total Knee Arthroplasty. BMC Musculoskelet. Disord. 2017, 18, 265. [Google Scholar] [CrossRef]
- Heim, C.E.; Yamada, K.J.; Fallet, R.; Odvody, J.; Schwarz, D.M.; Lyden, E.R.; Anderson, M.J.; Alter, R.; Vidlak, D.; Hartman, C.W.; et al. Orthopaedic Surgery Elicits a Systemic Anti-Inflammatory Signature. J. Clin. Med. 2020, 9, 2123. [Google Scholar] [CrossRef]
- Lv, Y.; Liu, N.; Li, Y.; Wu, J.; Zheng, J.; Li, X.; Zeng, M. Coagulation Dysfunction in Patients with Liver Cirrhosis and Splenomegaly and Its Countermeasures: A Retrospective Study of 1522 Patients. Dis. Markers 2023, 2023, 5560560. [Google Scholar] [CrossRef]
- Wikkelsø, A.J.; Edwards, H.M.; Afshari, A.; Stensballe, J.; Langhoff-Roos, J.; Albrechtsen, C.; Ekelund, K.; Hanke, G.; Secher, E.L.; Sharif, H.F.; et al. Pre-Emptive Treatment with Fibrinogen Concentrate for Postpartum Haemorrhage: Randomized Controlled Trial†. BJA Br. J. Anaesth. 2015, 114, 623–633. [Google Scholar] [CrossRef]
- Basili, S.; Raparelli, V.; Violi, F. The Coagulopathy of Chronic Liver Disease: Is There a Causal Relationship with Bleeding? Yes. Eur. J. Intern. Med. 2010, 21, 62–64. [Google Scholar] [CrossRef]
- Çakır, T.; Cingi, A.; Yeğen, C. Coagulation Dynamics and Platelet Functions in Obstructive Jaundiced Patients. J. Gastroenterol. Hepatol. 2009, 24, 748–751. [Google Scholar] [CrossRef]
- Cangemi, R.; Raparelli, V.; Talerico, G.; Basili, S.; Violi, F.; Giuseppe, P.; Felicia, D.; Ostilio, P.V.; Daniela, S.; Dario, D.M.; et al. Hypoalbuminemia and Risk of Portal Vein Thrombosis in Cirrhosis. Gastro Hep Adv. 2024, 3, 646–653. [Google Scholar] [CrossRef]
- Zealley, I. Retrospective Studies—Utility and Caveats. J. R. Coll. Physicians Edinb. 2021, 51, 106–110. [Google Scholar] [CrossRef]
| Factors | Scale (%) | Statistic | p | |
|---|---|---|---|---|
| Age | Less than 41 Years | 65 (16.00) | 1.958 a | 0.006 * |
| 41–60 Years | 245 (60.00) | |||
| Older than 60 Years | 98 (24.00) | |||
| Right Lower Extremity Strength | Outside the Reference Range | 109 (26.72) | 2.234 b | 0.029 * |
| Reference Range | 296 (72.54) | |||
| Missing | 3 (0.74) | |||
| ADL score | 100 | 90 (22.06) | 3.538 a | 0.03 * |
| 61–99 | 271 (66.42) | |||
| 41–60 | 25 (6.13) | |||
| Less than 41 | 14 (3.43) | |||
| Missing | 8 (1.96) | |||
| C-reactive Protein | Reference Range | 330 (80.88) | −2.514 b | 0.016 * |
| Outside the Reference Range | 61 (14.95) | |||
| Missing | 17 (4.17) | |||
| Thrombin Time | Reference Range | 124 (30.39) | −2.393 b | 0.019 * |
| Outside the Reference Range | 278 (68.14) | |||
| Missing | 6 (1.47) | |||
| Prothrombin Activity | Reference Range | 364 (89.22) | 2.582 b | 0.013 * |
| Outside the Reference Range | 38 (9.31) | |||
| Missing | 6 (1.47) | |||
| ESR | Reference Range | 304 (74.51) | −2.519 b | 0.015 * |
| Outside the Reference Range | 94 (23.04) | |||
| Missing | 10 (2.45) | |||
| Item | Bias Regression Coefficient | Standard Error | Standard Regression Coefficient | t | p |
|---|---|---|---|---|---|
| Constant | 32.232 | 20.832 | 1.547 | 0.123 | |
| Right Lower Extremity Strength | 1.203 | 0.431 | 0.134 | 2.794 | 0.005 |
| ADL score | −0.046 | 0.013 | −0.182 | −3.481 | 0.001 |
| ESR | 0.040 | 0.016 | 0.149 | 2.589 | 0.010 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, N.; Wang, X.; Yang, M.; Wang, X.; Dou, X. Bayesian Analysis of Length of Stay Determinants in ERAS-Guided Hip Arthroplasty. Healthcare 2025, 13, 777. https://doi.org/10.3390/healthcare13070777
Yao N, Wang X, Yang M, Wang X, Dou X. Bayesian Analysis of Length of Stay Determinants in ERAS-Guided Hip Arthroplasty. Healthcare. 2025; 13(7):777. https://doi.org/10.3390/healthcare13070777
Chicago/Turabian StyleYao, Nan, Xiaoyan Wang, Meng Yang, Xinglei Wang, and Xinman Dou. 2025. "Bayesian Analysis of Length of Stay Determinants in ERAS-Guided Hip Arthroplasty" Healthcare 13, no. 7: 777. https://doi.org/10.3390/healthcare13070777
APA StyleYao, N., Wang, X., Yang, M., Wang, X., & Dou, X. (2025). Bayesian Analysis of Length of Stay Determinants in ERAS-Guided Hip Arthroplasty. Healthcare, 13(7), 777. https://doi.org/10.3390/healthcare13070777





