The Association Between Craniofacial Morphological Parameters and the Severity of Obstructive Sleep Apnea: A Multivariate Analysis Using the Apnea–Hypopnea Index and Nocturnal Oxygen Desaturation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Samples
2.2. PSG
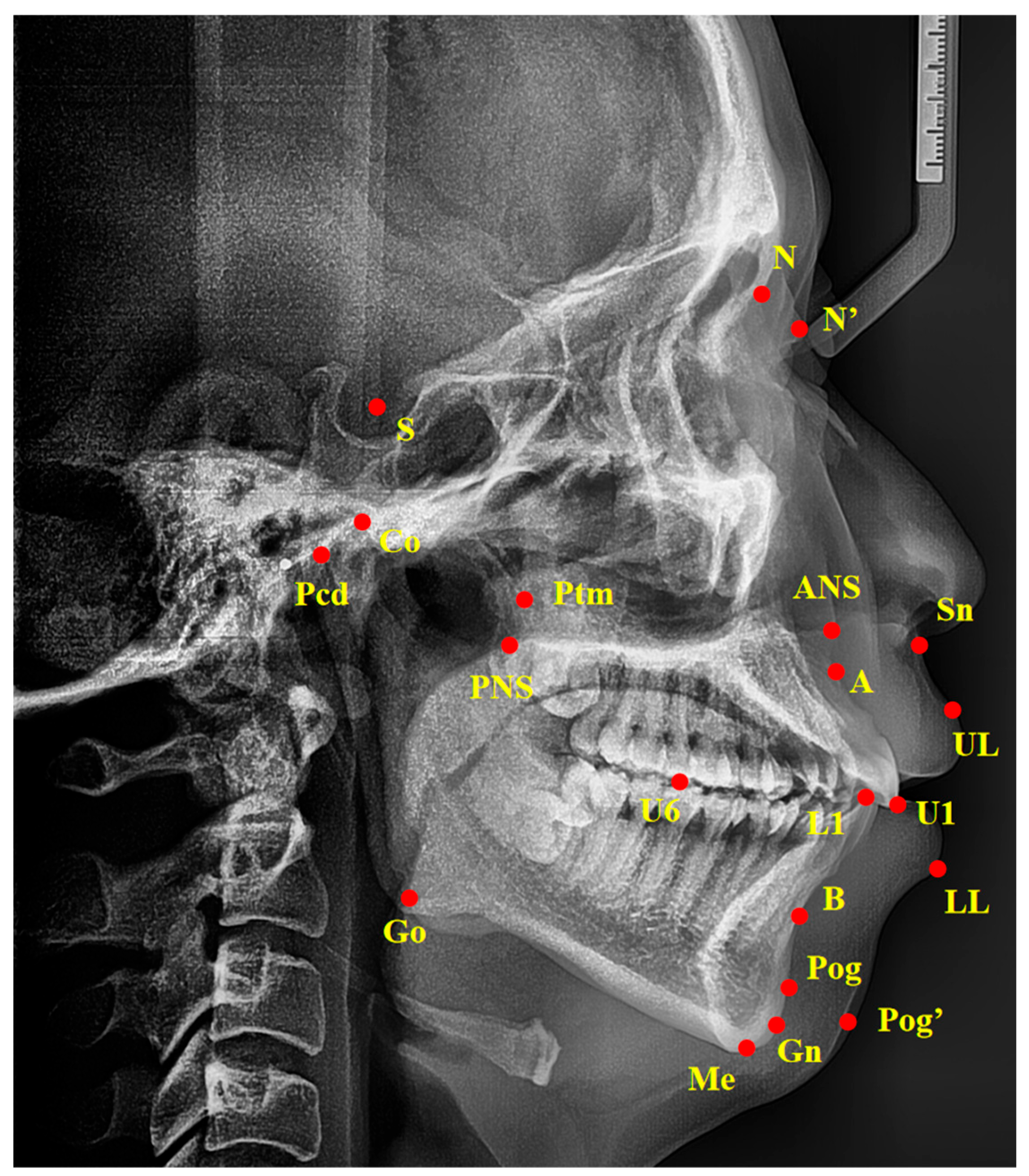
2.3. Cephalometric Analysis
2.4. Statistical Analysis
3. Results
3.1. Demographic Data
3.2. Correlation Analysis Between Craniofacial Cephalometric Parameters and the Severity of OSA in All Subjects Regardless of Gender
3.3. Correlation Analysis Between Craniofacial Cephalometric Parameters and the Severity of OSA in Male and Female Individuals
3.4. Multiple Regression Analysis Between Cephalometric Parameters and OSA Severity Indicators in the Male and Female Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patel, S.R. Obstructive Sleep Apnea. Ann. Intern. Med. 2019, 171, itc81–itc96. [Google Scholar] [CrossRef] [PubMed]
- Benjafield, A.V.; Ayas, N.T.; Eastwood, P.R.; Heinzer, R.; Ip, M.S.M.; Morrell, M.J.; Nunez, C.M.; Patel, S.R.; Penzel, T.; Pépin, J.L.; et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: A literature-based analysis. Lancet Respir. Med. 2019, 7, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Al Lawati, N.M.; Patel, S.R.; Ayas, N.T. Epidemiology, risk factors, and consequences of obstructive sleep apnea and short sleep duration. Prog. Cardiovasc. Dis. 2009, 51, 285–293. [Google Scholar] [CrossRef]
- Magnusdottir, S.; Hill, E.A. Prevalence of obstructive sleep apnea (OSA) among preschool aged children in the general population: A systematic review. Sleep. Med. Rev. 2024, 73, 101871. [Google Scholar] [CrossRef]
- Ouahchi, Y.; Mejbri, M.; Mediouni, A.; Hedhli, A.; Ouahchi, I.; El Euch, M.; Toujani, S.; Dhahri, B. The Resolution of Obstructive Sleep Apnea in a Patient with Goiter after Total Thyroidectomy: A Case Report. Reports 2024, 7, 29. [Google Scholar] [CrossRef]
- Fernandez-Mendoza, J.; He, F.; Calhoun, S.L.; Vgontzas, A.N.; Liao, D.; Bixler, E.O. Association of Pediatric Obstructive Sleep Apnea with Elevated Blood Pressure and Orthostatic Hypertension in Adolescence. JAMA Cardiol. 2021, 6, 1144–1151. [Google Scholar] [CrossRef]
- Smith, D.L.; Gozal, D.; Hunter, S.J.; Philby, M.F.; Kaylegian, J.; Kheirandish-Gozal, L. Impact of sleep disordered breathing on behaviour among elementary school-aged children: A cross-sectional analysis of a large community-based sample. Eur. Respir. J. 2016, 48, 1631–1639. [Google Scholar] [CrossRef]
- Lévy, P.; Kohler, M.; McNicholas, W.T.; Barbé, F.; McEvoy, R.D.; Somers, V.K.; Lavie, L.; Pépin, J.L. Obstructive sleep apnoea syndrome. Nat. Rev. Dis. Primers 2015, 1, 15015. [Google Scholar] [CrossRef]
- Yeghiazarians, Y.; Jneid, H.; Tietjens, J.R.; Redline, S.; Brown, D.L.; El-Sherif, N.; Mehra, R.; Bozkurt, B.; Ndumele, C.E.; Somers, V.K. Obstructive Sleep Apnea and Cardiovascular Disease: A Scientific Statement from the American Heart Association. Circulation 2021, 144, e56–e67. [Google Scholar] [CrossRef]
- Lv, R.; Liu, X.; Zhang, Y.; Dong, N.; Wang, X.; He, Y.; Yue, H.; Yin, Q. Pathophysiological mechanisms and therapeutic approaches in obstructive sleep apnea syndrome. Signal Transduct. Target. Ther. 2023, 8, 218. [Google Scholar] [CrossRef]
- Randerath, W.; Verbraecken, J.; de Raaff, C.A.L.; Hedner, J.; Herkenrath, S.; Hohenhorst, W.; Jakob, T.; Marrone, O.; Marklund, M.; McNicholas, W.T.; et al. European Respiratory Society guideline on non-CPAP therapies for obstructive sleep apnoea. Eur. Respir. Rev. 2021, 30, 210200. [Google Scholar] [CrossRef] [PubMed]
- Jo, J.H.; Park, J.W.; Jang, J.H.; Chung, J.W. Hyoid bone position as an indicator of severe obstructive sleep apnea. BMC Pulm. Med. 2022, 22, 349. [Google Scholar] [CrossRef] [PubMed]
- Armalaite, J.; Lopatiene, K. Lateral teleradiography of the head as a diagnostic tool used to predict obstructive sleep apnea. Dentomaxillofacial Radiol. 2016, 45, 20150085. [Google Scholar] [CrossRef]
- Signorelli, L.; Patcas, R.; Peltomäki, T.; Schätzle, M. Radiation dose of cone-beam computed tomography compared to conventional radiographs in orthodontics. J. Orofac. Orthop. 2016, 77, 9–15. [Google Scholar] [CrossRef]
- Neelapu, B.C.; Kharbanda, O.P.; Sardana, H.K.; Balachandran, R.; Sardana, V.; Kapoor, P.; Gupta, A.; Vasamsetti, S. Craniofacial and upper airway morphology in adult obstructive sleep apnea patients: A systematic review and meta-analysis of cephalometric studies. Sleep. Med. Rev. 2017, 31, 79–90. [Google Scholar] [CrossRef]
- Finke, H.; Drews, A.; Engel, C.; Koos, B. Craniofacial risk factors for obstructive sleep apnea-systematic review and meta-analysis. J. Sleep. Res. 2024, 33, e14004. [Google Scholar] [CrossRef]
- Gottlieb, D.J.; Punjabi, N.M. Diagnosis and Management of Obstructive Sleep Apnea: A Review. Jama 2020, 323, 1389–1400. [Google Scholar] [CrossRef]
- Mediano, O.; Barceló, A.; de la Peña, M.; Gozal, D.; Agustí, A.; Barbé, F. Daytime sleepiness and polysomnographic variables in sleep apnoea patients. Eur. Respir. J. 2007, 30, 110–113. [Google Scholar] [CrossRef]
- Asano, K.; Takata, Y.; Usui, Y.; Shiina, K.; Hashimura, Y.; Kato, K.; Saruhara, H.; Yamashina, A. New index for analysis of polysomnography, ‘integrated area of desaturation’, is associated with high cardiovascular risk in patients with mild to moderate obstructive sleep apnea. Respiration 2009, 78, 278–284. [Google Scholar] [CrossRef]
- Wang, L.; Wei, D.H.; Zhang, J.; Cao, J. Time Under 90% Oxygen Saturation and Systemic Hypertension in Patients with Obstructive Sleep Apnea Syndrome. Nat. Sci. Sleep. 2022, 14, 2123–2132. [Google Scholar] [CrossRef]
- Pae, E.K.; Lowe, A.A.; Sasaki, K.; Price, C.; Tsuchiya, M.; Fleetham, J.A. A cephalometric and electromyographic study of upper airway structures in the upright and supine positions. Am. J. Orthod. Dentofacial Orthop. 1994, 106, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Duarte, R.L.M.; Magalhães-da-Silveira, F.J.; Gozal, D. Screening for obstructive sleep apnea: Comparing the American Academy of Sleep Medicine proposed criteria with the STOP-Bang, NoSAS, and GOAL instruments. J. Clin. Sleep. Med. 2023, 19, 1239–1246. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Guo, Y.; Yang, C.; Zhou, Y.; Lin, Y.; Cheng, F.; Quan, S.; Feng, Q.; Li, J. Artificial intelligence system for automated landmark localization and analysis of cephalometry. Dentomaxillofacial Radiol. 2023, 52, 20220081. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Fujimoto, K.; Urushibata, K.; Matsuzawa, Y.; Kubo, K. Cephalometric analysis in obese and nonobese patients with obstructive sleep apnea syndrome. Chest 2003, 124, 212–218. [Google Scholar] [CrossRef]
- Pevernagie, D.A.; Gnidovec-Strazisar, B.; Grote, L.; Heinzer, R.; McNicholas, W.T.; Penzel, T.; Randerath, W.; Schiza, S.; Verbraecken, J.; Arnardottir, E.S. On the rise and fall of the apnea-hypopnea index: A historical review and critical appraisal. J. Sleep. Res. 2020, 29, e13066. [Google Scholar] [CrossRef]
- Malhotra, A.; Ayappa, I.; Ayas, N.; Collop, N.; Kirsch, D.; McArdle, N.; Mehra, R.; Pack, A.I.; Punjabi, N.; White, D.P.; et al. Metrics of sleep apnea severity: Beyond the apnea-hypopnea index. Sleep. 2021, 44, zsab030. [Google Scholar] [CrossRef]
- Gabryelska, A.; Chrzanowski, J.; Sochal, M.; Kaczmarski, P.; Turkiewicz, S.; Ditmer, M.; Karuga, F.F.; Czupryniak, L.; Białasiewicz, P. Nocturnal Oxygen Saturation Parameters as Independent Risk Factors for Type 2 Diabetes Mellitus among Obstructive Sleep Apnea Patients. J. Clin. Med. 2021, 10, 3770. [Google Scholar] [CrossRef]
- Fernandes, E.R.; Pires, G.N.; Andersen, M.L.; Tufik, S.; Rosa, D.S. Oxygen saturation as a predictor of inflammation in obstructive sleep apnea. Sleep Breath. 2022, 26, 1613–1620. [Google Scholar] [CrossRef]
- Park, J.W.; Almeida, F.R. Disparities in oxygen saturation and hypoxic burden levels in obstructive sleep apnoea patient’s response to oral appliance treatment. J. Oral. Rehabil. 2022, 49, 633–643. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, X.; Li, N.; Wang, Y.; Xu, X.; Guo, J. Craniofacial and upper airway morphological characteristics associated with the presence and severity of obstructive sleep apnea in Chinese children. Front. Pediatr. 2023, 11, 1124610. [Google Scholar] [CrossRef]
- Johal, A.; Conaghan, C. Maxillary morphology in obstructive sleep apnea: A cephalometric and model study. Angle Orthod. 2004, 74, 648–656. [Google Scholar] [PubMed]
- Schwendicke, F.; Chaurasia, A.; Arsiwala, L.; Lee, J.H.; Elhennawy, K.; Jost-Brinkmann, P.G.; Demarco, F.; Krois, J. Deep learning for cephalometric landmark detection: Systematic review and meta-analysis. Clin. Oral. Investig. 2021, 25, 4299–4309. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.W.; Park, J.H.; Moon, J.H.; Yu, Y.; Kim, H.; Her, S.B.; Srinivasan, G.; Aljanabi, M.N.A.; Donatelli, R.E.; Lee, S.J. Automated identification of cephalometric landmarks: Part 2-Might it be better than human? Angle Orthod. 2020, 90, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Sadek, M.; Alaskari, O.; Hamdan, A. Accuracy of web-based automated versus digital manual cephalometric landmark identification. Clin. Oral. Investig. 2024, 28, 621. [Google Scholar] [CrossRef]
- Behrents, R.G.; Shelgikar, A.V.; Conley, R.S.; Flores-Mir, C.; Hans, M.; Levine, M.; McNamara, J.A.; Palomo, J.M.; Pliska, B.; Stockstill, J.W.; et al. Obstructive sleep apnea and orthodontics: An American Association of Orthodontists White Paper. Am. J. Orthod. Dentofacial Orthop. 2019, 156, 13–28.e11. [Google Scholar] [CrossRef]
- Trzepizur, W.; Blanchard, M.; Ganem, T.; Balusson, F.; Feuilloy, M.; Girault, J.M.; Meslier, N.; Oger, E.; Paris, A.; Pigeanne, T.; et al. Sleep Apnea-Specific Hypoxic Burden, Symptom Subtypes, and Risk of Cardiovascular Events and All-Cause Mortality. Am. J. Respir. Crit. Care Med. 2022, 205, 108–117. [Google Scholar] [CrossRef]
- Bixler, E.O.; Vgontzas, A.N.; Lin, H.M.; Ten Have, T.; Rein, J.; Vela-Bueno, A.; Kales, A. Prevalence of sleep-disordered breathing in women: Effects of gender. Am. J. Respir. Crit. Care Med. 2001, 163, 608–613. [Google Scholar] [CrossRef]
- Chang, E.T.; Wang, H.M.; Lai, H.L. Gender differences in obstructive sleep apnea syndrome. Eur. J. Intern. Med. 2016, 33, e9–e10. [Google Scholar] [CrossRef]
- Senaratna, C.V.; Perret, J.L.; Lodge, C.J.; Lowe, A.J.; Campbell, B.E.; Matheson, M.C.; Hamilton, G.S.; Dharmage, S.C. Prevalence of obstructive sleep apnea in the general population: A systematic review. Sleep. Med. Rev. 2017, 34, 70–81. [Google Scholar] [CrossRef]
- Huang, L.; Gao, X. The interaction of obesity and craniofacial deformity in obstructive sleep apnea. Dentomaxillofacial Radiol. 2021, 50, 20200425. [Google Scholar] [CrossRef]
- Cho, S.H.; Jeon, J.Y.; Jang, K.S.; Kim, S.Y.; Kim, K.R.; Ryu, S.; Hwang, K.G. Gender-specific cephalometric features related to obesity in sleep apnea patients: Trilogy of soft palate-mandible-hyoid bone. Maxillofac. Plast. Reconstr. Surg. 2019, 41, 58. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.P.; Ayappa, I.A.; Caples, S.M.; Kimoff, R.J.; Patel, S.R.; Harrod, C.G. Treatment of Adult Obstructive Sleep Apnea with Positive Airway Pressure: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep. Med. 2019, 15, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Kapur, V.K.; Auckley, D.H.; Chowdhuri, S.; Kuhlmann, D.C.; Mehra, R.; Ramar, K.; Harrod, C.G. Clinical Practice Guideline for Diagnostic Testing for Adult Obstructive Sleep Apnea: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep. Med. 2017, 13, 479–504. [Google Scholar] [CrossRef] [PubMed]
- Lyons-Coleman, M.; Bates, C.; Barber, S. Obstructive sleep apnoea and the role of the dental team. Br. Dent. J. 2020, 228, 681–685. [Google Scholar] [CrossRef]
- Dicus Brookes, C.C.; Boyd, S.B. Controversies in Obstructive Sleep Apnea Surgery. Oral. Maxillofac. Surg. Clin. N. Am. 2017, 29, 503–513. [Google Scholar] [CrossRef]
- Hamoda, M.M.; Kohzuka, Y.; Almeida, F.R. Oral Appliances for the Management of OSA: An Updated Review of the Literature. Chest 2018, 153, 544–553. [Google Scholar] [CrossRef]
- Xie, B.; Zhang, L.; Lu, Y. The role of rapid maxillary expansion in pediatric obstructive sleep apnea: Efficacy, mechanism and multidisciplinary collaboration. Sleep. Med. Rev. 2023, 67, 101733. [Google Scholar] [CrossRef]
| Measurements | Description |
|---|---|
| Angular measurements (°) | |
| SNA | Angle between point S and A at N |
| SNB | Angle between point S and B at N |
| ANB | Angle between point A and B at N |
| PP-FH | Angle between PP plane and FH plane |
| PP-GoGn | Angle between PP plane and line Go–Gn |
| OP-SN | Angle between occlusal plane and SN plane |
| MP-SN | Angle between mandibular plane and SN plane |
| FH-MP | Angle between FH plane and mandibular plane |
| SGn-FH | Angle between line S–Gn and FH plane |
| NBa-PtGn | Angle between line N–Ba and line Pt–Gn |
| U1-L1 | Angle between the long axis of upper incisors and lower incisors |
| U1-SN | Angle between the long axis of upper incisors and SN plane |
| U1-NA | Angle between the long axis of upper incisors and line N–A |
| L1-NB | Angle between the long axis of lower incisors and line N–B |
| L1-FH | Angle between the long axis of lower incisors and FH plane |
| Z-Angle | Angle between line Z-line and FH plane |
| FH-N’Pog’ | Angle between FH plane and line N’Pog’ |
| N’-Sn-Pog’ | Angle between point N’ and Pog’ at Sn |
| Linear measurements (mm) | |
| Ptm-A | Distance between Ptm and A |
| Ptm-S | Distance between Ptm and S |
| Go-Pog | Distance between Go and Pog |
| Go-Co | Distance between Go and Co |
| Pcd-S | Distance between Pcd and S |
| N-ANS | Distance between N and ANS |
| ANS-Me | Distance between ANS and Me |
| S-Go | Distance between S and Go |
| U1-NA | Distance between U1 and line N–A |
| L1-NB | Distance between L1 and line N–B |
| U1-APo | Distance between U1 and line A–Pog |
| L1-APo | Distance between L1 and line A–Pog |
| U6-Ptm | Distance between U6 and Ptm |
| U1-PP | Distance between U1 and PP plane |
| U6-PP | Distance between U6 and PP plane |
| L1-MP | Distance between L1 and mandibular plane |
| L6-MP | Distance between L6 and mandibular plane |
| UL-EP | Distance between UL and EP plane |
| LL-EP | Distance between LL and EP plane |
| Male | Female | Total | p-Value (Male vs. Female) | |
|---|---|---|---|---|
| Number | 67 | 45 | 112 | - |
| Age | 28.8 ± 7.6 | 27.8 ± 6.8 | 28.4 ± 7.3 | 0.469 |
| Polysomnography parameters | ||||
| AHI | 11.8 (7.20, 19.0) | 7.40 (5.20, 11,1) | 10.0 (6.00, 17.9) | 0.001 ** |
| LSaO2 (%) | 86.0 (80.0, 90.0) | 89.0 (85.0, 92.0) | 87.0 (82.0, 91.0) | 0.019 * |
| Cephalometric parameters | ||||
| SNA | 80.9 ± 3.7 | 82.0 ± 3.6 | 81.3 ± 3.7 | 0.095 |
| SNB | 74.1 ± 5.0 | 73.6 ± 4.7 | 73.9 ± 4.9 | 0.534 |
| ANB | 6.7 (5.1, 7.9) | 8.5 ± 3.1 | 7.2 (5.8, 8.8) | <0.001 *** |
| Ptm-A | 44.6 ± 3.4 | 43.8 ± 3.9 | 44.3 ± 3.6 | 0.303 |
| Ptm-S | 17.6 ± 3.6 | 18.5 ± 2.7 | 18.0 ± 3.3 | 0.185 |
| PP-FH | 3.2 ± 3.0 | 2.6 ± 3.3 | 3.0 ± 3.1 | 0.272 |
| PP-GoGn | 28.8 ± 8.0 | 32.3 ± 8.9 | 30.2 ± 8.5 | 0.028 * |
| OP-SN | 21.4 (17.2, 25.6) | 22.6 (18.1, 28.0) | 21.7 (18.0, 26.3) | 0.320 |
| Go-Pog | 67.1 ± 6.4 | 64.8 ± 8.0 | 66.2 ± 7.2 | 0.092 |
| Go-Co | 55.2 ± 6.1 | 51.1 ± 6.5 | 54.4 (50.5, 57.5) | 0.001 ** |
| Pcd-S | 16.3 ± 3.2 | 14.6 ± 2.6 | 15.6 ± 3.1 | 0.003 |
| MP-SN | 40.8 ± 10.0 | 44.6 ± 9.3 | 42.3 ± 9.8 | 0.044 * |
| FH-MP | 33.6 ± 9.8 | 36.8 ± 9.7 | 34.9 ± 9.9 | 0.098 |
| SGn-FH | 68.3 ± 5.5 | 69.2 ± 5.2 | 68.6 ± 5.3 | 0.388 |
| NBa-PtGn | 82.4 ± 7.4 | 79.3 ± 7.7 | 81.2 ± 7.6 | 0.037 * |
| N-ANS | 54.7 ± 3.8 | 52.0 (50.9, 54.4) | 53.4 (51.2, 56.1) | 0.001 ** |
| ANS-Me | 64.8 ± 7.3 | 63.2 ± 8.5 | 64.2 ± 7.8 | 0.297 |
| S-Go | 74.9 ± 7.7 | 69.9 ± 7.2 | 72.9 ± 7.8 | <0.001 *** |
| S-Go/N-Me | 62.8 ± 5.5 | 60.3 ± 4.4 | 61.8 ± 5.2 | 0.013 * |
| ANS-Me/N-Me | 54.1 ± 2.4 | 54.3 ± 3.3 | 54.2 ± 2.8 | 0.723 |
| U1-L1 | 121 ± 11.8 | 117 (110, 124) | 120 ± 11.7 | 0.214 |
| U1-SN | 99.3 ± 9.0 | 98.3 ± 8.8 | 98.9 ± 8.9 | 0.562 |
| U1-NA (mm) | 4.4 ± 3.7 | 4.4 ± 3.8 | 4.4 ± 3.7 | 0.941 |
| U1-NA (°) | 18.5 ± 8.8 | 16.7 ± 7.4 | 17.8 ± 8.2 | 0.267 |
| L1-NB (mm) | 9.0 ± 3.2 | 10.5 ± 3.6 | 9.62 ± 3.4 | 0.022 * |
| L1-NB (°) | 34.3 ± 7.6 | 36.5 ± 6.5 | 35.2 ± 7.2 | 0.114 |
| L1-FH | 47.0 ± 10.4 | 44.9 ± 7.6 | 46.1 ± 9.4 | 0.243 |
| U1-APo | 10.4 ± 3.7 | 12.6 ± 4.2 | 11.3 ± 4.1 | 0.004 ** |
| L1-APo | 4.9 ± 3.8 | 6.0 ± 3.7 | 5.3 ± 3.8 | 0.117 |
| U6-Ptm | 20.5 ± 4.2 | 19.0 ± 3.8 | 19.9 ± 4.1 | 0.057 |
| U1-PP | 30.8 ± 3.1 | 31.3 ± 3.7 | 31.0 ± 3.3 | 0.452 |
| U6-PP | 23.8 ± 3.3 | 23.5 ± 3.1 | 23.7 ± 3.2 | 0.568 |
| L1-MP | 43.0 ± 4.4 | 43.2 ± 5.0 | 43.1 ± 4.6 | 0.757 |
| L6-MP | 34.1 ± 3.6 | 33.0 ± 4.0 | 33.6 ± 3.8 | 0.118 |
| UL-EP | 2.96 ± 3.2 | 5.06 ± 3.0 | 3.8 ± 3.3 | <0.001 *** |
| LL-EP | 3.57 ± 3.7 | 6.10 ± 3.4 | 4.6 ± 3.8 | <0.001 *** |
| Z-Angle | 54.3 ± 13.5 | 48.5 ± 11.0 | 52.0 ± 12.8 | 0.018 * |
| FH-N’Pog’ | 84.3 ± 6.2 | 83.6 ± 5.2 | 84.0 ± 5.8 | 0.549 |
| N’-SN-Pog’ | 154.0 ± 7.5 | 152.0 ± 6.7 | 153.0 ± 7.2 | 0.094 |
| AHI | LSaO2 | |||
|---|---|---|---|---|
| r | p | r | p | |
| SNA | −0.195 | 0.039 | 0.132 | 0.166 |
| SNB | −0.248 | 0.008 ** | 0.131 | 0.167 |
| ANB | 0.107 | 0.261 | −0.016 | 0.865 |
| Ptm-A | 0.046 | 0.633 | 0.015 | 0.878 |
| Ptm-S | −0.081 | 0.394 | −0.087 | 0.363 |
| PP-FH | 0.205 | 0.030 * | −0.041 | 0.665 |
| PP-GoGn | −0.133 | 0.162 | 0.04 | 0.674 |
| OP-SN | 0.003 | 0.977 | 0.075 | 0.431 |
| Go-Pog | −0.148 | 0.121 | 0.038 | 0.689 |
| Go-Co | 0.044 | 0.648 | −0.122 | 0.201 |
| Pcd-S | 0.169 | 0.075 | −0.039 | 0.681 |
| MP-SN | 0.006 | 0.952 | −0.015 | 0.878 |
| FH-MP | −0.012 | 0.899 | 0.016 | 0.866 |
| SGn-FH | 0.101 | 0.291 | −0.016 | 0.863 |
| NBa-PtGn | −0.1 | 0.294 | 0.1 | 0.292 |
| N-ANS | 0.134 | 0.158 | −0.121 | 0.202 |
| ANS-Me | −0.096 | 0.315 | −0.039 | 0.685 |
| S-Go | 0.055 | 0.567 | −0.106 | 0.265 |
| S-Go/N-Me | 0.083 | 0.387 | −0.051 | 0.594 |
| ANS-Me/N-Me | −0.18 | 0.058 | 0.015 | 0.877 |
| U1-L1 | 0.209 | 0.027 * | −0.114 | 0.230 |
| U1-SN | −0.211 | 0.025 * | 0.102 | 0.283 |
| U1-NA (mm) | −0.123 | 0.195 | 0.043 | 0.654 |
| U1-NA (°) | −0.084 | 0.381 | 0.017 | 0.860 |
| L1-NB (mm) | −0.12 | 0.209 | 0.052 | 0.583 |
| L1-NB (°) | −0.258 | 0.006 ** | 0.137 | 0.150 |
| L1-FH | 0.071 | 0.455 | −0.062 | 0.517 |
| U1-APo | −0.056 | 0.560 | 0.031 | 0.747 |
| L1-APo | −0.239 | 0.011 * | 0.078 | 0.415 |
| U6-Ptm | −0.073 | 0.447 | −0.026 | 0.748 |
| U1-PP | −0.069 | 0.467 | −0.014 | 0.885 |
| U6-PP | 0.014 | 0.885 | −0.104 | 0.273 |
| L1-MP | −0.01 | 0.919 | −0.078 | 0.413 |
| L6-MP | 0.053 | 0.582 | −0.12 | 0.206 |
| UL-EP | −0.006 | 0.946 | −0.001 | 0.988 |
| LL-EP | −0.131 | 0.170 | 0.115 | 0.226 |
| Z-Angle | 0.017 | 0.858 | −0.092 | 0.335 |
| FH-N’Pog’ | −0.127 | 0.182 | 0.011 | 0.909 |
| N’-SN-Pog’ | −0.01 | 0.916 | −0.09 | 0.347 |
| Male | Female | |||
|---|---|---|---|---|
| r | p | r | p | |
| SNA | −0.12 | 0.335 | −0.192 | 0.205 |
| SNB | −0.193 | 0.117 | −0.325 | 0.029 * |
| ANB | 0.168 | 0.173 | 0.265 | 0.078 |
| Ptm-A | 0.097 | 0.436 | −0.152 | 0.318 |
| Ptm-S | −0.052 | 0.677 | −0.047 | 0.760 |
| PP-FH | 0.062 | 0.616 | 0.259 | 0.086 |
| PP-GoGn | −0.044 | 0.726 | −0.125 | 0.412 |
| OP-SN | 0.068 | 0.585 | 0.007 | 0.963 |
| Go-Pog | −0.13 | 0.296 | −0.222 | 0.144 |
| Go-Co | 0.028 | 0.823 | −0.115 | 0.453 |
| Pcd-S | 0.089 | 0.475 | 0.026 | 0.866 |
| MP-SN | 0.065 | 0.604 | −0.007 | 0.965 |
| FH-MP | 0.024 | 0.846 | −0.028 | 0.857 |
| SGn-FH | 0.08 | 0.522 | 0.163 | 0.286 |
| NBa-PtGn | −0.127 | 0.307 | −0.186 | 0.221 |
| N-ANS | 0.109 | 0.379 | −0.01 | 0.946 |
| ANS-Me | 0.001 | 0.990 | −0.296 | 0.048 * |
| S-Go | 0.018 | 0.886 | −0.195 | 0.200 |
| S-Go/N-Me | −0.019 | 0.879 | 0.027 | 0.859 |
| ANS-Me/N-Me | −0.065 | 0.598 | −0.256 | 0.090 |
| U1-L1 | 0.25 | 0.041 * | 0.149 | 0.330 |
| U1-SN | −0.284 | 0.020 * | −0.132 | 0.386 |
| U1-NA (mm) | −0.134 | 0.278 | −0.112 | 0.463 |
| U1-NA (°) | −0.185 | 0.135 | −0.029 | 0.848 |
| L1-NB (mm) | −0.079 | 0.524 | −0.061 | 0.689 |
| L1-NB (°) | −0.199 | 0.107 | −0.311 | 0.037 * |
| L1-FH | 0.045 | 0.721 | 0.075 | 0.623 |
| U1-APo | −0.003 | 0.979 | 0.01 | 0.950 |
| L1-APo | −0.105 | 0.399 | −0.324 | 0.030 * |
| U6-Ptm | −0.022 | 0.858 | −0.238 | 0.115 |
| U1-PP | 0.02 | 0.875 | −0.187 | 0.220 |
| U6-PP | 0.122 | 0.324 | −0.19 | 0.211 |
| L1-MP | 0.114 | 0.360 | −0.189 | 0.214 |
| L6-MP | 0.102 | 0.409 | −0.236 | 0.119 |
| UL-EP | 0.187 | 0.129 | −0.055 | 0.720 |
| LL-EP | −0.037 | 0.766 | −0.105 | 0.492 |
| Z-Angle | −0.029 | 0.817 | −0.095 | 0.534 |
| FH-N’Pog’ | −0.071 | 0.567 | −0.2 | 0.188 |
| N’-SN-Pog’ | 0.036 | 0.772 | 0.112 | 0.465 |
| Male | Female | |||
|---|---|---|---|---|
| r | p | r | p | |
| SNA | 0.075 | 0.548 | 0.134 | 0.381 |
| SNB | 0.008 | 0.951 | 0.36 | 0.015 * |
| ANB | 0.036 | 0.775 | −0.319 | 0.033 * |
| Ptm-A | −0.092 | 0.458 | 0.235 | 0.120 |
| Ptm-S | −0.111 | 0.371 | −0.11 | 0.470 |
| PP-FH | 0.071 | 0.570 | −0.137 | 0.370 |
| PP-GoGn | 0.064 | 0.608 | −0.091 | 0.551 |
| OP-SN | 0.129 | 0.299 | −0.056 | 0.717 |
| Go-Pog | −0.089 | 0.473 | 0.274 | 0.069 |
| Go-Co | −0.229 | 0.062 | 0.174 | 0.252 |
| Pcd-S | 0.021 | 0.865 | 0.037 | 0.811 |
| MP-SN | −0.009 | 0.945 | −0.119 | 0.437 |
| FH-MP | 0.067 | 0.587 | −0.099 | 0.519 |
| SGn-FH | 0.128 | 0.301 | −0.262 | 0.082 |
| NBa-PtGn | 0.013 | 0.918 | 0.341 | 0.022 * |
| N-ANS | −0.161 | 0.193 | 0.054 | 0.727 |
| ANS-Me | −0.186 | 0.131 | 0.191 | 0.210 |
| S-Go | −0.183 | 0.139 | 0.23 | 0.128 |
| S-Go/N-Me | −0.002 | 0.988 | 0.067 | 0.661 |
| ANS-Me/N-Me | −0.111 | 0.371 | 0.13 | 0.394 |
| U1-L1 | −0.14 | 0.257 | −0.072 | 0.637 |
| U1-SN | 0.015 | 0.903 | 0.247 | 0.102 |
| U1-NA (mm) | −0.064 | 0.609 | 0.167 | 0.273 |
| U1-NA (°) | −0.053 | 0.670 | 0.208 | 0.171 |
| L1-NB (mm) | 0.087 | 0.483 | −0.098 | 0.524 |
| L1-NB (°) | 0.136 | 0.274 | 0.098 | 0.521 |
| L1-FH | −0.146 | 0.238 | 0.125 | 0.413 |
| U1-APo | 0.033 | 0.792 | −0.101 | 0.508 |
| L1-APo | −0.005 | 0.969 | 0.097 | 0.525 |
| U6-Ptm | −0.18 | 0.144 | 0.325 | 0.030 * |
| U1-PP | −0.077 | 0.538 | 0.065 | 0.670 |
| U6-PP | −0.276 | 0.024 * | 0.148 | 0.332 |
| L1-MP | −0.184 | 0.137 | 0.029 | 0.852 |
| L6-MP | −0.178 | 0.150 | 0.103 | 0.501 |
| UL-EP | −0.004 | 0.977 | −0.173 | 0.256 |
| LL-EP | 0.054 | 0.662 | 0.079 | 0.604 |
| Z-Angle | −0.157 | 0.204 | 0.099 | 0.518 |
| FH-N’Pog’ | −0.166 | 0.179 | 0.268 | 0.075 |
| N’-SN-Pog’ | −0.3 | 0.014 * | 0.229 | 0.130 |
| Gender | Variables | B | t | p | VIF | R2 | F |
|---|---|---|---|---|---|---|---|
| Male | L1-NB (°) | −0.745 | −2.407 | 0.019 | 1.395 | 0.124 | 2.984 * |
| U1-NA (°) | −0.337 | −1.462 | 0.149 | 1.017 | |||
| UL-EP | 1.025 | 1.392 | 0.169 | 1.390 | |||
| Female | SNB | −1.028 | −1.568 | 0.125 | 1.684 | 0.218 | 2.795 * |
| ANS-Me/N-Me | −1.644 | −1.902 | 0.064 | 1.404 | |||
| L1-NB (°) | −0.141 | −0.359 | 0.722 | 1.137 | |||
| U6-Ptm | 0.327 | 0.376 | 0.709 | 1.929 |
| Gender | Variables | B | t | p | VIF | R2 | F |
|---|---|---|---|---|---|---|---|
| U6-PP | −1.119 | −1.831 | 0.072 | 4.588 | 0.202 | 3.084 * | |
| Go-Co | −0.049 | −0.237 | 0.813 | 1.766 | |||
| Male | ANS-Me | 0.989 | 2.837 | 0.006 | 7.038 | ||
| L1-MP | −0.932 | −2.494 | 0.015 | 3 | |||
| N’-Sn-Pog’ | −0.526 | −2.894 | 0.005 | 2.037 | |||
| SNB | 0.231 | 0.694 | 0.492 | 3.958 | 0.469 | 6.881 ** | |
| ANB | −1.361 | −3.608 | 0.001 | 2.205 | |||
| Female | Go-Pog | −0.022 | −0.123 | 0.903 | 3.159 | ||
| U6-Ptm | 0.59 | 1.998 | 0.053 | 2.022 | |||
| SGn-FH | 0.471 | 1.791 | 0.081 | 2.958 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Z.; Wu, J.; Wu, L.; Hong, H. The Association Between Craniofacial Morphological Parameters and the Severity of Obstructive Sleep Apnea: A Multivariate Analysis Using the Apnea–Hypopnea Index and Nocturnal Oxygen Desaturation. Healthcare 2025, 13, 913. https://doi.org/10.3390/healthcare13080913
Dong Z, Wu J, Wu L, Hong H. The Association Between Craniofacial Morphological Parameters and the Severity of Obstructive Sleep Apnea: A Multivariate Analysis Using the Apnea–Hypopnea Index and Nocturnal Oxygen Desaturation. Healthcare. 2025; 13(8):913. https://doi.org/10.3390/healthcare13080913
Chicago/Turabian StyleDong, Zhili, Jinmei Wu, Liping Wu, and Hong Hong. 2025. "The Association Between Craniofacial Morphological Parameters and the Severity of Obstructive Sleep Apnea: A Multivariate Analysis Using the Apnea–Hypopnea Index and Nocturnal Oxygen Desaturation" Healthcare 13, no. 8: 913. https://doi.org/10.3390/healthcare13080913
APA StyleDong, Z., Wu, J., Wu, L., & Hong, H. (2025). The Association Between Craniofacial Morphological Parameters and the Severity of Obstructive Sleep Apnea: A Multivariate Analysis Using the Apnea–Hypopnea Index and Nocturnal Oxygen Desaturation. Healthcare, 13(8), 913. https://doi.org/10.3390/healthcare13080913







