Lokomat vs. Conventional Therapy—Impact on Gait Symmetry in Hemiparetic Patients: Preliminary Clinical Study
Abstract
1. Introduction
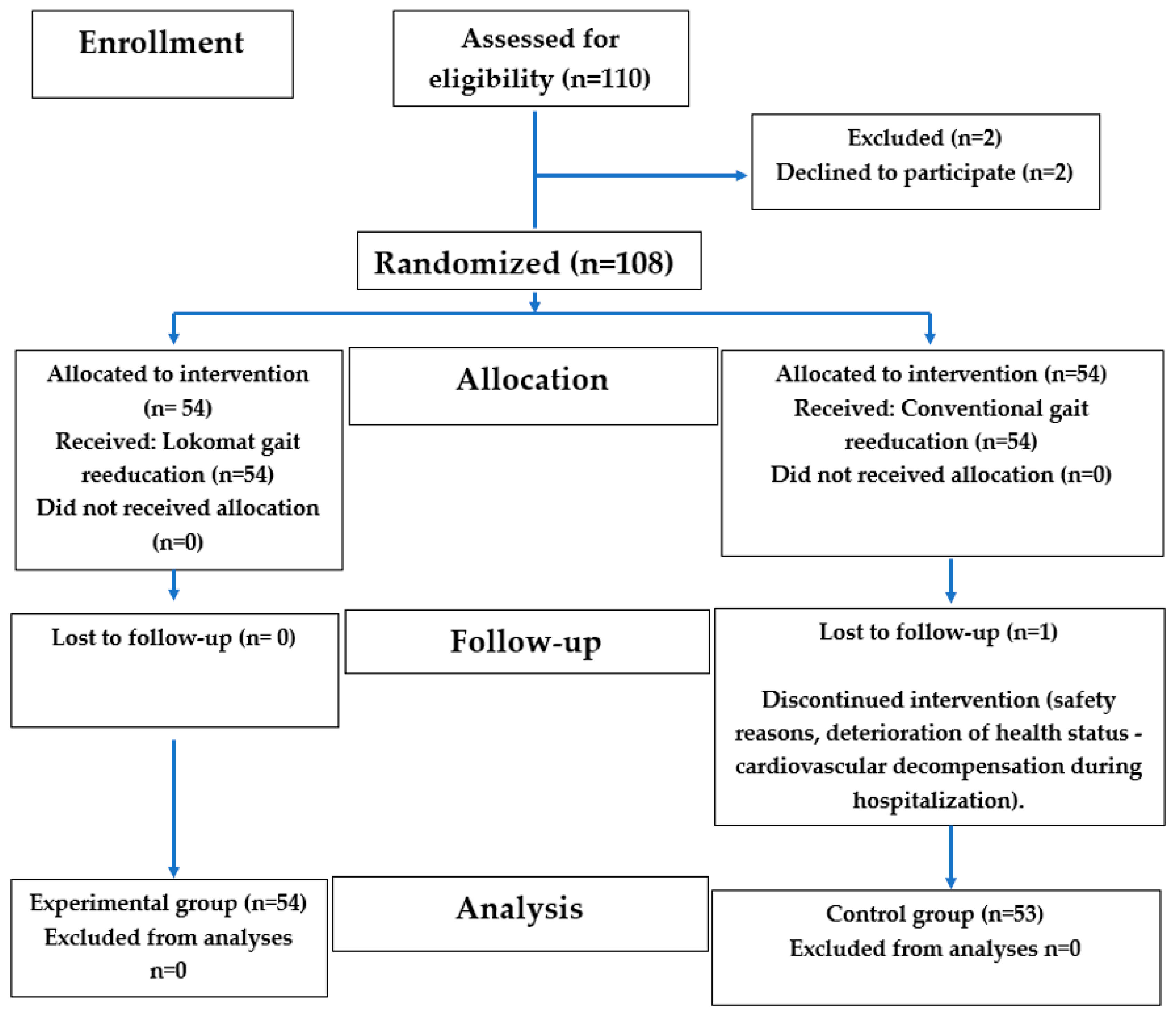
2. Materials and Methods
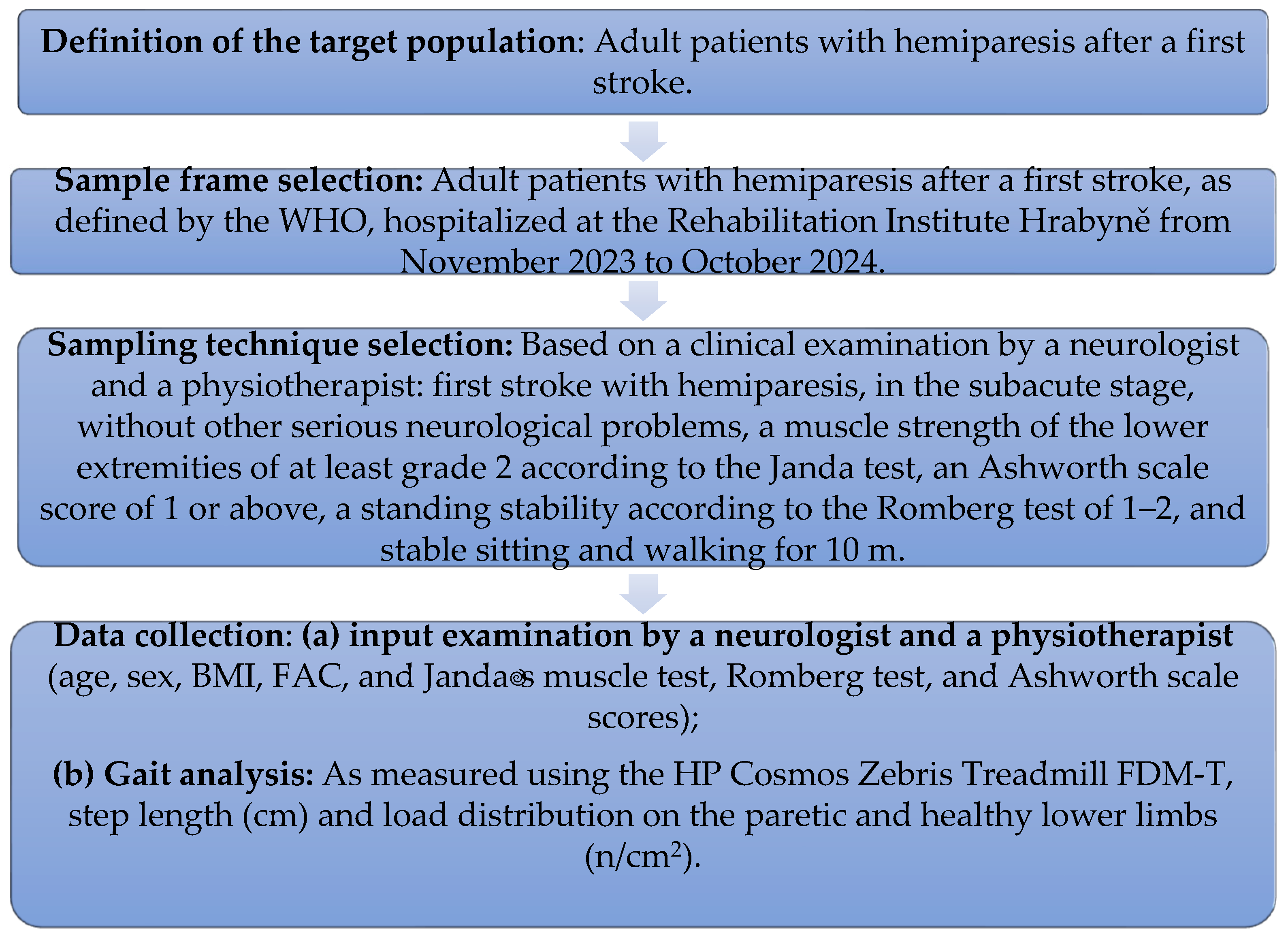
2.1. The Study Design
2.2. The Study Population
2.3. Comprehensive Neurorehabilitation
2.3.1. Treatment Groups
2.3.2. The Control Group
2.4. Measurements
2.5. Statistical Analysis
3. Results
3.1. The Stride Length Analysis
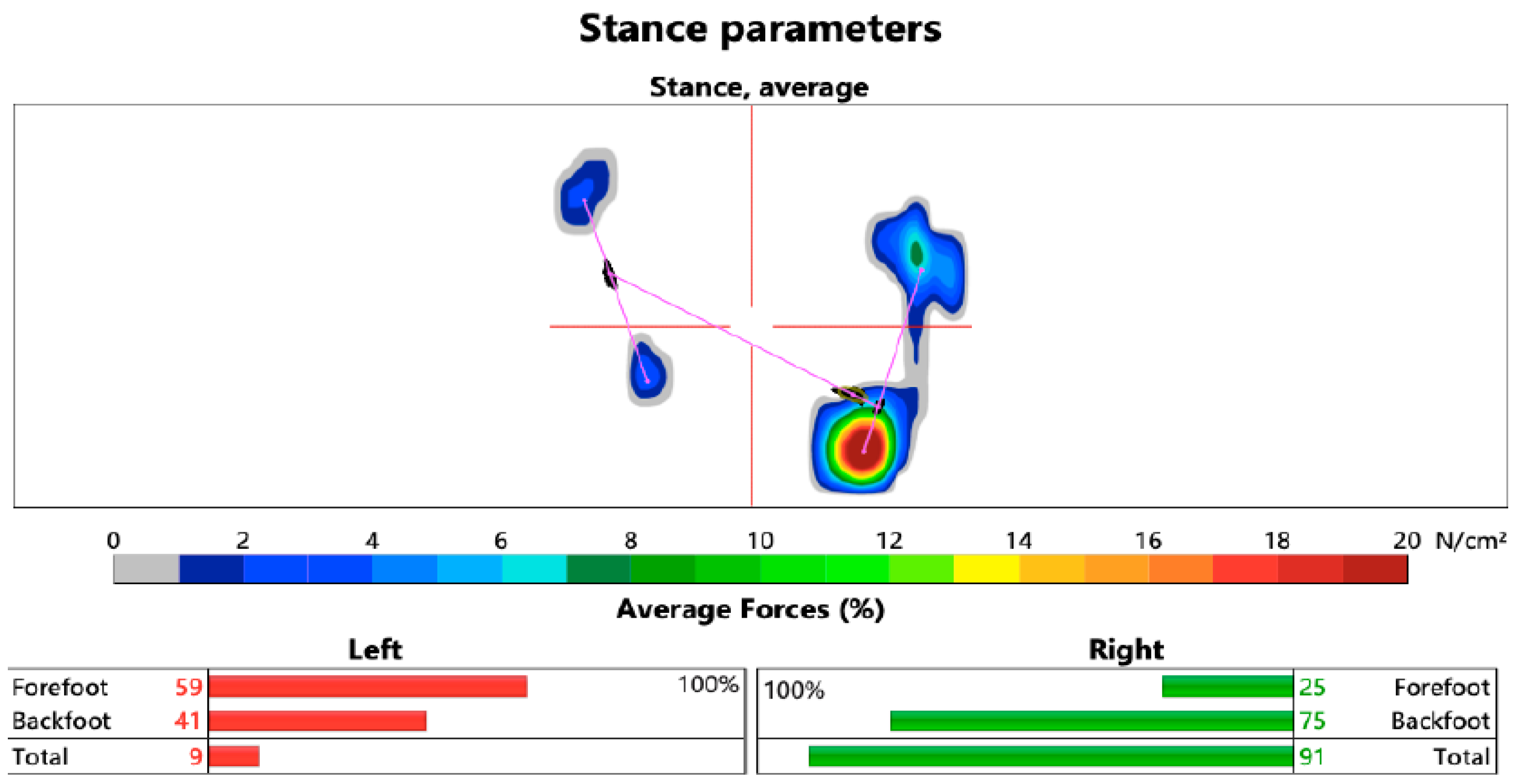
3.2. The Limb Load Analysis
4. Discussion
This Study’s Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Čuj, J. Coordination Changes in the Selected Remote Part of the Motion System When Walking in Flat Shoes and High Heels. Ph.D. Thesis, Univerzita Karlova, Praha, Czech Republic, 2021; pp. 21–23. [Google Scholar]
- Schuna, J.M.; Tudor-Locke, C. Step by step: Accumulated knowledge and future directions of step-defined ambulatory activity. Res. Exerc. Epidemiol. 2012, 14, 107–116. Available online: https://www.researchgate.net/publication/243464770_Step_by_step_Accumulated_knowledge_and_future_directions_of_step-defined_ambulatory_activity (accessed on 6 January 2025).
- Kračmar, B.; Chrástková, M.; Bačáková, R.; Bílý, M.; Horyna, R.; Škopek, M.; Zbořilová, M.; Čuříková, L.; Busta, J.; Fanta, O.; et al. Phylogeny of Human Locomotion; Karolinum: Praha, Czech Republic, 2016; p. 464. [Google Scholar]
- Pirker, W.; Katzenschlager, R. Gait disorders in adults and the elderly. Wien. Klin. Wochenschr. 2017, 129, 81–95. [Google Scholar] [CrossRef]
- Valouchová, P.; Kolář, P.; Bitnar, P.; Dyrhonová, O.; Horáček, O.; Kříž, J.; Adámková, M.; Černý, R.; Babková, L.; Čumpelík, J.; et al. Vyšetření posturálních funkcí-Chůze. In Rehabilitace V Klinické Praxi; Galén: Praha, Czech Republic, 2020; pp. 48–50. [Google Scholar]
- Hazari, A.; Maiya, A.G.; Nagda, T.V. Kinematics and Kinetics of Gait. In Conceptual Biomechanics and Kinesiology; Springer: Singapore, 2021; pp. 181–196. [Google Scholar]
- Marchetti, G.F.; Whitney, S.L.; Blatt, P.J.; Morris, L.O.; Vance, J.M. Temporal and spatial characteristics of gait during performance of the Dynamic Gait Index in people with and people without balance or vestibular disorders. Phys. Ther. 2008, 88, 640–651. [Google Scholar] [CrossRef] [PubMed]
- Gross, M.J.; Fetto, J.; Rosen, E. Diagnostic of Movement System; Portál: Bratislava, Slovakia, 2013; p. 600.
- Jandova, S.; Gajdoš, M.; Čuj, J.; Demjanovič Kendrova, L.; Mikuľákova, W. Gait Cycle of Slow Walking in High Heels. Health Probl. Civiliz. 2025, 19, 47–55. Available online: https://www.termedia.pl/GAIT-CYCLE-OF-SLOW-WALKING-IN-HIGH-HEELS,99,52415,1,1.html (accessed on 11 January 2025). [CrossRef]
- Maillard, B.; Boutaayamou, M.; Cassol, H.; Pirnay, L.; Kaux, J.F. Gait Analysis of Hemiparetic Adult Patients with a Quadripod Cane and a Rolling Cane. Healthcare 2024, 12, 464. [Google Scholar] [CrossRef]
- Wang, Y.; Mukaino, M.; Ohtsuka, K.; Otaka, Y.; Tanikawa, H.; Matsuda, F.; Tsuchiyama, K.; Yamada, J.; Saitoh, E. Gait characteristics of post-stroke hemiparetic patients with different walking speeds. Int. J. Rehabil. Res. 2020, 43, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Francisco, G.E.; Zhou, P. Post-stroke Hemiplegic Gait: New Perspective and Insights. Front. Physiol. 2018, 9, 1021. [Google Scholar] [CrossRef]
- Beyaert, C.; Vasa, R.; Frykberg, G.E. Gait post-stroke: Pathophysiology and rehabilitation strategies. Neurophysiol. Clin. 2015, 45, 335–355. [Google Scholar] [CrossRef]
- Nechvátal, P.; Laská, K.; Kendrová, L.; Čuj, J. Effect of constraint-induced movement therapy (CIMT) in patients with hemiparesis in the chronic stage of disease. Rehabilitácia 2015, 4, 195–202. Available online: https://www.researchgate.net/publication/301633478_Effect_of_constraint-induced_movement_therapy_CIMT_in_patients_with_hemiparesis_in_the_chronic_stage_of_disease (accessed on 11 December 2024).
- Douglas, G. The Detailed Neurologic Examination in Adults; UpToDate: Waltham, MA, USA, 2022. [Google Scholar]
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; de Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C.; et al. Heart disease and stroke statistics-2017 update: A report from the American Heart. Circulation 2017, 135, e146–e603. [Google Scholar] [CrossRef]
- Li, M.; Tian, S.; Sun, L.; Chen, X. Gait Analysis for Post-Stroke Hemiparetic Patient by Multi-Features Fusion Method. Sensers 2019, 19, 1737. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.C.; Rao, N.; Muthukrishnan, S.; Aruin, A.S. A textured insole improves gait symmetry in individuals with stroke. Disabil. Rehabil. 2017, 40, 2798–2802. [Google Scholar] [CrossRef]
- Solanki, D.; Lahiri, U. Design of instrumented shoes for gait characterization: A usability study with healthy and post-stroke hemiplegic individuals. Front. Neurosci. 2018, 12, 459. [Google Scholar] [CrossRef] [PubMed]
- Husemann, B.; Müller, F.; Krewer, C.; Heller, S.; Koenig, E. Effects of locomotion training with assistance of a robot-driven gait orthosis in hemiparetic patients after stroke: A randomized controlled pilot study. Stroke 2007, 38, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Flašková, M. Rehabilitation After Ischemic Stroke. Standard Procedure No. 0128, Issued by the Ministry of Health of the Slovak Republic § 45 par. 1 Letter c) of Act 576/2004 Coll. on Healthcare, Services Related to the Provision of Healthcare and on Amendments and Supplements to Certain Acts, as Amended. 2021. Available online: https://www.google.com.hk/url?sa=t&source=web&rct=j&opi=89978449&url=https://www.health.gov.sk/Zdroje%3F/Sources/dokumenty/SDTP/standardy/17-03-2021/Rehabilitacia-po-ischemickej-cievnej-mozgovej-prihode.pdf&ved=2ahUKEwi3tsaWyOCMAxUvbfUHHbcfAe0QFnoECB8QAQ&usg=AOvVaw0rISOlH4LAWUCE_V8XkUqu (accessed on 4 December 2024).
- Kozel, M.; Škrečková, G.; Lukáčová, E.; Grus, C. Monitoring and evaluation of flat-feet in children of pre-school age and younger school age. Fizjoterapia Pol. 2023, 4, 144–148. Available online: https://www.researchgate.net/publication/375140794_Monitoring_and_evaluation_of_flat-feet_in_children_of_pre-school_age_and_younger_school_age (accessed on 4 December 2024). [CrossRef]
- Baronchelli, F.; Zucchella, C.; Serrao, M.; Intiso, D.; Bartolo, M. The Effect of Robotic Assisted Gait Training with Lokomat® on Balance Control After Stroke: Systematic Review and Meta-Analysis. Front. Neurol. 2021, 12, 661815. [Google Scholar] [CrossRef]
- Hidler, J.; Nichols, D.; Pelliccio, M.; Brady, K.; Campbell, D.D.; Kahn, J.H.; Hornby, T.G. Multicenter randomized clinical trial evaluating the effectiveness of the Lokomat in subacute stroke. Neurorehabil. Neural Repair 2009, 23, 5–13. [Google Scholar] [CrossRef]
- Westlake, K.P.; Patten, C. Pilot study of Lokomat versus manual-assisted treadmill training for locomotor recovery post-stroke. J. Neuroeng. Rehabil. 2009, 6, 18. [Google Scholar] [CrossRef]
- Oh, W.; Park, C.; Oh, S.; You, S.H. Stage 2: Who Are the Best Candidates for Robotic Gait Training Rehabilitation in Hemiparetic Stroke? J. Clin. Med. 2021, 10, 5715. [Google Scholar] [CrossRef]
- van Kammen, K.; Boonstra, A.M.; van der Woude, L.H.V.; Visscher, C.; Reinders-Messelink, H.A.; den Otter, R. Lokomat guided gait in hemiparetic stroke patients: The effects of training parameters on muscle activity and temporal symmetry. Disabil. Rehabil. 2020, 42, 2977–2985. [Google Scholar] [CrossRef]
- Riener, R.; Lünenburger, L.; Maier, I.C.; Colombo, G. Locomotor training in subjects with sensori-motor deficits: An overview of the robotic gait orthosis Lokomat. J. Healthc. Eng. 2010, 2, 197–216. Available online: https://www.researchgate.net/publication/244922976_Locomotor_Training_in_Subjects_with_SensoriMotor_Deficits_An_Overview_of_the_Robotic_Gait_Orthosis_Lokomat (accessed on 11 January 2025). [CrossRef]
- Kim, H.Y.; You, J.S.H. A Review of Robot-Assisted Gait Training in Stroke Patients. Brain Neurorehabilit. 2017, 10, e9. [Google Scholar] [CrossRef]
- Chen, G.; Patten, C.; Kothari, D.H.; Zajac, F.E. Gait deviations associ-ated with post-stroke hemiparesis: Improvement duringtreadmill walking using weight support, speed, supportstiffness, and handrail hold. Gait Posture 2005, 22, 57–62. [Google Scholar] [CrossRef]
- Hesse, S.; Helm, B.; Krajnik, J.; Gregoric, M.; Mauritz, K.H. Treadmill training with par-tial body weight support: Influence of body weight releaseon the gait of hemiparetic patients. J. Neurol. Rehab. 1997, 11, 15–20. [Google Scholar]
- Hesse, S.; Konrad, M.; Uhlenbrock, D. Treadmill walking withpartial body weight support versus floor walking in hemi-paretic subjects. Arch. Phys. Med. Rehabil. 1999, 80, 421–427. [Google Scholar] [CrossRef]
- Burnfield, J.M.; Buster, T.W.; Goldman, A.J.; Corbridge, L.M.; Harper-Hanigan, K. Partial bodyweight support treadmill training speed influences pareticand non-paretic leg muscle activation, stride characteristics, and ratings of perceived exertion during acute strokerehabilitation. Hum. Mov. Sci. 2016, 47, 16–28. [Google Scholar] [CrossRef]
- Lamontagne, A.; Fung, J. Faster is better. Stroke 2004, 35, 2543–2548. [Google Scholar] [CrossRef] [PubMed]
- Hocoma. Lokomat® User Script. Available online: https://knowledge.hocoma.com/wp-content/uploads/2019/03/Lokomat_User_Script_EN_20180322_MDRJan23.pdf (accessed on 11 January 2025).
- Zebris FDM-T. Measuring System for Gait and Stance Analysis; Specifications and Hardware User Manual; Zebris Medical GmbH: Isny, Germany, 2013; Available online: https://www.zebris.de/fileadmin/Editoren/zebris-PDF-Manuals/Medizin/Hardware/Alte_Versionen/FDM-T_Hardware-Manual_130702_en.pdf (accessed on 12 January 2025).
- Nesi, B.; Taviani, A.; D’Auria, L.; Bardelli, R.; Zuccarello, G.; Platano, D.; Benedetti, M.G.; Benvenuti, F. The Relationship between Gait Velocity and Walking Pattern in Hemiplegic Patients. Appl. Sci. 2023, 13, 934. [Google Scholar] [CrossRef]
- Center for Scientific and Technical Information SR (CVTI SR). World Stroke Day: Time is key. Available online: https://vedanadosah.cvtisr.sk/zdravie/svetovy-den-cievnych-mozgovych-prihod-cas-hra-klucovu-ulohu/ (accessed on 13 March 2025).
- Bonnyaud, C.; Pradon, D.; Boudarham, J.; Robertson, J.; Vuillerme, N.; Roche, N. Effects of gait training using a robotic constraint (Lokomat®) on gait kinematics and kinetics in chronic stroke patients. J. Rehabil. Med. 2014, 46, 132–138. [Google Scholar] [CrossRef]
- Belda-Lois, J.M.; Mena-del Horno, S.; Bermejo-Bosch, I.; Moreno, J.C.; Pons, J.L.; Farina, D.; Iosa, M.; Molinari, M.; Tamburella, F.; Ramos, A.; et al. Rehabilitation of gait after stroke: A review towards a top-down approach. J. Neuroeng. Rehabil. 2011, 13, 8–66. [Google Scholar] [CrossRef]
- Peurala, S.H.; Tarkka, I.M.; Pitkanen, K.; Sivenius, J. The effectiveness of body weight supported gait training and floor walking in patients with chronic stroke. Arch. Phys. Med. Rehabil. 2005, 85, 1557–1564. [Google Scholar] [CrossRef]
- van Dellen, F.; Labruyčre, R. Settings matter: A scoping review on parameters in robot-assisted gait therapy identifies the importance of reporting standards. J. Neuroeng. Rehabil. 2022, 19, 40. [Google Scholar] [CrossRef]
- Mehrholz, J.; Thomas, S.; Kugler, J.; Pohl, M.; Elsner, B. Electromechanicalassisted training for walking after stroke. Cochrane Database Syst. Rev. 2020, 10, 10. [Google Scholar]
- Nam, Y.G.; Lee, J.W.; Park, J.W.; Lee, H.J.; Nam, K.Y.; Park, J.H.; Yu, C.S.; Choi, M.R.; Kwon, B.S. Effects of electromechanical exoskeleton-assisted gait training on walking ability of stroke patients: A randomized controlled trial. Arch. Phys. Med. Rehabil. 2019, 100, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Bang, D.H.; Shin, W.S. Effects of robot-assisted gait training on spatiotemporal gait parameters and balance in patients with chronic stroke: A randomized controlled pilot trial. NeuroRehabilitation 2016, 38, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Wallard, L.; Dietrich, G.; Kerlirzin, Y.; Bredin, J. Effects of robotic gait rehabilitation on biomechanical parameters in the chronic hemiplegic patients. Neurophysiol. Clin. 2015, 45, 215–219. [Google Scholar] [CrossRef]
- Mayr, A.; Quirbach, E.; Picelli, A.; Kofler, M.; Smania, N.; Saltuari, L. Early robot-assisted gait retraining in non-ambulatory patients with stroke: A single blind randomized controlled trial. Eur. J. Phys. Rehabil. Med. 2019, 54, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Hornby, T.G.; Campbell, D.D.; Kahn, J.H.; Demott, T.; Moore, J.L.; Roth, H.R. Enhanced gait-related improvements after therapist- versus roboticassisted locomotor training in subjects with chronic stroke. Stroke 2008, 39, 1786–1792. [Google Scholar] [CrossRef]
- Hsiao, H.Y.; Grayz, V.L.; Borrelli, J.; Rogers, M.W. Biomechanical control of paretic lower limb during imposed weight transfer in individuals post-stroke. J. Neuroeng. Rehabil. 2020, 17, 140. [Google Scholar] [CrossRef]
- Kusofsky, A.; Apel, I.; Hirschfeld, H. Reachingliftingplacing task during standing after stroke: Coordination among ground forces, ankle muscle activity, and hand movement. Arch. Phys. Med. Rehabil. 2001, 82, 650–660. [Google Scholar] [CrossRef]
- Lomaglio, M.J.; Eng, J.J. Muscle strength and weightbearing symmetry relate to sittostand performance in individuals with stroke. Gait Posture 2005, 22, 126–131. [Google Scholar] [CrossRef]
- Marigold, D.S.; Eng, J.J. The relationship of asymmetric weightbearing with postural sway and visual reliance in stroke. Gait Posture 2006, 23, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.M.; Eng, J.J. Symmetry in vertical ground reaction force is accompanied by symmetry in temporal but not distance variables of gait in persons with stroke. Gait Posture 2003, 18, 23–28. [Google Scholar] [CrossRef] [PubMed]
- De Haart, M.; Geurts, A.C.; Dault, M.C.; Nienhuis, B.; Duysens, J. Restoration of weightshifting capacity in patients with postacute stroke: A rehabilitation cohort study. Arch. Phys. Med. Rehabil. 2005, 86, 755–762. [Google Scholar] [CrossRef]
- Mercer, V.S.; Freburger, J.K.; Chang, S.H.; Purser, J.L. Measurement of paretic lowerextremity loading and weight transfer after stroke. Phys. Ther. 2009, 89, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Li, S. Stroke Recovery Is a Journey: Prediction and Potentials of Motor Recovery after a Stroke from a Practical Perspective. Life 2023, 13, 2061. [Google Scholar] [CrossRef]
- Mayo Clinic. Stroke rehabilitation: What to Expect as You Recover. Available online: https://www.mayoclinic.org/diseases-conditions/stroke/in-depth/stroke-rehabilitation/art-20045172 (accessed on 13 March 2025).
- Dromerick, A.W.; Geed, S.; Barth, J.; Brady, K.; Giannetti, M.L.; Mitchell, A.; Edwardson, M.A.; Tan, M.T.; Zhou, Y.; Newport, E.L.; et al. Critical Period After Stroke Study (CPASS): A phase II clinical trial testing an optimal time for motor recovery after stroke in humans. Proc. Natl. Acad. Sci. USA 2021, 118, e2026676118. [Google Scholar] [CrossRef]
- Dobkin Bruce, H. Strategies for stroke rehabilitation. Lancet Neurol. 2004, 3, 528–536. [Google Scholar] [CrossRef]
- Morone, G.; Bragoni, M.; Iosa, M.; De Angelis, D.; Venturiero, V.; Coiro, P.; Pratesi, L.; Paolucci, S. Who may benefit from robotic-assisted gait training? Neurorehabil. Neural Repair 2011, 25, 636–644. [Google Scholar] [CrossRef]
- Kelley, C.P.; Childress, J.; Boake, C.; Noser, E.A. Over-ground and robotic-assisted locomotor training in adults with chronic stroke: A blinded randomized clinical trial. Disabil. Rehabil. Assist. Technol. 2013, 8, 161–168. [Google Scholar] [CrossRef]

| Experimental Group | |||
|---|---|---|---|
| Mean ± SD | n | % | |
| Age | 64.31 | - | - |
| Sex | - | M = 12; F = 42 | M = 22.22; F = 77.78 |
| Hemiparesis dx. | - | 32 | 59.3 |
| Hemiparesis sin. | - | 22 | 40.7 |
| BMI | 27.85 ± 3.8 | ||
| Time since stroke (in days) | 36.3 ± 7.3 | ||
| Romberg test score | 2.06 ± 0.2 | ||
| Muscle strength DK according to the Janda test | 3.19 ± 0.6 | ||
| FAC | 3.04 ± 0.6 | ||
| Ashworth scale score | 0.61 ± 0.8 | ||
| Time spent on complex neurorehabilitation (in hours) | 28.44 ± 1.8 1536 total | ||
| Time on the Lokomat during hospitalization (in minutes) | 332.67 ± 12.3 17,964 total | ||
| Control group | |||
| Age | 61.23 | - | - |
| Sex | - | M = 28; F = 25 | M = 52.83; F = 47.17 |
| Hemiparesis dx. | - | 29 | 54.72 |
| Hemiparesis sin. | - | 24 | 45.28 |
| BMI | 29.53 ± 3.6 | ||
| Time since stroke | 38.3 ± 9.8 | ||
| Romberg test score | 3.43 ± 0.7 | ||
| Janda test score DK | 3.34 ± 0.5 | ||
| FAC | 3.06 ± 0.6 | ||
| Ashworth scale score | 0.53 ± 0.7 | ||
| Time spent on complex neurorehabilitation (in hours) | 28.72 ± 1.4 1522 total | ||
| Time spent on conventional gait training during hospitalization (in minutes) | 317 ± 14 16,801 total | ||
| Parameter: Step | Mean | Median | SD | p | Effect Size | |
|---|---|---|---|---|---|---|
| Experimental group | Stride length before rehabilitation | 73.3 | 78.0 | 29.4 | - | |
| Stride length after rehabilitation | 79.0 | 74.0 | 26.8 | - | ||
| Deviation from ideal length before | 24.7 | 27.5 | 15.5 | - | ||
| Deviation from ideal length after | 22.1 | 20.5 | 15.8 | - | ||
| Difference in deviation | 2.6 | 3.0 | 16.2 | 0.191 • | 0.133 | |
| Control group | Stride length before rehabilitation | 67.5 | 66 | 24.0 | - | |
| Stride length after rehabilitation | 76.1 | 75 | 27.6 | - | ||
| Deviation from ideal length before | 20.1 | 19.5 | 14.2 | - | ||
| Deviation from ideal length after | 21.3 | 15.5 | 17.6 | - | ||
| Difference in deviation | −1.2 | 1.0 | 16.2 | 0.492 • | 0.003 | |
| Comparison | Deviations in lengths between groups | - | - | - | 0.432 ▫ | 0.234 |
| Parameter: Load | Mean | Median | SD | p | Effect Size | |
|---|---|---|---|---|---|---|
| Experimental group | Deviation from ideal load before | 6.5 | 5.1 | 5.6 | - | |
| Deviation from ideal load after | 5.4 | 4.1 | 4.6 | - | ||
| Difference in deviation | 1.0 | 0 | 0.1 | 0.057 • | 0.241 | |
| Control group | Deviation from ideal load before | 8.7 | 5.1 | 10.9 | - | |
| Deviation from ideal load after | 6.3 | 5.1 | 6.7 | - | ||
| Difference in deviation | 2.3 | 1 | 8.1 | 0.042 • | 0.237 | |
| Comparison | Load deviations between groups | - | - | - | 0.432 ▫ | 0.015 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Potašová, M.; Mačej, P.; Moraučíková, E.; Baňárová, P.S.; Kutiš, P. Lokomat vs. Conventional Therapy—Impact on Gait Symmetry in Hemiparetic Patients: Preliminary Clinical Study. Healthcare 2025, 13, 929. https://doi.org/10.3390/healthcare13080929
Potašová M, Mačej P, Moraučíková E, Baňárová PS, Kutiš P. Lokomat vs. Conventional Therapy—Impact on Gait Symmetry in Hemiparetic Patients: Preliminary Clinical Study. Healthcare. 2025; 13(8):929. https://doi.org/10.3390/healthcare13080929
Chicago/Turabian StylePotašová, Marina, Peter Mačej, Eva Moraučíková, Patrícia Shtin Baňárová, and Peter Kutiš. 2025. "Lokomat vs. Conventional Therapy—Impact on Gait Symmetry in Hemiparetic Patients: Preliminary Clinical Study" Healthcare 13, no. 8: 929. https://doi.org/10.3390/healthcare13080929
APA StylePotašová, M., Mačej, P., Moraučíková, E., Baňárová, P. S., & Kutiš, P. (2025). Lokomat vs. Conventional Therapy—Impact on Gait Symmetry in Hemiparetic Patients: Preliminary Clinical Study. Healthcare, 13(8), 929. https://doi.org/10.3390/healthcare13080929






