2. Institutional Approach/Protocol
Prior to the administration of any anesthetic, all patients should be screened for MH through a complete medical and family history analysis. This may not be possible in emergency situations. The initial signs of MH may occur at any time following the administration of a triggering agent, including immediately following the induction of general anesthesia or at any point during the maintenance phase for the anesthetic. As previously mentioned, the earliest clinical signs include an increase in the end-tidal carbon dioxide and tachycardia. As these findings are much more frequently a result of inadequate anesthesia and hypoventilation, respectively, the anesthesiologist must maintain a high level of suspicion for MH. If the anesthesiologist feels that MH is probable, or if there is no alternative diagnosis to explain the patient’s clinical findings, they should immediately discontinue any triggering agents, notify the surgeon, hyperventilate with 100% inspired oxygen, increase fresh gas flow to >10 L/min, and trigger our multidisciplinary response. If available, charcoal filters should also be placed on the inspiratory and expiratory limbs of the anesthesia circuit. As MH is a potentially lethal disorder, a well-coordinated multidisciplinary approach is valuable in ensuring a timely and organized response.
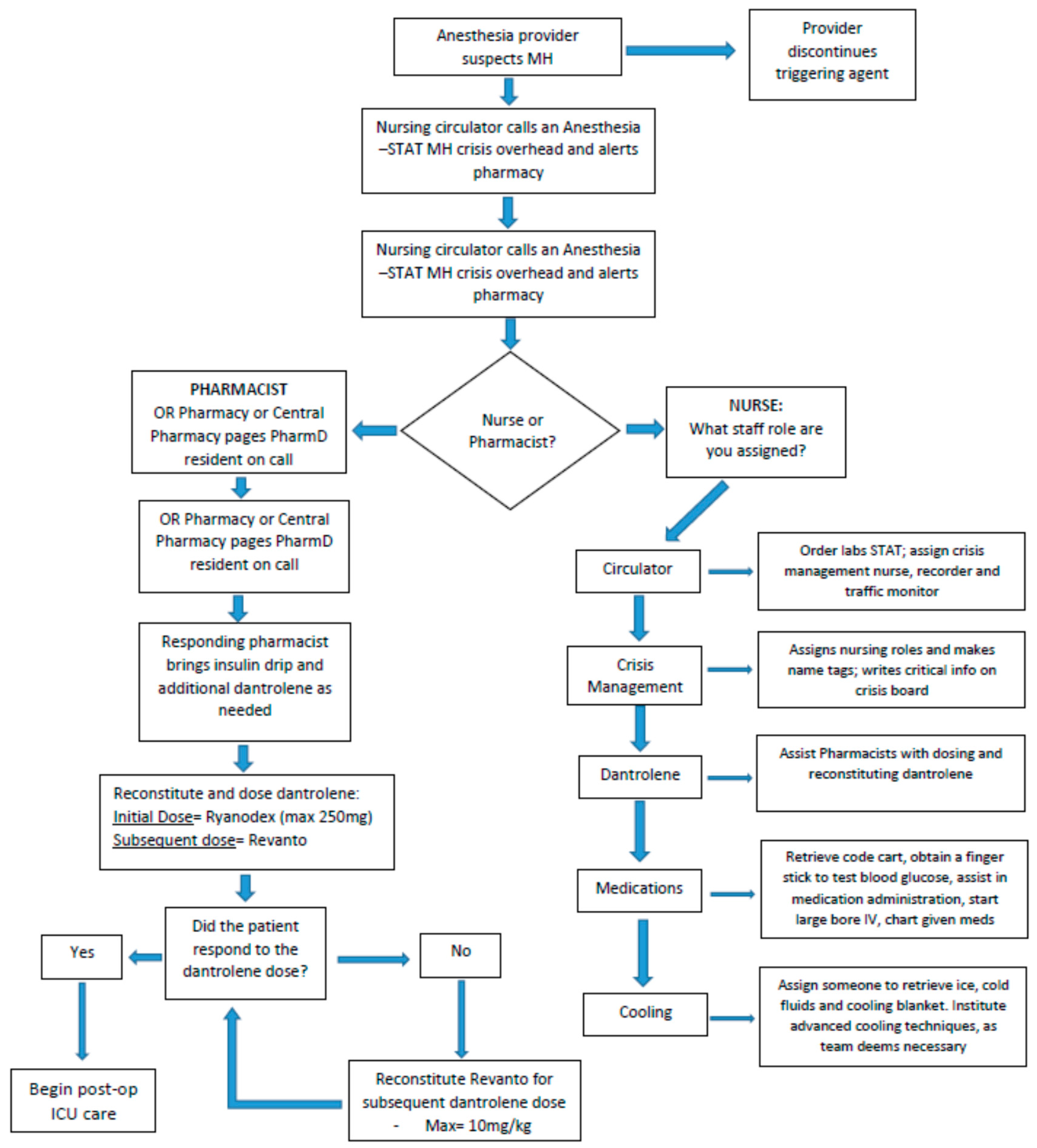
Figure 1 demonstrates the sequence of events initiated at our institution when MH is suspected.
An “anesthesia stat-MH crisis” is called out over the intercom to alert operating room (OR) staff including anesthesiologists, nurses and pharmacists to respond and assist in treating the patient. The anesthesiologist will serve as the primary leader for the resuscitation response and ensure that all aspects of patient care are accounted for. The primary OR nurse will retrieve the MH cart (contents shown in
Table 1) from the adjoining storage area and bring it into the OR. Color-coded cards corresponding to tasks or roles are assigned to responding personnel. These roles include a registered nurse (RN) circulator, cooling nurse, medication nurse, dantrolene nurse/pharmacist and crisis management nurse. Attached to each card is a bag of supplies specific to the individual’s role. The RN circulator may assign additional MH Team roles as needed. The cooling nurse procures ice and is prepared to implement advanced cooling as indicated. Cooling techniques at our institution include ice bags at the groin, axilla and neck; cooling blankets; and cold saline, as indicated. The medication nurse starts a large bore IV and works with the pharmacist to calculate the appropriate dantrolene dose. The pharmacist double-checks all drug dosing and assists with medication documentation, as well as ensuring the order of dantrolene products are utilized in the correct order to maximize efficiency and cost-effectiveness. Additionally, the pharmacists help to procure regular insulin and dextrose if needed for the treatment of hyperkalemia. Without all of these providers assessing and participating in the care of the patient, these cases would be extremely laborious. Having a multidisciplinary team attend to an MH crisis allows for the rapid control of a patient’s symptoms and to potentially stabilize them quickly.
Two types of dantrolene are contained in our MH cart, one vial of Ryanodex and nine vials of Revonto, in addition to the 10 vials of nonbacteriostatic sterile water (nine 100 mL vials and one 20 mL vial). The Ryanodex is used for the first dose, and the nine vials of Revonto are provided for any necessary subsequent dosing. Ryanodex is a lyophilized powder form of dantrolene containing 250 mg per vial, which costs around USD 2500.00. Revonto is also a lyophilized powder but in contrast only contains 20 mg of dantrolene per vial, costing around USD 60.00 a vial. To reconstitute Ryanodex, only 5 mL of sterile water is required. When reconstituting Revonto, 60 mL is needed per vial. Ryanodex should be used for the first dose because of its ease of use and need to reconstitute fewer vials. For an average 80 kg patient, 10 vials of Revonto and 600 mL of sterile water would be required to reconstitute an initial dose. Other components of the MH cart are listed below in
Table 1 and follow MHAUS recommendations [
7].
Once the patient’s MH symptoms and the patient are clinically stabilized, post-operative critical care and intensive care unit (ICU) admission are initiated. Patient allergies are updated to include likely triggering agents as a placeholder for future operations and hospital visits as a safety measure. When appropriate, patient and family are counseled on the importance of notifying anesthesia providers about MH history and avoiding triggering agents.
3. Case
With the consent of the patient, we present a case of a 22-year-old male admitted with an open right intercondylar fracture of the distal humerus after getting his arm caught in a steel press. In the emergency department, the patient received intravenous cefazolin, morphine, hydromorphone and a Tetanus/Diptheria/Pertussis (Tdap) vaccine. He was taken to the OR on the same day for an irrigation and debridement, as well as closed reduction of the open distal humerus fracture. General anesthesia was induced with lidocaine, fentanyl and propofol. Rocuronium was used for neuromuscular blockade. During the procedure, he was maintained on sevoflurane. No complications were noted during or after initial surgery. The following day, the patient was scheduled for a definitive internal fixation of his distal humerus fracture. General anesthesia was again induced with lidocaine, fentanyl and proprofol. Succinylcholine was administered to facilitate endotracheal intubation. The patient was maintained on isoflurane, and intermittent dosing of rocuronium was used to facilitate neuromuscular blockade. Approximately 30 min into the procedure, during the placement of an additional intravenous line, unexpected resistance was noted. Upon closer examination, his extremities were found to be rigid. A quick assessment of his vitals showed that he was tachycardic, with a heart rate of 160 bpm; hypercapnic, with an end-tidal CO2 of 62 mmHg; and hypertensive, with systolic blood pressures >160 mmHg (baseline blood pressure was 130/82 mmHg at preoperative evaluation). A temperature-sensing catheter was placed in the bladder, and the patient was found to be normothermic at 37.4 °C. Despite the normothermia, malignant hyperthermia was suspected. The isoflurane was discontinued, and charcoal filters were placed in the circuit. Nitrous oxide was used to maintain general anesthesia, and a malignant hyperthermia response was initiated and allowed for additional responders to arrive at the patient’s bedside within minutes.
The patient quickly received an initial bolus of 187.5 mg of Ryanodex (2.5 mg/kg). Additionally, 20 mg of IV push esmolol was administered to treat his tachycardia but with a negligible response. Over the next 35 min, the patient received 80 mg of Revonto via intermittent 20 mg doses. These doses were administered to treat persistent and intermittent symptoms of MH.
The non-pharmacological measures taken include ice packs applied to the axilla and the placement of cooling blankets. The patient responded to the dantrolene with marked reductions in heart rate, muscle rigidity and end-tidal carbon dioxide (EtCO2). While not elevated, the patient’s temperature remained normothermic. The surgical procedure was aborted, and the patient was transferred to the ICU for close monitoring, with care being assumed by the ICU intensivists.
In the ICU, the patient continued to receive Revonto 80 mg (~1 mg/kg) IV Q 6 h for 24 h. During this time period, the patient’s lactate fell from 3.2 mmol/L at its peak to 0.6 mmol/L (
Figure 2). His creatinine kinase (CK) peaked at 16,505 units/L and decreased to 7887 units/L prior to discharge (
Figure 3). The patient’s serum creatinine (SCr) was also elevated at 1.44 mg/dL and trended back down to his assumed baseline.
In light of the etiology and triggering factor in this case, one may incorrectly assume it was precipitated by succinylcholine alone, since the patient previously received sevoflurane without incident. However, the literature suggests that different inhaled anesthetics may trigger MH at different rates, and his initial sevoflurane exposure was not sufficient [
8]. Furthermore, studies have shown that a triggering inhalation agent plus the use of succinylcholine may cause a more marked response than a single agent [
9].
After the patient was stabilized, the case was discussed with the mother, who had also experienced MH in the past; however, she was not aware that this was a hereditary disease. The patient’s family was educated regarding the risks of MH and the potential for genetic predisposition within the family. An allergy was also added to the patient’s chart for future potential cases. The patient was extubated that evening. Four days into the patient’s admission, he received an open reduction internal fixation (ORIF) of his distal humerus. Total IV anesthesia (TIVA) was used with continuous infusion of propofol and intermittent dosing of fentanyl, dexmedetomidine and rocuronium throughout the case. Aside from the CK, lactate and SCr, the patient’s lab results all remained normal, and the patient progressed to his baseline function. The patient was discharged home on post-operative day 3 from the index surgery, with follow up after 2 weeks with the orthopedic service. Through the utilization of the institution’s protocol, all providers were aware of their roles within the team and were able to quickly perform their assigned duties. This allowed delays to be reduced for the rapid control of the patient’s MH. Without the swift initiation of an MH protocol, it is possible that patients could experience a lethal outcome.