SUMAMOS EXCELENCIA® Project: Results of the Implementation of Best Practice in a Spanish National Health System (NHS)
Abstract
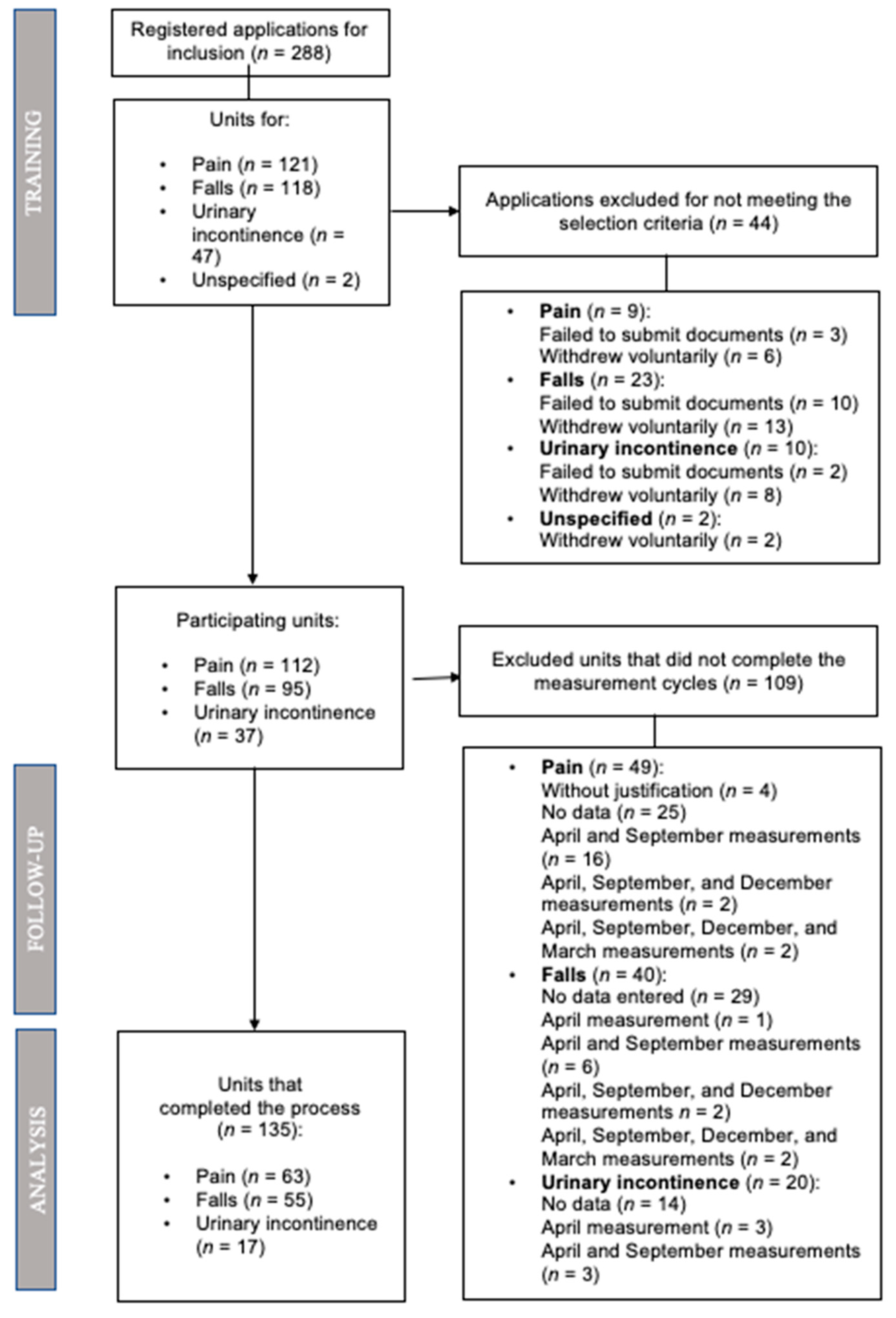
:1. Introduction
Hypothesis
2. Materials and Methods
2.1. Design
2.2. Setting and Participants
2.2.1. Inclusion Criteria for the Units
2.2.2. Inclusion Criteria for Patients
2.2.3. Recruitment of Study Subjects and Sample Size
2.3. Intervention
2.4. Measurements
2.5. Data collection
2.6. Data Analysis
3. Results
3.1. Description of the Sample
3.2. Primary Outcome: Implementation of a Model for the Assessment of Pain, Risk of Falling and Urinary Incontinence
3.3. Secondary Outcome: Efficacy of the Implementation Model in the Provision of Care Plans and Patient Education
3.4. Impact of the Intervention
3.5. Barriers Detected during Measurement Cycles and Planned Actions
4. Discussion
4.1. Baseline Situation of the Degree of Compliance with the Recommendations in the Participating Units and Final Levels of Compliance to the Best Practice Criteria
4.2. Assessment Indicators, Barriers and Actions in the Implementation Process
4.3. Facilitators in Implementation Projects
4.4. Clinical Audits and Feedback as Implementation Strategies
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kreindler, S.A. What if implementation is not the problem? Exploring the missing links between knowledge and action. Int. J. Health Plann. Manag. 2016, 31, 208–226. [Google Scholar] [CrossRef]
- Squires, J.E.; Graham, I.D.; Hutchinson, A.M.; Michie, S.; Francis, J.J.; Sales, A.; Brehaut, J.; Curran, J.; Ivers, N.; Lavis, J.; et al. Identifying the domains of context important to implementation science: A study protocol. Implement. Sci. 2015, 10, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Squires, J.E.; Estabrooks, C.A.; Gustavsson, P.; Wallin, L. Individual determinants of research utilization by nurses: A systematic review update. Implement. Sci. 2011, 6, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Mc Goldrick, E.L.; Crawford, T.; Brown, J.A.; Groom, K.M.; Crowther, C.A. Identifying the barriers and enablers in the implementation of the New Zealand and Australian Antenatal Corticosteroid Clinical Practice Guidelines. BMC Health Serv. Res. 2016, 16, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Meijers, J.M.M.; Janssen, M.A.P.; Cummings, G.G.; Wallin, L.; Estabrooks, C.A.; Halfens, R.Y.G. Assessing the relationships between contextual factors and research utilization in nursing: Systematic literature review. J. Adv. Nurs. 2006, 55, 622–635. [Google Scholar] [CrossRef] [PubMed]
- Neta, G.; Brownson, R.C.; Chambers, D.A. Opportunities for Epidemiologists in Implementation Science: A Primer. Am. J. Epidemiol. 2018, 187, 899–910. [Google Scholar] [CrossRef] [PubMed]
- Wallin, L. Knowledge translation and implementation research in nursing. Int. J. Nurs. Stud. 2009, 46, 576–587. [Google Scholar] [CrossRef]
- Flodgren, G.; Deane, K.; Dickinson, H.O.; Kirk, S.; Alberti, H.; Beyer, F.R.; Brown, J.G.; Penney, T.L.; Summerbell, C.D.; Eccles, M.P. Interventions to change the behaviour of health professionals and the organisation of care to promote weight reduction in overweight and obese people. Cochrane Database Syst. Rev. 2010. [Google Scholar] [CrossRef] [PubMed]
- Munten, G.; van den Bogaard, J.; Cox, K.; Garretsen, H.; Bongers, I. Implementation of evidence-based practice in nursing using action research: A review. Worldviews Evid. Based Nurs. 2010, 7, 135–157. [Google Scholar] [CrossRef] [Green Version]
- Powell, B.J.; Mcmillen, J.C.; Proctor, E.K.; Carpenter, R.; Griffey, R.T.; Bunger, A.C.; Glass, J.E.; York, J.L. A Compilation of Strategies for Implementing Clinical Innovations in Health and Mental Health. Med. Care Res. Rev. 2012, 69, 123–157. [Google Scholar] [CrossRef] [Green Version]
- Davies, B.; Edwards, N.; Ploeg, J.; Virani, T. Insights about the process and impact of implementing nursing guidelines on delivery of care in hospitals and community settings. BMC Health Serv. Res. 2008, 8, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Mickan, S.; Burls, A.; Glasziou, P. Patterns of ‘leakage’ in the utilisation of clinical guidelines: A systematic review. Postgrad. Med. J. 2011, 87, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Weller, C.D.; Richards, C.; Turnour, L.; Patey, A.M.; Russell, G.; Team, V. Barriers and enablers to the use of venous leg ulcer clinical practice guidelines in Australian primary care: A qualitative study using the theoretical domains framework. Int. J. Nurs. Stud. 2020, 103, 103503. [Google Scholar] [CrossRef] [PubMed]
- Almazrou, S.H.; Alfaifi, S.I.; Alfaifi, S.H.; Hakami, L.E.; Al-Aqeel, S.A. Barriers to and Facilitators of Adherence to Clinical Practice Guidelines in the Middle East and North Africa Region: A Systematic Review. Healthcare 2020, 8, 564. [Google Scholar] [CrossRef] [PubMed]
- Grimshaw, J.; Eccles, M.; Tetroe, J. Implementing clinical guidelines: Current evidence and future implications. J. Contin. Educ. Health Prof. 2004, 24 (Suppl. S1), S31–S37. [Google Scholar] [CrossRef]
- Esposito, P. Clinical audit, a valuable tool to improve quality of care: General methodology and applications in nephrology. World J. Nephrol. 2014, 3, 249. [Google Scholar] [CrossRef] [PubMed]
- Wentland, B.A.; Hinderer, K.A. A Nursing Research and Evidence-Based Practice Fellowship Program in a Magnet®-designated pediatric medical center. Appl. Nurs. Res. 2020, 55, 151287. [Google Scholar] [CrossRef]
- Ivers, N.; Jamtvedt, G.; Flottorp, S.; Young, J.M.; Odgaard-Jensen, J.; French, S.D.; O’Brien, M.A.; Johansen, M.; Grimshaw, J.; Oxman, A.D. Audit and feedback: Effects on professional practice and healthcare outcomes. Cochrane Database Syst. Rev. 2012, 2012. [Google Scholar] [CrossRef]
- Sinuff, T.; Muscedere, J.; Rozmovits, L.; Dale, C.M.; Scales, D.C. A qualitative study of the variable effects of Audit and feedback in the ICU. BMJ Qual. Saf. 2015, 24, 393–399. [Google Scholar] [CrossRef]
- Schalk, D.; Bijl, M.; Halfens, R.; Hollands, L.; Cummings, G. Interventions aimed at improving the nursing work environment: A systematic review. Implement. Sci. 2010, 5, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pearson, A.; Jordan, Z.; Munn, Z. Translational Science and Evidence-Based Healthcare: A Clarification and Reconceptualization of How Knowledge Is Generated and Used in Healthcare. Nurs. Res. Pr. 2012, 2012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daniels, J.F. Purposeful and timely nursing rounds: A best practice implementation project Key dates Executive summary. JBI Database Sytematic Rev. Implement. Rep. 2016, 14, 248–267. [Google Scholar] [CrossRef] [PubMed]
- Martın-Losada, L.; Huerta, M.; González, N.; Ortega, I.; Cuenca, J.N. Managing urinary incontinence in older people in hospital: A best practice implementation project. JBI Database Sytematic Rev. Implement. Rep. 2020, 18, 243–255. [Google Scholar] [CrossRef]
- Szymaniak, S. Accurate falls risk assessment and interventions for preventing falls in patients in the acute care setting within a private hospital in a large capital city: A best practice implementation project. JBI Database Sytematic Rev. Implement. Rep. 2015, 13, 386–406. [Google Scholar] [CrossRef]
- Holmes, B.J.; Best, A.; Davies, H.; Hunter, D.; Kelly, M.P.; Marshall, M.; Rycroft-Malone, J. Mobilising knowledge in complex health systems: A call to action. Evid. Policy 2017, 13, 539–560. [Google Scholar] [CrossRef]
- Ruzafa-Martínez, M.; González-María, E.; Moreno-Casbas, T.; Del Rio Faes, C.; Albornos-Muñoz, L.; Escandell-García, C. Proyecto de implantación de Guías de Buenas Prácticas en España 2011–2016. Enferm. Clin. 2016, 21, 275–283. [Google Scholar] [CrossRef]
- Albornos-Muñoz, L.; González-María, E.; Moreno-Casbas, T. Implantación de guías de buenas prácticas en España. Programa de centros comprometidos con la excelencia en cuidados. MedUNAB 2015, 17, 163–169. [Google Scholar] [CrossRef]
- Moreno-Casbas, T.; González-María, E.; Albornos-Muñoz, L.; Grinspun, D. Getting guidelines into practice: Lessons learned as Best Practice Spotlight Organization host. Int. J. Evid. Based. Healthc. 2019, 17, S15–S17. [Google Scholar] [CrossRef]
- Escobar-Aguilar, G.; Moreno-Casbas, M.; González-María, E.; Martínez-Gimeno, M.; Sánchez-Pablo, C.; Orts-Cortés, I. The SUMAMOS EXCELENCIA Project. J. Adv. Nurs. 2019, jan.13988. [Google Scholar] [CrossRef]
- Schyve, P. Leadership in Healthcare Organizations: A Guide to Joint Commission Leadership Standards; The Governance Institute: San Diego, CA, USA, 2009. [Google Scholar]
- Torralba, A.; Miquel, A.; Darba, J. Situación actual del dolor crónico en España: Iniciativa “Pain Proposal”. Rev. Soc. Esp. Dolorspañola Dolor 2014, 21, 16–22. [Google Scholar] [CrossRef]
- Langley, P.C.; Ruiz-Iban, M.A.; Molina, J.T.; De Andres, J.; Castellón, J.R. The prevalence, correlates and treatment of pain in Spain. J. Med. Econ. 2011, 14, 367–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joint Commission International. Joint Commission International Accreditation Standards for Hospital; Joint Commision International: Oakbrook Terrace, IL, USA, 2017. [Google Scholar]
- Aoki, Y.; Brown, H.W.; Brubaker, L.; Cornu, J.N.; Daly, J.O.; Cartwright, R. Urinary incontinence in Muslim women. Nurs. Times 2008, 104, 49–52. [Google Scholar] [CrossRef] [Green Version]
- Savas, S.; Saka, B.; Akın, S.; Tasci, I.; Tasar, P.T.; Tufan, A.; Yavuzer, H.; Balci, C.; Sezgin, G.; Karan, M.A.; et al. The prevalence and risk factors for urinary incontinence among inpatients, a multicenter study from Turkey. Arch. Gerontol. Geriatr. 2020, 90, 104122. [Google Scholar] [CrossRef]
- Moehrer, B.; Hextall, A.; Jackson, S. Oestrogens for urinary incontinence in women. Cochrane Database Syst. Rev. 2003. [Google Scholar] [CrossRef]
- Abrams, P.; Cardozo, L.; Fall, M.; Griffiths, D.; Rosier, P.; Ulmsten, U.; Van Kerrebroeck, P.; Victor, A.; Wein, A. The standardisation of terminology in lower urinary tract function: Report from the standardisation sub-committee of the International Continence Society. Urology 2003, 61, 37–49. [Google Scholar] [CrossRef]
- McKay, M.A.; Todd-Magel, C.; Copel, L. Factors associated with the risk for falls in PACE participants. Geriatr. Nurs. 2020, 41, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Saiz-Vinuesa, M.D.; Muñoz-Mansilla, E.; Muñoz-Serrano, T.; Córcoles-Jiménez, M.P.; Ruiz-García, M.V.; Fernández-Pallarés, P.; Herreros-Sáez, L.; Calero-Yáñez, F. Implantación de una guía de buenas prácticas para la prevención de caídas: Percepción de los pacientes hospitalizados y sus cuidadores. Rev. Calid. Asist. 2016, 31, 329–337. [Google Scholar] [CrossRef] [PubMed]
- National Guideline Clearinghouse; Agency for Health Care Policy and Research. Urinary Incontinence in the Long Term Care Setting; American Medical Directors Association: Columbia, MA, USA, 2012. [Google Scholar]
- Registered Nurses’ Association of Ontario. Valoración y Manejo del Dolor, Tercera Edición; Registered Nurses’ Association of Ontario: Toronto, CA, USA, 2013. [Google Scholar]
- Moreno-Casbas, T. Perspectives: Implementation strategies to adopt and integrate evidence-based nursing. What are we doing? J. Res. Nurs. 2015, 20, 729–733. [Google Scholar] [CrossRef] [Green Version]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. lme4: Linear mixed-effects models using Eigen and S4. R package version 1.1-8. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.; Christensen, R. lmerTest package: Tests in linear mixed effects models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef] [Green Version]
- R Core Team R: A Language and Environment for Statistical Computing Disponible en. Available online: http://www.r-project.org/ (accessed on 22 May 2019).
- Ang, E.; Chow, Y.L. General pain assessment among patients with cancer in an acute care setting: A best practice implementation project. Int. J. Evid. Based Healthc. 2010, 8, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Xing, W.; Zhang, Y.; Gu, C.; Lizarondo, L. Pelvic floor muscle training for the prevention of urinary incontinence in antenatal and postnatal women: A best practice implementation project. JBI Database Sytematic Rev. Implement. Rep. 2017, 15, 567–583. [Google Scholar] [CrossRef]
- Stevens, B.J.; Yamada, J.; Estabrooks, C.A.; Stinson, J.; Campbell, F.; Scott, S.D.; Cummings, G. Pain in hospitalized children: Effect of a multidimensional knowledge translation strategy on pain process and clinical outcomes. Pain 2014, 155, 60–68. [Google Scholar] [CrossRef]
- Johnson, M.; Kelly, L.; Siric, K.; Tran, D.; Overs, B. Improving falls risk screening and prevention using an e-learning approach. J. Nurs. Manag. 2015, 23, 910–919. [Google Scholar] [CrossRef]
- Dulko, D.; Hertz, E.; Beck, S.; Mooney, K. Implementation of cancer pain guidelines by acute care nurse practitioners using an audit and feedback strategy. J. Am. Acad. Nurse Pract. 2010, 22, 45–55. [Google Scholar] [CrossRef]
- Lau, R.; Stevenson, F.; Ong, B.N.; Dziedzic, K.; Treweek, S.; Eldridge, S.; Everitt, H.; Kennedy, A.; Qureshi, N.; Rogers, A.; et al. Achieving change in primary care-causes of the evidence to practice gap: Systematic reviews of reviews. Implement. Sci. 2016, 11, 2–39. [Google Scholar] [CrossRef] [Green Version]
- Muñoz Jiménez, D. From evidence-based nursing to healthcare practice: The evaluation of results as an integrating element. Enferm. Clin. 2018, 28, 149–153. [Google Scholar] [CrossRef]
- Baker, R.; Camosso-Stefinovic, J.; Gillies, C.; Shaw, E.; Cheater, F.; Flottorp, S.; Robertson, N. Tailored interventions to overcome identified barriers to change: Effects on professional practice and health care outcomes. Cochrane Database Syst. Rev. 2010. [Google Scholar] [CrossRef] [Green Version]
- Salter, K.L.; Kothari, A. Using realist evaluation to open the black box of knowledge translation: A state-of-the-art review. Implement. Sci. 2014, 9, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Christina, V.; Baldwin, K.; Biron, A.; Emed, J.; Lepage, K. Factors influencing the effectiveness of audit and feedback: Nurses’ perceptions. J. Nurs. Manag. 2016, 24, 1080–1087. [Google Scholar] [CrossRef]
- Chaillet, N.; Dubé, E.; Dugas, M.; Audibert, F.; Tourigny, C.; Fraser, W.D.; Dumont, A. Evidence-based strategies for implementing guidelines in obstetrics: A systematic review. Obstet. Gynecol. 2006, 108, 1234–1245. [Google Scholar] [CrossRef]
- Dingwall, L.; McLafferty, E. Do nurses promote urinary continence in hospitalized older people? An exploratory study. J. Clin. Nurs. 2006, 15, 1276–1286. [Google Scholar] [CrossRef]
- Trad, W.; Flowers, K.; Caldwell, J.; Sousa, M.S.; Vigh, G.; Lizarondo, L.; Gaudin, J.; Hooper, D.; Parker, D. Nursing assessment and management of incontinence among medical and surgical adult patients in a tertiary hospital: A best practice implementation project. JBI Database Syst. Rev. Implement. Rep. 2019, 17, 2578–2590. [Google Scholar] [CrossRef]

| Type of Unit | Pain | Fall Prevention | Urinary Incontinence | Total | |||
|---|---|---|---|---|---|---|---|
| Medical | 19 | 30 | 9 | 58 | |||
| Surgical | 14 | 5 | 1 | 20 | |||
| Medical-surgical | 13 | 8 | 0 | 21 | |||
| Critical care | 8 | 0 | 0 | 8 | |||
| Primary care/outpatient care | 2 | 7 | 3 | 12 | |||
| Maternal and childcare | 2 | 0 | 1 | 3 | |||
| Other (emergency care and delivery care) | 4 | 1 | 0 | 5 | |||
| Nursing home | 1 | 4 | 3 | 8 | |||
| Total | 63 | 55 | 17 | 135 | |||
| Complete cycle 9 month | 9 | 6 | 4 | 9 | |||
| Complete cycle 12 month | 54 | 49 | 13 | 116 | |||
| Mean age | 55.2 (SD: 23.13) | 75.93 (SD: 14.13) | 69.12 (SD: 17.55) | 66.75 (SD: 18.27) | |||
| Sex | |||||||
| Male | 5661 | 49.5% | 4462 | 55.0% | 1074 | 41.8% | 11,197 |
| Female | 5595 | 48.9% | 3617 | 44.6% | 1481 | 57.7% | 10,693 |
| Missing data | 184 | 1.6% | 27 | 0.3% | 13 | 0.5% | 224 |
| Patients per cycle | n | n | n | N | |||
| Cycle 0 (April-baseline) | 2153 | 1608 | 481 | 4242 | |||
| Cycle 1 (September-baseline) | 1851 | 1372 | 447 | 3670 | |||
| Cycle 2 (December-3 months) | 2050 | 1565 | 448 | 4063 | |||
| Cycle 3 (March-6 months) | 2040 | 1334 | 417 | 3791 | |||
| Cycle 4 (June-9 months) | 2075 | 1243 | 400 | 3718 | |||
| Cycle 5 (September-12 months-optional) | 1271 | 984 | 375 | 2630 | |||
| TOTAL | 11,440 | 8106 | 2568 | 22,114 | |||
| Issues | Cycle | |||||
|---|---|---|---|---|---|---|
| Pain | 0 | 1 | 2 | 3 | 4 | 5 |
| Assessment | 59.9 [57.8–62.1] | 57.8 [55.5–60.2] | 61.7 [59.6–63.9] | 73.6 [71.7–75.6] | 70.2 [68.2–72.2] | 71.6 [69.1–74.2] |
| Care plan | 51.1 [47.6–54.7] | 55.1 [51.3–59.0] | 56.7 [53.3–60.1] | 69.3 [66.3–72.3] | 74.0 [71.1–77.0] | 73.1 [69.8–76.4] |
| Patient education | 12.2 [9.90–14.6] | 16.9 [14.0–19.8] | 30.0 [26.9–33.1] | 50.8 [47.6–53.9] | 65.5 [62.4–68.7] | 68.4 [64.9–72.0] |
| Comprehensive assessment | 69.6 [66.3–72.9] | 59.3 [55.5–63.1] | 74.1 [71.1–77.1] | 83.9 [81.5–86.2] | 84.5 [82.1–86.9] | 78.7 [75.5–81.8] |
| Fall prevention | ||||||
| Risk assessment | 56.8 [54.4–59.3] | 56.1 [53.4–58.8] | 69.8 [67.5–72.1] | 78.3 [76–80.5] | 76.6 [74.2–79.0] | 87.8 [85.7–89.9] |
| Care plan | 65.8 [61.9–69.7] | 67.3 [63.2–71.5] | 82.3 [79.5–85] | 77.5 [74.3–80.6] | 82.6 [79.4–85.8] | 84.5 [81.4–87.6] |
| Restraining measures | 39.4 [36.8–42.0] | 35.8 [33.0–38.6] | 41.2 [38.5–43.8] | 41.4 [38.6–44.3] | 39.5 [36.5–42.5] | 33.9 [30.7–37.1] |
| Urinary incontinence | ||||||
| Assessment | 43.4 [38.9–47.9] | 41.7 [37.1–46.3] | 52.7 [48.1–57.4] | 62.3 [57.6–67.1] | 72.3 [67.9–76.8] | 62.2 [57.2–67.2] |
| Care plan | 68.5 [57.8–79.1] | 56.5 [44.8–68.2] | 63.6 [54.4–72.7] | 66.7 [57.6–75.7] | 86.2 [79.9–92.5] | 80.9 [72.9–88.8] |
| Patient education | 41.1 [29.8–52.4] | 33.3 [22.0–44.7] | 25.7 [17.4–34.1] | 24.8 [16.3–33.2] | 70.5 [62.1–79.0] | 60.9 [50.9–70.8] |
| Issues | Pain | Fall Prevention | Urinary Incontinence | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Adjusted OR for Evaluation | z-Value | p-Value | Adjusted OR for Evaluation | z-Value | p-Value | Adjusted OR for Evaluation | z-Value | p-Value | |
| Primary outcome | |||||||||
| Intercept | 1.33 [0.78–2.28] | 1.036 | 0.300 | 0.60 [0.29–1.25] | −1.356 | 0.175 | 0.43 [0.12–1.57] | −1.271 | 0.204 |
| Cycle | 1.30 [1.26–1.35] | 15.877 | < 0.001 | 1.50 [1.44–1.56] | 20.493 | < 0.001 | 1.41 [1.32–1.49] | 10.869 | < 0.001 |
| Sex (female) | 1.05 [0.95–1.17] | 0.998 | 0.318 | 1.02 [0.91–1.15] | 0.396 | 0.692 | 0.99 [0.80–1.22] | −0.104 | 0.917 |
| Age (years) | 1.00 [1.00–1.00] | 0.626 | 0.531 | 1.02 [1.01–1.02] | 6.583 | < 0.001 | 1.03 [1.02–1.04] | 7.161 | < 0.001 |
| Secondary outcome 1 | |||||||||
| Intercept | 1.61 [0.45–5.71] | 0.731 | 0.465 | 1.12 [0.31–3.95] | 0.169 | 0.866 | 7.8 [0.89–68.74] | 1.851 | 0.064 |
| Cycle | 1.85 [1.72–1.99] | 16.526 | < 0.001 | 1.57 [1.45–1.69] | 11.521 | < 0.001 | 1.50 [1.27–1.77] | 4.718 | < 0.001 |
| Sex (female) | 1.31 [1.06–1.62] | 2.525 | 0.012 | 1.02 [0.81–1.28] | 0.165 | 0.869 | 1.48 [0.85–2.56] | 1.391 | 0.164 |
| Age (years) | 0.99 [0.98–0.99] | −4.386 | < 0.001 | 1.01 [1.00–1.02] | 1.944 | 0.052 | 0.98 [0.96–1.00] | −1.632 | 0.103 |
| Secondary outcome 2 | |||||||||
| Intercept | 0.07 [0.02–0.21] | −4.855 | < 0.001 | 0.00 [0.00–0.01] | −11.832 | < 0.001 | 1.04 [0.11–9.82] | 0.038 | 0.969 |
| Cycle | 2.82 [2.6–3.05] | 25.295 | < 0.001 | 1.09 [1.05–1.14] | 4.407 | < 0.001 | 1.74 [1.47–2.05] | 6.435 | < 0.001 |
| Sex (female) | 1.01 [0.83–1.22] | 0.06 | 0.952 | 1.11 [0.97–1.27] | 1.551 | 0.121 | 1.96 [1.08–3.56] | 2.203 | 0.028 |
| Age (years) | 1.00 [0.99–1.00] | −1.793 | 0.073 | 1.06 [1.05–1.07] | 16.415 | < 0.001 | 0.97 [0.94–0.99] | −2.819 | 0.005 |
| Secondary outcome 3 | |||||||||
| Intercept | 4.13 [1.14–15.06] | 2.152 | 0.0314 | ||||||
| Cycle | 1.85 [1.71–1.99] | 15.989 | < 0.001 | ||||||
| Sex (female) | 0.77 [0.62–0.96] | −2.29 | 0.022 | ||||||
| Age (years) | 0.99 [0.99–1.00] | −1.997 | 0.046 | ||||||
| Barriers Identified | Actions Undertaken | ||||||
|---|---|---|---|---|---|---|---|
| Type of Barrier | No. | % | Institutional Support | Tool Development | Patient-Family Education | Training of Professionals | |
| Context | |||||||
| Lack of tool for computer registration | 66 | 30.3% | - | 58 | 1 | 7 | |
| Lack of action procedures | 55 | 25.2% | 3 | 21 | 10 | 21 | |
| Type of patient | 10 | 4.6% | - | 1 | 1 | 8 | |
| Time | 2 | 0.9% | 1 | - | - | 1 | |
| Difficulty in handling tools | 1 | 0.5% | - | - | - | 1 | |
| Individual | |||||||
| Incomplete records | 43 | 19.7% | - | 5 | - | 38 | |
| Lack of training | 36 | 16.5% | - | - | 2 | 34 | |
| Lack of interest | 5 | 2.3% | 1 | - | - | 4 | |
| Result | 218 | % | 2.3% | 39.0% | 6.4% | 52.3% | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Gimeno, M.-L.; Fernández-Martínez, N.; Escobar-Aguilar, G.; Moreno-Casbas, M.-T.; Brito-Brito, P.-R.; Caperos, J.-M. SUMAMOS EXCELENCIA® Project: Results of the Implementation of Best Practice in a Spanish National Health System (NHS). Healthcare 2021, 9, 374. https://doi.org/10.3390/healthcare9040374
Martínez-Gimeno M-L, Fernández-Martínez N, Escobar-Aguilar G, Moreno-Casbas M-T, Brito-Brito P-R, Caperos J-M. SUMAMOS EXCELENCIA® Project: Results of the Implementation of Best Practice in a Spanish National Health System (NHS). Healthcare. 2021; 9(4):374. https://doi.org/10.3390/healthcare9040374
Chicago/Turabian StyleMartínez-Gimeno, María-Lara, Nélida Fernández-Martínez, Gema Escobar-Aguilar, María-Teresa Moreno-Casbas, Pedro-Ruyman Brito-Brito, and Jose-Manuel Caperos. 2021. "SUMAMOS EXCELENCIA® Project: Results of the Implementation of Best Practice in a Spanish National Health System (NHS)" Healthcare 9, no. 4: 374. https://doi.org/10.3390/healthcare9040374






