Measurements of Serum Mac-2-Binding Protein Glycosylation Isomer and Shear Wave Velocity in Health Checkups Are Useful in Screening for Non-Alcoholic Steatohepatitis
Abstract
1. Introduction
2. Material and Methods
2.1. Subjects
2.2. Complete Medical Checkup Basic Tests
2.3. Non-Invasive Liver Fibrosis Tests
2.4. Statistical Analysis
3. Results
3.1. Subject Characteristics
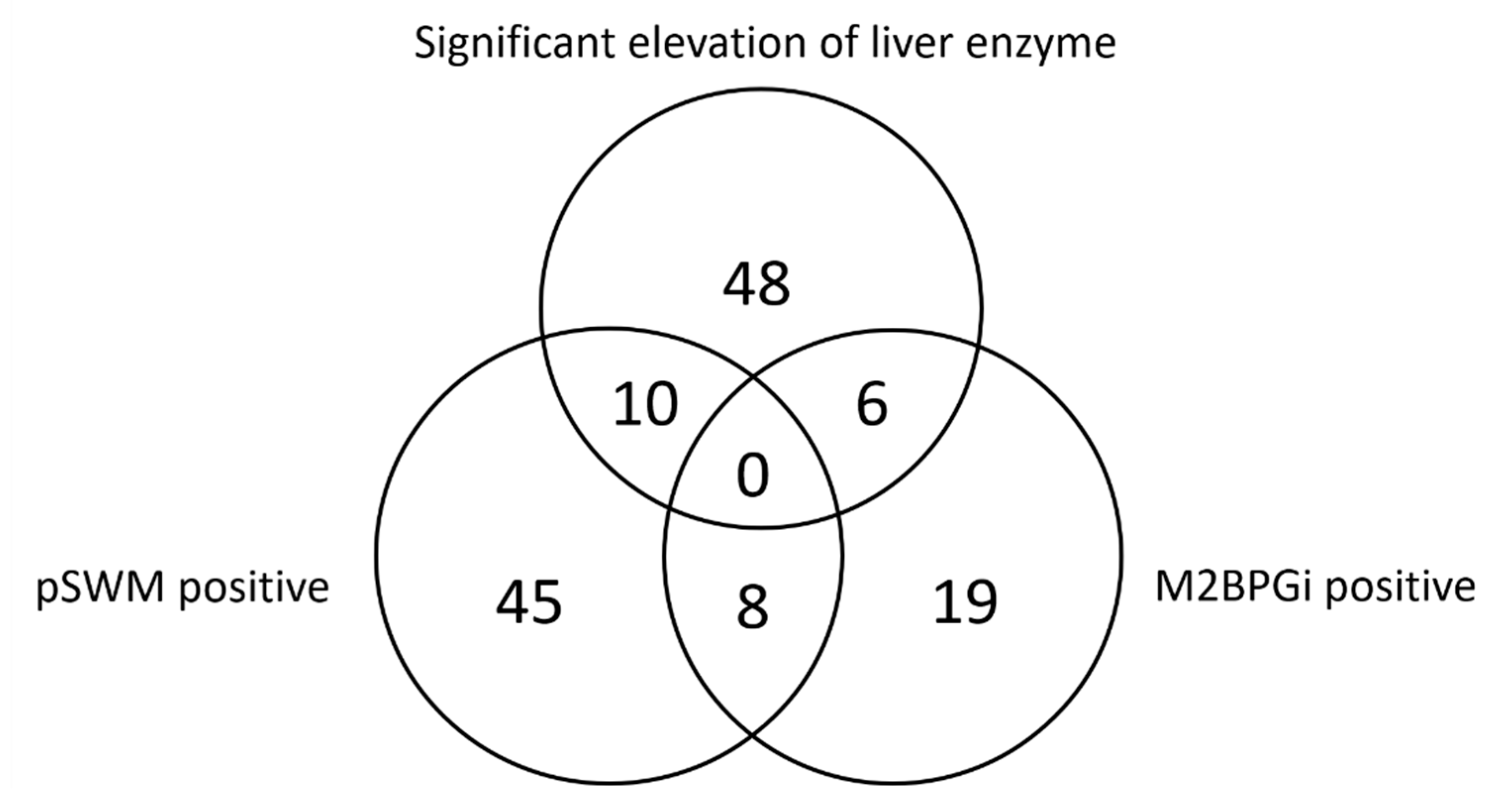
3.2. Outcomes after Follow-Up Survey
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Watanabe, S.; Hashimoto, E.; Ikejima, K.; Uto, H.; Ono, M.; Sumida, Y.; Seike, M.; Takei, Y.; Takehara, T.; Tokushige, K.; et al. Evidence-based clinical practice guidelines for nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Hepatol. Res. 2015, 45, 363–377. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver; European Association for the Study of Diabetes. EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef] [PubMed]
- Mofrad, P.; Contos, M.J.; Haque, M.; Sargeant, C.; Fisher, R.A.; Luketic, V.A.; Sterling, R.K.; Shiffman, M.L.; Stravitz, R.T.; Sanyal, A.J. Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology 2003, 37, 1286–1292. [Google Scholar] [CrossRef] [PubMed]
- Amarapurkar, D.N.; Patel, N.D. Clinical spectrum and natural history of non-alcoholic steatohepatitis with normal alanine aminotransferase values. Trop. Gastroenterol. 2004, 25, 130–134. [Google Scholar] [PubMed]
- Dulai, P.S.; Singh, S.; Patel, J.; Soni, M.; Prokop, L.J.; Younossi, Z.; Sebastiani, G.; Ekstedt, M.; Hagstrom, H.; Nasr, P.; et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: Systematic review and meta-analysis. Hepatology 2017, 65, 1557–1565. [Google Scholar] [CrossRef]
- Yoneda, M.; Imajo, K.; Takahashi, H.; Ogawa, Y.; Eguchi, Y.; Sumida, Y.; Yoneda, M.; Kawanaka, M.; Saito, S.; Tokushige, K.; et al. Clinical strategy of diagnosing and following patients with nonalcoholic fatty liver disease based on invasive and noninvasive methods. J. Gastroenterol. 2018, 53, 181–196. [Google Scholar] [CrossRef]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef] [PubMed]
- McPherson, S.; Hardy, T.; Dufour, J.F.; Petta, S.; Romero-Gomez, M.; Allison, M.; Oliveira, C.P.; Francque, S.; Van Gaal, L.; Schattenberg, J.M.; et al. Age as a Confounding Factor for the Accurate Non-Invasive Diagnosis of Advanced NAFLD Fibrosis. Am. J. Gastroenterol. 2017, 112, 740–751. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, N.; Kurosaki, M.; Loomba, R.; Izumi, N. Clinical Utility of Mac-2 Binding Protein Glycosylation Isomer in Chronic Liver Diseases. Ann. Lab. Med. 2021, 41, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, N.; Higuchi, M.; Kurosaki, M.; Kirino, S.; Osawa, L.; Watakabe, K.; Wang, W.; Okada, M.; Shimizu, T.; Takaura, K.; et al. Wisteria floribunda agglutinin-positive mac-2 binding protein as an age-independent fibrosis marker in nonalcoholic fatty liver disease. Sci. Rep. 2019, 9, 10109. [Google Scholar] [CrossRef] [PubMed]
- Sporea, I.; Bota, S.; Peck-Radosavljevic, M.; Sirli, R.; Tanaka, H.; Iijima, H.; Badea, R.; Lupsor, M.; Fierbinteanu-Braticevici, C.; Petrisor, A.; et al. Acoustic Radiation Force Impulse elastography for fibrosis evaluation in patients with chronic hepatitis C: An international multicenter study. Eur. J. Radiol. 2012, 81, 4112–4118. [Google Scholar] [CrossRef] [PubMed]
- Castera, L.; Friedrich-Rust, M.; Loomba, R. Noninvasive Assessment of Liver Disease in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 2019, 156, 1264–1281.e4. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Adams, L.A. Advances in non-invasive assessment of hepatic fibrosis. Gut 2020, 69, 1343–1352. [Google Scholar] [CrossRef] [PubMed]
- Cassinotto, C.; Boursier, J.; de Ledinghen, V.; Lebigot, J.; Lapuyade, B.; Cales, P.; Hiriart, J.B.; Michalak, S.; Bail, B.L.; Cartier, V.; et al. Liver stiffness in nonalcoholic fatty liver disease: A comparison of supersonic shear imaging, FibroScan, and ARFI with liver biopsy. Hepatology 2016, 63, 1817–1827. [Google Scholar] [CrossRef] [PubMed]
- Friedrich-Rust, M.; Nierhoff, J.; Lupsor, M.; Sporea, I.; Fierbinteanu-Braticevici, C.; Strobel, D.; Takahashi, H.; Yoneda, M.; Suda, T.; Zeuzem, S.; et al. Performance of Acoustic Radiation Force Impulse imaging for the staging of liver fibrosis: A pooled meta-analysis. J. Viral Hepat. 2012, 19, e212–e219. [Google Scholar] [CrossRef] [PubMed]
- Nierhoff, J.; Chavez Ortiz, A.A.; Herrmann, E.; Zeuzem, S.; Friedrich-Rust, M. The efficiency of acoustic radiation force impulse imaging for the staging of liver fibrosis: A meta-analysis. Eur. Radiol. 2013, 23, 3040–3053. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, N.; Kurosaki, M.; Takahashi, Y.; Itakura, Y.; Kirino, S.; Inada, K.; Yamashita, K.; Sekiguchi, S.; Hayakawa, Y.; Osawa, L.; et al. Wisteria floribunda Agglutinin-Positive Mac-2 Binding Protein as a Screening Tool for Significant Liver Fibrosis in Health Checkup. Int. J. Mol. Sci. 2020, 22, 40. [Google Scholar] [CrossRef]
- Nah, E.H.; Cho, S.; Kim, S.; Kim, H.S.; Cho, H.I. Diagnostic performance of Mac-2 binding protein glycosylation isomer (M2BPGi) in screening liver fibrosis in health checkups. J. Clin. Lab. Anal. 2020, 34, e23316. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamai, H.; Okamura, J.; Ohoshi, T.; Wakasaki, H. Measurements of Serum Mac-2-Binding Protein Glycosylation Isomer and Shear Wave Velocity in Health Checkups Are Useful in Screening for Non-Alcoholic Steatohepatitis. Healthcare 2021, 9, 523. https://doi.org/10.3390/healthcare9050523
Tamai H, Okamura J, Ohoshi T, Wakasaki H. Measurements of Serum Mac-2-Binding Protein Glycosylation Isomer and Shear Wave Velocity in Health Checkups Are Useful in Screening for Non-Alcoholic Steatohepatitis. Healthcare. 2021; 9(5):523. https://doi.org/10.3390/healthcare9050523
Chicago/Turabian StyleTamai, Hideyuki, Jumpei Okamura, Takashi Ohoshi, and Hisao Wakasaki. 2021. "Measurements of Serum Mac-2-Binding Protein Glycosylation Isomer and Shear Wave Velocity in Health Checkups Are Useful in Screening for Non-Alcoholic Steatohepatitis" Healthcare 9, no. 5: 523. https://doi.org/10.3390/healthcare9050523
APA StyleTamai, H., Okamura, J., Ohoshi, T., & Wakasaki, H. (2021). Measurements of Serum Mac-2-Binding Protein Glycosylation Isomer and Shear Wave Velocity in Health Checkups Are Useful in Screening for Non-Alcoholic Steatohepatitis. Healthcare, 9(5), 523. https://doi.org/10.3390/healthcare9050523





