Preliminary Assessment of Muscle Activity and Muscle Characteristics during Training with Powered Robotic Exoskeleton: A Repeated-Measures Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.3. Exoskeleton
2.4. Training Procedure
2.5. Outcome Measure
2.5.1. Muscle Activity
2.5.2. Muscle Characteristics
2.6. Statistical Analysis
3. Results
3.1. General Characteristic of Participants
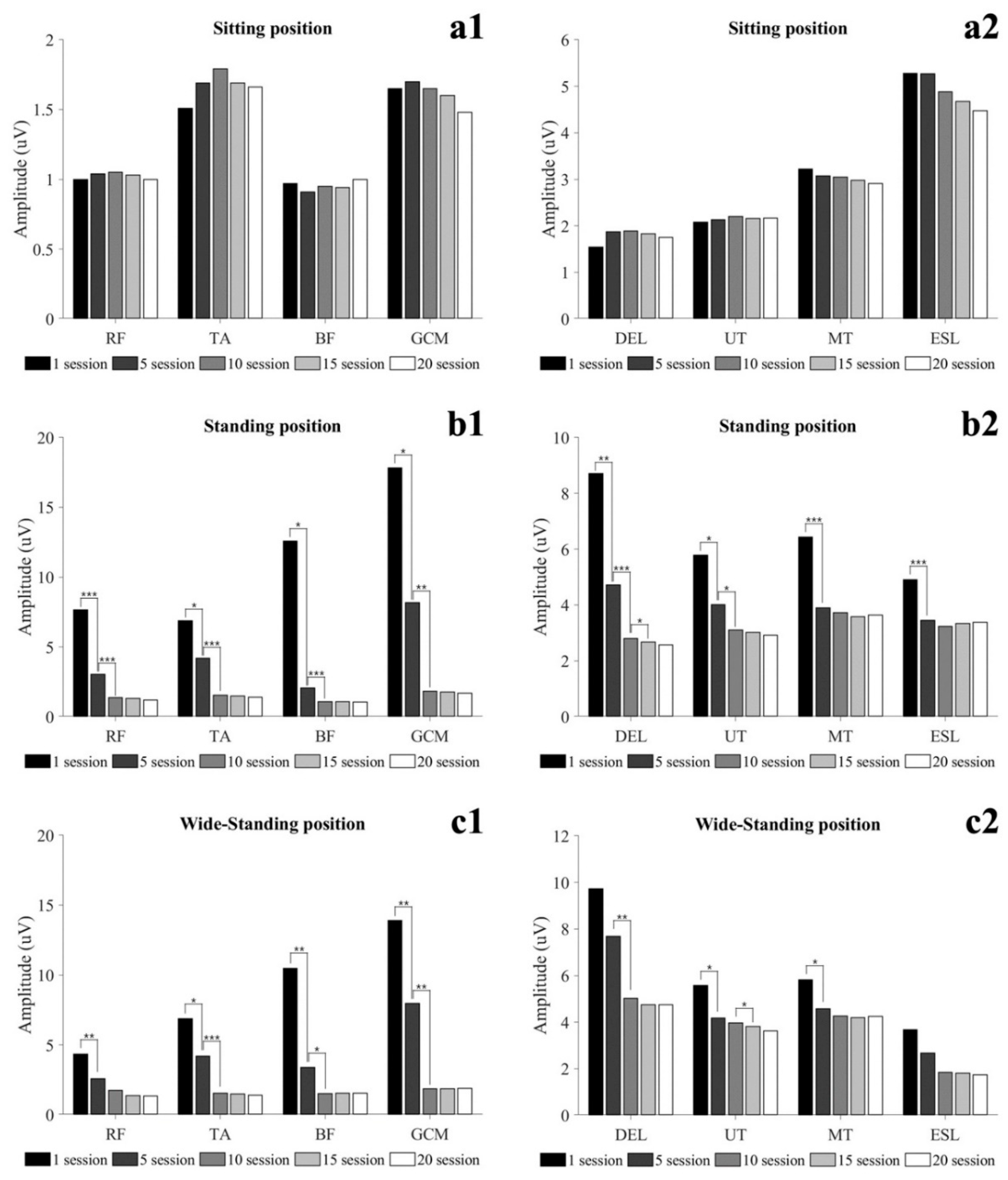
3.2. Muscle Activity
3.2.1. Static Condition
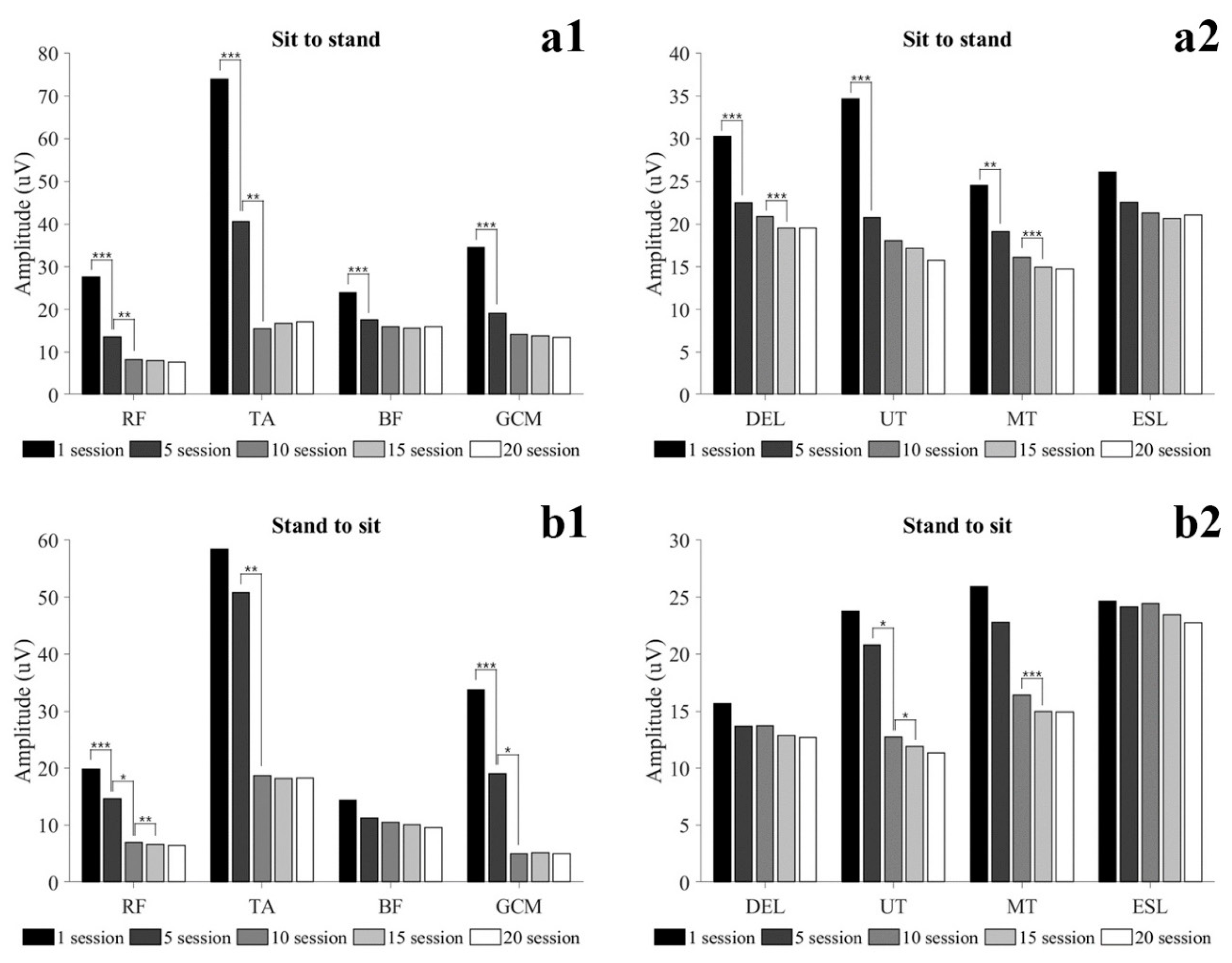
3.2.2. Dynamic Condition
3.3. Muscle Characteristic
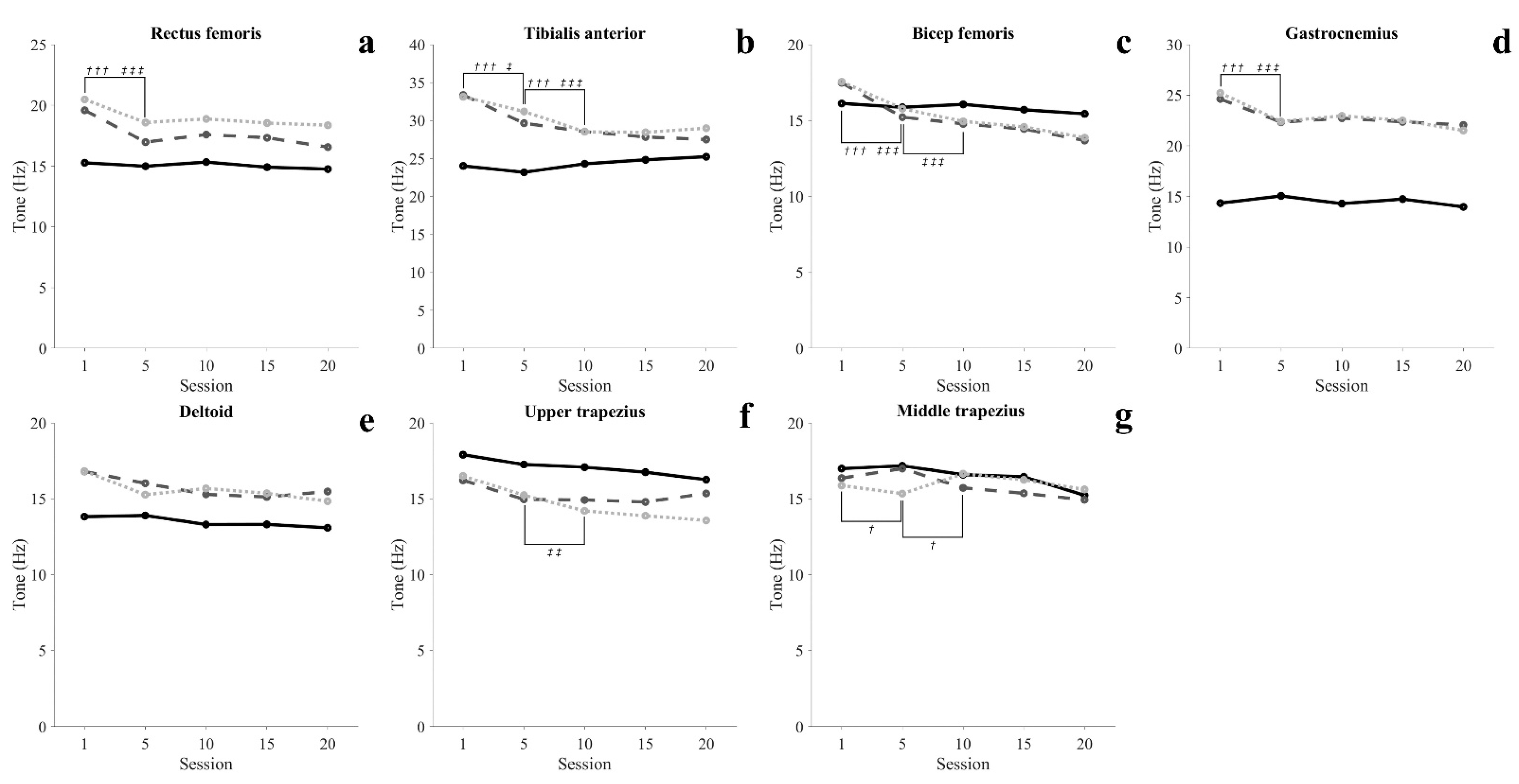
3.3.1. Muscle Tone
3.3.2. Muscle Stiffness
3.3.3. Muscle Elasticity
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Variables | 1 Session | 5 Session | 10 Session | 15 Session | 20 Session | η2 | p-Value |
|---|---|---|---|---|---|---|---|
| Sitting (uV) | |||||||
| Rectus femoris | 1.00 (0.22) | 1.04 (0.25) | 1.05 (0.27) | 1.03 (0.28) | 1.00 (0.34) | 0.049 | 0.493 |
| Tibialis anterior | 1.51 (0.72) | 1.69 (0.88) | 1.79 (0.98) | 1.69 (0.87) | 1.66 (0.99) | 0.127 | 0.133 |
| Biceps femoris | 0.97 (0.30) | 0.91 (0.25) | 0.95 (0.29) | 0.94 (0.29) | 1.00 (0.43) | 0.072 | 0.324 |
| Gastrocnemius | 1.65 (0.54) | 1.70 (0.51) | 1.65 (0.44) | 1.60 (0.45) | 1.48 (0.45) | 0.133 | 0.117 |
| Deltoid | 1.54 (0.36) | 1.87 (0.75) | 1.89 (0.51) | 1.83 (0.54) | 1.75 (0.56) | 0.147 | 0.099 |
| Upper trapezius | 2.08 (0.26) | 2.13 (0.56) | 2.20 (0.31) | 2.16 (0.49) | 2.17 (0.58) | 0.020 | 0.822 |
| Middle trapezius | 3.22 (0.97) | 3.07 (0.82) | 3.05 (1.10) | 2.98 (0.85) | 2.91 (1.20) | 0.079 | 0.289 |
| Erector spine | 5.28 (3.71) | 5.27 (3.98) | 4.88 (4.47) | 4.67 (4.02) | 4.47 (4.17) | 0.037 | 0.501 |
| Standing (uV) | |||||||
| Rectus femoris | 7.66 (4.82) | 3.04 (1.39) *** | 1.37 (0.42) ††† | 1.32 (0.40) | 1.20 (0.33) | 0.629 | <0.001 |
| Tibialis anterior | 6.88 (5.62) | 4.19 (1.79) * | 1.54 (0.61) ††† | 1.49 (0.58) | 1.41 (0.64) | 0.502 | 0.001 |
| Biceps femoris | 12.58 (17.14) | 2.05 (0.87) * | 1.07 (0.20) ††† | 1.05 (0.17) | 1.06 (0.30) | 0.320 | 0.018 |
| Gastrocnemius | 17.82 (18.54) | 8.17 (8.05) * | 1.82 (0.60) †† | 1.76 (0.54) | 1.70 (0.62) | 0.422 | 0.003 |
| Deltoid | 8.71 (4.01) | 4.72 (1.46) ** | 2.80 (1.14) ††† | 2.67 (1.02) ‡ | 2.41 (1.06) | 0.715 | <0.001 |
| Upper trapezius | 5.78 (1.89) | 4.02 (1.56) * | 3.10 (0.80) † | 3.02 (0.98) | 2.92 (1.23) | 0.592 | <0.001 |
| Middle trapezius | 6.43 (2.55) | 3.90 (0.40) *** | 3.72 (0.39) | 3.58 (0.44) | 3.64 (0.73) | 0.569 | <0.001 |
| Erector spine | 4.90 (2.68) | 3.45 (2.09) *** | 3.24 (1.95) | 3.34 (2.24) | 3.38 (2.67) | 0.439 | <0.001 |
| Wide-standing (uV) | |||||||
| Rectus femoris | 4.33 (4.10) | 2.59 (2.69) ** | 1.74 (1.13) | 1.37 (0.74) | 1.34 (0.70) | 0.317 | 0.014 |
| Tibialis anterior | 10.48 (8.80) | 3.38 (2.53) * | 1.50 (0.56) ††† | 1.53 (0.65) | 1.54 (0.75) | 0.504 | 0.001 |
| Biceps femoris | 11.27 (16.77) | 1.76 (0.87) ** | 1.34 (0.64) † | 1.30 (0.60) | 1.20 (0.53) | 0.273 | 0.031 |
| Gastrocnemius | 13.91 (11.06) | 7.98 (6.32) ** | 1.87 (0.79) †† | 1.87 (0.94) | 1.90 (1.08) | 0.537 | <0.001 |
| Deltoid | 9.73 (4.57) | 7.69 (2.53) | 5.02 (1.87) †† | 4.74 (1.79) | 4.74 (2.42) | 0.394 | 0.002 |
| Upper trapezius | 5.57 (1.88) | 4.18 (1.33) * | 3.96 (0.92) | 3.82 (1.00) ‡ | 3.63 (1.21) | 0.392 | 0.003 |
| Middle trapezius | 5.81 (2.16) | 4.58 (1.35) * | 4.26 (0.77) | 4.20 (0.81) | 4.24 (1.49) | 0.313 | 0.007 |
| Erector spine | 3.68 (3.38) | 2.68 (1.76) | 1.85 (0.38) | 1.80 (0.36) | 1.74 (0.56) | 0.218 | 0.041 |
| Variables | 1 Session | 5 Session | 10 Session | 15 Session | 20 Session | η2 | p-Value |
|---|---|---|---|---|---|---|---|
| Sit to stand (uV) | |||||||
| Rectus femoris | 27.65 (11.55) | 13.57 (7.46) *** | 8.29 (3.66) †† | 8.05 (3.80) | 7.65 (3.91) | 0.738 | <0.001 |
| Tibialis anterior | 73.96 (47.36) | 40.66 (34.04) *** | 15.56 (8.96) †† | 16.80 (10.97) | 17.08 (12.51) | 0.633 | <0.001 |
| Biceps femoris | 23.92 (15.16) | 17.62 (10.71) *** | 15.91 (8.69) | 15.66 (8.92) | 15.93 (10.89) | 0.457 | <0.001 |
| Gastrocnemius | 34.58 (24.33) | 19.12 (13.06) *** | 14.08 (5.61) | 13.73 (5.60) | 13.48 (6.29) | 0.459 | 0.002 |
| Deltoid | 30.28 (11.00) | 22.48 (6.75) *** | 20.88 (3.52) | 19.50 (3.21) ‡‡‡ | 19.49 (7.07) | 0.526 | <0.001 |
| Upper trapezius | 34.65 (11.22) | 20.77 (7.40) *** | 18.07 (4.78) | 17.17 (5.63) | 15.75 (5.30) | 0.617 | <0.001 |
| Middle trapezius | 24.55 (12.49) | 19.10 (9.69) ** | 16.12 (4.45) | 14.97 (4.25) ‡‡‡ | 14.74 (4.42) | 0.413 | 0.001 |
| Erector spine | 26.06 (14.19) | 22.58 (9.16) | 21.30 (6.64) | 20.65 (6.49) | 21.08 (8.62) | 0.207 | 0.040 |
| Stand to sit (uV) | |||||||
| Rectus femoris | 19.86 (13.26) | 14.66 (12.06) *** | 7.01 (3.41) † | 6.69 (3.39) ‡‡ | 6.51 (3.44) | 0.515 | <0.001 |
| Tibialis anterior | 58.38 (48.35) | 50.81 (53.80) | 18.73 (19.75) †† | 18.17 (19.28) | 18.27 (21.02) | 0.559 | <0.001 |
| Biceps femoris | 14.41 (4.13) | 11.28 (3.37) | 10.49 (5.01) | 10.11 (4.60) | 9.57 (4.81) | 0.191 | 0.051 |
| Gastrocnemius | 33.79 (21.95) | 19.05 (22.08) *** | 5.09 (1.77) † | 5.23 (1.93) | 5.08 (2.11) | 0.544 | <0.001 |
| Deltoid | 15.66 (6.55) | 13.68 (9.32) | 13.71 (5.34) | 12.87 (5.04) | 12.70 (6.58) | 0.099 | 0.210 |
| Upper trapezius | 23.76 (19.13) | 20.82 (11.90) | 12.71 (6.00) † | 11.91 (5.32) ‡ | 11.36 (5.16) | 0.290 | 0.015 |
| Middle trapezius | 25.92 (22.19) | 22.81 (16.93) | 16.40 (9.57) | 14.99 (8.73) ‡‡‡ | 14.95 (10.25) | 0.228 | 0.038 |
| Erector spine | 24.67 (16.14) | 24.16 (15.29) | 24.43 (14.14) | 23.44 (14.26) | 22.78 (15.70) | 0.020 | 0.733 |
| Variables | 1 Session | 5 Session | 10 Session | 15 Session | 20 Session | η2 | p-Value |
|---|---|---|---|---|---|---|---|
| Sitting (Hz) | |||||||
| Rectus femoris | 15.27 (0.63) | 14.99 (0.50) | 15.33 (0.73) | 14.91 (0.95) | 14.75 (3.89) | 0.021 | 0.609 |
| Tibialis anterior | 24.02 (3.27) | 23.18 (3.08) | 24.30 (3.32) | 24.83 (3.53) | 25.23 (4.37) | 0.130 | 0.125 |
| Biceps femoris | 16.12 (2.41) | 15.87 (1.76) | 16.06 (2.12) | 15.71 (1.97) | 15.44 (3.24) | 0.039 | 0.530 |
| Gastrocnemius | 14.34 (1.66) | 15.05 (1.80) | 14.29 (0.96) | 14.74 (1.51) | 13.97 (2.90) | 0.062 | 0.373 |
| Deltoid | 13.82 (0.83) | 13.90 (1.23) | 13.30 (1.03) | 13.31 (1.38) | 13.09 (3.17) | 0.069 | 0.322 |
| Upper trapezius | 17.90 (0.78) | 17.26 (0.59) | 17.08 (1.03) | 16.75 (1.19) | 16.26 (2.86) | 0.166 | 0.079 |
| Middle trapezius | 16.99 (0.72) | 17.17 (1.03) | 16.59 (1.32) | 16.45 (1.30) | 15.23 (2.77) | 0.200 | 0.051 |
| Standing (Hz) | |||||||
| Rectus femoris | 19.59 (2.19) | 16.97 (1.21) *** | 17.59 (1.98) | 17.33 (1.66) | 16.56 (2.87) | 0.311 | 0.005 |
| Tibialis anterior | 33.35 (2.41) | 29.64 (3.37) *** | 28.54 (2.96) ††† | 27.83 (3.11) | 27.49 (5.50) | 0.467 | <0.001 |
| Biceps femoris | 17.48 (1.90) | 15.22 (1.22) *** | 14.78 (0.56) | 14.44 (0.94) | 13.68 (3.35) | 0.405 | 0.001 |
| Gastrocnemius | 24.62 (1.60) | 22.33 (0.62) *** | 22.73 (1.40) | 22.37 (1.95) | 22.06 (3.91) | 0.223 | 0.030 |
| Deltoid | 16.80 (1.79) | 16.02 (2.31) | 15.29 (2.37) | 15.12 (2.21) | 15.48 (4.75) | 0.129 | 0.135 |
| Upper trapezius | 16.22 (1.45) | 14.96 (0.69) | 14.92 (0.92) | 14.79 (1.09) | 15.35 (2.83) | 0.151 | 0.084 |
| Middle trapezius | 16.36 (1.32) | 16.99 (1.87) * | 15.72 (1.27) † | 15.36 (1.46) | 14.93 (3.23) | 0.197 | 0.040 |
| Wide-standing (Hz) | |||||||
| Rectus femoris | 20.48 (2.11) | 18.58 (1.89) *** | 18.88 (2.26) | 18.54 (2.11) | 18.36 (3.51) | 0.222 | 0.029 |
| Tibialis anterior | 33.13 (4.15) | 31.19 (2.88) * | 28.52 (1.87) ††† | 28.45 (2.00) | 28.99 (5.60) | 0.351 | 0.002 |
| Biceps femoris | 17.55 (1.44) | 15.78 (1.10) *** | 14.94 (0.67) ††† | 14.58 (0.96) | 13.87 (3.13) | 0.446 | <0.001 |
| Gastrocnemius | 25.23 (2.40) | 22.40 (1.11) *** | 22.96 (1.20) | 22.51 (1.60) | 21.52 (4.32) | 0.299 | 0.006 |
| Deltoid | 16.82 (2.37) | 15.27 (1.48) | 15.68 (1.75) | 15.36 (1.63) | 14.84 (2.72) | 0.162 | 0.056 |
| Upper trapezius | 16.50 (2.39) | 15.24 (0.96) | 14.20 (0.84) †† | 13.88 (1.02) | 13.57 (2.87) | 0.341 | 0.003 |
| Middle trapezius | 15.87 (0.95) | 15.34 (0.93) | 16.65 (0.92) | 16.25 (0.90) | 15.61 (2.93) | 0.116 | 0.168 |
| Variables | 1 Session | 5 Session | 10 Session | 15 Session | 20 Session | η2 | p-Value |
|---|---|---|---|---|---|---|---|
| Sitting (N/m) | |||||||
| Rectus femoris | 270.01 (12.25) | 264.49 (12.37) | 279.41 (14.23) | 262.11 (18.76) | 256.81 (53.39) | 0.114 | 0.174 |
| Tibialis anterior | 513.28 (91.50) | 528.81 (89.06) | 514.48 (87.40) | 516.15 (99.95) | 497.98 (141.07) | 0.042 | 0.463 |
| Biceps femoris | 263.97 (32.51) | 261.38 (34.43) | 255.33 (33.34) | 253.67 (34.32) | 242.71 (63.49) | 0.086 | 0.261 |
| Gastrocnemius | 232.08 (22.11) | 228.41 (17.10) | 230.18 (20.99) | 227.56 (24.36) | 231.09 (55.77) | 0.006 | 0.840 |
| Deltoid | 222.64 (17.36) | 230.27 (18.24) | 213.37 (26.87) | 209.76 (17.10) | 207.16 (38.09) | 0.166 | 0.063 |
| Upper trapezius | 294.40 (15.16) | 285.33 (15.88) | 292.81 (22.76) | 286.56 (24.69) | 276.04 (54.07) | 0.080 | 0.281 |
| Middle trapezius | 291.12 (28.77) | 305.19 (16.63) | 301.87 (30.47) | 301.00 (43.53) | 302.71 (50.82) | 0.043 | 0.556 |
| Standing (N/m) | |||||||
| Rectus femoris | 400.99 (71.61) | 302.31 (24.27) *** | 330.65 (39.88) †† | 320.65 (39.00) ‡ | 307.43 (68.13) | 0.525 | <0.001 |
| Tibialis anterior | 889.74 (108.39) | 759.43 (136.05) *** | 700.93 (129.42) †† | 673.03 (142.88) ‡ | 673.92 (211.43) | 0.538 | <0.001 |
| Biceps femoris | 318.46 (80.94) | 270.64 (38.11) * | 247.79 (30.19) † | 245.01 (33.12) | 242.34 (50.43) | 0.369 | 0.001 |
| Gastrocnemius | 559.65 (81.38) | 450.33 (41.33) *** | 436.00 (56.68) | 430.88 (61.12) | 433.41 (85.84) | 0.461 | <0.001 |
| Deltoid | 286.26 (52.00) | 264.71 (46.63) | 267.97 (65.48) | 261.55 (59.89) | 260.66 (81.61) | 0.094 | 0.227 |
| Upper trapezius | 259.90 (31.01) | 235.24 (33.13) | 252.12 (26.44) | 248.20 (32.06) | 240.12 (54.55) | 0.072 | 0.331 |
| Middle trapezius | 274.33 (21.15) | 307.30 (53.72) | 267.29 (30.94) | 262.24 (34.01) | 255.10 (75.96) | 0.191 | 0.058 |
| Wide-standing (N/m) | |||||||
| Rectus femoris | 411.48 (67.10) | 346.49 (43.44) *** | 346.84 (38.64) | 339.63 (36.98) | 325.73 (54.49) | 0.463 | <0.001 |
| Tibialis anterior | 941.58 (184.07) | 842.82 (138.11) *** | 717.02 (121.66) ††† | 704.91 (132.77) | 700.11 (209.44) | 0.549 | <0.001 |
| Biceps femoris | 334.29 (56.76) | 288.70 (41.35) ** | 270.92 (37.42) | 271.78 (36.27) | 250.80 (51.97) | 0.443 | <0.001 |
| Gastrocnemius | 580.98 (104.10) | 448.66 (50.98) | 456.38 (37.59) | 455.36 (42.77) | 446.14 (103.84) | 0.486 | <0.001 |
| Deltoid | 291.97 (54.510) | 250.63 (37.72) ** | 262.56 (41.83) | 259.54 (39.65) | 239.23 (48.97) | 0.279 | 0.005 |
| Upper trapezius | 275.36 (51.78) | 257.31 (18.52) | 236.81 (10.98) †† | 234.18 (18.00) | 222.20 (52.03) | 0.310 | 0.003 |
| Middle trapezius | 267.57 (25.93) | 261.10 (29.66) | 294.31 (18.53) | 289.07 (16.96) | 285.64 (65.12) | 0.142 | 0.118 |
| Variables | 1 Session | 5 Session | 10 Session | 15 Session | 20 Session | η2 | p-Value |
|---|---|---|---|---|---|---|---|
| Sitting (LogD) | |||||||
| Rectus femoris | 0.93 (0.05) | 0.89 (0.06) | 0.89 (0.08) | 0.87 (0.06) | 0.86 (0.14) | 0.101 | 0.202 |
| Tibialis anterior | 0.68 (0.08) | 0.70 (0.05) | 0.70 (0.09) | 0.67 (0.08) | 0.63 (0.12) | 0.154 | 0.082 |
| Biceps femoris | 1.02 (0.15) | 1.06 (0.16) | 1.05 (0.11) | 1.06 (0.13) | 1.02 (0.23) | 0.037 | 0.532 |
| Gastrocnemius | 1.13 (0.18) | 1.13 (0.17) | 1.14 (0.11) | 1.11 (0.17) | 1.09 (0.37) | 0.019 | 0.671 |
| Deltoid | 1.04 (0.08) | 1.04 (0.12) | 1.09 (0.12) | 1.04 (0.10) | 1.04 (0.24) | 0.040 | 0.496 |
| Upper trapezius | 0.91 (0.11) | 0.92 (0.12) | 0.91 (0.12) | 0.87 (0.10) | 0.87 (0.20) | 0.062 | 0.374 |
| Middle trapezius | 0.90 (0.08) | 0.95 (0.07) | 0.91 (0.08) | 0.92 (0.10) | 0.93 (0.16) | 0.080 | 0.285 |
| Standing (LogD) | |||||||
| Rectus femoris | 0.96 (0.07) | 0.94 (0.10) | 0.94 (0.12) | 0.91 (0.12) | 0.88 (0.18) | 0.125 | 0.139 |
| Tibialis anterior | 0.63 (0.12) | 0.65 (0.10) | 0.67 (0.12) | 0.65 (0.12) | 0.62 (0.16) | 0.082 | 0.279 |
| Biceps femoris | 1.13 (0.15) | 1.17 (0.14) | 1.15 (0.18) | 1.13 (0.19) | 1.06 (0.23) | 0.115 | 0.154 |
| Gastrocnemius | 1.00 (0.18) | 1.01 (0.17) | 1.07 (0.15) | 1.06 (0.15) | 0.99 (0.28) | 0.101 | 0.203 |
| Deltoid | 1.07 (0.09) | 1.02 (0.12) | 1.04 (0.14) | 1.01 (0.13) ‡‡ | 0.96 (0.18) | 0.181 | 0.038 |
| Upper trapezius | 0.99 (0.17) | 1.00 (0.17) | 0.94 (0.17) † | 0.92 (0.19) ‡ | 0.84 (0.19) | 0.261 | 0.013 |
| Middle trapezius | 1.03 (0.23) | 1.01 (0.11) | 1.01 (0.17) | 0.97 (0.14) | 0.92 (0.25) | 0.103 | 0.189 |
| Wide-standing (LogD) | |||||||
| Rectus femoris | 1.06 (0.15) | 1.19 (0.14) | 1.15 (0.14) | 1.13 (0.15) | 1.07 (0.25) | 0.173 | 0.067 |
| Tibialis anterior | 0.55 (0.18) | 0.59 (0.15) | 0.60 (0.14) | 0.50 (0.09) ‡ | 0.46 (0.05) | 0.233 | 0.036 |
| Biceps femoris | 1.18 (0.14) | 1.20 (0.15) | 1.20 (0.12) | 1.19 (0.11) | 1.17 (0.30) | 0.007 | 0.882 |
| Gastrocnemius | 0.76 (0.11) | 0.81 (0.05) | 0.83 (0.04) | 0.83 (0.05) | 0.83 (0.15) | 0.113 | 0.158 |
| Deltoid | 0.99 (0.10) | 0.94 (0.09) | 0.91 (0.08) | 0.88 (0.08) ‡ | 0.85 (0.20) | 0.236 | 0.026 |
| Upper trapezius | 0.99 (0.10) | 0.95 (0.09) | 0.89 (0.07) † | 0.87 (0.06) ‡ | 0.87 (0.21) | 0.200 | 0.038 |
| Middle trapezius | 1.23 (0.16) | 1.26 (0.18) | 1.06 (0.15) ††† | 1.04 (0.15) | 0.99 (0.22) | 0.528 | <0.001 |
References
- Rahimi-Movaghar, V.; Saadat, S.; Rasouli, M.R.; Ganji, S.; Bs, M.G.; Zarei, M.; Vaccaro, A.R. Prevalence of spinal cord injury in Tehran, Iran. J. Spinal Cord Med. 2009, 32, 428–431. [Google Scholar] [CrossRef] [Green Version]
- Knútsdóttir, S.; Thórisdóttir, H.; Sigvaldason, K.; Jónsson, H., Jr.; Björnsson, A.; Ingvarsson, P. Epidemiology of traumatic spinal cord injuries in Iceland from 1975 to 2009. Spinal Cord 2012, 50, 123–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- New, P.W.; Baxter, D.; Farry, A.; Noonan, V.K. Estimating the incidence and prevalence of traumatic spinal cord injury in Australia. Arch. Phys. Med. Rehabil. 2015, 96, 76–83. [Google Scholar] [CrossRef]
- New, P.W.; Farry, A.; Baxter, D.; Noonan, V.K. Prevalence of non-traumatic spinal cord injury in Victoria, Australia. Spinal Cord 2013, 51, 99–102. [Google Scholar] [CrossRef] [Green Version]
- Jain, N.B.; Higgins, L.D.; Katz, J.N.; Garshick, E. Association of Shoulder pain with the use of mobility devices in persons with chronic spinal cord injury. PM&R 2010, 2, 896–900. [Google Scholar] [CrossRef]
- Hayes, S.C.; Wilcox, C.R.J.; White, H.S.F.; Vanicek, N. The effects of robot assisted gait training on temporal-spatial characteristics of people with spinal cord injuries: A systematic review. J. Spinal Cord Med. 2018, 41, 529–543. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, N.; Zhou, M.; Lu, Y.; Li, F. Bibliometric analysis of global research on the rehabilitation of spinal cord injury in the past two decades. Ther. Clin. Risk Manag. 2018, 15, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dijkers, M.P.; Akers, K.; Dieffenbach, S.; Galen, S.S. Systematic reviews of clinical benefits of exoskeleton use for gait and mobility in neurologic disorders: A tertiary study. Arch. Phys. Med. Rehabil. 2021, 102, 300–313. [Google Scholar] [CrossRef] [PubMed]
- Esquenazi, A.; Talaty, M.; Packel, A.; Saulino, M. The ReWalk powered exoskeleton to restore ambulatory function to individuals with thoracic-level motor-complete spinal cord injury. Am. J. Phys. Med. Rehabil. 2012, 91, 911–921. [Google Scholar] [CrossRef] [Green Version]
- Kolakowsky-Hayner, S.A. Safety and feasibility of using the EksoTM bionic exoskeleton to aid ambulation after spinal cord injury. J. Spine 2013, 4. [Google Scholar] [CrossRef]
- Farris, R.J.; Quintero, H.A.; Murray, S.A.; Ha, K.H.; Hartigan, C.; Goldfarb, M. A Preliminary assessment of legged mobility provided by a lower limb exoskeleton for persons with paraplegia. IEEE Trans. Neural Syst. Rehabil. Eng. 2014, 22, 482–490. [Google Scholar] [CrossRef] [Green Version]
- Bass, A.; Morin, S.N.; Vermette, M.; Aubertin-Leheudre, M.; Gagnon, D. Incidental bilateral calcaneal fractures following overground walking with a wearable robotic exoskeleton in a wheelchair user with a chronic spinal cord injury: Is zero risk possible? Osteoporos. Int. 2020, 31, 1007–1011. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.H.; Zhu, F.; Patel, N.; Afzal, T.; Kern, M.; Francisco, G.E. Combining robotic exoskeleton and body weight unweighing technology to promote walking activity in tetraplegia following SCI: A case study. J. Spinal Cord Med. 2020, 43, 126–129. [Google Scholar] [CrossRef]
- Okawara, H.; Sawada, T.; Matsubayashi, K.; Sugai, K.; Tsuji, O.; Nagoshi, N.; Matsumoto, M.; Nakamura, M. Gait ability required to achieve therapeutic effect in gait and balance function with the voluntary driven exoskeleton in patients with chronic spinal cord injury: A clinical study. Spinal Cord 2020, 58, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Finley, M.A.; Rodgers, M.M. Prevalence and identification of shoulder pathology in athletic and nonathletic wheelchair users with shoulder pain: A pilot study. J. Rehabil. Res. Dev. 2004, 41, 395–402. [Google Scholar] [CrossRef] [Green Version]
- Samuelsson, K.A.M.; Tropp, H.; Gerdle, B. Shoulder pain and its consequences in paraplegic spinal cord-injured, wheelchair users. Spinal Cord 2004, 42, 41–46. [Google Scholar] [CrossRef] [Green Version]
- Alm, M.; Saraste, H.; Norrbrink, C. Shoulder pain in persons with thoracic spinal cord injury: Prevalence and characteristics. J. Rehabil. Med. 2008, 40, 277–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brose, S.W.; Boninger, M.; Fullerton, B.; McCann, T.; Collinger, J.; Impink, B.G.; Dyson-Hudson, T. Shoulder ultrasound abnormalities, physical examination findings, and pain in manual wheelchair users with spinal cord injury. Arch. Phys. Med. Rehabil. 2008, 89, 2086–2093. [Google Scholar] [CrossRef] [PubMed]
- Sylos-Labini, F.; La Scaleia, V.; D’Avella, A.; Pisotta, I.; Tamburella, F.; Scivoletto, G.; Molinari, M.; Wang, S.; Wang, L.; van Asseldonk, E.; et al. EMG patterns during assisted walking in the exoskeleton. Front. Hum. Neurosci. 2014, 8, 423. [Google Scholar] [CrossRef]
- Anton, H.A.; Miller, W.C.; Townson, A.F.; Imam, B.; Silverberg, N.; Forwell, S. The course of fatigue after acute spinal cord injury. Spinal Cord 2017, 55, 94–97. [Google Scholar] [CrossRef]
- Cudeiro-Blanco, J.; Onate-Figuérez, A.; Soto-León, V.; Coy, J.A.; Mordillo-Mateos, L.; Brocalero-Camacho, A.; Esclarin-Ruz, A.; Rotondi, M.; Aguilar, J.; Arias, P.; et al. Prevalence of fatigue and associated factors in a spinal cord injury population: Data from an internet-based and face-to-face surveys. J. Neurotrauma 2017, 34, 2335–2341. [Google Scholar] [CrossRef] [PubMed]
- Lay, B.; Sparrow, W.; Hughes, K.M.; O’Dwyer, N. Practice effects on coordination and control, metabolic energy expenditure, and muscle activation. Hum. Mov. Sci. 2002, 21, 807–830. [Google Scholar] [CrossRef]
- Sköld, C.; Levi, R.; Seiger, Å. Spasticity after traumatic spinal cord injury: Nature, severity, and location. Arch. Phys. Med. Rehabil. 1999, 80, 1548–1557. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.-G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.-Y.; Choi, J.-D.; Kim, S.-Y.; Oh, D.-W.; Kim, J.-K.; Park, J.-W. Comparison between muscle activation measured by electromyography and muscle thickness measured using ultrasonography for effective muscle assessment. J. Electromyogr. Kinesiol. 2014, 24, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Padua, D.A.; Bell, D.R.; Clark, M.A. Neuromuscular characteristics of individuals displaying excessive medial knee displacement. J. Athl. Train. 2012, 47, 525–536. [Google Scholar] [CrossRef] [Green Version]
- Cosme-Trindade, D.C.; Baldisserotto, S.M.; Loss, J.F.; Shinkai, R.S.A. Duration and sequence of muscular activation in dentate individuals and complete denture wearers during simulation of activities of daily living. Eur. J. Oral Sci. 2019, 127, 222–231. [Google Scholar] [CrossRef] [PubMed]
- MacLean, K.F.E.; Dickerson, C.R. Kinematic and EMG analysis of horizontal bimanual climbing in humans. J. Biomech. 2019, 92, 11–18. [Google Scholar] [CrossRef]
- Pozzi, F.; Plummer, H.A.; Sanchez, N.; Lee, Y.; Michener, L.A. Electromyography activation of shoulder and trunk muscles is greater during closed chain compared to open chain exercises. J. Electromyogr. Kinesiol. 2019, 102306. [Google Scholar] [CrossRef]
- Šarabon, N.; Marusic, J.; Marković, G.; Kozinc, Ž. Kinematic and electromyographic analysis of variations in Nordic hamstring exercise. PLoS ONE 2019, 14, e0223437. [Google Scholar] [CrossRef]
- Chuang, L.-L.; Wu, C.-Y.; Lin, K.-C. Reliability, validity, and responsiveness of myotonometric measurement of muscle tone, elasticity, and stiffness in patients with stroke. Arch. Phys. Med. Rehabil. 2012, 93, 532–540. [Google Scholar] [CrossRef]
- Pruyn, E.C.; Watsford, M.L.; Murphy, A.J. Validity and reliability of three methods of stiffness assessment. J. Sport Health Sci. 2016, 5, 476–483. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.; Kim, M.; Lee, H. The measurement of stiffness for major muscles with shear wave elastography and myoton: A quantitative analysis study. Diagnostics 2021, 11, 524. [Google Scholar] [CrossRef]
- Murray, R.F.; Asghari, A.; Egorov, D.D.; Rutkowski, S.B.; Siddall, P.J.; Soden, R.J.; Ruff, R. Impact of spinal cord injury on self-perceived pre- and postmorbid cognitive, emotional and physical functioning. Spinal Cord 2007, 45, 429–436. [Google Scholar] [CrossRef] [Green Version]
- John, L.T.; Cherian, B.; Babu, A. Postural control and fear of falling in persons with low-level paraplegia. J. Rehabil. Res. Dev. 2010, 47, 497. [Google Scholar] [CrossRef] [PubMed]
- Gavronski, G.; Veraksitš, A.; Vasar, E.; Maaroos, J. Evaluation of viscoelastic parameters of the skeletal muscles in junior triathletes. Physiol. Meas. 2007, 28, 625–637. [Google Scholar] [CrossRef] [PubMed]
- Kocur, P.; Grzeskowiak, M.; Wiernicka, M.; Goliwas, M.; Lewandowski, J.; Lochynski, D. Effects of aging on mechanical properties of sternocleidomastoid and trapezius muscles during transition from lying to sitting position—A cross-sectional study. Arch. Gerontol. Geriatr. 2017, 70, 14–18. [Google Scholar] [CrossRef]
- Horak, F.B.; Wrisley, D.M.; Frank, J. The Balance Evaluation Systems Test (BESTest) to differentiate balance deficits. Phys. Ther. 2009, 89, 484–498. [Google Scholar] [CrossRef]
- Di Fabio, R.P.; Emasithi, A. Aging and the mechanisms underlying head and postural control during voluntary motion. Phys. Ther. 1997, 77, 458–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Runge, C.F.; Shupert, C.L.; Horak, F.B.; Zajac, F.E. Ankle and hip postural strategies defined by joint torques. Gait Posture 1999, 10, 161–170. [Google Scholar] [CrossRef]
- Neumann, D.A. Kinesiology of the Musculoskeletal System: Foundations for Rehabilitation, 3rd ed.; Elsevier: St Louis, MO, USA, 2017. [Google Scholar]
- Spurrs, R.W.; Murphy, A.J.; Watsford, M.L. The effect of plyometric training on distance running performance. Eur. J. Appl. Physiol. 2003, 89, 1–7. [Google Scholar] [CrossRef]
- Kuitunen, S.; Kyröläinen, H.; Avela, J.; Komi, P.V. Leg stiffness modulation during exhaustive stretch-shortening cycle exercise. Scand. J. Med. Sci. Sports 2007, 17, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Watsford, M.L.; Murphy, A.J.; McLachlan, K.A.; Bryant, A.L.; Cameron, M.L.; Crossley, K.M.; Makdissi, M. A Prospective study of the relationship between lower body stiffness and hamstring injury in professional Australian rules footballers. Am. J. Sports Med. 2010, 38, 2058–2064. [Google Scholar] [CrossRef] [PubMed]



| Parameters | Value (N = 16) | |
|---|---|---|
| Gender, male/female (n) ∗ | 7/9 | |
| Age (year) † | 24.88 ± 2.03 | |
| Body height (cm) † | 166.06 ± 8.00 | |
| Body mass (kg) † | 59.63 ± 8.71 | |
| Body mass index (kg/m2) † | 21.51 ± 1.69 | |
| Total leg length (cm) † | Rt. | 85.69 ± 5.48 |
| Lt. | 85.59 ± 5.67 | |
| Thigh length (cm) † | Rt. | 37.21 ± 2.48 |
| Lt. | 37.16 ± 2.54 | |
| Tibia length (cm) † | Rt. | 37.21 ± 2.60 |
| Lt. | 37.15 ± 2.43 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-H.; Shin, H.-J.; Cho, H.-Y. Preliminary Assessment of Muscle Activity and Muscle Characteristics during Training with Powered Robotic Exoskeleton: A Repeated-Measures Study. Healthcare 2021, 9, 1003. https://doi.org/10.3390/healthcare9081003
Kim S-H, Shin H-J, Cho H-Y. Preliminary Assessment of Muscle Activity and Muscle Characteristics during Training with Powered Robotic Exoskeleton: A Repeated-Measures Study. Healthcare. 2021; 9(8):1003. https://doi.org/10.3390/healthcare9081003
Chicago/Turabian StyleKim, Sung-Hyeon, Ho-Jin Shin, and Hwi-Young Cho. 2021. "Preliminary Assessment of Muscle Activity and Muscle Characteristics during Training with Powered Robotic Exoskeleton: A Repeated-Measures Study" Healthcare 9, no. 8: 1003. https://doi.org/10.3390/healthcare9081003
APA StyleKim, S.-H., Shin, H.-J., & Cho, H.-Y. (2021). Preliminary Assessment of Muscle Activity and Muscle Characteristics during Training with Powered Robotic Exoskeleton: A Repeated-Measures Study. Healthcare, 9(8), 1003. https://doi.org/10.3390/healthcare9081003







