Review of Current Spinal Robotic Orthoses
Abstract
:1. Background
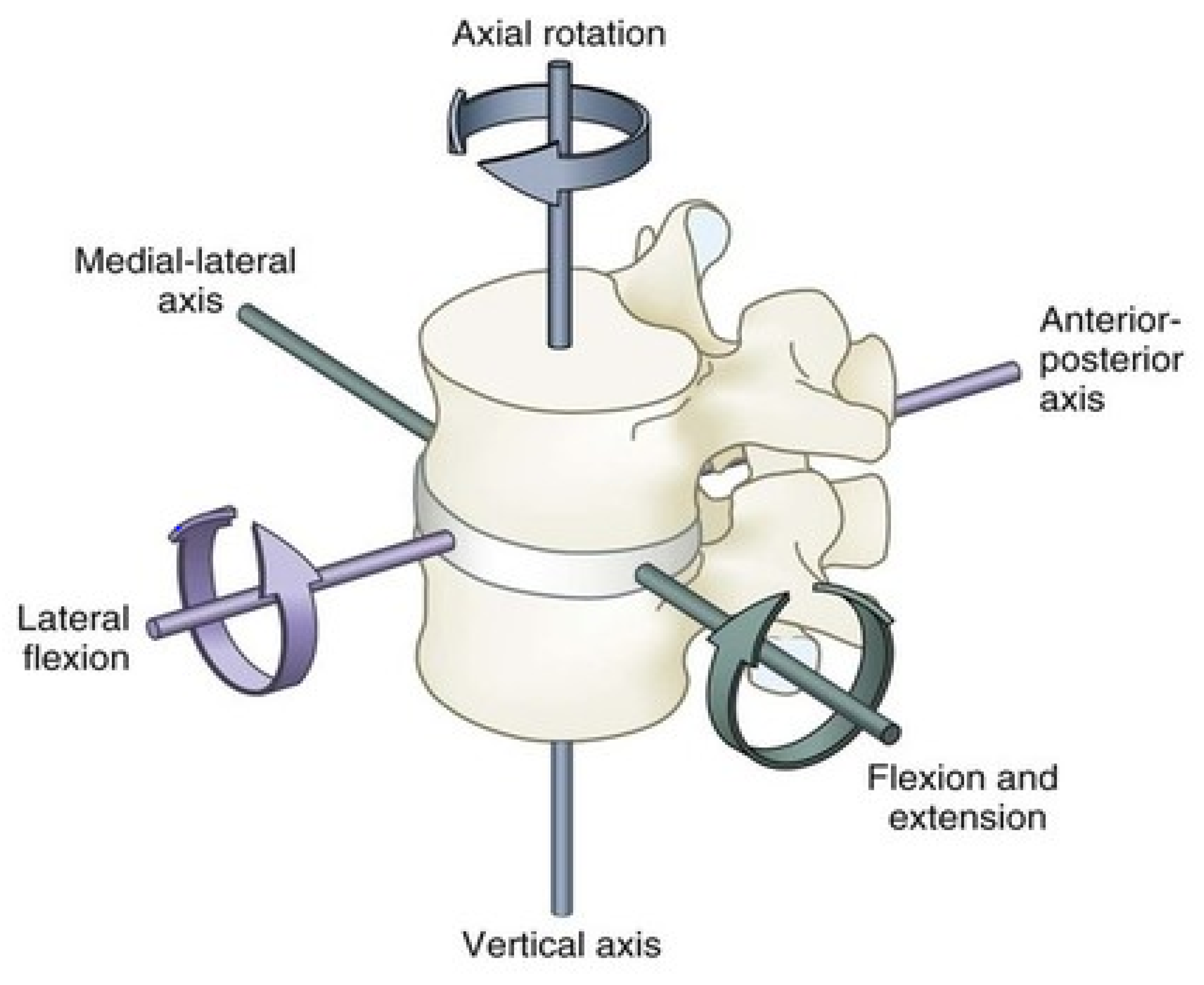
Biomechanics of the Spine
2. Passive Spine Braces in the Treatment of Osteoporotic Spine Fractures (OSF)
Limitations with Traditional Spine Braces in OSF
3. Active Spine Braces
3.1. State of the Art
- (i)
- Classification of Devices: Active vs. Passive
- (ii)
- Support of Different Body Parts
- (iii)
- Human Anthropometry
- (iv)
- Early Exoskeletons
- (v)
- Application of Exoskeletons
3.2. Overview of Spinal Exoskeletons
- (i)
- Current Spinal Exoskeletons
- (ii)
- Taxonomy of Devices
4. Design Considerations for Wearable Spinal Robots
- (a)
- Lumbar spinal forces
- (b)
- Materials and Wearability
- (c)
- Recent Design Concepts for Exoskeletons
- (d)
- Sensors and Controls of Exoskeletons
5. Discussion
- (i)
- Shortcomings and Limitations of Current Spinal Exoskeletons
- (ii)
- Suggested design guidelines
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OSF | Osteoporotic spine fractures |
| ADLs | Activities of daily living |
| QOL | Quality of life |
| ROM | Range of movements |
| TLSO | Thoracolumbar spinal orthosis |
| TLS | Thoracolumbar sacral |
| DOF | Degrees of freedom |
| BLEEX | Berkeley Lower Extremity Exoskeleton |
| EMG | Electromyography |
| HAL | Hybrid Assistive Limb |
| FDA | Food and Drug Aministration |
| PLAD | Personal Lift Augmentation Device |
| Bndr | Bending Non-Demand Return |
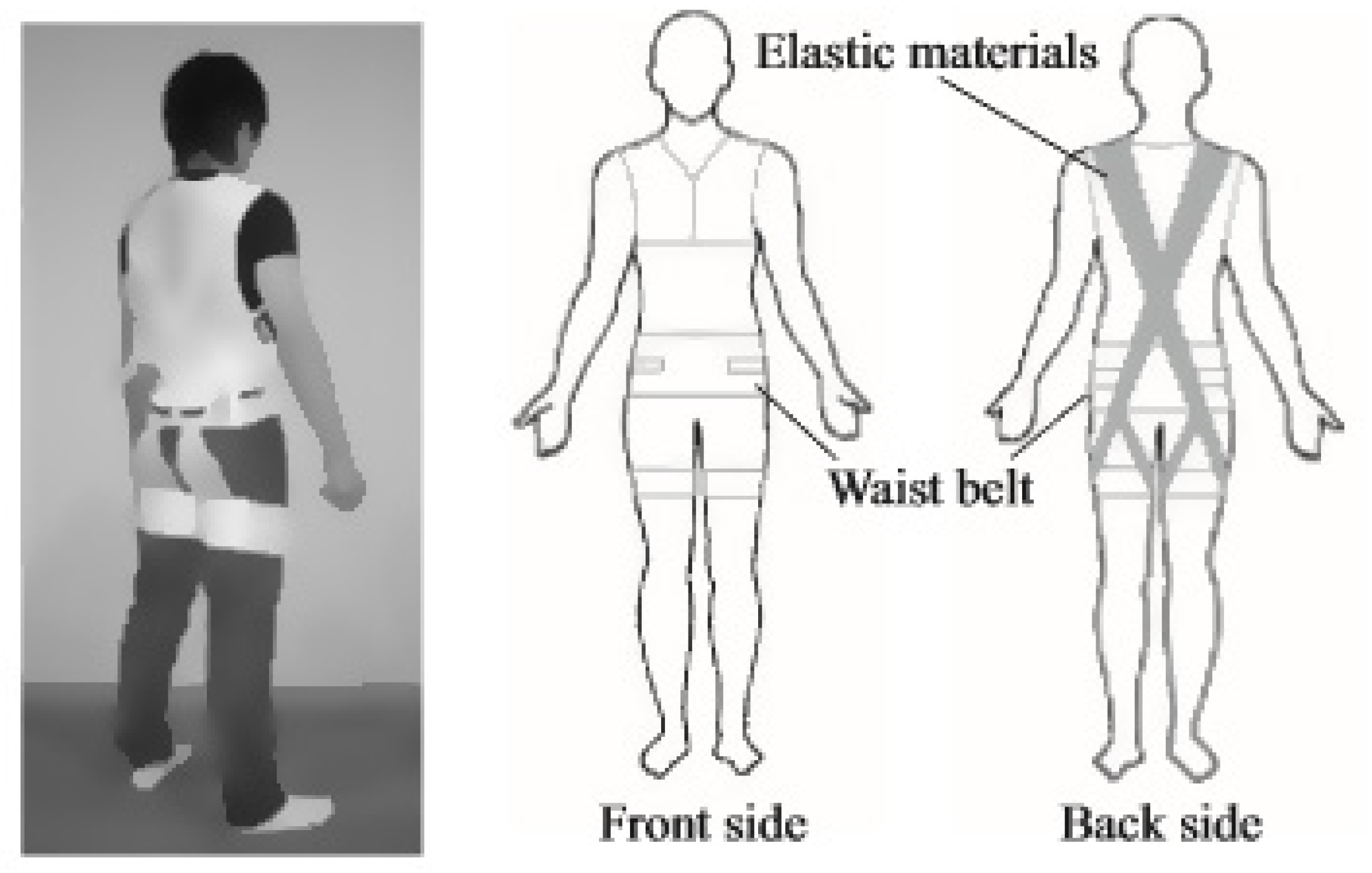
| SSL | Smart Suit Lite |
| SEA | Series Elastic Actuators |
| WHO | World Health Organization |
References
- United Nations Population Division. Available online: https://www.un.org/en/development/desa/population/publications/pdf/ageing/WPA2017_Highlights.pdf (accessed on 10 January 2021).
- International Osteoporosis Federation Data and Publications: Facts and Statistics. Available online: https://www.osteoporosis.foundation/facts-statistics (accessed on 10 January 2021).
- Kim, H.-J.; Yi, J.-M.; Cho, H.-G.; Chang, B.-S.; Lee, C.-K.; Kim, J.H.; Yeom, J.S. Comparative Study of the Treatment Outcomes of Osteoporotic Compression Fractures without Neurologic Injury Using a Rigid Brace, a Soft Brace, and no Brace: A Prospective Randomized Controlled Non-inferiority Trial. J. Bone Jt. Surg. Am. Vol. 2014, 96, 1959–1966. [Google Scholar] [CrossRef] [Green Version]
- Oleksik, A.M.; Ewing, S.; Shen, W.; Van Schoor, N.M.; Lips, P. Impact of Incident Vertebral Fractures on Health-related Quality of Life (HRQOL) in Postmenopausal Women with Prevalent Vertebral Fractures. Osteoporos. Int. 2005, 16, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Seo, K.; Hyung, S.; Shim, Y.; Lim, S.-C. Compact Hip-Force Sensor for a Gait-Assistance Exoskeleton System. Sensors 2018, 18, 566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christophy, M.; Senan, N.A.F.; Lotz, J.C.; O’Reilly, O.M. A Musculoskeletal Model for the Lumbar Spine. Biomech. Modeling Mechanobiol. 2012, 11, 19–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, M.A. The Biomechanics of Back Pain. Acupunct. Med. 2004, 22, 178–188. [Google Scholar] [CrossRef]
- Sinaki, M.; Nwaogwugwu, N.C.; Phillips, B.E.; Mokri, M.P. Effect of Gender, Age, and Anthropometry on Axial and Appendicular Muscle Strength. Am. J. Phys. Med. Rehabil. 2001, 80, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Miyakoshi, N.; Hongo, M.; Maekawa, S.; Ishikawa, Y.; Shimada, Y.; Itoi, E. Back Extensor Strength and Lumbar Spinal Mobility are Predictors of Quality of Life in Patients with Postmenopausal Osteoporosis. Osteoporos. Int. 2007, 18, 1397–1403. [Google Scholar] [CrossRef] [PubMed]
- Cafolla, D.; Marco, C. Design and Simulation of Humanoid Spine. Mech. Mach. Sci. 2014, 24, 585–593. [Google Scholar]
- Kim, H.K.; Zhang, Y. Estimation of Lumbar Spinal Loading and Trunk Muscle Forces during Asymmetric Lifting Tasks: Application of Whole-body Musculoskeletal Modelling in OpenSim. Ergonomics 2017, 60, 563–576. [Google Scholar] [CrossRef]
- Shirazi-Adl, A.; Ahmed, A.M.; Shrivastava, S.C. Mechanical Response of a Lumbar Motion Segment in Axial Torque Alone and Combined with Compression. Spine 1986, 11, 914–927. [Google Scholar] [CrossRef]
- Schultz, A.B.; Haderspeck-Grib, K.; Sinkora, G.; Warwick, D.N. Quantitative Studies of the Flexion-relaxation Phenomenon in the Back Muscles. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 1985, 3, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Blattert, T.R.; Schnake, K.J.; Gonschorek, O.; Gercek, E.; Hartmann, F.; Katscher, S.; Mörk, S.; Morrison, R.; Müller, M.; Partenheimer, A.; et al. Nonsurgical and Surgical Management of Osteoporotic Vertebral Body Fractures: Recommendations of the Spine Section of the German Society for Orthopaedics and Trauma (DGOU). Glob. Spine J. 2018, 8, 50S–55S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyles, K.W. Management of Patients with Vertebral Compression Fractures. Pharmacotherapy 1999, 19, 21S–24S. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.Z.; Lee, J.H. Effect of Brace to Osteoporotic Vertebral Fracture: A Meta-Analysis. J. Korean Med. Sci. 2016, 31, 1641–1649. [Google Scholar] [CrossRef] [Green Version]
- Pfeifer, M.; Begerow, B.; Minne, H.W. Effects of a New Spinal Orthosis on Posture, Trunk Strength, and Quality of Life in Women with Postmenopausal Osteoporosis. A Randomized Trial. Am. J. Phys. Med. Rehabil. 2004, 83, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Law, S.-W.; Cheng, J.; Kee, H.-M.; Wong, M.S. Comparison Study on the Efficacy of SpinoMed® and Soft Lumbar Orthosis for Osteoporotic Vertebral Fracture. Prosthet. Orthot. Int. 2015, 39, 270–276. [Google Scholar] [CrossRef] [PubMed]
- The Effect of Posture Support Corset on Balance, Quality of Life, Dorsal Kyphosis in Patients with Kyphosis due to Osteoporosis. Available online: https://www.researchgate.net/publication/288156692_The_effect_of_posture_support_corset_on_balance_quality_of_life_dorsal_kyphosis_in_patients_with_kyphosis_due_to_osteoporosis (accessed on 10 January 2021).
- Chang, V.; Holly, L.H. Bracing for Thoracolumbar Fractures. Neurosurg. Focus 2014, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alcalá-Cerra, G.; Díaz-Becerra, C.; Fernandes-Joaquim, A.; Moscote-Salazar, L.R.; Paternina-Caicedo, A.J. Orthosis for Thoracolumbar Burst Fractures without Neurologic Deficit: A Systematic Review of Prospective Randomized Controlled Trials. J. Craniovertebral Junction Spine 2014, 5, 25–32. [Google Scholar] [CrossRef]
- Prather, H.; Watson, J.O.; Gilula, L.A. Nonoperative Management of Osteoporotic Vertebral Compression Fractures. Injury 2007, 38 (Suppl. S3), 40–48. [Google Scholar] [CrossRef]
- Slavici, A.; Rauschmann, M.; Fleege, C. Conservative Management of Osteoporotic Vertebral Fractures: An Update. Eur. J. Trauma Emerg. Surg. 2016, 43, 19–26. [Google Scholar] [CrossRef]
- Agabegi, S.S.; Asghar, F.A.; Herkowitz, H.N. Spinal Orthoses. J. Am. Acad. Orthop. Surg. 2010, 18, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Lantz, S.A.; Schultz, A.B. Lumbar Spine Orthosis Wearing. I. Restriction of Gross Body Motions. Spine 1986, 11, 834–837. [Google Scholar] [CrossRef] [PubMed]
- Longo, U.G.; Loppini, M.; Denaro, L.; Maffulli, N.; Denaro, V. Osteoporotic Vertebral Fractures: Current Concepts of Conservative Care. Br. Med Bull. 2012, 102, 171–189. [Google Scholar] [CrossRef] [Green Version]
- Esses, S.I.; McGuire, R.; Jenkins, J.; Finkelstein, J.; Woodard, E.; Watters, W.C.; Goldberg, M.J.; Keith, M.; Turkelson, C.M.; Wies, J.L.; et al. The Treatment of Symptomatic Osteoporotic Spinal Compression Fractures. J. Am. Acad. Orthop. Surg. 2011, 19, 176–182. [Google Scholar] [CrossRef] [Green Version]
- Möller, A.; Hasserius, R.; Redlund-Johnell, I.; Ohlin, A.; Karlsson, M.K. Nonoperatively Treated Burst Fractures of the Thoracic and Lumbar Spine in Adults: A 23- to 41-year follow-up. Spine 2007, 7, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Valentin, G.H.; Pedersen, L.N.; Maribo, T. Wearing an Active Spinal Orthosis Improves Back Extensor Strength in Women with Osteoporotic Vertebral Fractures. Prosthet. Orthot. Int. 2014, 38, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Sinaki, M.; Itoi, E.; Wahner, H.; Wollan, P.; Gelzcer, R.; Mullan, B.; Collins, D.; Hodgson, S. Stronger Back Muscles Reduce the Incidence of Vertebral Fractures: A Prospective 10 year Follow-up of Postmenopausal Women. Bone 2002, 30, 836–841. [Google Scholar] [CrossRef]
- Dionyssiotis, Y.; Trovas, G.; Thoma, S.; Lyritis, G.; Papaioannou, N. Prospective Study of Spinal Orthoses in Women. Prosthet. Orthot. Int. 2015, 39, 487–495. [Google Scholar] [CrossRef]
- Newman, M.; Lowe, C.M.; Barker, K.L. Spinal Orthoses for Vertebral Osteoporosis and Osteoporotic Vertebral Fracture: A Systematic Review. Arch. Phys. Med. Rehabil. 2016, 97, 1013–1025. [Google Scholar] [CrossRef]
- Rzewuska, M.; Ferreira, M.; McLachlan, A.J.; Machado, G.C.; Maher, C.G. The Efficacy of Conservative Treatment of Osteoporotic Compression Fractures on Acute Pain Relief: A Systematic Review with Meta-analysis. Eur. Spine J. 2015, 24, 702–714. [Google Scholar] [CrossRef]
- Influence of Spinal Orthosis on Gait and Physical Functioning in Women with Postmenopausal Osteoporosis. Available online: https://www.researchgate.net/publication/51851552_Influence_of_spinal_orthosis_on_gait_and_physical_functioning_in_women_with_postmenopausal_osteoporosis (accessed on 10 January 2021).
- Yagn, N. Apparatus for Facilitating Walking, Running, and Jumping. United States Patent and Trademark Office. U.S. Patent 420,179. U.S. Patent 438,830, 28 January 1890. [Google Scholar]
- Gilbert, K.E.; Callan, P.C. Hardiman I Prototype Project; Schenectady, G.E., Ed.; Tech. Rep. S-68-1081; General Electric Company: Boston, MA, USA, 1968. [Google Scholar]
- Fick, B.R.; Makinson, J.B. Hardiman I Prototype for Machine Augmentation of Human Strength and Endurance: Final Report; Schenectady, G.E., Ed.; Tech. Rep. S-71-1056; General Electric Company: Boston, MA, USA, 1971. [Google Scholar]
- Moore, J.A. Pitman: A Powered Exoskeleton Suit for the Infantryman; Tech. Rep. LA-10761-MS; Los Alamos National Laboratory: Los Alamos, NM, USA, 1986. [Google Scholar]
- Rosheim, M.E. Man-amplifying exoskeleton. Proc. Spie Mob. Robot. IV 1990, 1195, 402–411. [Google Scholar]
- Zoss, A.B.; Kazerooni, H.; Chu, A. Biomechanical design of the Berkeley Lower Extremity Exoskeleton (BLEEX). IEEE/Asme Trans. Mechatron. 2006, 11, 128–138. [Google Scholar] [CrossRef]
- Chu, A.; Kazerooni, H.; Zoss, A. On the Biomimetic Design of the Berkeley Lower Extremity Exoskeleton (BLEEX). In Proceedings of the IEEE International Conference on Robotics and Autonomation, Barcelona, Spain, 18–22 April 2005; pp. 4345–4352. [Google Scholar]
- Zoss, A.; Kazerooni, H. Design of an Electrically Actuated Lower Extremity Exoskeleton. Adv. Robot. 2006, 20, 967–988. [Google Scholar] [CrossRef] [Green Version]
- Walsh, C.; Paluska, D.; Pasch, K.; Grand, W.; Valiente, A.; Herr, H. Development of a Lightweight, Underactuated Exoskeleton for Load-carrying Augmentation. In Proceedings of the IEEE International Conference on Robotics and Automation, Orlando, FL, USA, 15–19 May 2006; pp. 3485–3491. [Google Scholar]
- Valiente, A. Design of a Quasi-passive Parallel Leg Exoskeleton to Augment Load Carrying for Walking. Master’s Thesis, Massachusetts Institute of Technology, Boston, MA, USA, August 2005. [Google Scholar]
- Exoskeletons for Industrial Application and their Potential Effects on Physical Work Load. Available online: https://www.tandfonline.com/doi/abs/10.1080/00140139.2015.1081988?journalCode=terg20 (accessed on 10 January 2021).
- Kawamoto, H.; Lee, S.; Kanbe, S.; Sankai, Y. Power Assist Method for HAL-3 using EMG-based Feedback Controller. In Proceedings of the IEEE International Conference on Systems, Man, and Cybernetics, Washington, DC, USA, 8 October 2003; pp. 1648–1653. [Google Scholar]
- Rewalk. Available online: http://www.rewalk.com (accessed on 10 January 2021).
- Mekki, M.; Delgado, A.D.; Fry, A.; Putrino, D.; Huang, V. Robotic Rehabilitation and Spinal Cord Injury: A Narrative Review. Neurotherapeutics 2018, 15, 604–617. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Noritsugu, T.; Takaiwa, M.; Sasaki, D. Design of Wearable Power Assist Wear for Low Back Support Using Pneumatic Actuators. Int. J. Autom. 2013, 7, 228–236. [Google Scholar] [CrossRef] [Green Version]
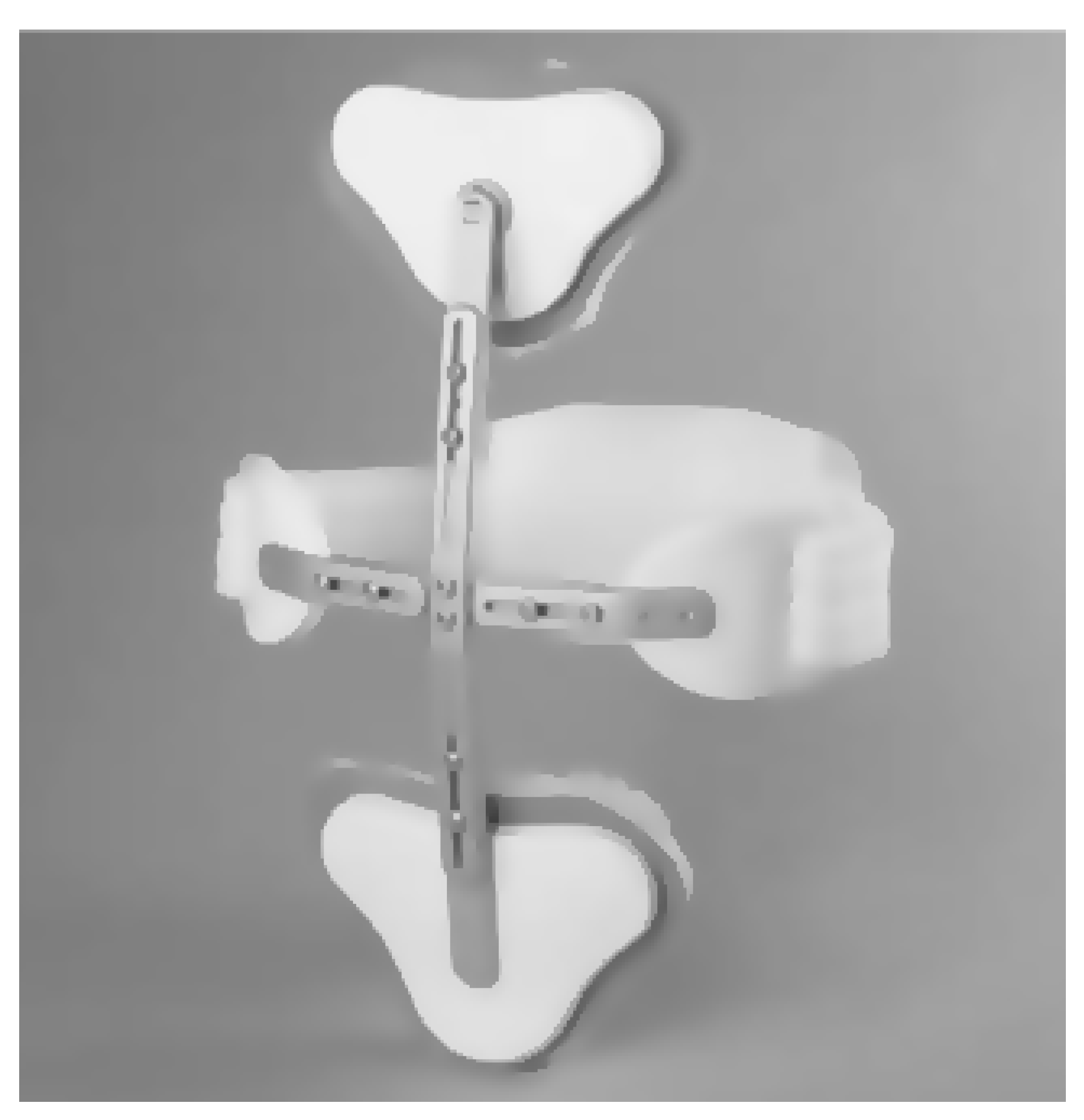
- Zhang, H.; Kadrolkar, A.; Sup, F.C. Design and Preliminary Evaluation of a Passive Spine Exoskeleton. J. Med. Devices 2016, 10, 011002. [Google Scholar] [CrossRef]
- De Rijcke, L.; Näf, M.; Rodriguez-Guerrero, C.; Graimann, B.; Houdijk, H.; Van Dieën, J.; Mombaur, K.; Russold, M.; Sarabon, N.; Babič, J.; et al. SPEXOR: Towards a Passive Spinal Exoskeleton. In Proceedings of the 2nd International Symposium on Wearable Robotics, WeRob2016, Segovia, Spain, 18–21 October 2016; pp. 325–329. [Google Scholar]
- Naf, M.B.; De Rijcke, L.; Guerrero, C.R.; Millard, M.; VanderBorght, B.; Lefeber, D. Towards Low Back Support with a Passive Biomimetic Exo-spine. IEEE Int. Conf. on Rehabil. Robot. 2017, 2017, 1165–1170. [Google Scholar]
- Johnson, A.P.; Gorsic, M.; Regmi, Y.; Davidson, B.S.; Dai, B.; Novak, D. Design and Pilot Evaluation of a Reconfigurable Spinal Exoskeleton. In Proceedings of the IEEE Engineering in Medicine and Biology Society, Honolulu, HI, USA, 18–21 July 2018; pp. 1731–1734. [Google Scholar]
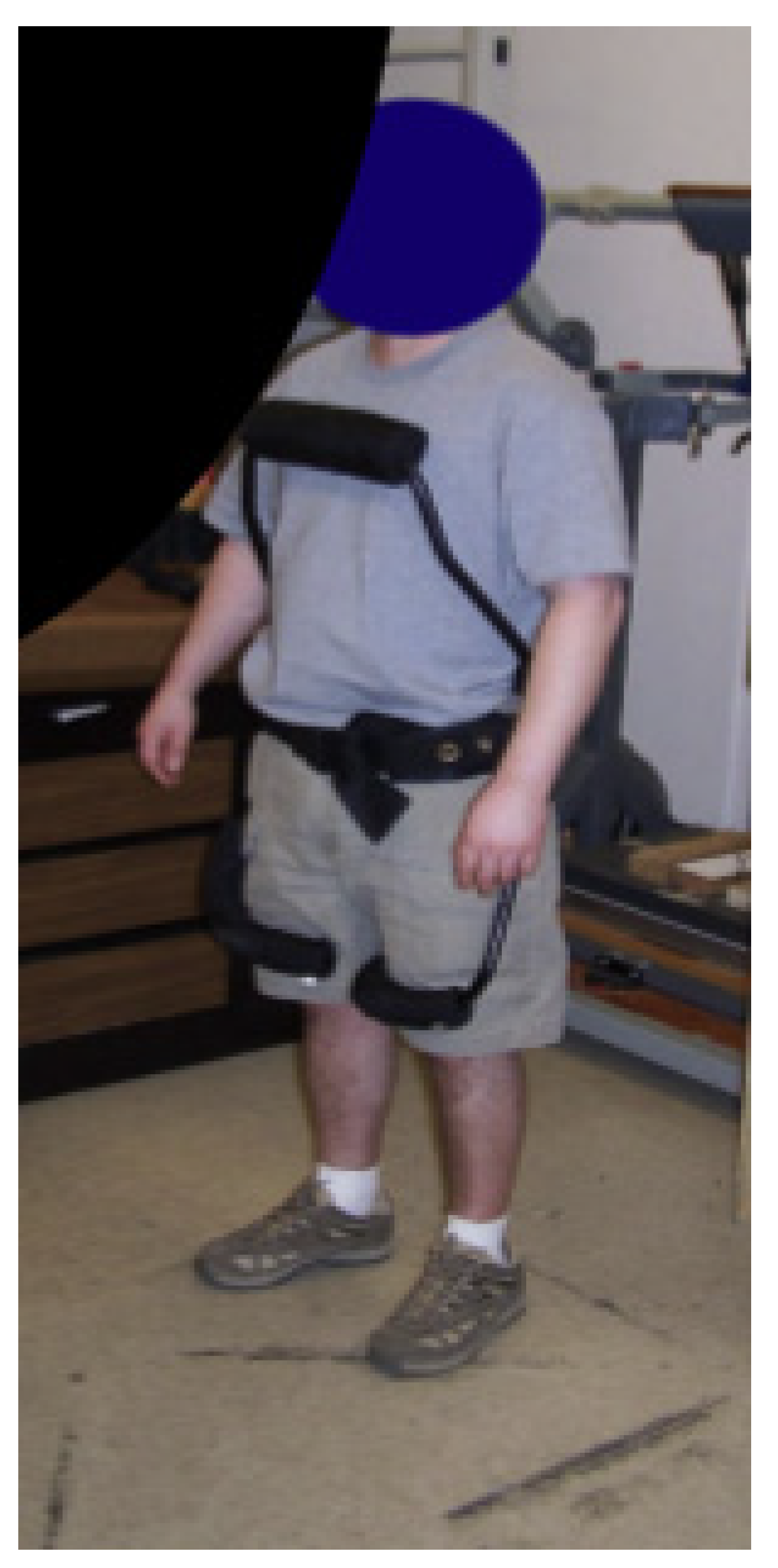
- Sadler, E.M.; Graham, R.B.; Stevenson, J.M. The Personal Lift-assist Device and Lifting Technique: A Principal Component Analysis. Ergonomics 2011, 54, 392–402. [Google Scholar] [CrossRef]
- Frost, D.M.; Abdoli-Eramaki, M.; Stevenson, J.M. PLAD (Personal Lift Assistive Device) Stiffness Affects the Lumbar Flexion/extension Moment and the Posterior Chain EMG during Symmetrical Lifting Tasks. J. Electromyogr. Kinesiol. 2009, 19, 403–412. [Google Scholar] [CrossRef]
- Abdoli-Eramaki, M.; Stevenson, J.M.; Reid, S.A.; Bryant, T.J. Mathematical and Empirical Proof of Principle for an On-body Personal Lift Augmentation Device (PLAD). J. Biomech. 2007, 40, 1694–1700. [Google Scholar] [CrossRef]
- Imamura, Y.; Tanaka, T.; Suzuki, Y.; Takizawa, K.; Yamanaka, M. Analysis of Trunk Stabilization Effect by Passive Power-assist Device. J. Robot. Mechatron. 2014, 26, 791–798. [Google Scholar] [CrossRef]
- Ulrey, B.L.; Fathallah, F.A. Subject-specific, Whole-body Models of the Stooped Posture with a Personal Weight Transfer Device. J. Electromyogr. Kinesiol. 2013, 23, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Ulrey, B.L.; Fathallah, F.A. Effect of a Personal Weight Transfer Device on Muscle Activities and Joint Flexions in the Stooped Posture. J. Electromyogr. Kinesiol. 2013, 23, 195–205. [Google Scholar] [CrossRef]
- Bosch, T.; Van Eck, J.; Knitel, K.; De Looze, M. The Effects of a Passive Exoskeleton on Muscle Activity, Discomfort and Endurance Time in Forward Bending Work. Appl. Ergon. 2016, 54, 212–217. [Google Scholar] [CrossRef]
- Koopman, A.S.; Kingma, I.; Faber, G.S.; De Looze, M.P.; Van Dieën, J.H. Effects of a Passive Exoskeleton on the Mechanical Loading of the Low Back in Static Holding Tasks. J. Biomech. 2018, 83, 97–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.S.W.; Chow, D.H.K. Effects of Backpack Load on Critical Changes of Trunk Muscle Activation and Lumbar Spine Loading during Walking. Ergonomics 2018, 61, 553–565. [Google Scholar] [CrossRef]
- Muramatsu, Y.; Umehara, H.; Kobayashi, H. Improvement and Quantitative Performance Estimation of the Back Support Muscle Suit. In Proceedings of the IEEE Engineering in Medicine and Biology Society (EMBC) 35th Annual International Conference, Osaka, Japan, 3–7 July 2013; pp. 2844–2849. [Google Scholar]
- Park, J.-H.; Stegall, P.R.; Roye, D.P.; Agrawal, S.K. Robotic Spine Exoskeleton (RoSE): Characterizing the 3-D Stiffness of the Human Torso in the Treatment of Spine Deformity. IEEE Trans. Neural Syst. Rehabil. Eng. 2018, 26, 1026–1035. [Google Scholar] [CrossRef] [PubMed]
- Huysamen, K.; De Looze, M.; Bosch, T.; Caldwell, D.G.; Toxiri, S.; O’Sullivan, L.W. Assessment of an Active Industrial Exoskeleton to Aid Dynamic Lifting and Lowering Manual Handling Tasks. Appl. Ergon. 2018, 68, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, J.P.; McGill, S.M. Low Back Joint Loading and Kinematics during Standing and Unsupported Sitting. Ergonomics 2001, 44, 280–294. [Google Scholar] [CrossRef]
- Aissaoui, R.; Lacoste, M.; Dansereau, J. Analysis of Sliding and Pressure Distribution during a Repositioning of Persons in a Simulator Chair. IEEE Trans. Neural Syst. Rehabil. Eng. 2001, 9, 215–224. [Google Scholar] [CrossRef]
- Goh, J.-H.; Thambyah, A.; Bose, K. Effects of Varying Backpack Loads on Peak Forces in the Lumbosacral Spine during Walking. Clin. Biomech. 1998, 13, S26–S31. [Google Scholar] [CrossRef]
- Youssef, K.M.; Zaddach, A.J.; Niu, C.; Irving, D.L.; Koch, C. A Novel Low-Density, High-Hardness, High-entropy Alloy with Close-packed Single-phase Nanocrystalline Structures. Mater. Res. Lett. 2014, 3, 95–99. [Google Scholar] [CrossRef] [Green Version]
- Schiele, A.; van der Helm, F. Influence of Attachment Pressure and Kinematic Configuration on pHRI with Wearable Robots. Appl. Bionics Biomech. 2009, 6, 157–173. [Google Scholar] [CrossRef] [Green Version]
- Schiele, A. Ergonomics of Exoskeletons: Objective Performance Metrics. In Proceedings of the EuroHaptics Conference on Haptic Interfaces for Virtual Environment and Teleoperator Systems, Salt Lake City, UT, USA, 18–20 March 2009; pp. 103–108. [Google Scholar]
- Lee, Y.; Kim, Y.-J.; Lee, J.; Lee, M.; Choi, B.; Kim, J.; Park, Y.J.; Choi, J. Biomechanical Design of a Novel Flexible Exoskeleton for Lower Extremities. IEEE/ASME Trans. Mechatron. 2017, 22, 2058–2069. [Google Scholar] [CrossRef]
- Lee, Y.; Choi, B.; Lee, J.; Lee, M.; Roh, S.-G.; Kim, J.; Choi, H.; Kim, Y.-J. Flexible Sliding Frame for Gait Enhancing Mechatronic System (GEMS). In Proceedings of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; pp. 598–602. [Google Scholar]
- Yan, T.; Cempini, M.; Oddo, C.M.; Vitiello, N. Review of Assistive Strategies in Powered Lower-limb Orthoses and Exoskeletons. Robot. Auton. Syst. 2015, 64, 120–136. [Google Scholar] [CrossRef]
- Aguirre-Ollinger, G.; Colgate, J.E.; Peshkin, M.A.; Goswami, A. Active-Impedance Control of a Lower-Limb Assistive Exoskeleton. In Proceedings of the 2007 IEEE 10th International Conference on Rehabilitation Robotics, Noordwijk, The Netherlands, 13–15 June 2007. [Google Scholar]
- Chen, G.; Chan, C.K.; Guo, Z.; Yu, H. Review of Lower Extremity Assistive Robotic Exoskeletons in Rehabilitation Therapy. Crit. Rev. Biomed. Eng. 2013, 41, 343–363. [Google Scholar] [CrossRef]
- Tucker, M.R.; Olivier, L.; Pagel, A.; Bleuler, H.; Bouri, M.; Lambercy, O.; Millan, J.D.R.; Riener, R.; Vallery, H.; Gassert, R. Control Strategies for Active Lower Extremity Prosthetics and Orthotics: A Review. J. Neuroeng. Rehabil. 2015, 12, 1. [Google Scholar] [CrossRef] [Green Version]
- Wade, S.W.; Strader, C.; Fitzpatrick, L.A.; Anthony, M.S.; O’Malley, C.D. Estimating Prevalence of Osteoporosis: Examples from Industrialized Countries. Arch. Osteoporos. 2014, 9, 182. [Google Scholar] [CrossRef]
- Prather, H.; Hunt, D.; Watson, J.O.; Gilula, L.A. Conservative Care for Patients with Osteoporotic Vertebral Compression Fractures. Phys. Med. Rehabil. Clin. N. Am. 2007, 18, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Cafolla, D.; Chen, I.-M.; Ceccarelli, M.M. An experimental characterization of human torso motion. Front. Mech. Eng. 2015, 10, 311–325. [Google Scholar] [CrossRef]











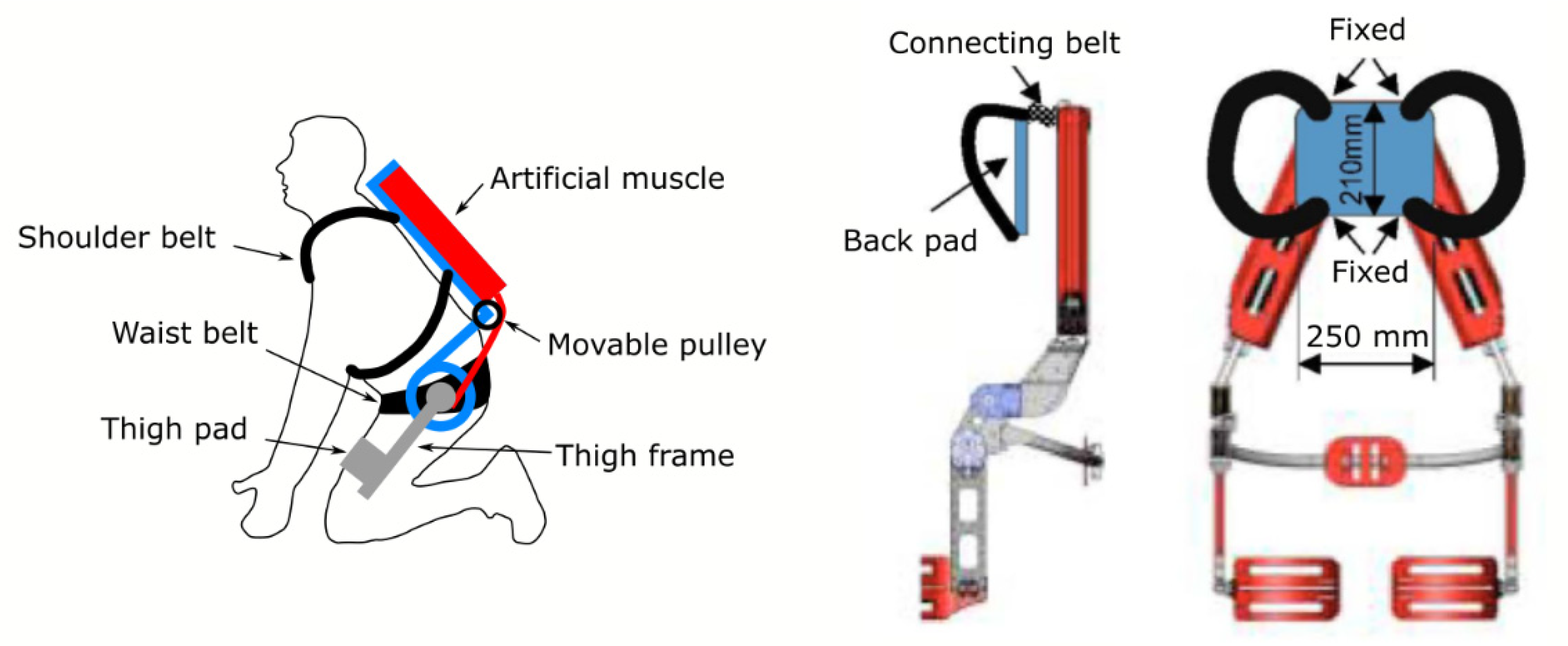

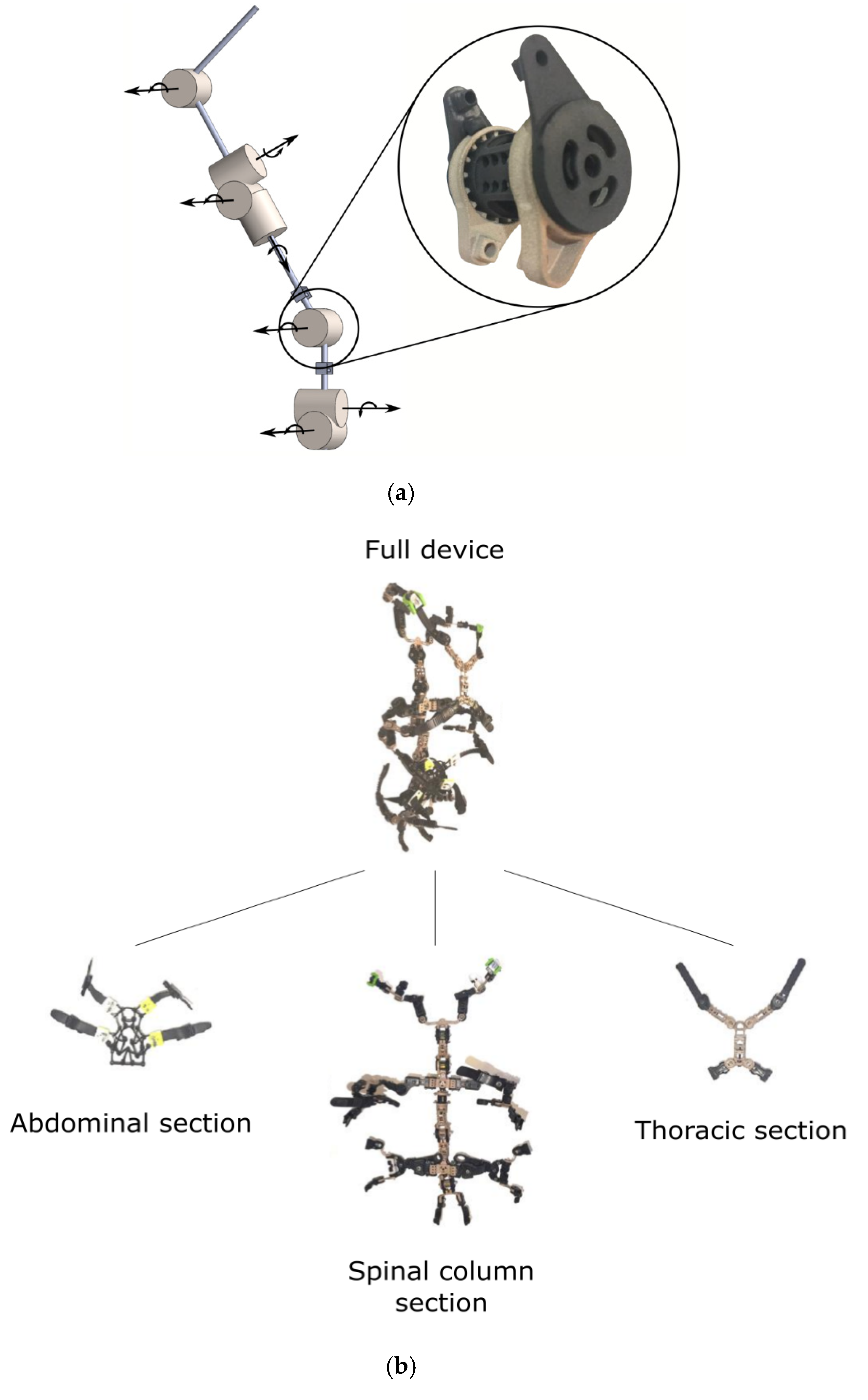

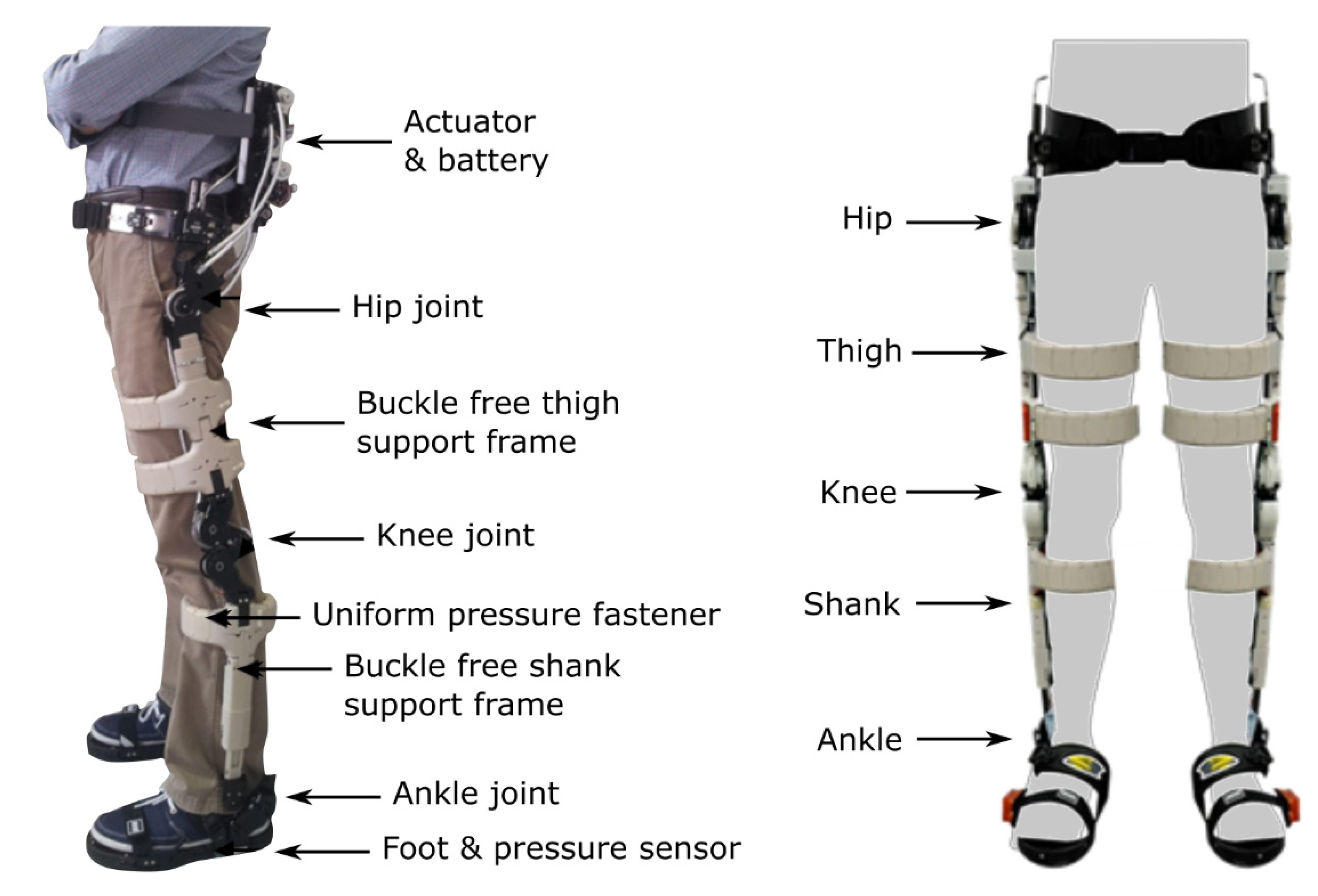

| Current Devices | Soft Exo-Suits | Rigid Exoskeletons |
|---|---|---|
| Passive Device | LAEVO (Springs) PLAD (Elastic Bands) SSL (Elastic Bands) Passive Spine Exoskeleton (Springs & Tension Cables) | SPEXOR (Flexible Beams) BNDR (Stiff Torsional Springs) TLS Exoskeleton (Viscoelastic Couplings) |
| Active Device | N/A | Muscle Suit (McKibben Muscle) |
| Current Wearable Spinal Robots | Original Intention of Design | Active Device | Passive Devices: Soft Exo-Suits | Passive Devices: Rigid Exoskeletons | Unique Difference in the Design | How Does It Help |
|---|---|---|---|---|---|---|
| Muscle Suit | Industrial load carrying, reduce lower back strain | McKibben muscle | - | - | Only wearable spinal exoskeleton with active actuator | Up to 40% reduction in EMGs of posterior spinal muscles at weight-lifting tasks |
| TLS exoskeleton ** | Industrial load carrying, reduce lower back strain | - | - | Viscoelastic couplings | Allows independent control of each joint’s resistance and range of motion at the thoracolumbar spine | Up to 30% reduction in EMGs of posterior spinal muscles |
| SPEXOR | Industrial and commercial load carrying, reduce lower back strain, lower back pain prevention | - | - | Flexible beams | Aside from flexible lower back support, it also consists of compensating hip module and a passive hip torque source | Awaiting official test results |
| BNDR | Industrial load carrying, reduce lower back strain | - | - | Stiff torsional springs | Consists of stiff anterior chest and anterior thigh piece to limit torso or hip flexions | 13.5% reduction of lumbar spinal compressive force and 12.5% reduction of anterior-posterior shearing forces at the L5/S1 level |
| LAEVO | Industrial load carrying, reduce lower back strain | - | Springs | - | Transfer forces from the lower back to the chest and thigh pads | 35–38% reduction in the posterior spinal muscle EMGs at assembly tasks |
| PLAD | Industrial load carrying, reduce lower back strain | - | Elastic bands | - | One of the earlier designs—transferring of load from lower back to lower limbs | Up to 40% reduction of posterior spinal muscle EMGs at lifting tasks, and 23–29% reduction of lumbar spine compressive forces |
| SSL | Medical nursing care (patient transfer), reduce lower back strain | - | Elastic bands | - | Additional elastic belt around the torso for stabilization during flexion and extension of lower back | 24.4% reduction in EMGs of the posterior lumbar spinal muscles |
| Passive Spine Exoskeleton * | For prevention to reduce lower back and shoulder injuries during load carrying | - | Springs and tension cables | - | Based on the push–pull strategy of external assistance | 20% reduction in EMGs of posterior spinal muscles |
| RoSE | Research measures 3D stiffness of torso | - | - | - | - | - |
| Limitations | Possible Improvement Strategy |
|---|---|
| Psychological fear of social isolation and being labelled as “disabled” | Wearable exo-suit designs, with soft tight fitting, stretchable materials like elastomers. External batteries (power sources)/ actuators can employ a plug-and-play design when required. |
| Not accounting for the contribution of hip joints during flexion and extension of spine | Provide adequate thigh and lumbar lower back support reactive forces. Actuators acting on hip as the center of rotation for active assistance of lumbar flexion extension a compensating hip module. |
| Overall weight of the wearable exoskeleton | Ideally < 3% of total body weight, in order to reduce peak lumbosacral compression forces. Carbon fiber could be the ideal material. Radiolucent material ideal for X-ray films. |
| Unpredictable forces generated from actuators | Not desirable in injured bony spinal vertebrae for OSF. |
| Lack of detection of spinal parameters | Attachment of lightweight IMUs to the robotic spinal brace for impedance control for OSF patients. |
| Kinematics Rom Per DoF | Lumbar Spine ROM [6]: Flexion 40–60 Degrees Extension 20–35 Degrees Lateral Bending 15–20 Degrees Axial Rotation 3–18 Degrees |
|---|---|
| Ergonomics Max weight of device Body attachment Materials | < 3% body weight [62] Upper body to hips Soft materials as paddings for comfortable fit Lightweight stiff materials for the main framework for effective force transmission |
| Actuation and Control Active DoF Passive DoF Forces to be generated Actuation and Power Supply Sensors Controls | Flexion and extension Prevention and limitation of lateral bending, rotation (most pain generation from rotation after spinal fracture) At least 300N (the mean extensor strength of the back muscles) [8] Ability to resist up to 700N of force at a fully flexed posture [13] Lightweight and with high power-to-weight ratio Portable Lightweight and efficient power supply (e.g., batteries) Orientation sensors to measure inclination of the spine; possibly pressure sensors at the interface between the device and the body, for contact pressure measurement, hence, this can also derive the underlying 3D stiffness of the spinal column. Increase assistance force based on inclination of the spine |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mak, S.K.D.; Accoto, D. Review of Current Spinal Robotic Orthoses. Healthcare 2021, 9, 70. https://doi.org/10.3390/healthcare9010070
Mak SKD, Accoto D. Review of Current Spinal Robotic Orthoses. Healthcare. 2021; 9(1):70. https://doi.org/10.3390/healthcare9010070
Chicago/Turabian StyleMak, Siu Kei David, and Dino Accoto. 2021. "Review of Current Spinal Robotic Orthoses" Healthcare 9, no. 1: 70. https://doi.org/10.3390/healthcare9010070
APA StyleMak, S. K. D., & Accoto, D. (2021). Review of Current Spinal Robotic Orthoses. Healthcare, 9(1), 70. https://doi.org/10.3390/healthcare9010070






