A Fast and Effective System for Detection of Neonatal Jaundice with a Dynamic Threshold White Balance Algorithm
Abstract
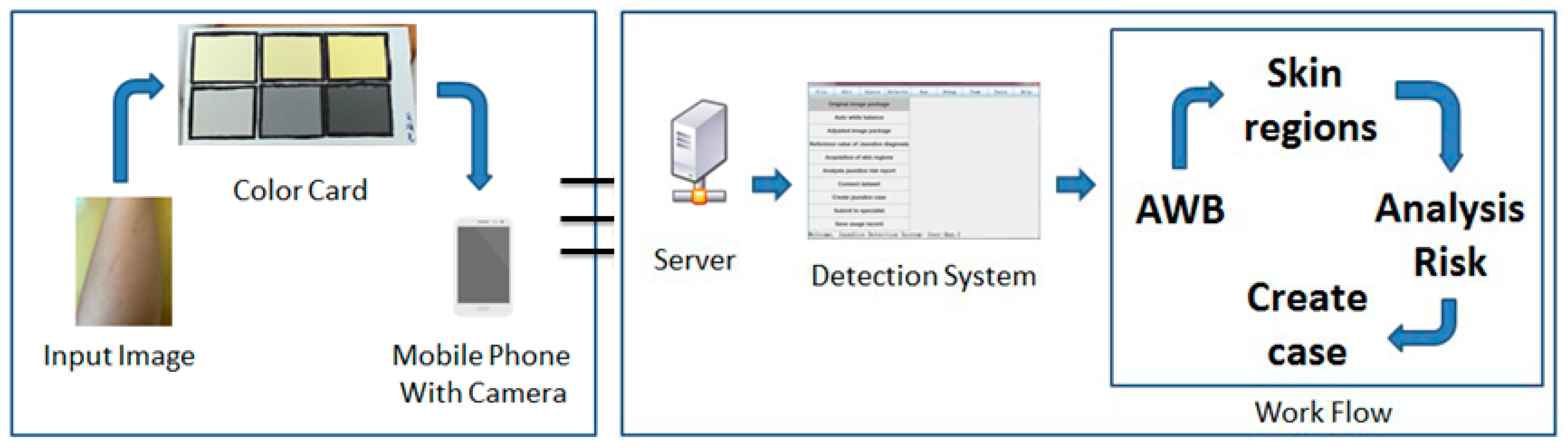
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Proposed White Balance Method
2.2.1. Filtering of Feature Pixels in UV Space
2.2.2. Dynamical Feature Pixels Searching
2.2.3. Feature Pixels Optimization
3. Experimental Results and Discussion
3.1. Quantitative Measurement of White Balance Method
3.2. Comparisons with the State-of-the-Art Approaches
3.3. Research Contributions and Limitations
4. Conclusions and Future Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tuncer, O.; Yeşilmen, O.; Kaya, A.; Aktar, F.; Akıl, M.; Gülmehmed, F. The Factors Affecting on Newborn Jaundice. City 2017, 239, 33–37. [Google Scholar]
- De Greef, L.; Goel, M.; Seo, M.J.; Larson, E.C.; Stout, J.W.; Taylor, J.A.; Patel, S.N. Bilicam: Using mobile phones to monitor newborn jaundice. In Proceedings of the 2014 ACM International Joint Conference on Pervasive and Ubiquitous Computing, Seattle, WA, USA, 13–17 September 2014; pp. 331–342. [Google Scholar]
- Crigler, J.F.; Najjar, V.A. Congenital familial nonhemolytic jaundice with kernicterus. Pediatrics 1952, 10, 169–180. [Google Scholar]
- Bhutani, V.K.; Stark, A.R.; Lazzeroni, L.; Poland, R.; Gourley, G.R.; Kazmierczak, S.; Meloy, L.; Burgos, A.E.; Hall, J.Y.; Stevenson, D.K. Predischarge screening for severe neonatal hyperbilirubinemia identifies infants who need phototherapy. J. Pediatr. 2013, 162, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Maisels, M.J.; McDonagh, A.F. Phototherapy for neonatal jaundice. N. Engl. J. Med. 2008, 358, 920–928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golandaj, J.A.; Kampli, M.S.; Hallad, J.S. Prevalence, care-seeking behaviors and treatment cost for neonatal morbidities in Karnataka (India). J. Humanit. Appl. Soc. Sci. 2019, 1, 115–131. [Google Scholar] [CrossRef]
- National Collaborating Centre for Women’s and Children’s Health. Neonatal Jaundice: Clinical Guideline; National for Health and Clinical Excellence (NICE): London, UK, 2010. [Google Scholar]
- Bhutani, V.K.; Johnson, L.; Sivieri, E.M. Predictive ability of a predischarge hour-specific serum bilirubin for subsequent significant hyperbilirubinemia in healthy term and near-term newborns. Pediatrics 1999, 103, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Ogundele, M.O.; Halliday, J.; Weir, P. Implementation of a prolonged neonatal jaundice protocol supported by electronic software. Clin. Gov. Int. J. 2010, 15, 179–190. [Google Scholar] [CrossRef]
- Agu, E.; Pedersen, P.; Strong, D.; Tulu, B.; He, Q.; Wang, L.; Li, Y. The smartphone as a medical device: Assessing enablers, benefits and challenges. In Proceedings of the 2013 IEEE International Workshop of Internet-of-Things Networking and Control (IoT-NC), New Orleans, LA, USA, 24 June 2013; pp. 48–52. [Google Scholar]
- Hsu, W.Y. Automatic left ventricle recognition, segmentation and tracking in cardiac ultrasound image sequences. IEEE Access 2019, 7, 140524–140533. [Google Scholar] [CrossRef]
- Consolvo, S.; Klasnja, P.; McDonald, D.W.; Avrahami, D.; Froehlich, J.; LeGrand, L.; Libby, R.; Mosher, K.; Landay, J.A. Flowers or a robot army? Encouraging awareness & activity with personal, mobile displays. In Proceedings of the 10th International Conference on Ubiquitous Computing, Seoul, Korea, 21–24 September 2008; pp. 54–63. [Google Scholar]
- Chen, C.M.; Wang, J.Y.; Lin, Y.C. A visual interactive reading system based on eye tracking technology to improve digital reading performance. Electron. Libr. 2019. [Google Scholar] [CrossRef]
- Hsu, W.Y.; Chung, C.J. A Novel Eye Center Localization Method for Head Poses with Large Rotations. IEEE Trans. Image Process. 2021, 30, 1369–1381. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.B.; Hung, W.Y.; Hsu, W.Y. A Fast and Effective System for Analysis of Optokinetic Waveforms with a Low-Cost Eye Tracking Device. Healthcare 2021, 9, 10. [Google Scholar] [CrossRef]
- Harrop, N.; Hex, N.; Tuggey, J.; Wright, D.; Malin, R. Telemedicine in care homes in Airedale, Wharfedale and Craven. Clin. Gov. Int. J. 2015, 20, 146–154. [Google Scholar]
- Hsu, W.Y. Automatic Compensation for Defects of Laser Reflective Patterns in Optics-Based Auto-Focusing Microscopes. IEEE Sens. J. 2020, 4, 2034–2044. [Google Scholar] [CrossRef]
- Fadhil, A. Beyond patient monitoring: Conversational agents role in telemedicine & healthcare support for home-living elderly individuals. arXiv preprint 2018, arXiv:1803.06000. [Google Scholar]
- Li, H. Target infrared radiation calculation model and method based on finite element analysis method in infrared photoelectric detection system. Sens. Rev. 2017, 37, 26–32. [Google Scholar] [CrossRef]
- Hsu, W.Y.; Sun, Y.N. EEG-based motor imagery analysis using weighted wavelet transform features. J. Neurosci. Methods 2009, 176, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, L.; Hassan, M.; Benner, D. Changing paradigms in the long-term care market. Int. J. Pharm. Healthc. Mark. 2012, 6, 267–278. [Google Scholar] [CrossRef]
- Hsu, W.Y.; Chung, C.J. A Novel Eye Center Localization Method for Multiview Faces. Pattern Recognit. 2021, 119, 108078. [Google Scholar] [CrossRef]
- Hsu, W.Y.; Lin, W.Y. Ratio-and-Scale-Aware YOLO for Pedestrian Detection. IEEE Trans. Image Process. 2021, 30, 934–947. [Google Scholar] [CrossRef] [PubMed]
- Swindal, J.C. The International encyclopedia of ethics. Choice Rev. 2013, 51, 51-0021, online. [Google Scholar]
- Weng, C.C.; Chen, H.; Fuh, C.S. A novel automatic white balance method for digital still cameras. In Proceedings of the 2005 IEEE International Symposium on Circuits and Systems (ISCAS), Kobe, Japan, 23–26 May 2005; Volume 4, pp. 3801–3804. [Google Scholar] [CrossRef]
- Sharma, G.; Wu, W.; Dalal, E.N. The CIEDE2000 Color-Difference Formula: Implementation Notes, Supplementary Test Data, and Mathematical Observations. Available online: https://onlinelibrary.wiley.com/doi/epdf/10.1002/col.20070 (accessed on 15 August 2021).
- Garud, H.; Ray, A.K.; Mahadevappa, M.; Chatterjee, J.; Mandal, S. A fast auto white balance scheme for digital pathology. In Proceedings of the IEEE-EMBS International Conference on Biomedical and Health Informatics (BHI), Valencia, Spain, 1–4 June 2014; pp. 153–156. [Google Scholar] [CrossRef]
- Gijsenij, A.; Gevers, T.; Van De Weijer, J. Physics-based edge evaluation for improved color constancy. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Miami, FL, USA, 20–25 June 2009; pp. 581–588. [Google Scholar] [CrossRef]









| Group | Bilirubin Level (mg/dL) | Number | ||
|---|---|---|---|---|
| AVG | MAX | MIN | ||
| High risk | 18.34 | 22.7 | 15.4 | 20 |
| Low risk | 8.6 | 14.3 | 0.6 | 18 |
| Color Temperature | ||||||
|---|---|---|---|---|---|---|
| 28 K | 32 K | 40 K | 48 K | 56 K | 65 K | |
| Origin Image | 22.16 | 20.63 | 19.62 | 19.21 | 18.71 | 18.85 |
| Our Method | 20.36 | 19.18 | 18.70 | 18.39 | 18.17 | 18.35 |
| Method | Color Temperature | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 28 K vs. 32 K | 28 K vs. 40 K | 28 K vs. 48 K | 28 K vs. 56 K | 28 K vs. 65 K | 32 K vs. 40 K | 32 K vs. 48 K | 32 K vs. 56 K | 32 K vs. 65 K | 40 K vs. 48 K | 40 K vs. 56 K | 40 K vs. 65 K | 48 K vs. 56 K | 48 K vs. 65 K | 56 K vs. 65K | Mean | |
| WPR [27] | 4.18 | 5.78 | 7.36 | 8.18 | 8.22 | 3.63 | 5.01 | 5.56 | 6.28 | 3.71 | 4.17 | 4.97 | 3.16 | 3.89 | 2.80 | 5.13 |
| WGE [28] | 3.65 | 5.15 | 6.21 | 6.65 | 6.62 | 3.51 | 4.46 | 4.71 | 4.70 | 3.43 | 3.40 | 3.78 | 2.91 | 2.93 | 2.51 | 4.31 |
| DH [25] | 4.46 | 5.61 | 6.41 | 6.66 | 7.36 | 3.64 | 4.34 | 4.61 | 5.57 | 4.45 | 4.65 | 5.44 | 3.62 | 3.83 | 3.88 | 4.97 |
| Ours | 3.57 | 4.50 | 5.56 | 5.97 | 6.04 | 3.11 | 3.95 | 4.23 | 4.44 | 3.09 | 3.10 | 3.44 | 2.66 | 2.65 | 2.64 | 3.93 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, W.-Y.; Cheng, H.-C. A Fast and Effective System for Detection of Neonatal Jaundice with a Dynamic Threshold White Balance Algorithm. Healthcare 2021, 9, 1052. https://doi.org/10.3390/healthcare9081052
Hsu W-Y, Cheng H-C. A Fast and Effective System for Detection of Neonatal Jaundice with a Dynamic Threshold White Balance Algorithm. Healthcare. 2021; 9(8):1052. https://doi.org/10.3390/healthcare9081052
Chicago/Turabian StyleHsu, Wei-Yen, and Han-Chang Cheng. 2021. "A Fast and Effective System for Detection of Neonatal Jaundice with a Dynamic Threshold White Balance Algorithm" Healthcare 9, no. 8: 1052. https://doi.org/10.3390/healthcare9081052
APA StyleHsu, W.-Y., & Cheng, H.-C. (2021). A Fast and Effective System for Detection of Neonatal Jaundice with a Dynamic Threshold White Balance Algorithm. Healthcare, 9(8), 1052. https://doi.org/10.3390/healthcare9081052






