A Feasibility and Efficacy Randomized Controlled Trial of Two Exercise Programs in Severe AECOPD Patients with Resting Hypoxemia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
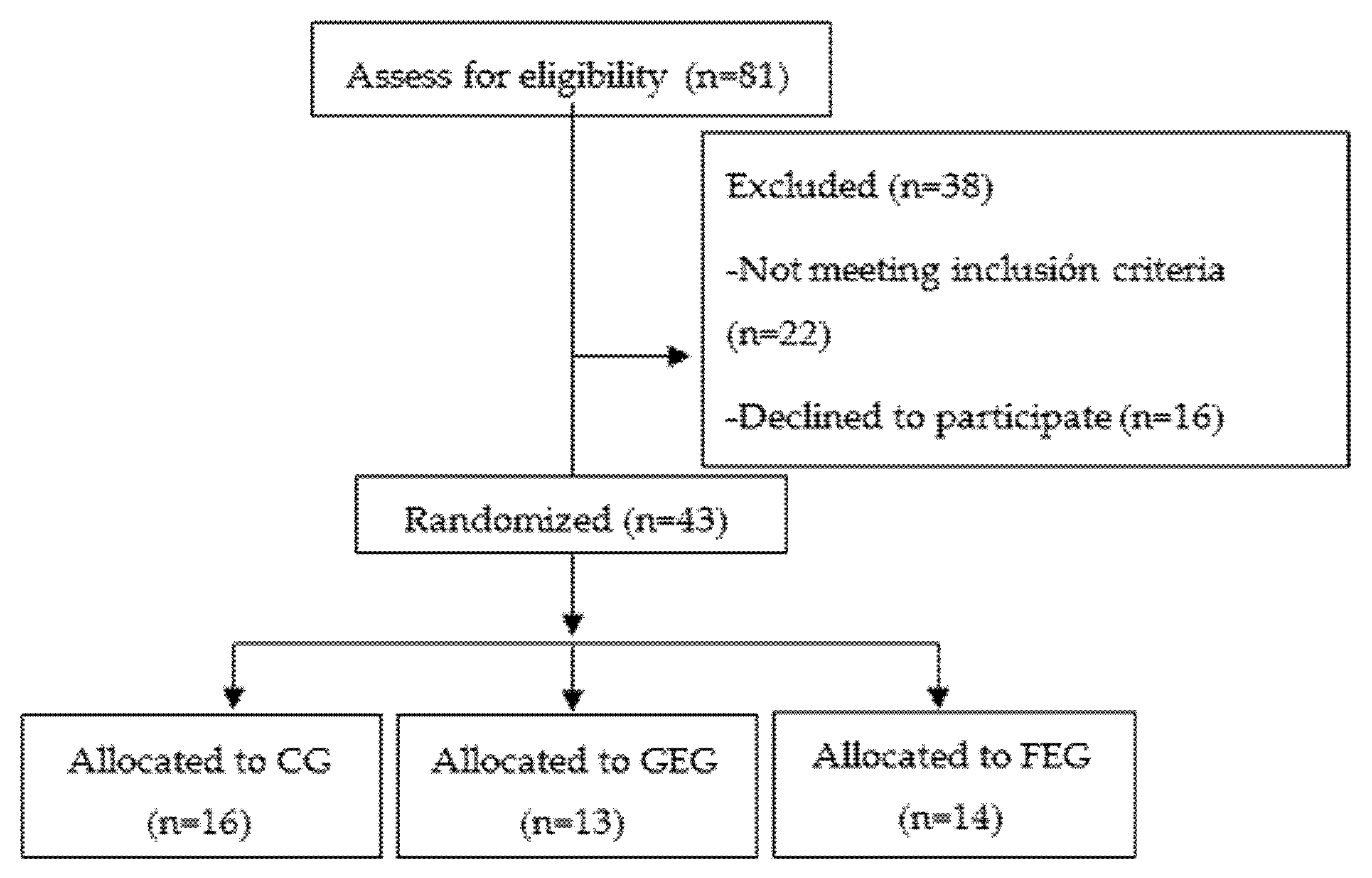
2.2. Randomization Procedure
2.3. Patients
2.4. Evaluation
2.4.1. Lower Limb Strength
2.4.2. Balance
2.5. Health Related Quality of Life
2.6. Adverse Events
2.7. Adherence
2.8. Interventions
Statistical Analysis
2.9. Sample Size Calculation
3. Results
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Wells, J.M.; Estepar, R.S.J.; McDonald, M.-L.N.; Bhatt, S.P.; Diaz, A.A.; Bailey, W.C.; Jacobson, F.L.; Dransfield, M.T.; Washko, G.R.; The COPDGene Investigators; et al. Clinical, physiologic, and radiographic factors contributing to development of hypoxemia in moderate to severe COPD: A cohort study. BMC Pulm. Med. 2016, 16, 169. [Google Scholar] [CrossRef] [Green Version]
- Gibson, G.J. Clinical Tests of Respiratory Function, 3rd ed.; Hodder Arnold: London, UK, 2009. [Google Scholar]
- Riley, C.M.; Sciurba, F.C. Diagnosis and Outpatient Management of Chronic Obstructive Pulmonary Disease: A Review. JAMA 2019, 321, 786–797. [Google Scholar] [CrossRef]
- McNicholas, W.; Kent, B.D.; Mitchell, P.D. Hypoxemia in patients with COPD: Cause, effects, and disease progression. Int. J. Chronic Obstr. Pulm. Dis. 2011, 6, 199–208. [Google Scholar] [CrossRef] [Green Version]
- Barberà, J.; Roca, J.; Ferrer, A.; Félez, M.; Díaz, O.; Roger, N.; Rodriguez-Roisin, R. Mechanisms of worsening gas exchange during acute exacerbations of chronic obstructive pulmonary disease. Eur. Respir. J. 1997, 10, 1285–1291. [Google Scholar] [CrossRef] [Green Version]
- Decramer, M.; Lacquet, L.M.; Fagard, R.; Rogiers, P. Corticosteroids contribute to muscle weakness in chronic airflow obstruction. Am. J. Respir. Crit. Care Med. 1994, 150, 11–16. [Google Scholar] [CrossRef]
- Kim, V.; Benditt, J.O.; Wise, R.; Sharafkhaneh, A. Oxygen therapy in chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc. 2008, 5, 513–518. [Google Scholar] [CrossRef]
- Van Helvoort, H.A.C.; Heijdra, Y.F.; Heunks, L.M.A.; Meijer, P.L.M.; Ruitenbeek, W.; Thijs, H.M.H.; Dekhuijzen, P.N.R. Supplemental oxygen prevents exercise-induced oxidative stress in muscle-wasted patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2006, 173, 1122–1129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rochester, C.L.; Vogiatzis, I.; Holland, A.E.; Lareau, S.C.; Marciniuk, D.D.; Puhan, M.; Spruit, M.A.; Masefield, S.C.; Casaburi, R.; Clini, E.; et al. An official american thoracic society/european respiratory society policy statement: Enhancing implementation, use, and delivery of pulmonary rehabilitation. Am. J. Respir. Crit. Care Med. 2015, 192, 1373–1386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabinovich, R.A.; Ardite, E.; Troosters, T.; Carbó, N.; Alonso, J.; De Suso, J.M.G.; Vilaró, J.; Barberà, J.A.; Polo, M.F.; Argilés, J.M.; et al. Reduced muscle redox capacity after endurance training in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2001, 164, 1114–1118. [Google Scholar] [CrossRef]
- Casanova, C.; Cote, C.; Marin, J.M.; Pinto-Plata, V.; de Torres, J.P.; Aguirre-Jaíme, A.; Vassaux, C.; Celli, B.R. Distance and oxygen desaturation during the 6-min walk test as predictors of long-term mortality in patients with COPD. Chest 2008, 134, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Neunhaeuserer, D.; Steidle-Kloc, E.; Weiss, G.; Kaiser, B.; Niederseer, D.; Hartl, S.; Tschentscher, M.; Egger, A.; Schönfelder, M.; Lamprecht, B.; et al. Supplemental oxygen during high-intensity exercise training in nonhypoxemic chronic obstructive pulmonary disease. Am. J. Med. 2016, 129, 1185–1193. [Google Scholar] [CrossRef]
- Andrianopoulos, V.; Franssen, F.M.; Peeters, J.P.; Ubachs, T.J.; Bukari, H.; Groenen, M.; Burtin, C.; Vogiatzis, I.; Wouters, E.F.; Spruit, M.A. Exercise-induced oxygen desaturation in COPD patients without resting hypoxemia. Respir. Physiol. Neurobiol. 2014, 190, 40–46. [Google Scholar] [CrossRef]
- Cobos-Carbó, A.; Augustovski, F. Declaración CONSORT 2010: Actualización de la lista de comprobación para informar ensayos clínicos aleatorizados de grupos paralelos. Med. Clín. 2011, 137, 213–215. [Google Scholar] [CrossRef] [Green Version]
- American Thoracic Society. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. Am. J. Respir Crit. Care Med. 1995, 152, S77–S121. [Google Scholar]
- Rodriguez-Roisin, R. Toward a consensus definition for COPD exacerbations. Chest 2000, 117, 398S–401S. [Google Scholar] [CrossRef] [Green Version]
- Narewski, E.R.; Blackford, A.L.; Lammi, M.R.; Fuhlbrigge, A.L.; Soler, X.; Albert, R.; Criner, G.J. Clinical differences in COPD patients with variable patterns of hypoxemia. Chronic Obstr. Pulm. Dis. J. COPD Found. 2018, 5, 167–176. [Google Scholar] [CrossRef]
- Mallery, L.H.; Macdonald, E.A.; Hubley-Kozey, C.L.; Earl, M.E.; Rockwood, K.; Macknight, C. The feasibility of performing resistance exercise with acutely ill hospitalized older adults. BMC Geriatr. 2003, 3, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; Van Der Grinten, C.P.M.; Gustafsson, P.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef] [Green Version]
- Jones, P.; Quirk, F.; Baveystock, C. The St George’s respiratory questionnaire. Respir. Med. 1991, 85, 25–31. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [Green Version]
- Walker, P.P.; Burnett, A.; Flavahan, P.W.; Calverley, P.M.A. Lower limb activity and its determinants in COPD. Thorax 2008, 63, 683–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, H.; Yule, V.; Syddall, H.; Dennison, E.; Cooper, C.; Sayer, A.A. Is hand-held dynamometry useful for the measurement of quadriceps strength in older people? A comparison with the gold standard biodex dynamometry. Gerontology 2006, 52, 154–159. [Google Scholar] [CrossRef]
- Bohannon, R.W. Single limb stance times. A descriptive meta-analysis of data from individuals at least 60 years of age. Top. Geriatr. Rehabil. 2006, 22, 70–77. [Google Scholar] [CrossRef]
- EuroQol Group. EuroQol—A new facility for the measurement of health-related quality of life. Health Policy 1990, 16, 199–208. [Google Scholar] [CrossRef]
- Hurst, J.R.; Vestbo, J.; Anzueto, A.; Locantore, N.; Müllerová, H.; Tal-Singer, R.; Miller, B.; Lomas, D.A.; Agusti, A.; MacNee, W.; et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N. Engl. J. Med. 2010, 363, 1128–1138. [Google Scholar] [CrossRef] [Green Version]
- Sánchez, I.T.; Valenza, M.C.; Sáez-Roca, G.; Cabrera-Martos, I.; López-Torres, I.; Rodríguez-Torres, J. Results of a multimodal program during hospitalization in obese COPD exacerbated patients. COPD: J. Chronic Obstr. Pulm. Dis. 2015, 13, 19–25. [Google Scholar] [CrossRef]
- Valenza, M.C.; Torres-Sanchez, I.; Lopez-Lopez, L.; Cabrera-Martos, I.; Ortiz-Rubio, A.; Valenza-Demet, G. Effects of home-based neuromuscular electrical stimulation in severe chronic obstructive pulmonary disease patients: A randomized controlled clinical trial. Eur. J. Phys. Rehabil. Med. 2018, 54, 323–332. [Google Scholar] [CrossRef]
- Spruit, M.A.; Gosselink, R.; Troosters, T.; Kasran, A.; Gayan-Ramirez, G.; Bogaerts, P.; Bouillon, R.; Decramer, M. Muscle force during an acute exacerbation in hospitalised patients with COPD and its relationship with CXCL8 and IGF-I. Thorax 2003, 58, 752–756. [Google Scholar] [CrossRef] [Green Version]
- Pitta, F.; Troosters, T.; Probst, V.S.; Spruit, M.A.; Decramer, M.; Gosselink, R. Physical activity and hospitalization for exacerbation of COPD. Chest 2006, 129, 536–544. [Google Scholar] [CrossRef] [Green Version]
- Green, H.J.; Sutton, J.R.; Wolfel, E.E.; Reeves, J.T.; Butterfield, G.E.; Brooks, G.A. Altitude acclimatization and energy metabolic adaptations in skeletal muscle during exercise. J. Appl. Physiol. 1992, 73, 2701–2708. [Google Scholar] [CrossRef]
- Clanton, T.L.; Klawitter, P.F. Invited review: Adaptive responses of skeletal muscle to intermittent hypoxia: The known and the unknown. J. Appl. Physiol. 2001, 90, 2476–2487. [Google Scholar] [CrossRef] [Green Version]
- Bailey, D.M.; Davies, B.; Baker, J. Training in hypoxia: Modulation of metabolic and cardiovascular risk factors in men. Med. Sci. Sports Exerc. 2000, 32, 1058–1066. [Google Scholar] [CrossRef]
- Bernardi, L.; Passino, C.; Serebrovska, T.; Appenzeller, O. Respiratory and cardiovascular adaptations to progressive hypoxia. Eur. Hear. J. 2001, 22, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Reeves, J.T.; Wolfel, E.E.; Green, H.J.; Mazzeo, R.S.; Young, A.J.; Sutton, J.R.; Brooks, G.A. Oxygen transport during exercise at altitude and the lactate paradox: Lessons from Operation Everest II and Pikes Peak. Exerc. Sport Sci. Rev. 1992, 20, 275–296. [Google Scholar] [PubMed]
- Antezana, A.M.; Kacimi, R.; Le Trong, J.L.; Marchal, M.; Abousahl, I.; DuBray, C.; Richalet, J.P. Adrenergic status of humans during prolonged exposure to the altitude of 6542 m. J. Appl. Physiol. 1994, 76, 1055–1059. [Google Scholar] [CrossRef]
- Burtscher, M.; Haider, T.; Domej, W.; Linser, T.; Gatterer, H.; Faulhaber, M.; Pocecco, E.; Ehrenburg, I.; Tkatchuk, E.; Koch, R.; et al. Intermittent hypoxia increases exercise tolerance in patients at risk for or with mild COPD. Respir. Physiol. Neurobiol. 2009, 165, 97–103. [Google Scholar] [CrossRef]
- Sánchez, I.T.; Valenza, M.C.; Cabrera-Martos, I.; López-Torres, I.; Benítez-Feliponi, Á.; Conde-Valero, A. Effects of an exercise intervention in frail older patients with chronic obstructive pulmonary disease hospitalized due to an exacerbation: A randomized controlled trial. COPD J. Chronic Obstr. Pulm. Dis. 2016, 14, 37–42. [Google Scholar] [CrossRef]
- Lopez-Lopez, L.; Sánchez, I.T.; Rodriguez-Torres, J.; Cabrera-Martos, I.; Cahalin, L.P.; Valenza, M.C. Randomized feasibility study of twice a day functional electrostimulation in patients with severe chronic obstructive pulmonary disease hospitalized for acute exacerbation. Physiother. Theory Pract. 2019, 26, 1–8. [Google Scholar] [CrossRef]
- Troosters, T.; Probst, V.S.; Crul, T.; Pitta, F.; Gayan-Ramirez, G.; Decramer, M.; Gosselink, R. Resistance training prevents deterioration in quadriceps muscle function during acute exacerbations of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2010, 181, 1072–1077. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, M.K.; Brooks, D.; Goldstein, R.S. Deficits in postural control in individuals with COPD—Emerging evidence for an important secondary impairment. Multidiscip. Respir. Med. 2010, 5, 417–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozalevli, S.; Ilgin, D.; Narin, S.; Akkoclu, A. Association between disease-related factors and balance and falls among the elderly with COPD: A cross-sectional study. Aging Clin. Exp. Res. 2011, 23, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Sibley, K.M.; Voth, J.; Munce, S.E.; Straus, S.E.; Jaglal, S.B. Chronic disease and falls in community-dwelling Canadians over 65 years old: A population-based study exploring associations with number and pattern of chronic conditions. BMC Geriatr. 2014, 14, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, K.; Mathur, S.; Roig, M.; Janaudis-Ferreira, T.; Robles, P.; Dolmage, T.E.; Goldstein, R. Neuromuscular electrostimulation for chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2013, 5, 010821. [Google Scholar] [CrossRef]
- Gordon, C.S.; Waller, J.W.; Cook, R.M.; Cavalera, S.L.; Lim, W.T.; Osadnik, C.R. Effect of pulmonary rehabilitation on symptoms of anxiety and depression in COPD. Chest 2019, 156, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.; Rebelo, P.; Paixão, C.; Jácome, C.; Cruz, J.; Martins, V.; Simão, P.; Brooks, D.; Marques, A. Minimal clinically important difference for quadriceps muscle strength in people with copd following pulmonary rehabilitation. COPD J. Chronic Obstr. Pulm. Dis. 2021, 18, 35–44. [Google Scholar] [CrossRef]
| Variables | CG (n = 15) | GEG (n = 13) | FEG (n = 14) | F |
|---|---|---|---|---|
| Age (years) | 70.98 ± 9.22 | 74.92 ± 7.07 | 75.80 ± 8.61 | 12.541 |
| Males (%) | 93.3 | 92.3 | 85.7 | - |
| BMI (Kg/m2) | 26.29 ± 3.69 | 25.41 ± 4.88 | 23.65 ± 6.25 | 6.954 |
| FEV1% | 33.41 ± 14.97 | 32.46 ± 12.31 | 37.12 ± 18.52 | 12.068 |
| FVC% | 41.25 ± 15.36 | 40.68 ± 12.55 | 43.58 ± 16.78 | 11.745 |
| SGRQ | 66.79 ± 13.58 | 63.80 ± 13.57 | 57.24 ± 11.61 | 2.582 |
| CCI | 4.77 ± 1.40 | 5.69 ± 1.65 | 2.80 ± 1.47 | 14.745 |
| HAD anxiety | 7.64 ± 5.17 | 7.46 ± 5.13 | 9.31 ± 4.90 | 2.587 |
| HAD depression | 8.13 ± 4.60 | 7.50 ± 4.70 | 7.00 ± 4.79 | 6.135 |
| HAD total | 15.92 ± 7.94 | 14.96 ± 8.60 | 16.31 ± 8.50 | 4.203 |
| Hospital stay (days) | 11.00 ± 5.29 | 10.33 ± 3.05 | 7.47 ± 3.50 | 8.745 |
| Variables | CG (n = 15) | GEG (n = 13) | FEG (n = 14) | F |
|---|---|---|---|---|
| Quadriceps strength (n) | 107.10 ± 38.03 | 102.91 ± 40.00 | 103.93 ± 13.28 | 1.197 |
| Balance (s) | 2.98 ± 4.72 | 2.15 ± 2.26 | 2.95 ± 1.99 | 3.685 |
| EQ-5D mobility | 1.84 ± 0.75 | 1.85 ± 0.49 | 1.59 ± 0.57 | 1.679 |
| EQ-5D personal care | 1.95 ± 0.58 | 1.62 ± 0.69 | 1.89 ± 0.29 | 2.796 |
| EQ-5D daily activities | 2.29 ± 0.68 | 1.91 ± 0.87 | 2.12 ± 0.63 | 2.158 |
| EQ-5D A/D | 1.82 ± 0.58 | 1.51 ± 0.68 | 1.37 ± 0.49 | 3.678 |
| EQ-5D pain | 2.07 ± 0.67 | 1.81 ± 0.67 | 2.18 ± 0.71 | 2.378 |
| EQ-5D VAS | 41.67 ± 15.23 | 51.23 ± 10.32 | 44.27 ± 10.23 | 5.247 |
| Variables | CG (n = 15) | GEG (n = 13) | FEG (n = 14) | F | |||
|---|---|---|---|---|---|---|---|
| Admission | Discharge | Admission | Discharge | Admission | Discharge | ||
| Quadriceps strength (n) | 107.10 ± 38.03 | 108.94 ± 69.95 | 102.91 ± 40.00 | 118.50 ± 31.64 ** | 103.93 ± 13.28 | 133.28 ± 30.41 ** | 11.046a,b,c |
| Balance (s) | 2.98 ± 4.72 | 3.12 ± 3.83 | 2.15 ± 2.26 | 4.84 ± 7.25 ** | 2.95 ± 1.99 | 4.11 ± 2.71 ** | 3.548a,b |
| EQ-5D mobility | 1.84 ± 0.75 | 1.51 ± 0.63 | 1.85 ± 0.49 | 1.04 ± 0.30 * | 1.59 ± 0.57 | 1.20 ± 0.62 * | 0.98ab |
| EQ-5D personal care | 1.95 ± 0.58 | 1.54 ± 0.82 | 1.62 ± 0.69 | 1.39 ± 0.71 | 1.89 ± 0.29 | 1.10 ± 0.59 * | 1.14abc |
| EQ-5D daily activities | 2.29 ± 0.68 | 1.91 ± 0.79 * | 1.91 ± 0.87 | 1.19 ± 0.31 * | 2.12 ± 0.63 | 1.08 ± 0.88 * | 1.87 |
| EQ-5D A/D | 1.82 ± 0.58 | 1.34 ± 0.39 * | 1.51 ± 0.68 | 1.79 ± 0.80 | 1.37 ± 0.49 | 1.29 ± 0.47 | 2.94b,c |
| EQ-5D pain | 2.07 ± 0.67 | 2.10 ± 0.81 | 1.81 ± 0.67 | 0.91 ± 0.34 * | 2.18 ± 0.71 | 0.79 ± 0.62 ** | 0.86b |
| EQ-5D VAS | 41.67 ± 15.23 | 54.14 ± 20.49 | 51.23 ± 10.32 | 79.97 ± 14.22 * | 44.27 ± 10.23 | 73.57 ± 13.25 * | 19.25ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-López, L.; Calvache-Mateo, A.; Rodríguez-Torres, J.; Granados-Santiago, M.; Ortiz-Rubio, A.; Valenza, M.C. A Feasibility and Efficacy Randomized Controlled Trial of Two Exercise Programs in Severe AECOPD Patients with Resting Hypoxemia. Healthcare 2021, 9, 1102. https://doi.org/10.3390/healthcare9091102
López-López L, Calvache-Mateo A, Rodríguez-Torres J, Granados-Santiago M, Ortiz-Rubio A, Valenza MC. A Feasibility and Efficacy Randomized Controlled Trial of Two Exercise Programs in Severe AECOPD Patients with Resting Hypoxemia. Healthcare. 2021; 9(9):1102. https://doi.org/10.3390/healthcare9091102
Chicago/Turabian StyleLópez-López, Laura, Andrés Calvache-Mateo, Janet Rodríguez-Torres, María Granados-Santiago, Araceli Ortiz-Rubio, and Marie Carmen Valenza. 2021. "A Feasibility and Efficacy Randomized Controlled Trial of Two Exercise Programs in Severe AECOPD Patients with Resting Hypoxemia" Healthcare 9, no. 9: 1102. https://doi.org/10.3390/healthcare9091102
APA StyleLópez-López, L., Calvache-Mateo, A., Rodríguez-Torres, J., Granados-Santiago, M., Ortiz-Rubio, A., & Valenza, M. C. (2021). A Feasibility and Efficacy Randomized Controlled Trial of Two Exercise Programs in Severe AECOPD Patients with Resting Hypoxemia. Healthcare, 9(9), 1102. https://doi.org/10.3390/healthcare9091102








