Umbelliferone-Based Fluorescent Probe for Selective Recognition of Hydrogen Sulfide and Its Bioimaging in Living Cells and Zebrafish
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Instruments
2.2. Synthesis of Probe 1
2.3. General Procedure for Absorption and Fluorescence Measurements
2.4. Cell Imaging
2.5. Zebrafish Imaging
3. Results and Discussion
3.1. Synthesis and Characterization
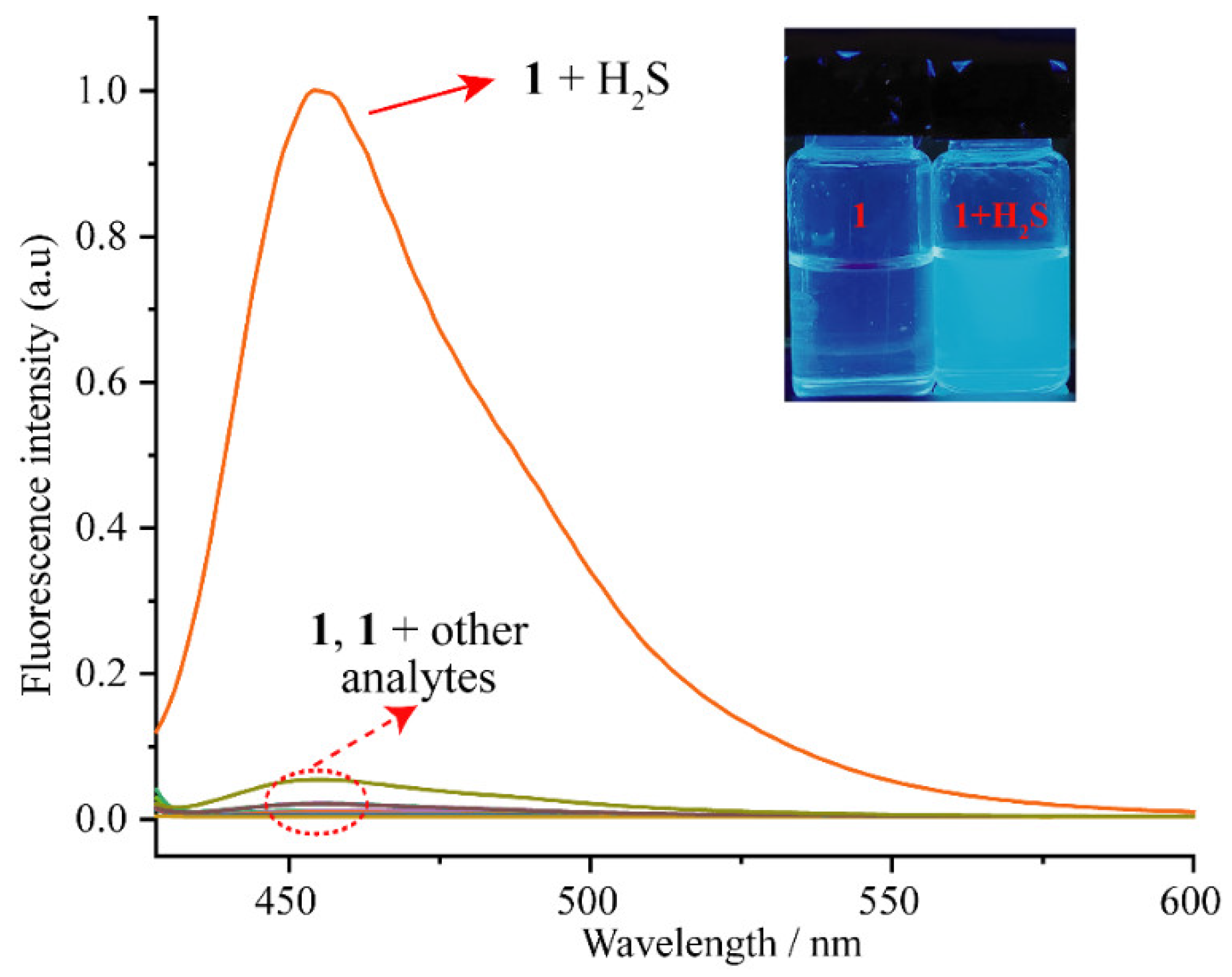
3.2. Optical Behavior of 1 to H2S
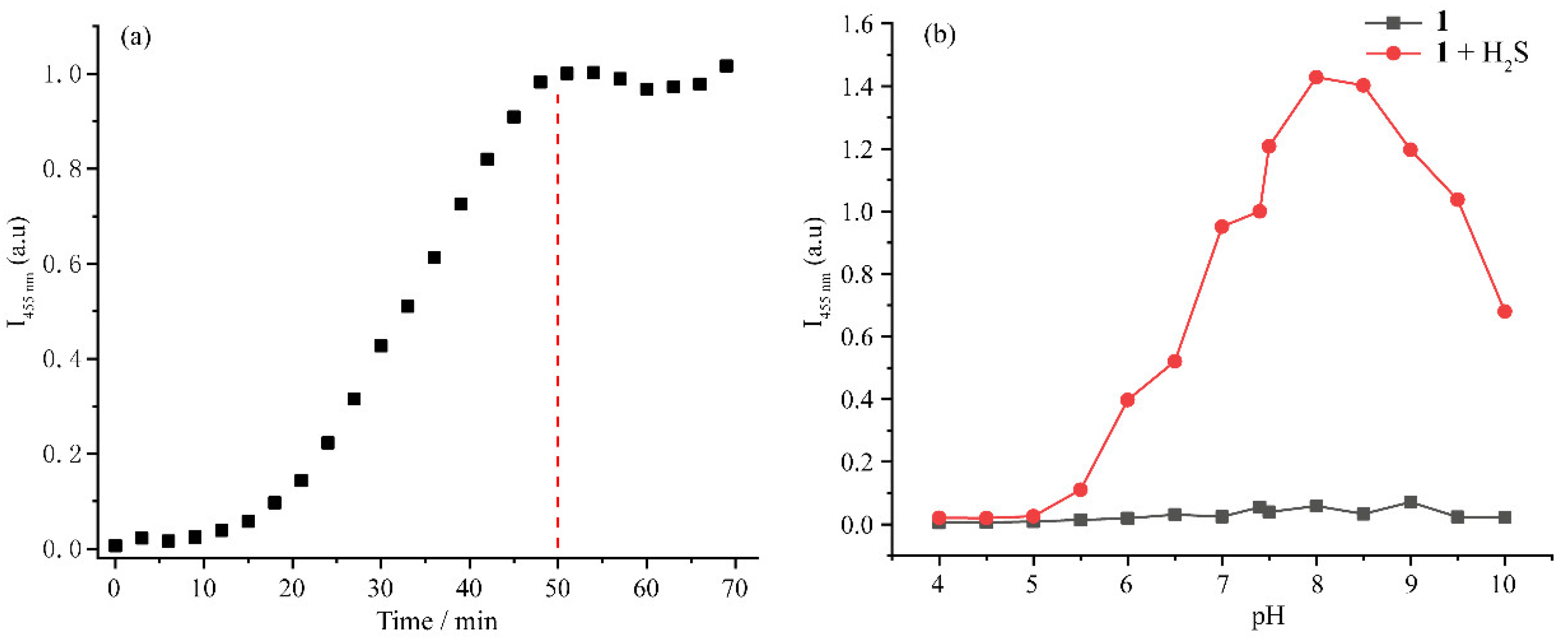
3.3. Time and pH-Dependent Fluorescence Response of 1 to H2S
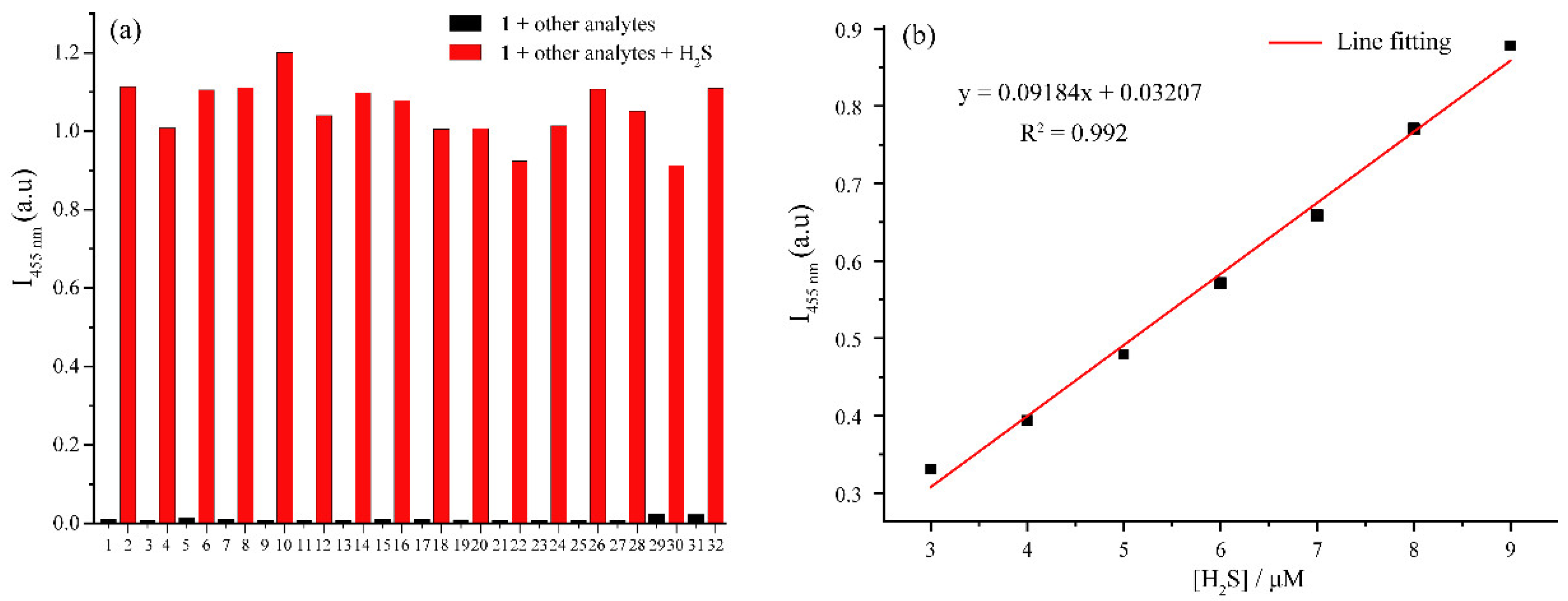
3.4. Selectivity and Detection Limit of 1 to H2S
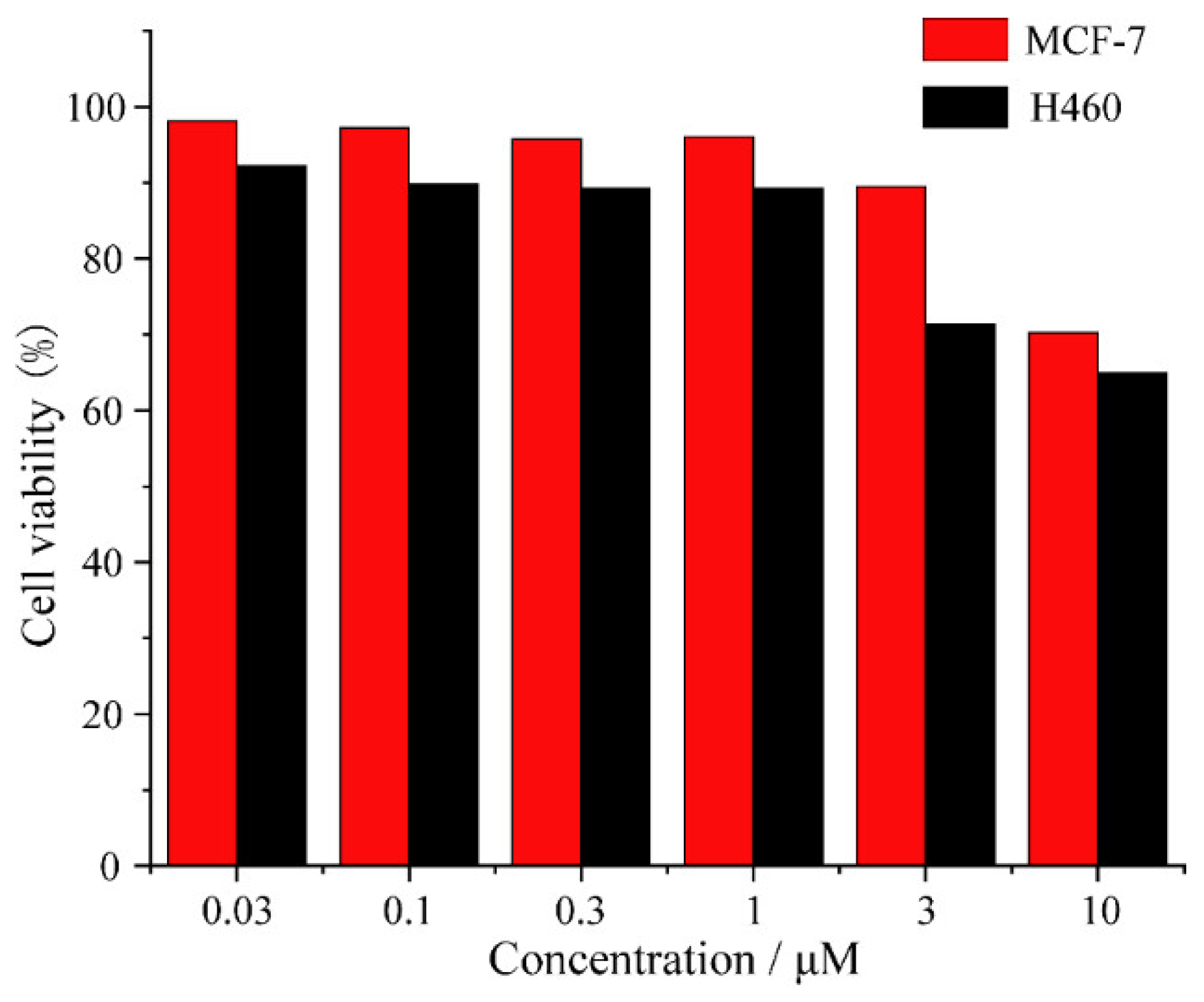
3.5. Cytotoxicity of 1
3.6. Imaging of H2S in Cells by 1
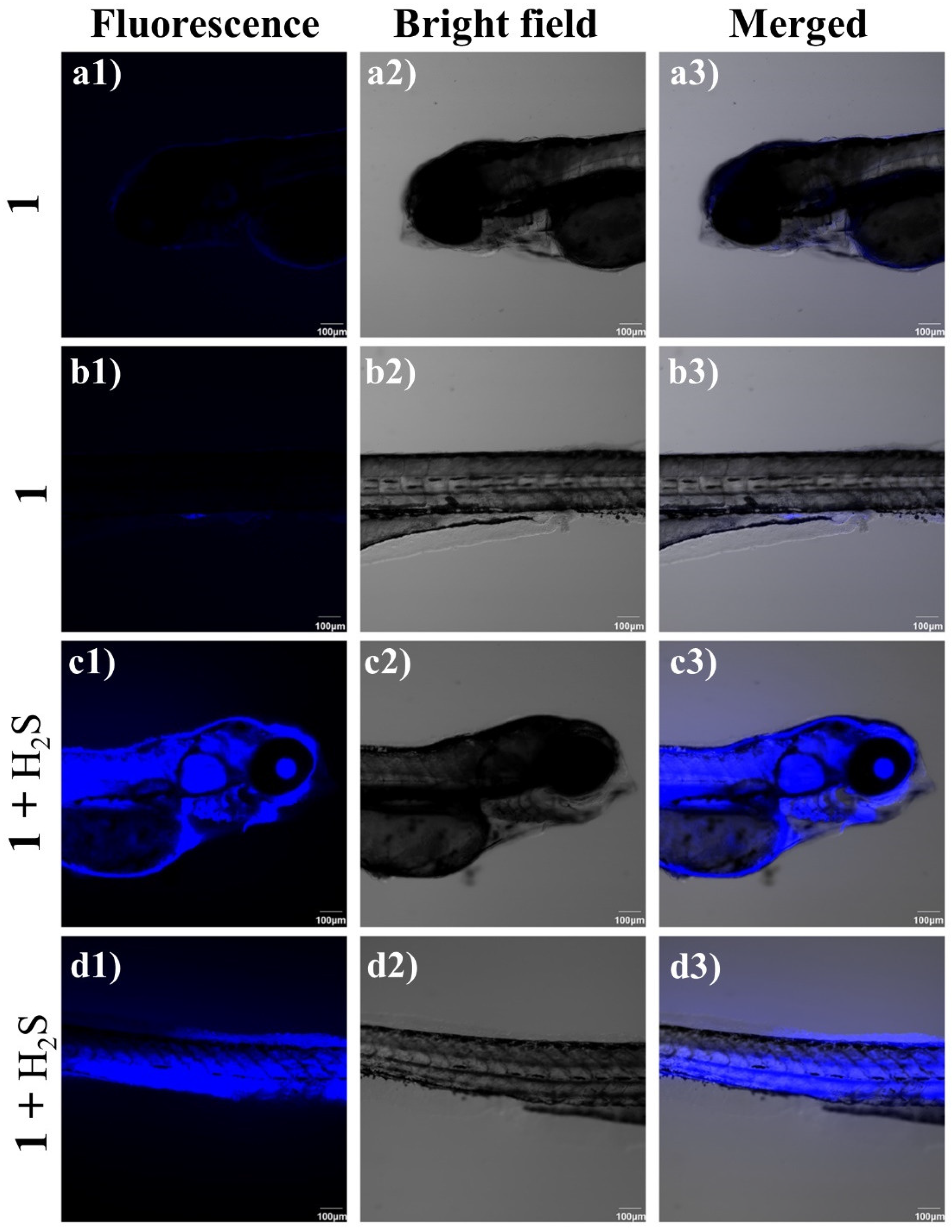
3.7. Imaging of H2S in Zebrafish by 1
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aroca, A.; Gotor, C.; Bassham, D.C.; Romero, L.C. Hydrogen Sulfide: From a Toxic Molecule to a Key Molecule of Cell Life. Antioxidants 2020, 9, 621. [Google Scholar] [CrossRef] [PubMed]
- Szabo, C. Hydrogen Sulfide, an Endogenous Stimulator of Mitochondrial Function in Cancer Cells. Cells 2021, 10, 220. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Li, G.; Yang, L. Role of H2S in pain: Growing evidences of mystification. Eur. J. Pharmacol. 2020, 883, 173322. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H. Hydrogen Sulfide (H2S) and Polysulfide (H2Sn) Signaling: The First 25 Years. Biomolecules 2021, 11, 896. [Google Scholar] [CrossRef]
- Yang, N.; Liu, Y.; Li, T.; Tuo, Q. Role of Hydrogen Sulfide in Chronic Diseases. DNA Cell Biol. 2019, 39, 187–196. [Google Scholar] [CrossRef]
- Casin, K.M.; Calvert, J.W. Harnessing the Benefits of Endogenous Hydrogen Sulfide to Reduce Cardiovascular Disease. Antioxidants 2021, 10, 383. [Google Scholar] [CrossRef]
- Lefer, D.J. A new gaseous signaling molecule emerges: Cardioprotective role of hydrogen sulfide. Proc. Natl. Acad. Sci. USA 2007, 104, 17907–17908. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Shang, Q.; Yao, J.; Ji, Y. Hydrogen sulfide: A gaseous signaling molecule modulates tissue homeostasis: Implications in ophthalmic diseases. Cell Death Dis. 2019, 10, 293. [Google Scholar] [CrossRef] [Green Version]
- Tan, B.H.; Wong, P.T.H.; Bian, J.-S. Hydrogen sulfide: A novel signaling molecule in the central nervous system. Neurochem. Int. 2010, 56, 3–10. [Google Scholar] [CrossRef]
- di Masi, A.; Ascenzi, P. H2S: A “Double face” molecule in health and disease. BioFactors 2013, 39, 186–196. [Google Scholar] [CrossRef]
- Sun, H.J.; Wu, Z.Y.; Nie, X.W.; Bian, J.S. Role of Endothelial Dysfunction in Cardiovascular Diseases: The Link between Inflammation and Hydrogen Sulfide. Front. Pharmacol. 2019, 10, 1568. [Google Scholar] [CrossRef] [Green Version]
- Quinzii, C.M.; Lopez, L.C. Abnormalities of hydrogen sulfide and glutathione pathways in mitochondrial dysfunction. J. Adv. Res. 2021, 27, 79–84. [Google Scholar] [CrossRef]
- Tamele, M.W.; Ryland, L.B.; McCoy, R.N. Simultaneous Determination of Hydrogen Sulfide and Mercaptans by Potentiometric Titration. Anal. Chem. 1960, 32, 1007–1011. [Google Scholar] [CrossRef]
- Ubuka, T.; Abe, T.; Kajikawa, R.; Morino, K. Determination of hydrogen sulfide and acid-labile sulfur in animal tissues by gas chromatography and ion chromatography. J. Chromatogr. B 2001, 757, 31–37. [Google Scholar] [CrossRef]
- Lee, J.; Lee, Y.J.; Ahn, Y.J.; Choi, S.; Lee, G.-J. A simple and facile paper-based colorimetric assay for detection of free hydrogen sulfide in prostate cancer cells. Sens. Actuators B Chem. 2018, 256, 828–834. [Google Scholar] [CrossRef]
- Xu, T.; Scafa, N.; Xu, L.-P.; Zhou, S.; Abdullah Al-Ghanem, K.; Mahboob, S.; Fugetsu, B.; Zhang, X. Electrochemical hydrogen sulfide biosensors. Analyst 2016, 141, 1185–1195. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Chen, G.; Lin, W.; Li, J.; Fang, L.; Wang, X.; Zhang, Y.; Chen, Y.; Lin, Z. Signal-On and Highly Sensitive Electrochemiluminescence Biosensor for Hydrogen Sulfide in Joint Fluid Based on Silver-Ion-Mediated Base Pairs and Hybridization Chain Reaction. Chemosensors 2022, 10, 250. [Google Scholar] [CrossRef]
- Xiao, P.; Liu, J.; Wang, Z.; Tao, F.; Yang, L.; Yuan, G.; Sun, W.; Zhang, X. A color turn-on fluorescent probe for real-time detection of hydrogen sulfide and identification of food spoilage. Chem. Commun. 2021, 57, 5012–5015. [Google Scholar] [CrossRef]
- Skorjanc, T.; Shetty, D.; Valant, M. Covalent Organic Polymers and Frameworks for Fluorescence-Based Sensors. ACS Sens. 2021, 6, 1461–1481. [Google Scholar] [CrossRef]
- Paganini, C.; Hettich, B.; Kopp, M.R.G.; Eördögh, A.; Capasso Palmiero, U.; Adamo, G.; Touzet, N.; Manno, M.; Bongiovanni, A.; Rivera-Fuentes, P.; et al. Rapid Characterization and Quantification of Extracellular Vesicles by Fluorescence-Based Microfluidic Diffusion Sizing. Adv. Healthc. Mater. 2022, 11, 2100021. [Google Scholar] [CrossRef]
- Jia, T.-T.; Li, Y.; Niu, H. Recent Progress in Fluorescent Probes for Diabetes Visualization and Drug Therapy. Chemosensors 2022, 10, 280. [Google Scholar] [CrossRef]
- Li, H.; An, Y.; Gao, J.; Yang, M.; Luo, J.; Li, X.; Lv, J.; Li, X.; Yuan, Z.; Ma, H. Recent Advances of Fluorescence Probes for Imaging of Ferroptosis Process. Chemosensors 2022, 10, 233. [Google Scholar] [CrossRef]
- Wu, L.; Sedgwick, A.C.; Sun, X.; Bull, S.D.; He, X.P.; James, T.D. Reaction-Based Fluorescent Probes for the Detection and Imaging of Reactive Oxygen, Nitrogen, and Sulfur Species. Acc. Chem. Res. 2019, 52, 2582–2597. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Nie, J.; Fang, W.; Yang, L.; Hu, Q.; Wang, Z.; Sun, J.Z.; Tang, B.Z. Sugar-Based Aggregation-Induced Emission Luminogens: Design, Structures, and Applications. Chem. Rev. 2020, 120, 4534–4577. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, W.; Chung, J.; Yin, J.; Yoon, J. Recent progress in fluorescent probes for bacteria. Chem. Soc. Rev. 2021, 50, 7725–7744. [Google Scholar] [CrossRef]
- Liu, C.; Zhu, H.; Zhang, Y.; Su, M.; Liu, M.; Zhang, X.; Wang, X.; Rong, X.; Wang, K.; Li, X.; et al. Recent advances in Golgi-targeted small-molecule fluorescent probes. Coord. Chem. Rev. 2022, 462, 214504. [Google Scholar] [CrossRef]
- Fang, Y.; Dehaen, W. Small-molecule-based fluorescent probes for f-block metal ions: A new frontier in chemosensors. Coord. Chem. Rev. 2021, 427, 213524. [Google Scholar] [CrossRef]
- Fang, Y.; Dehaen, W. Fluorescent Probes for Selective Recognition of Hypobromous Acid: Achievements and Future Perspectives. Molecules 2021, 26, 363. [Google Scholar] [CrossRef]
- Li, H.; Fang, Y.; Yan, J.; Ren, X.; Zheng, C.; Wu, B.; Wang, S.; Li, Z.; Hua, H.; Wang, P.; et al. Small-molecule fluorescent probes for H2S detection: Advances and perspectives. TrAC Trends Anal. Chem. 2021, 134, 116117. [Google Scholar] [CrossRef]
- Park, S.Y.; Yoon, S.A.; Cha, Y.; Lee, M.H. Recent advances in fluorescent probes for cellular antioxidants: Detection of NADH, hNQO1, H2S, and other redox biomolecules. Coord. Chem. Rev. 2021, 428, 213613. [Google Scholar] [CrossRef]
- Wang, J.; Huo, F.; Yue, Y.; Yin, C. A review: Red/near-infrared (NIR) fluorescent probes based on nucleophilic reactions of H2S since 2015. Luminescence 2020, 35, 1156–1173. [Google Scholar] [CrossRef] [PubMed]
- Zhong, K.; Zhou, S.; Yan, X.; Li, X.; Hou, S.; Cheng, L.; Gao, X.; Tang, L. A simple H2S fluorescent probe with long wavelength emission: Application in water, wine, living cells and detection of H2S gas. Dye. Pigment. 2020, 174, 108049. [Google Scholar] [CrossRef]
- Niu, P.; Liu, J.; Rong, Y.; Liu, X.; Wei, L. A fluorescent probe for selective and instantaneous detection of hydrogen sulfide in living cells and zebrafish. Tetrahedron 2021, 89, 132174. [Google Scholar] [CrossRef]
- Saha, S.; Roy, P.K.; Maity, K.; Mandal, M.; Biradha, K. Comparative Study of Nitro- and Azide-Functionalized ZnII-Based Coordination Polymers (CPs) as Fluorescent Turn-On Probes for Rapid and Selective Detection of H2S in Living Cells. Chem. Eur. J. 2022, 28, e202103830. [Google Scholar] [CrossRef]
- Pal, D.; Saha, S. Coumarins: An Important Phytochemical with Therapeutic Potential. In Plant-Derived Bioactives: Chemistry and Mode of Action; Swamy, M.K., Ed.; Springer: Singapore, 2020; pp. 205–222. [Google Scholar]
- Cao, D.; Liu, Z.; Verwilst, P.; Koo, S.; Jangjili, P.; Kim, J.S.; Lin, W. Coumarin-Based Small-Molecule Fluorescent Chemosensors. Chem. Rev. 2019, 119, 10403–10519. [Google Scholar] [CrossRef]
- Gerasimova, E.; Gazizullina, E.; Radosteva, E.; Ivanova, A. Antioxidant and Antiradical Properties of Some Examples of Flavonoids and Coumarins—Potentiometric Studies. Chemosensors 2021, 9, 112. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, H.; Zhao, B.; Liu, H.; Liu, Q.; Gao, Y. Highly Selective Recognition of Pyrophosphate by a Novel Coumarin-Iron(III) Complex and the Application in Living Cells. Chemosensors 2021, 9, 48. [Google Scholar] [CrossRef]
- Mazimba, O. Umbelliferone: Sources, chemistry and bioactivities review. Bull. Fac. Pharm. Cairo Univ. 2017, 55, 223–232. [Google Scholar] [CrossRef]
- Zheng, D.; Zhang, T.; Huang, Y.; Chen, H.; Li, Y.; Cao, Z.; Deng, Y.; Fang, Y.; Peng, C. Phenoxazine-conjugated-benzoeindolium as a novel mitochondria-targeted fluorescent probe for turn-on detection of sulfur dioxide and its derivatives in vivo. Microchem. J. 2022, 175, 107192. [Google Scholar] [CrossRef]
- Zheng, D.; Zhang, T.; Huang, J.; Wang, M.; Cao, Z.; Huang, Y.; Yang, Z.; Deng, Y.; Fang, Y. Indole-incorporated-benzoeindolium as a novel mitochondrial and ratiometric fluorescent probe for real-time tracking of SO2 derivatives in vivo and herb samples. Dye. Pigment. 2022, 198, 109973. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, R.; Dehaen, W.; Fang, Y.; Qian, S.; Ren, Y.; Cheng, F.; Guo, Y.; Guo, C.; Li, Y.; et al. Specific recognition, intracellular assay and detoxification of fluorescent curcumin derivative for copper ions. J. Hazard. Mater. 2021, 420, 126490. [Google Scholar] [CrossRef]
- Fang, Y.; Deng, Y.; Dehaen, W. Tailoring pillararene-based receptors for specific metal ion binding: From recognition to supramolecular assembly. Coord. Chem. Rev. 2020, 415, 213313. [Google Scholar] [CrossRef]
- Fang, Y.; Wang, M.; Shen, Y.; Zhang, M.; Cao, Z.; Deng, Y. Highly sensitive and selective recognition behaviour for fluoride based on a homoditopic curcumin-difluoroboron receptor. Inorg. Chim. Acta 2020, 503, 119413. [Google Scholar] [CrossRef]
- Zhang, Y.; Deng, Y.; Ji, N.; Zhang, J.; Fan, C.; Ding, T.; Cao, Z.; Li, Y.; Fang, Y. A rationally designed flavone-based ESIPT fluorescent chemodosimeter for highly selective recognition towards fluoride and its application in live-cell imaging. Dye. Pigment. 2019, 166, 473–479. [Google Scholar] [CrossRef]
- Dhivya, R.; Kavitha, V.; Gomathi, A.; Keerthana, P.; Santhalakshmi, N.; Viswanathamurthi, P.; Haribabu, J. Dinitrobenzene ether reactive turn-on fluorescence probes for the selective detection of H2S. Anal. Methods 2022, 14, 58–66. [Google Scholar] [CrossRef]
- Li, B.; Mei, H.; Wang, M.; Gu, X.; Hao, J.; Xie, X.; Xu, K. A near-infrared fluorescent probe for imaging of endogenous hydrogen sulfide in living cells and mice. Dye. Pigment. 2021, 189, 109231. [Google Scholar] [CrossRef]
- Ge, C.; Li, J.; Liu, L.; Liu, H.K.; Qian, Y. A self-immolated fluorogenic agent triggered by H2S exhibiting potential anti-glioblastoma activity. Analyst 2021, 146, 3510–3515. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, Y.; Luo, F.; Cao, Z.; Peng, C.; Dehaen, W. Umbelliferone-Based Fluorescent Probe for Selective Recognition of Hydrogen Sulfide and Its Bioimaging in Living Cells and Zebrafish. Chemosensors 2022, 10, 427. https://doi.org/10.3390/chemosensors10100427
Fang Y, Luo F, Cao Z, Peng C, Dehaen W. Umbelliferone-Based Fluorescent Probe for Selective Recognition of Hydrogen Sulfide and Its Bioimaging in Living Cells and Zebrafish. Chemosensors. 2022; 10(10):427. https://doi.org/10.3390/chemosensors10100427
Chicago/Turabian StyleFang, Yuyu, Fan Luo, Zhixing Cao, Cheng Peng, and Wim Dehaen. 2022. "Umbelliferone-Based Fluorescent Probe for Selective Recognition of Hydrogen Sulfide and Its Bioimaging in Living Cells and Zebrafish" Chemosensors 10, no. 10: 427. https://doi.org/10.3390/chemosensors10100427




