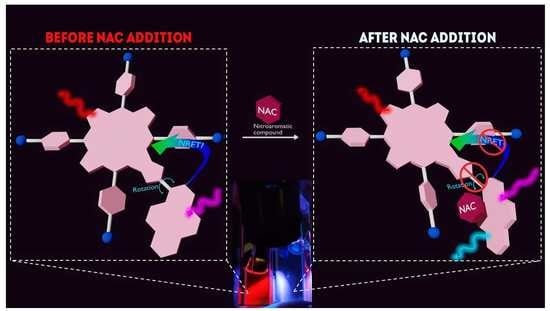
Unusual ‘Turn-on’ Ratiometric Response of Fluorescent Porphyrin-Pyrene Dyads to the Nitroaromatic Compounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. UV-Vis and Fluorescence Titration
2.3. “ZnPIP in PMMA” Film Preparation
2.4. Quantum Chemical Calculations
3. Results
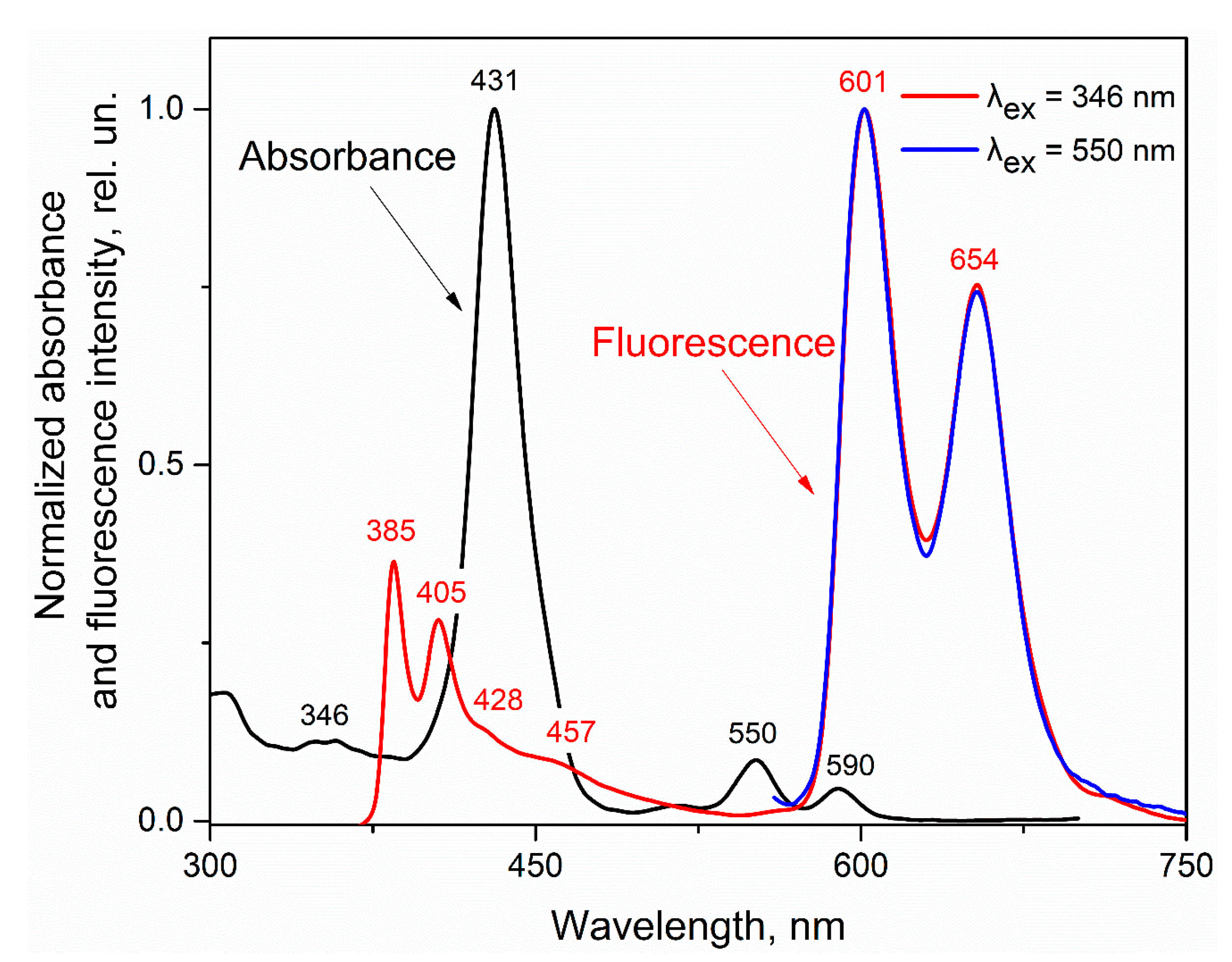
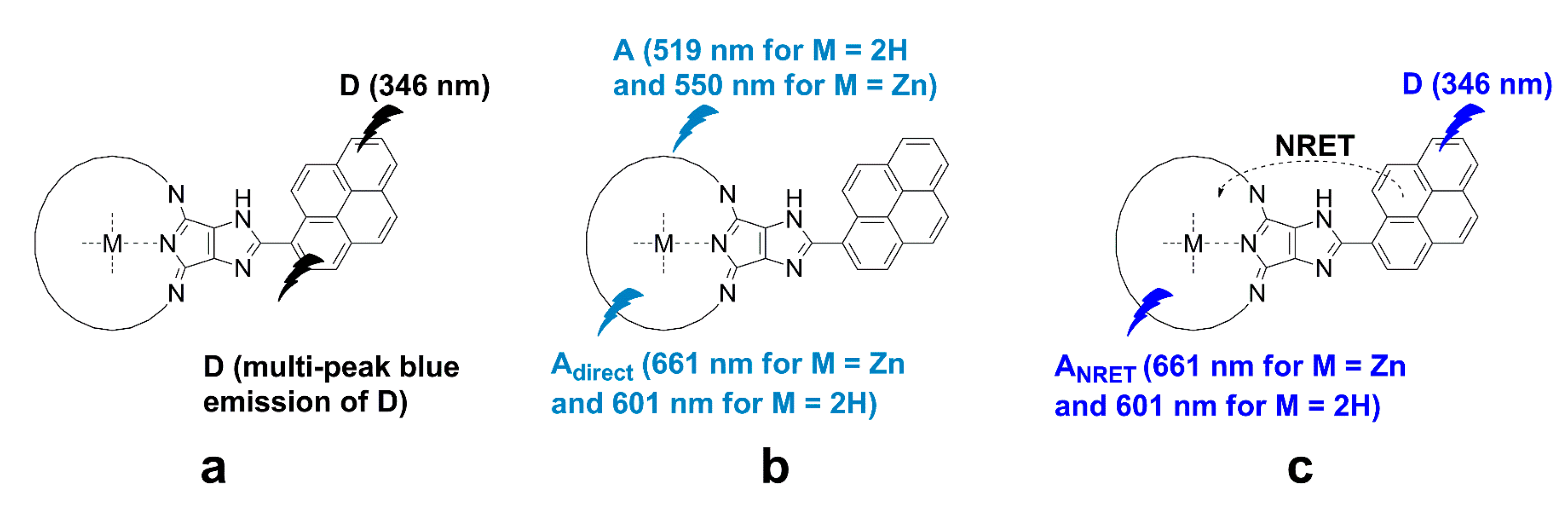
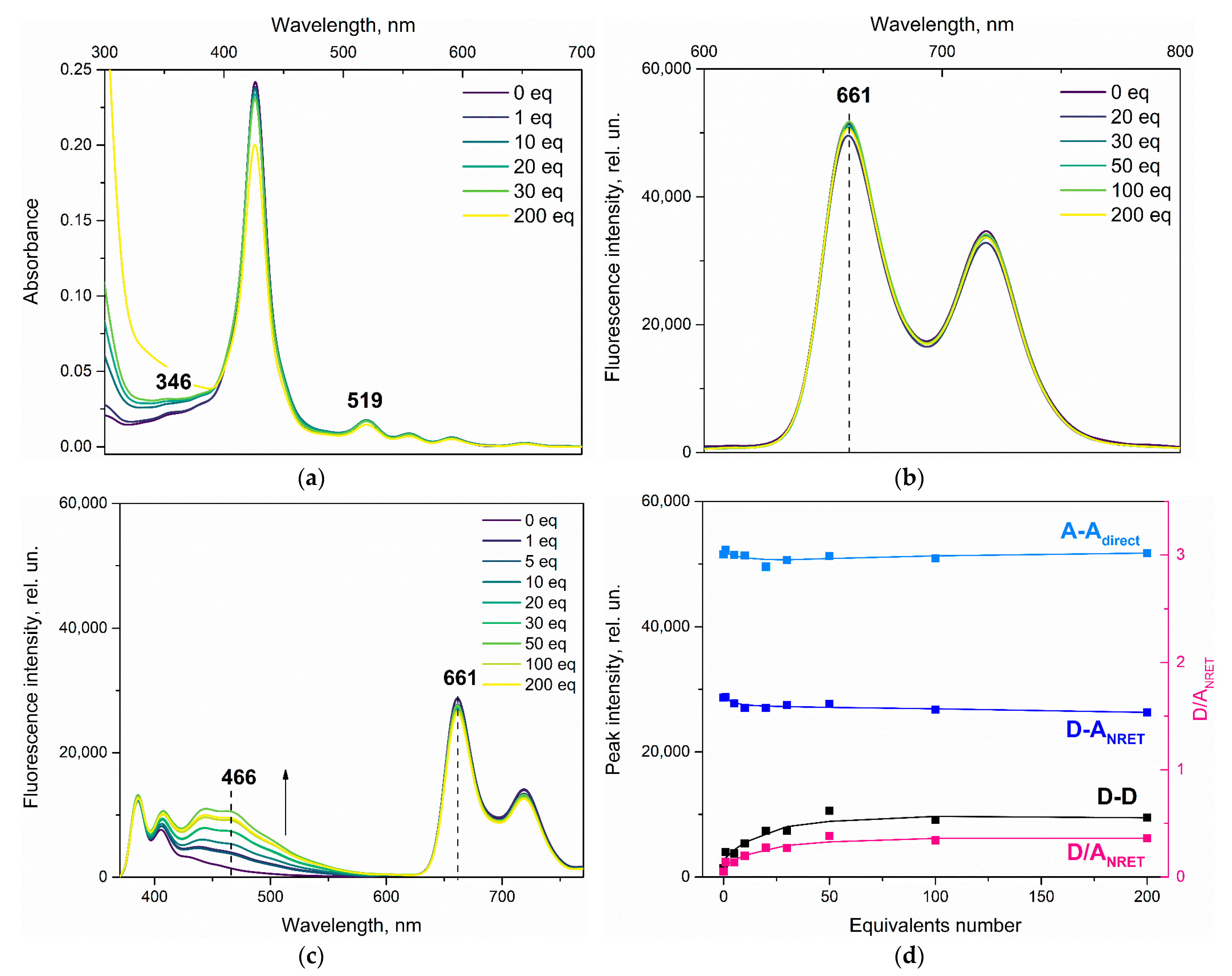
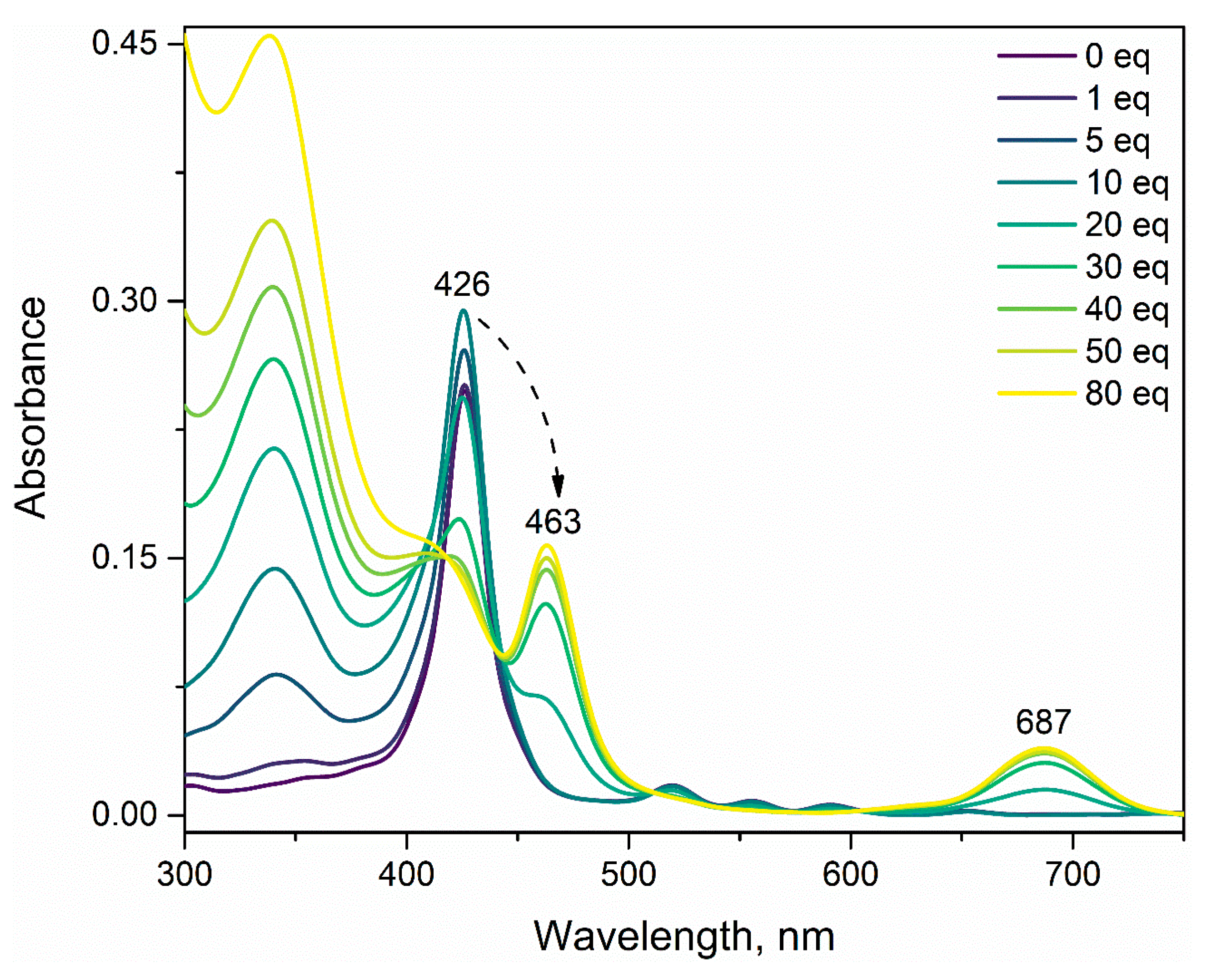
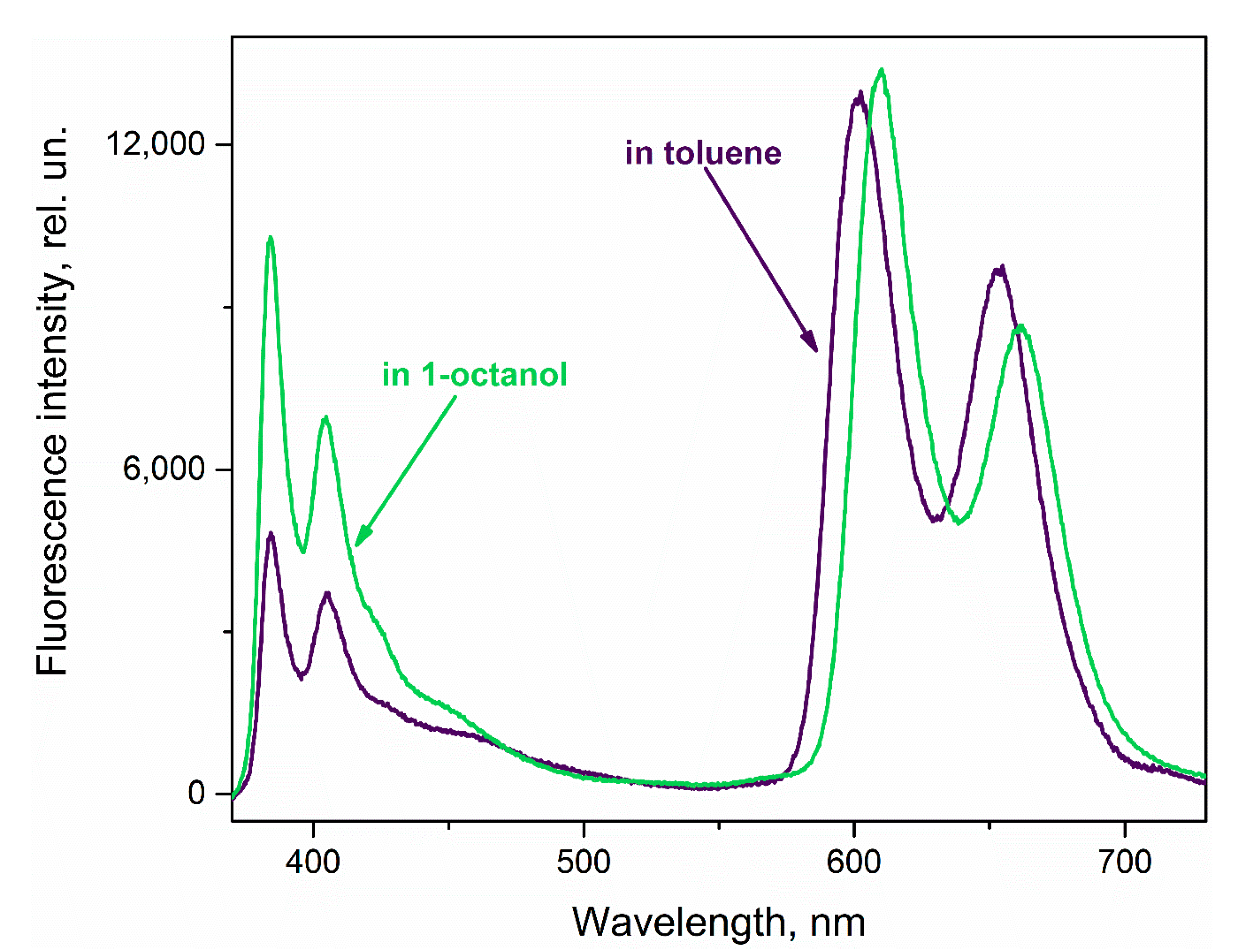
3.1. Effect of Nitrobenzene and Picric Acid on H2PIP
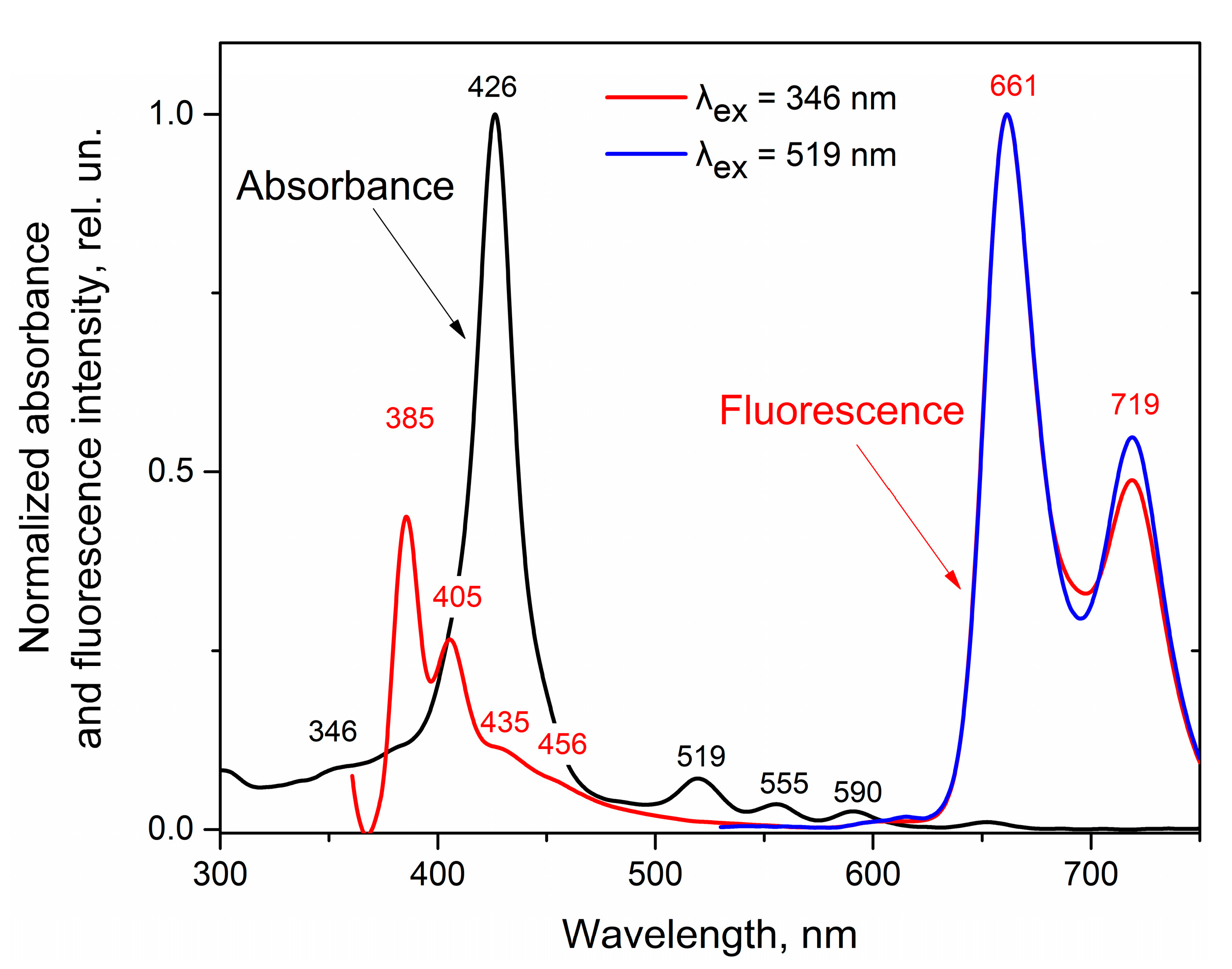
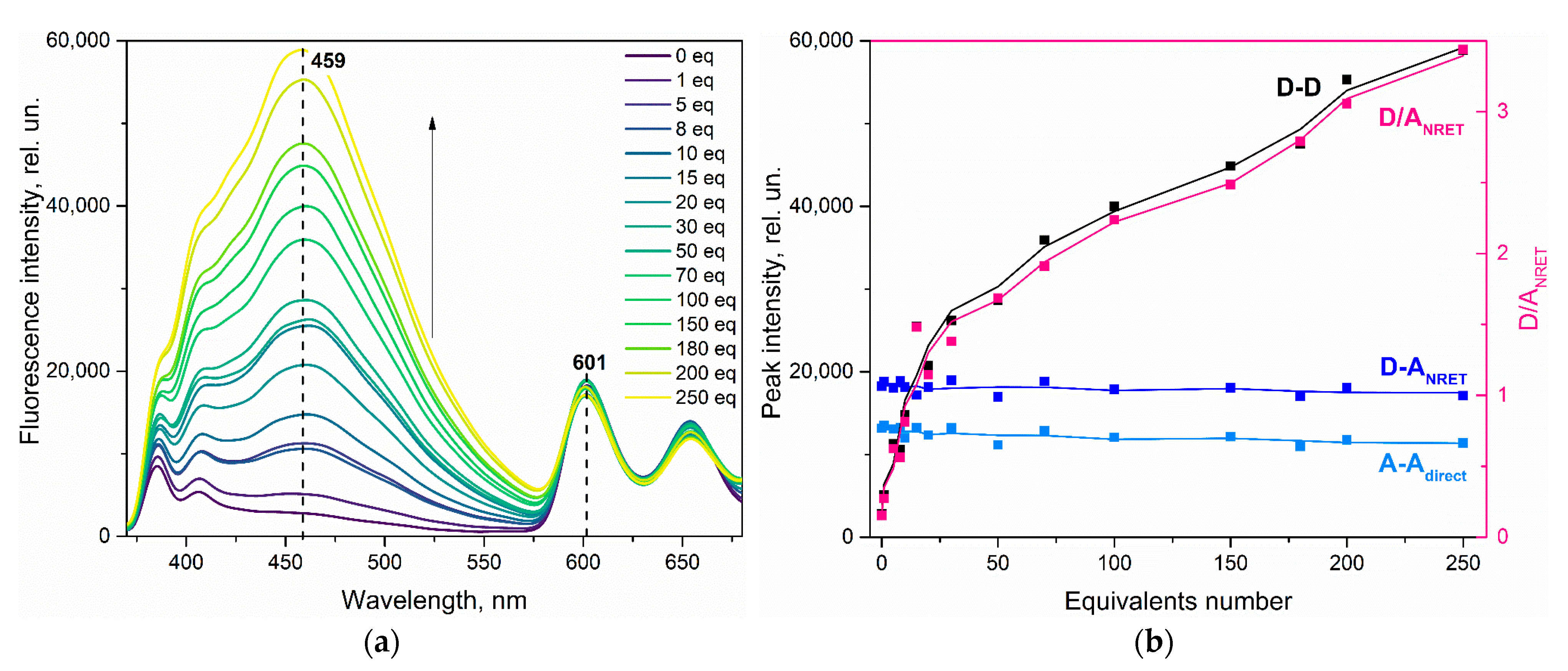
3.2. Effect of Nitrobenzene on ZnPIP
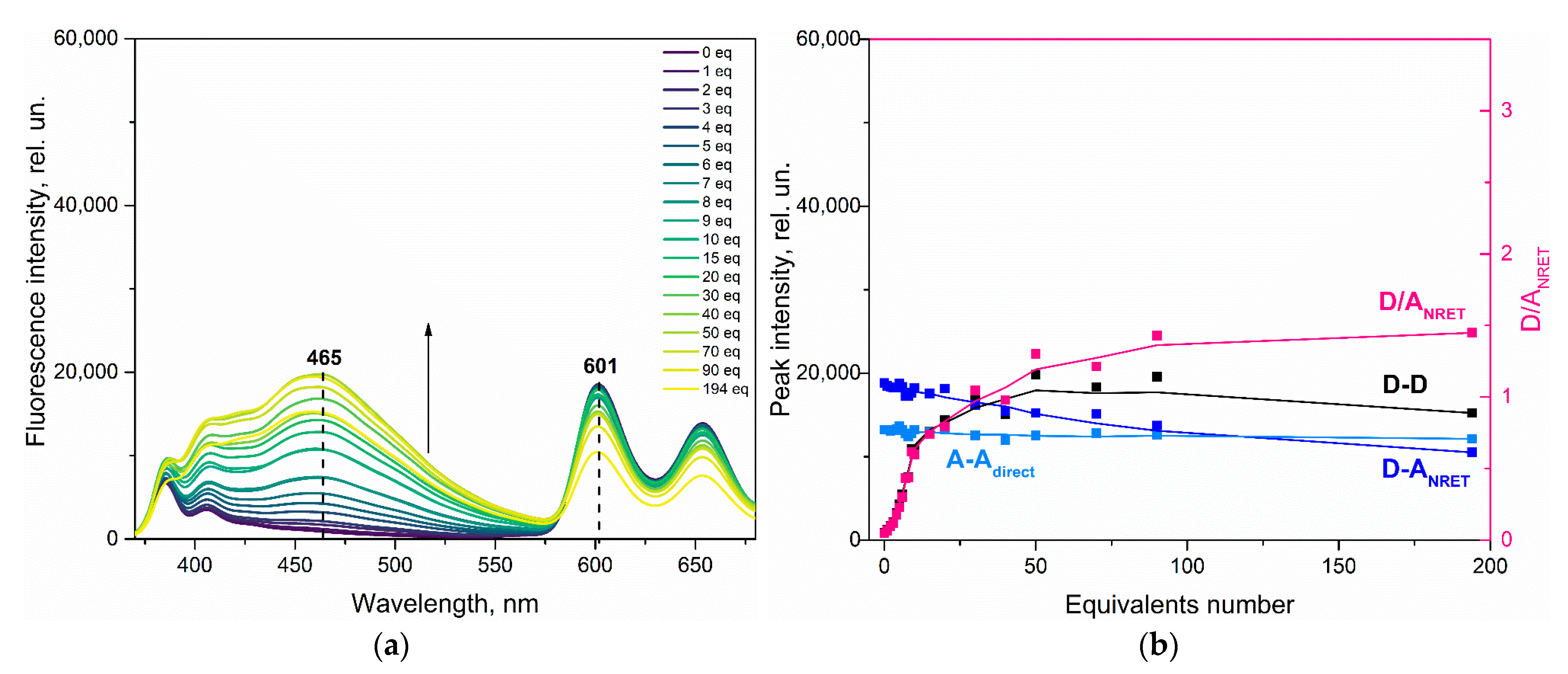
3.3. Effect of Picric Acid on ZnPIP
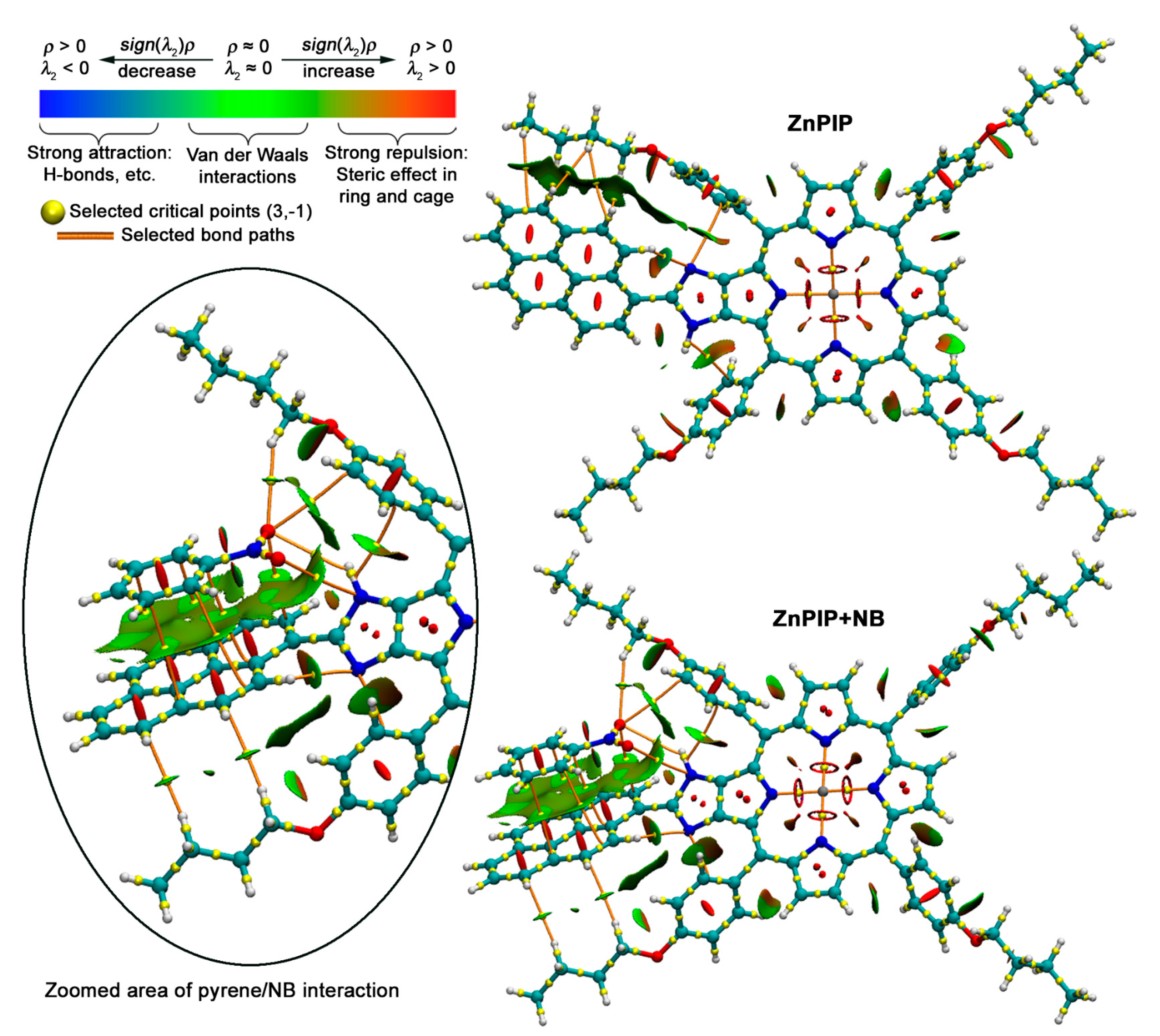
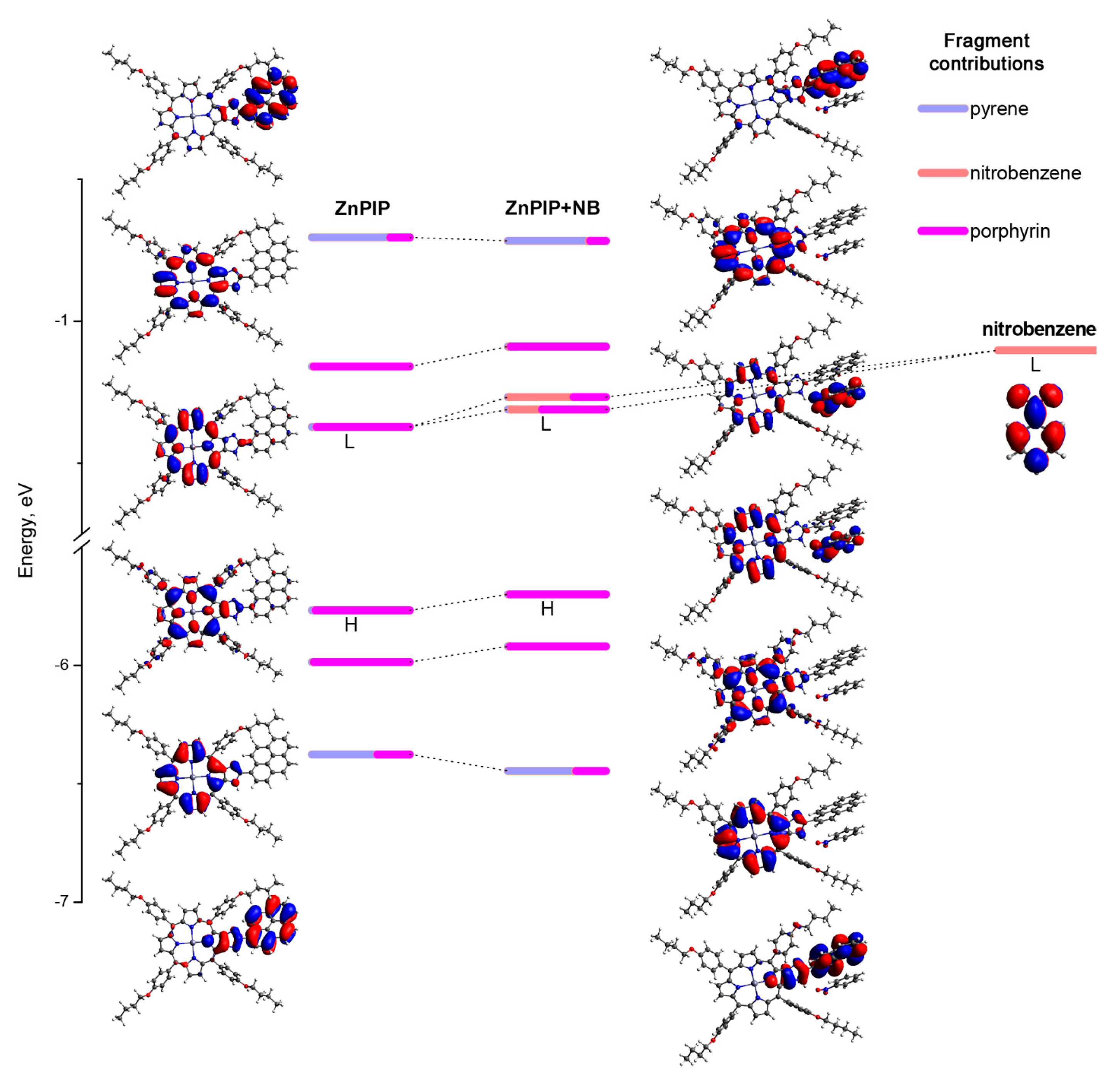
3.4. Possible Causes of the D Fluorescence Intensity Enhancement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kovacic, P.; Somanathan, R. Nitroaromatic Compounds: Environmental Toxicity, Carcinogenicity, Mutagenicity, Therapy and Mechanism. J. Appl. Toxicol. 2014, 34, 810–824. [Google Scholar] [CrossRef] [PubMed]
- Ju, K.-S.; Parales, R.E. Nitroaromatic Compounds, from Synthesis to Biodegradation. Microbiol. Mol. Biol. Rev. 2010, 74, 250–272. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, J. The Chemistry of Explosives; Royal Society of Chemistry: London, UK, 2011; p. 4. ISBN 978-1-84973-330-4. [Google Scholar]
- Spain, J.C. Biodegradation of Nitroaromatic Compounds. Annu. Rev. Microbiol. 1995, 49, 523–555. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, M.; Chaudhari, A. Microbial Remediation of Nitro-Aromatic Compounds: An Overview. J. Environ. Manag. 2007, 85, 496–512. [Google Scholar] [CrossRef]
- Boelsterli, U.; Ho, H.; Zhou, S.; Yeow Leow, K. Bioactivation and Hepatotoxicity of Nitroaromatic Drugs. Curr. Drug Metab. 2006, 7, 715–727. [Google Scholar] [CrossRef] [PubMed]
- Miliukiene, V.; Čenas, N. Cytotoxicity of Nitroaromatic Explosives and Their Biodegradation Products in Mice Splenocytes: Implications for Their Immunotoxicity. Z. Fur Nat. Sect. C J. Biosci. 2008, 63, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Zyryanov, G.V.; Kopchuk, D.S.; Kovalev, I.S.; Nosova, E.V.; Rusinov, V.L.; Chupakhin, O.N. Chemosensors for Detection of Nitroaromatic Compounds (Explosives). Russ. Chem. Rev. 2014, 83, 783. [Google Scholar] [CrossRef]
- Giannoukos, S.; Brkić, B.; Taylor, S.; Marshall, A.; Verbeck, G.F. Chemical Sniffing Instrumentation for Security Applications. Chem. Rev. 2016, 116, 8146–8172. [Google Scholar] [CrossRef]
- Akhgari, F.; Fattahi, H.; Oskoei, Y.M. Recent Advances in Nanomaterial-Based Sensors for Detection of Trace Nitroaromatic Explosives. Sens. Actuators B Chem. 2015, 221, 867–878. [Google Scholar] [CrossRef]
- Sylvia, J.M.; Janni, J.A.; Klein, J.D.; Spencer, K.M. Surface-Enhanced Raman Detection of 2,4-Dinitrotoluene Impurity Vapor as a Marker to Locate Landmines. Anal. Chem. 2000, 72, 5834–5840. [Google Scholar] [CrossRef]
- Stahl, D.C.; Tilotta, D.C. Screening Method for Nitroaromatic Compounds in Water Based on Solid-Phase Microextraction and Infrared Spectroscopy. Environ. Sci. Technol. 2001, 35, 3507–3512. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.E. Determination of Nitroaromatic, Nitramine, and Nitrate Ester Explosives in Soil by Gas Chromatography and an Electron Capture Detector. Talanta 2001, 54, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.S. Instrumentation for Trace Detection of High Explosives. Rev. Sci. Instrum. 2004, 75, 2499–2512. [Google Scholar] [CrossRef]
- Wong, M.H.; Giraldo, J.P.; Kwak, S.-Y.; Koman, V.B.; Sinclair, R.; Lew, T.T.S.; Bisker, G.; Liu, P.; Strano, M.S. Nitroaromatic Detection and Infrared Communication from Wild-Type Plants Using Plant Nanobionics. Nat. Mater. 2016, 16, 264–272. [Google Scholar] [CrossRef]
- Verbitskiy, E.V.; Rusinov, G.L.; Chupakhin, O.N.; Charushin, V.N. Design of Fluorescent Sensors Based on Azaheterocyclic Push-Pull Systems towards Nitroaromatic Explosives and Related Compounds: A Review. Dye. Pigment. 2020, 180, 108414. [Google Scholar] [CrossRef]
- Salinas, Y.; Mañez, R.M.; Marcos, M.D.; Sancenón, F.; Costero, A.M.; Parra, M.; Gil, S. Optical Chemosensors and Reagents to Detect Explosives. Chem. Soc. Rev. 2012, 41, 1261–1296. [Google Scholar] [CrossRef]
- Vora, M.; Panchal, M.; Dey, S.; Pandya, A.; Athar, M.; Verma, N.; Irfan, A.; Jain, V.K. Oxacalix[4]Arene Based Dual-Signalling Fluorimetric and Electrochemical Chemosensor for the Selective Detection of Nitroaromatic Compounds. J. Mol. Liq. 2022, 362, 119791. [Google Scholar] [CrossRef]
- Chhatwal, M.; Mittal, R.; Gupta, R.D.; Awasthi, S.K. Sensing Ensembles for Nitroaromatics. J. Mater. Chem. C 2018, 6, 12142–12158. [Google Scholar] [CrossRef]
- Brzechwa-Chodzyńska, A.; Drożdż, W.; Harrowfield, J.; Stefankiewicz, A.R. Fluorescent Sensors: A Bright Future for Cages. Coord. Chem. Rev. 2021, 434, 213820. [Google Scholar] [CrossRef]
- Kalva, N.; Tran, C.H.; Lee, M.W.; Augustine, R.; Lee, S.J.; Kim, I. Aggregation-Induced Emission-Active Hyperbranched Polymers Conjugated with Tetraphenylethylene for Nitroaromatic Explosive Detection. Dye. Pigment. 2021, 194, 109617. [Google Scholar] [CrossRef]
- Martínez-Máñez, R.; Sancenón, F. Fluorogenic and Chromogenic Chemosensors and Reagents for Anions. Chem. Rev. 2003, 103, 4419–4476. [Google Scholar] [CrossRef] [PubMed]
- Akkoc, E.; Karagoz, B. One Step Synthesis of Crosslinked Fluorescent Microspheres for the Effective and Selective Sensing of Explosives in Aqueous Media. Eur. Polym. J. 2022, 172, 111238. [Google Scholar] [CrossRef]
- Mauricio, F.G.M.; Silva, J.Y.R.; Talhavini, M.; Júnior, S.A.; Weber, I.T. Luminescent Sensors for Nitroaromatic Compound Detection: Investigation of Mechanism and Evaluation of Suitability of Using in Screening Test in Forensics. Microchem. J. 2019, 150, 104037. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer: Berlin/Heidelberg, Germany, 2006; pp. 1–954. [Google Scholar] [CrossRef]
- Gogoi, B.; Sarma, N. Sen Curcumin–Cysteine and Curcumin–Tryptophan Conjugate as Fluorescence Turn On Sensors for Picric Acid in Aqueous Media. ACS Appl. Mater. Interfaces 2015, 7, 11195–11202. [Google Scholar] [CrossRef] [PubMed]
- Sivaraman, G.; Vidya, B.; Chellappa, D. Rhodamine Based Selective Turn-on Sensing of Picric Acid. RSC Adv. 2014, 4, 30828–30831. [Google Scholar] [CrossRef]
- Lee, J.Y.; Root, H.D.; Ali, R.; An, W.; Lynch, V.M.; Bähring, S.; Kim, I.S.; Sessler, J.L.; Park, J.S. Ratiometric Turn-On Fluorophore Displacement Ensembles for Nitroaromatic Explosives Detection. J. Am. Chem. Soc. 2020, 142, 19579–19587. [Google Scholar] [CrossRef]
- Lin, Q.; Guan, X.W.; Fan, Y.Q.; Wang, J.; Liu, L.; Liu, J.; Yao, H.; Zhang, Y.M.; Wei, T.B. A Tripodal Supramolecular Sensor to Successively Detect Picric Acid and CN− through Guest Competitive Controlled AIE. New J. Chem. 2019, 43, 2030–2036. [Google Scholar] [CrossRef]
- Xu, Y.; Li, B.; Li, W.; Zhao, J.; Sun, S.; Pang, Y. “ICT-Not-Quenching„ near Infrared Ratiometric Fluorescent Detection of Picric Acid in Aqueous Media. Chem. Commun. 2013, 49, 4764–4766. [Google Scholar] [CrossRef]
- Li, L.; Cheng, J.; Liu, Z.; Song, L.; You, Y.; Zhou, X.; Huang, W. Ratiometric Luminescent Sensor of Picric Acid Based on the Dual-Emission Mixed-Lanthanide Coordination Polymer. ACS Appl. Mater. Interfaces 2018, 10, 44109–44115. [Google Scholar] [CrossRef]
- Taya, P.; Maiti, B.; Kumar, V.; De, P.; Satapathi, S. Design of a Novel FRET Based Fluorescent Chemosensor and Their Application for Highly Sensitive Detection of Nitroaromatics. Sens. Actuators B Chem. 2018, 255, 2628–2634. [Google Scholar] [CrossRef]
- Chen, X.; Ma, Y.; Chen, H.; Wang, F.; Kambam, S.; Wang, Y.; Mao, C. A Highly Sensitive and Selective Ratiometric Fluorescent Sensor for Zn2+ Ion Based on ICT and FRET. Dye. Pigment. 2014, 102, 301–307. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, M.Y.; Zheng, M.H.; Zhao, X.L.; Xie, Y.Z.; Jin, J.Y. 2-Pyridylthiazole Derivative as ICT-Based Ratiometric Fluorescent Sensor for Fe(III). Tetrahedron Lett. 2016, 57, 2399–2402. [Google Scholar] [CrossRef]
- Deore, P.S.; Coman, D.S.; Manderville, R.A. A Coumarin–Hemicyanine Hybrid as a Ratiometric Fluorescent Sensor of Microenvironment Proticity. Chem. Commun. 2019, 55, 3540–3543. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, H.; Li, F.; Chen, Y.; Kwok, R.T.K.; Huang, Y.; Zhang, J.; Hou, J.; Tang, B.Z. A Ratiometric Fluorescent Probe Based on AIEgen for Detecting HClO in Living Cells. Chem. Commun. 2020, 56, 14613–14616. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, D.; Peng, J.; Du, Q.; He, H. Ratiometric Fluorescence Sensing of Metal-Organic Frameworks: Tactics and Perspectives. Coord. Chem. Rev. 2020, 404, 213113. [Google Scholar] [CrossRef]
- Dhiman, S.; Ahmad, M.; Kumar, G.; Luxami, V.; Singh, P.; Kumar, S. Ratiometric Chemosensor for Differentiation of TNP from Other NACs Using Distinct Blue Fluorescence and Visualization of Latent Fingerprints. J. Mater. Chem. C 2021, 9, 1097–1106. [Google Scholar] [CrossRef]
- He, L.; Dong, B.; Liu, Y.; Lin, W. Fluorescent Chemosensors Manipulated by Dual/Triple Interplaying Sensing Mechanisms. Chem. Soc. Rev. 2016, 45, 6449–6461. [Google Scholar] [CrossRef]
- Kowerko, D.; Schuster, J.; Amecke, N.; Abdel-Mottaleb, M.; Dobrawa, R.; Würthner, F.; Von Borczyskowski, C. FRET and Ligand Related NON-FRET Processes in Single Quantum Dot-Perylene Bisimide Assemblies. Phys. Chem. Chem. Phys. 2010, 12, 4112–4123. [Google Scholar] [CrossRef]
- Shchepinov, M.S.; Korshun, V.A.; Egeland, R.D.; Southern, E.M. Tritylisation of Pyrene, Perylene and Coronene: A New Family of Switchable Fluorescent Labels. Tetrahedron Lett. 2000, 41, 4943–4948. [Google Scholar] [CrossRef]
- Hong, Y.; Kim, J.; Kim, W.; Kaufmann, C.; Kim, H.; Würthner, F.; Kim, D. Efficient Multiexciton State Generation in Charge-Transfer-Coupled Perylene Bisimide Dimers via Structural Control. J. Am. Chem. Soc. 2020, 142, 7845–7857. [Google Scholar] [CrossRef]
- Karuppannan, S.; Chambron, J.-C. Supramolecular Chemical Sensors Based on Pyrene Monomer–Excimer Dual Luminescence. Chem. Asian J. 2011, 6, 964–984. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, N.; Zhou, J.; Chang, T.; Fang, C.; Shangguan, D. A Turn-on Fluorescent Sensor for Zinc and Cadmium Ions Based on Perylene Tetracarboxylic Diimide. Analyst 2013, 138, 901–906. [Google Scholar] [CrossRef] [PubMed]
- Bhatta, S.R.; Mondal, B.; Vijaykumar, G.; Thakur, A. ICT–Isomerization-Induced Turn-On Fluorescence Probe with a Large Emission Shift for Mercury Ion: Application in Combinational Molecular Logic. Inorg. Chem. 2017, 56, 11577–11590. [Google Scholar] [CrossRef] [PubMed]
- Kathiravan, A.; Gowri, A.; Khamrang, T.; Kumar, M.D.; Dhenadhayalan, N.; Lin, K.-C.; Velusamy, M.; Jaccob, M. Pyrene-Based Chemosensor for Picric Acid—Fundamentals to Smartphone Device Design. Anal. Chem. 2019, 91, 13244–13250. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.L.; Kumar, A.M.; Dhir, A.; Krishnan, V. Selective and Sensitive Fluorescent Detection of Picric Acid by New Pyrene and Anthracene Based Copper Complexes. J. Fluoresc. 2016, 26, 2041–2046. [Google Scholar] [CrossRef]
- Rajasekaran, P.; Kannan, H.; Das, S.; Young, M.; Santra, S. Comparative Analysis of Copper and Zinc Based Agrichemical Biocide Products: Materials Characteristics, Phytotoxicity and in Vitro Antimicrobial Efficacy. AIMS Environ. Sci. 2016, 3, 439–455. [Google Scholar] [CrossRef]
- Sun, X.; Wang, Y.; Lei, Y. Fluorescence Based Explosive Detection: From Mechanisms to Sensory Materials. Chem. Soc. Rev. 2015, 44, 8019–8061. [Google Scholar] [CrossRef]
- Shanmugaraju, S.; Mukherjee, P.S. π-Electron Rich Small Molecule Sensors for the Recognition of Nitroaromatics. Chem. Commun. 2015, 51, 16014–16032. [Google Scholar] [CrossRef]
- Jeyasingh, V.; Murugesan, K.; Lakshminarayanan, S.; Selvapalam, N.; Das, G.; Piramuthu, L. Pyrene-Hydrazone-Π···Hole Coupled Turn-on Fluorescent and Naked- Eye Colorimetric Sensor for Cyanide: Role of Homogeneous π-Hole Dispersion in Anion Selectivity. J. Fluoresc. 2021, 31, 1303–1309. [Google Scholar] [CrossRef]
- Kowser, Z.; Jin, C.C.; Jiang, X.; Rahman, S.; Georghiou, P.E.; Ni, X.L.; Zeng, X.; Redshaw, C.; Yamato, T. Fluorescent Turn-on Sensors Based on Pyrene-Containing Schiff Base Derivatives for Cu2+ Recognition: Spectroscopic and DFT Computational Studies. Tetrahedron 2016, 72, 4575–4581. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Sheng, M.; Kang, Y.; Jia, B.; Li, W.; Fu, Y. A Novel Pyrene-Based Fluorescent Probe for Al3+ Detection. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 287, 122085. [Google Scholar] [CrossRef]
- Xu, Z.; Singh, N.J.; Lim, J.; Pan, J.; Kim, H.N.; Park, S.; Kim, K.S.; Yoon, J. Unique Sandwich Stacking of Pyrene-Adenine-Pyrene for Selective and Ratiometric Fluorescent Sensing of ATP at Physiological pH. J. Am. Chem. Soc. 2009, 131, 15528–15533. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.A.; Lee, J.; Lee, M.H. A Ratiometric Fluorescent Probe for Zn2+ Based on Pyrene-Appended Naphthalimide-Dipicolylamine. Sens. Actuators B Chem. 2018, 258, 50–55. [Google Scholar] [CrossRef]
- Hou, M.; Fan, L.; Fan, X.; Liang, X.; Zhang, W.; Ding, Y. Pyrene-Porphyrin Based Ratiometric Fluorescent Sensor Array for Discrimination of Glycosaminoglycans. Anal. Chim. Acta 2021, 1141, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Liu, W.; Zhuang, X.; Wu, J.; Zhang, H.; Wang, P. Ratiometric Fluorescence Sensor Based on a Pyrene Derivative and Quantification Detection of Heparin in Aqueous Solution and Serum. Anal. Chem. 2011, 83, 6559–6564. [Google Scholar] [CrossRef] [PubMed]
- Birin, K.P.; Abdulaeva, I.A.; Polivanovskaia, D.A.; Martynov, A.G.; Shokurov, A.V.; Gorbunova, Y.G.; Tsivadze, A.Y. Imidazoporphyrins with Appended Polycyclic Aromatic Hydrocarbons: To Conjugate or Not to Conjugate? Dye. Pigment. 2021, 186, 109042. [Google Scholar] [CrossRef]
- Zhao, M.; Deng, Z.; Tang, J.; Zhou, X.; Chen, Z.; Li, X.; Yang, L.; Ma, L.-J. 2-(1-Pyrenyl) Benzimidazole as a Ratiometric and “Turn-on” Fluorescent Probe for Iron(III) Ions in Aqueous Solution. Analyst 2016, 141, 2308–2312. [Google Scholar] [CrossRef]
- Armarego, W.L. Purification of Laboratory Chemicals; Butterworth-Heinemann: Oxford, UK, 2017; ISBN 0-12-805456-5. [Google Scholar]
- Brynn Hibbert, D.; Thordarson, P. The Death of the Job Plot, Transparency, Open Science and Online Tools, Uncertainty Estimation Methods and Other Developments in Supramolecular Chemistry Data Analysis. Chem. Commun. 2016, 52, 12792–12805. [Google Scholar] [CrossRef]
- Neese, F. Software Update: The ORCA Program System—Version 5.0. WIREs Comput. Mol. Sci. 2022, 12, e1606. [Google Scholar] [CrossRef]
- Grimme, S.; Hansen, A.; Ehlert, S.; Mewes, J.-M. R2SCAN-3c: A “Swiss Army Knife” Composite Electronic-Structure Method. J. Chem. Phys. 2021, 154, 064103. [Google Scholar] [CrossRef]
- Takano, Y.; Houk, K.N. Benchmarking the Conductor-like Polarizable Continuum Model (CPCM) for Aqueous Solvation Free Energies of Neutral and Ionic Organic Molecules. J. Chem. Theory Comput. 2005, 1, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Allouche, A.-R. Gabedit—A Graphical User Interface for Computational Chemistry Softwares. J. Comput. Chem. 2011, 32, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Yanai, T.; Tew, D.P.; Handy, N.C. A New Hybrid Exchange–Correlation Functional Using the Coulomb-Attenuating Method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef]
- Rassolov, V.A.; Pople, J.A.; Ratner, M.A.; Windus, T.L. 6-31G* Basis Set for Atoms K through Zn. J. Chem. Phys. 1998, 109, 1223–1229. [Google Scholar] [CrossRef]
- Marsh, D.F.; Mink, L.M. Microscale Synthesis and Electronic Absorption Spectroscopy of Tetraphenylporphyrin H2(TPP) and Metalloporphyrins ZnII(TPP) and NiII(TPP). J. Chem. Educ. 1996, 73, 1188–1190. [Google Scholar] [CrossRef]
- Lopes, J.M.S.; Sampaio, R.N.; Ito, A.S.; Batista, A.A.; Machado, A.E.H.; Araujo, P.T.; Neto, N.M.B. Evolution of Electronic and Vibronic Transitions in Metal(II) Meso-Tetra(4-Pyridyl)Porphyrins. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 215, 327–333. [Google Scholar] [CrossRef]
- Edwards, L.; Dolphin, D.H.; Gouterman, M.; Adler, A.D. Porphyrins XVII. Vapor Absorption Spectra and Redox Reactions: Tetraphenylporphins and Porphin. J. Mol. Spectrosc. 1971, 38, 16–32. [Google Scholar] [CrossRef]
- Khasanov, A.F.; Kopchuk, D.S.; Kovalev, I.S.; Taniya, O.S.; Giri, K.; Slepukhin, P.A.; Santra, S.; Rahman, M.; Majee, A.; Charushin, V.N.; et al. Extended Cavity Pyrene-Based Iptycenes for the Turn-off Fluorescence Detection of RDX and Common Nitroaromatic Explosives. New J. Chem. 2017, 41, 2309–2320. [Google Scholar] [CrossRef]
- Mondal, T.; Mondal, I.; Biswas, S.; Mane, M.V.; Panja, S.S. Mechanistic Insight into Selective Sensing of Hazardous Hg2+ and Explosive Picric Acid by Using a Pyrene-Azine-Hydroxyquinoline Framework in Differential Media. ChemistrySelect 2020, 5, 9336–9349. [Google Scholar] [CrossRef]
- Santra, D.C.; Bera, M.K.; Sukul, P.K.; Malik, S. Charge-Transfer-Induced Fluorescence Quenching of Anthracene Derivatives and Selective Detection of Picric Acid. Chem. A Eur. J. 2016, 22, 2012–2019. [Google Scholar] [CrossRef]
- Deshmukh, S.C.; Rana, S.; Shinde, S.V.; Dhara, B.; Ballav, N.; Talukdar, P. Selective Sensing of Metal Ions and Nitro Explosives by Efficient Switching of Excimer-to-Monomer Emission of an Amphiphilic Pyrene Derivative. ACS Omega 2016, 1, 371–377. [Google Scholar] [CrossRef]
- Gole, B.; Bar, A.K.; Mukherjee, P.S. Fluorescent Metal–Organic Framework for Selective Sensing of Nitroaromatic Explosives. Chem. Commun. 2011, 47, 12137–12139. [Google Scholar] [CrossRef]
- Ma, Y.; Li, H.; Peng, S.; Wang, L. Highly Selective and Sensitive Fluorescent Paper Sensor for Nitroaromatic Explosive Detection. Anal. Chem. 2012, 84, 8415–8421. [Google Scholar] [CrossRef]
- Shanmugaraju, S.; Joshi, S.A.; Mukherjee, P.S. Fluorescence and Visual Sensing of Nitroaromatic Explosives Using Electron Rich Discrete Fluorophores. J. Mater. Chem. 2011, 21, 9130–9138. [Google Scholar] [CrossRef]
- Ni, R.; Tong, R.B.; Guo, C.C.; Shen, G.L.; Yu, R.Q. An Anthracene/Porphyrin Dimer Fluorescence Energy Transfer Sensing System for Picric Acid. Talanta 2004, 63, 251–257. [Google Scholar] [CrossRef]
- Gouterman, M. Optical Spectra and Electronic Structure of Porphyrins and Related Rings. In The Porphyrins; Dolphin, D., Ed.; Academic Press: Cambridge, MA, USA, 1978; pp. 1–165. ISBN 978-0-12-220103-5. [Google Scholar]
- Wang, C.; Wamser, C.C. Hyperporphyrin Effects in the Spectroscopy of Protonated Porphyrins with 4-Aminophenyl and 4-Pyridyl Meso Substituents. J. Phys. Chem. A 2014, 118, 3605–3615. [Google Scholar] [CrossRef]
- Wamser, C.C.; Ghosh, A. The Hyperporphyrin Concept: A Contemporary Perspective. JACS Au 2022, 2, 1543–1560. [Google Scholar] [CrossRef]
- Vyšniauskas, A.; Qurashi, M.; Gallop, N.; Balaz, M.; Anderson, H.L.; Kuimova, M.K. Unravelling the Effect of Temperature on Viscosity-Sensitive Fluorescent Molecular Rotors. Chem. Sci. 2015, 6, 5773–5778. [Google Scholar] [CrossRef]
- Miao, W.; Yu, C.; Hao, E.; Jiao, L. Functionalized BODIPYs as Fluorescent Molecular Rotors for Viscosity Detection. Front. Chem. 2019, 7, 825. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-C.; Heo, J.; Woo, H.C.; Lee, J.-A.; Seo, Y.H.; Lee, C.-L.; Kim, S.; Kwon, O.-P. Fluorescent Molecular Rotors for Viscosity Sensors. Chem. A Eur. J. 2018, 24, 13706–13718. [Google Scholar] [CrossRef] [PubMed]
- Hoche, J.; Schmitt, H.-C.; Humeniuk, A.; Fischer, I.; Mitrić, R.; Röhr, M.I.S. The Mechanism of Excimer Formation: An Experimental and Theoretical Study on the Pyrene Dimer. Phys. Chem. Chem. Phys. 2017, 19, 25002–25015. [Google Scholar] [CrossRef]
- Kawai, T.; Ikegami, M.; Arai, T. Exciplex Formation between Pyrene and Guanine in Highly Polar Solvents. Chem. Commun. 2004, 10, 824–825. [Google Scholar] [CrossRef] [PubMed]
- Hamada, F.; Narita, M.; Kinoshita, K.; Makabe, A.; Osa, T. Synthesis and Fluorescent Molecular Sensing at Exciplex Emission of Pyrene- and Cyanobenzene-Modified γ-Cyclodextrins. J. Chem. Soc. Perkin Trans. 2 2001, 44, 388–394. [Google Scholar] [CrossRef]
- Bertocchi, M.J.; Zhang, X.-F.; Bajpai, A.; Moorthy, J.N.; Weiss, R.G. Fluorescence Quenching of Sterically-Graded Pyrene Molecules by N,N-Dialkylanilines. Exciplexes or Locally Excited States? J. Photochem. Photobiol. A Chem. 2018, 355, 467–478. [Google Scholar] [CrossRef]
- Rasheed, T.; Nabeel, F.; Rizwan, K.; Bilal, M.; Hussain, T.; Shehzad, S.A. Conjugated Supramolecular Architectures as State-of-the-Art Materials in Detection and Remedial Measures of Nitro Based Compounds: A Review. TrAC Trends Anal. Chem. 2020, 129, 115958. [Google Scholar] [CrossRef]
- Lewis, F.D.; Daublain, P.; Delos Santos, G.B.; Liu, W.; Asatryan, A.M.; Markarian, S.A.; Fiebig, T.; Raytchev, M.; Wang, Q. Dynamics and Mechanism of Bridge-Dependent Charge Separation in Pyrenylurea−Nitrobenzene π-Stacked Protophanes. J. Am. Chem. Soc. 2006, 128, 4792–4801. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.R.; Keinan, S.; Mori-Sánchez, P.; Contreras-García, J.; Cohen, A.J.; Yang, W. Revealing Noncovalent Interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef]
- Thordarson, P. Determining Association Constants from Titration Experiments in Supramolecular Chemistry. Chem. Soc. Rev. 2011, 40, 1305–1323. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shepeleva, I.I.; Birin, K.P.; Polivanovskaia, D.A.; Martynov, A.G.; Shokurov, A.V.; Tsivadze, A.Y.; Selektor, S.L.; Gorbunova, Y.G. Unusual ‘Turn-on’ Ratiometric Response of Fluorescent Porphyrin-Pyrene Dyads to the Nitroaromatic Compounds. Chemosensors 2023, 11, 43. https://doi.org/10.3390/chemosensors11010043
Shepeleva II, Birin KP, Polivanovskaia DA, Martynov AG, Shokurov AV, Tsivadze AY, Selektor SL, Gorbunova YG. Unusual ‘Turn-on’ Ratiometric Response of Fluorescent Porphyrin-Pyrene Dyads to the Nitroaromatic Compounds. Chemosensors. 2023; 11(1):43. https://doi.org/10.3390/chemosensors11010043
Chicago/Turabian StyleShepeleva, Irina I., Kirill P. Birin, Daria A. Polivanovskaia, Alexander G. Martynov, Alexander V. Shokurov, Aslan Yu. Tsivadze, Sofiya L. Selektor, and Yulia G. Gorbunova. 2023. "Unusual ‘Turn-on’ Ratiometric Response of Fluorescent Porphyrin-Pyrene Dyads to the Nitroaromatic Compounds" Chemosensors 11, no. 1: 43. https://doi.org/10.3390/chemosensors11010043
APA StyleShepeleva, I. I., Birin, K. P., Polivanovskaia, D. A., Martynov, A. G., Shokurov, A. V., Tsivadze, A. Y., Selektor, S. L., & Gorbunova, Y. G. (2023). Unusual ‘Turn-on’ Ratiometric Response of Fluorescent Porphyrin-Pyrene Dyads to the Nitroaromatic Compounds. Chemosensors, 11(1), 43. https://doi.org/10.3390/chemosensors11010043