Energy-Efficient Chemiresistive Sensor Array Based on SWCNT Networks, WO3 Nanochannels and SWCNT-Pt Heterojunctions for NH3 Detection against the Background Humidity
Abstract
1. Introduction
2. Materials and Methods
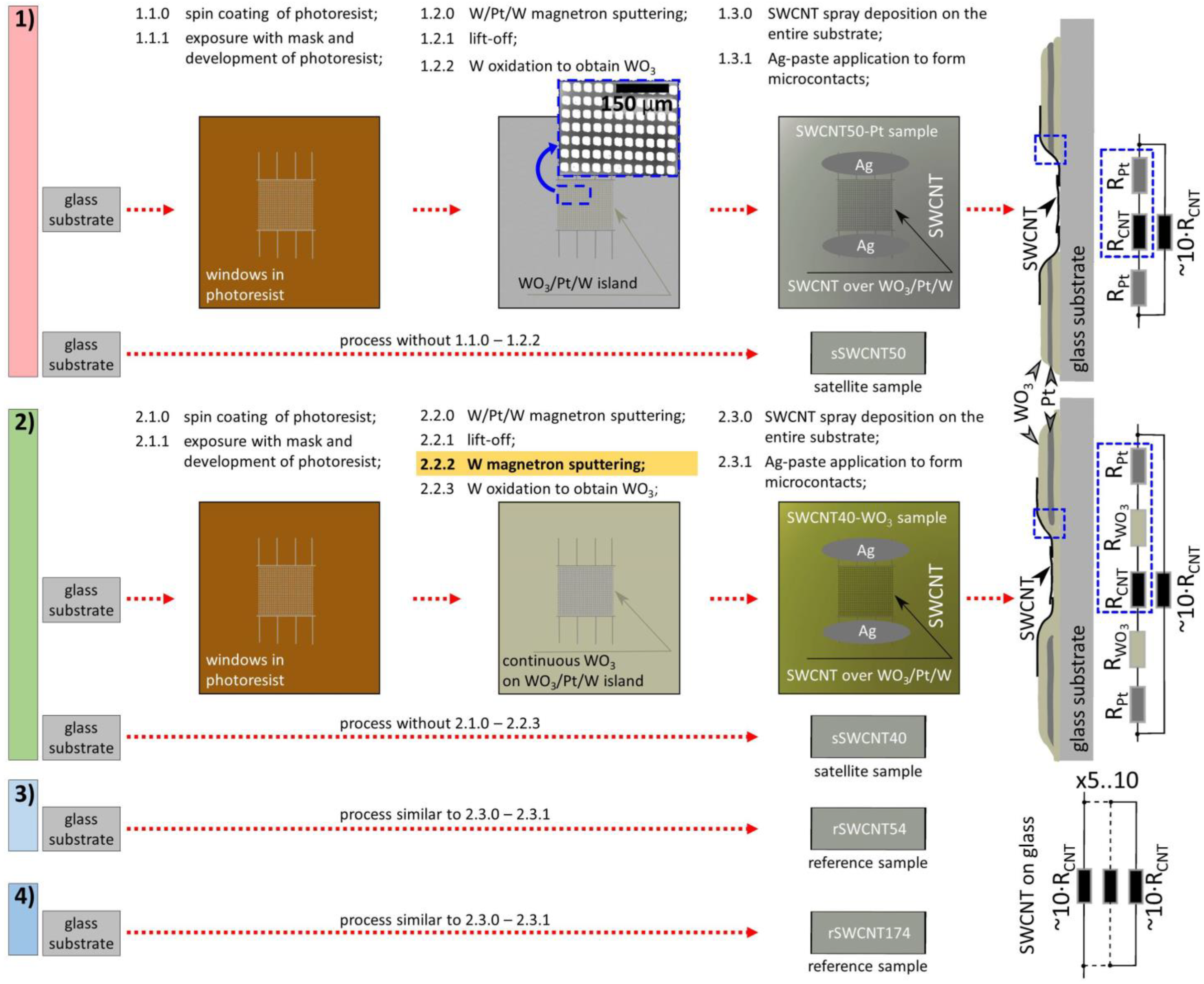
2.1. Fabrication of Structures under Study
2.2. Material Characterization Methods
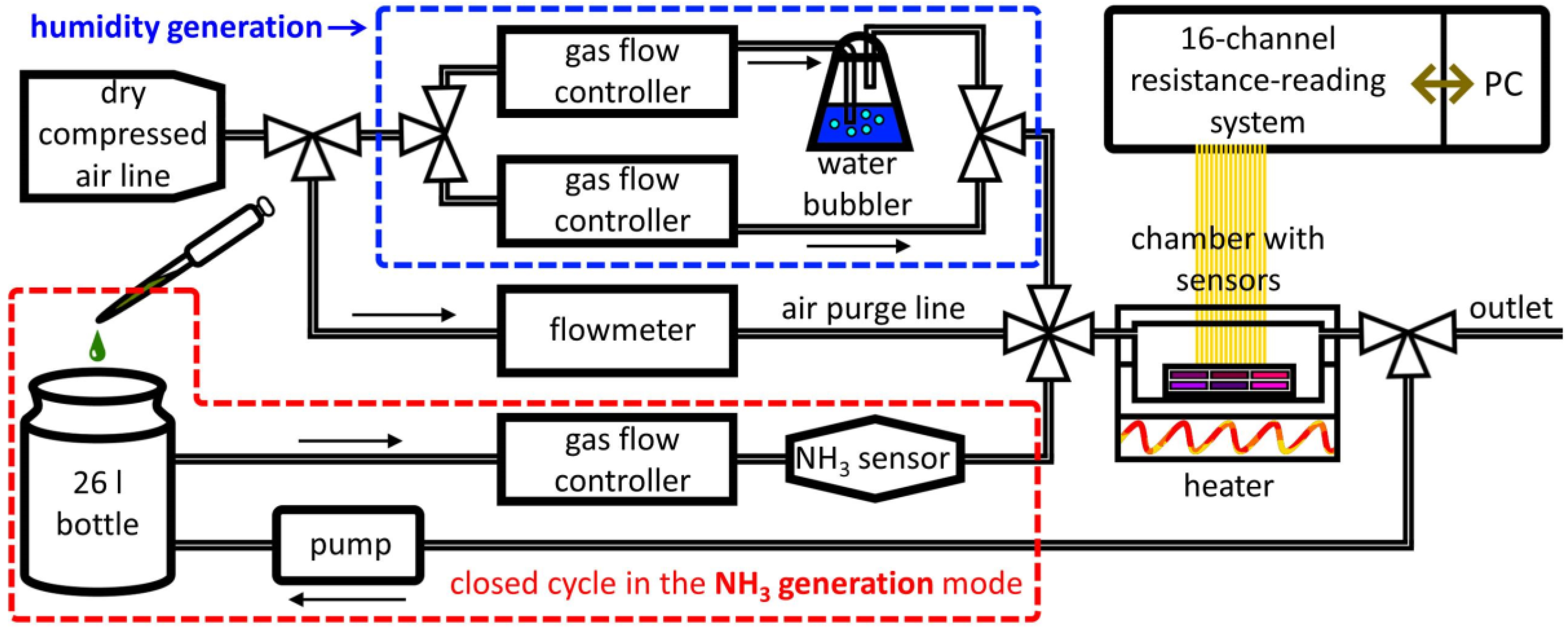
2.3. Gas-Sensing Measurements
2.4. Processing of Sensor Responses
3. Results and Discussion
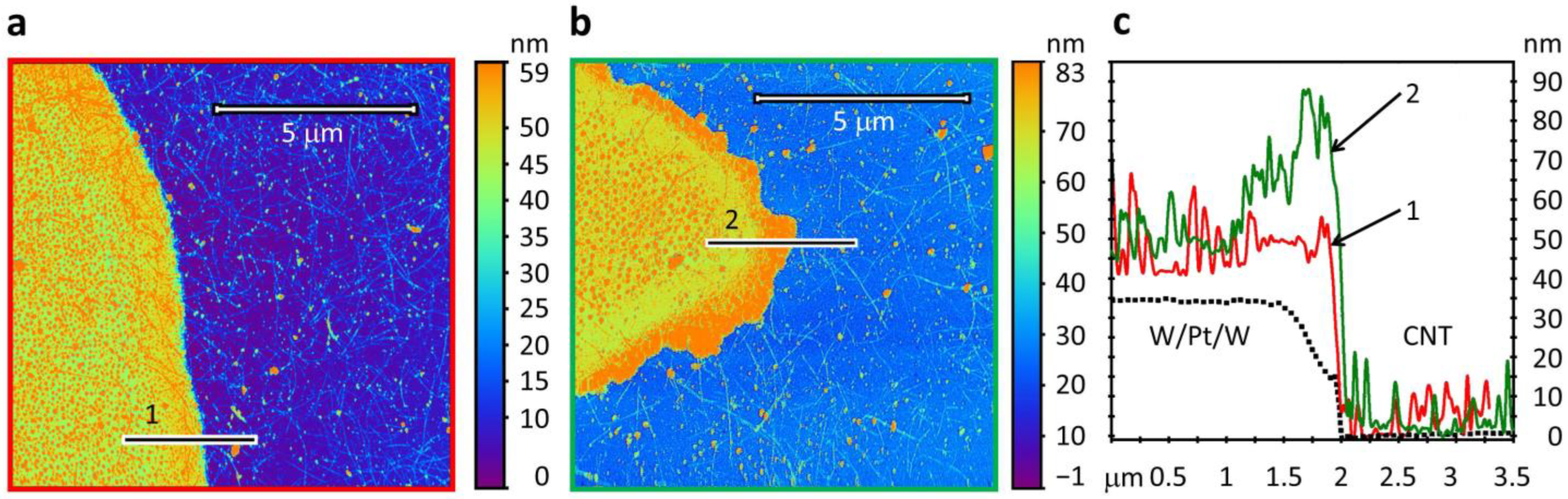
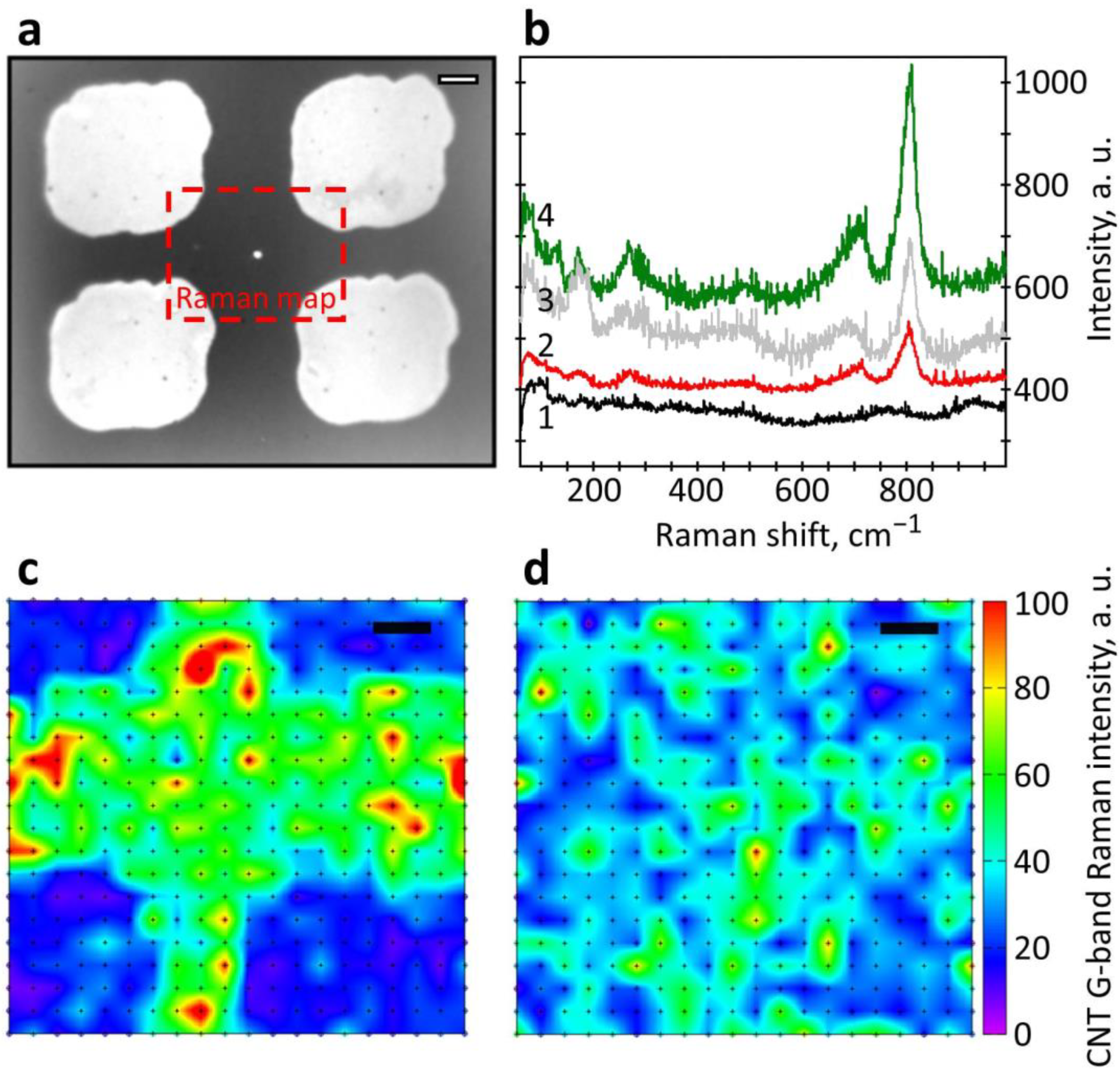
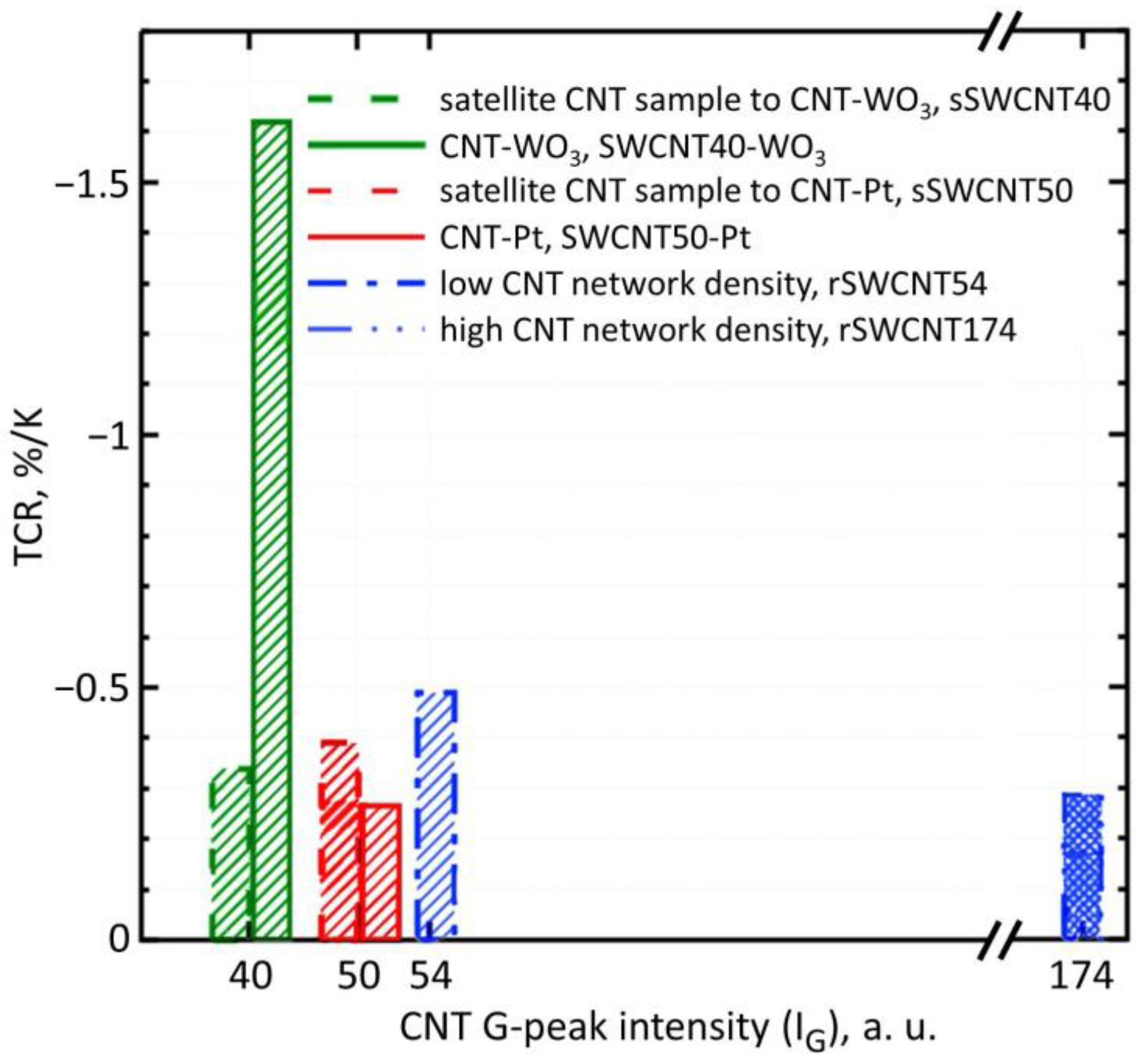
3.1. Characterization of the Sensor Structures Layers Parameters
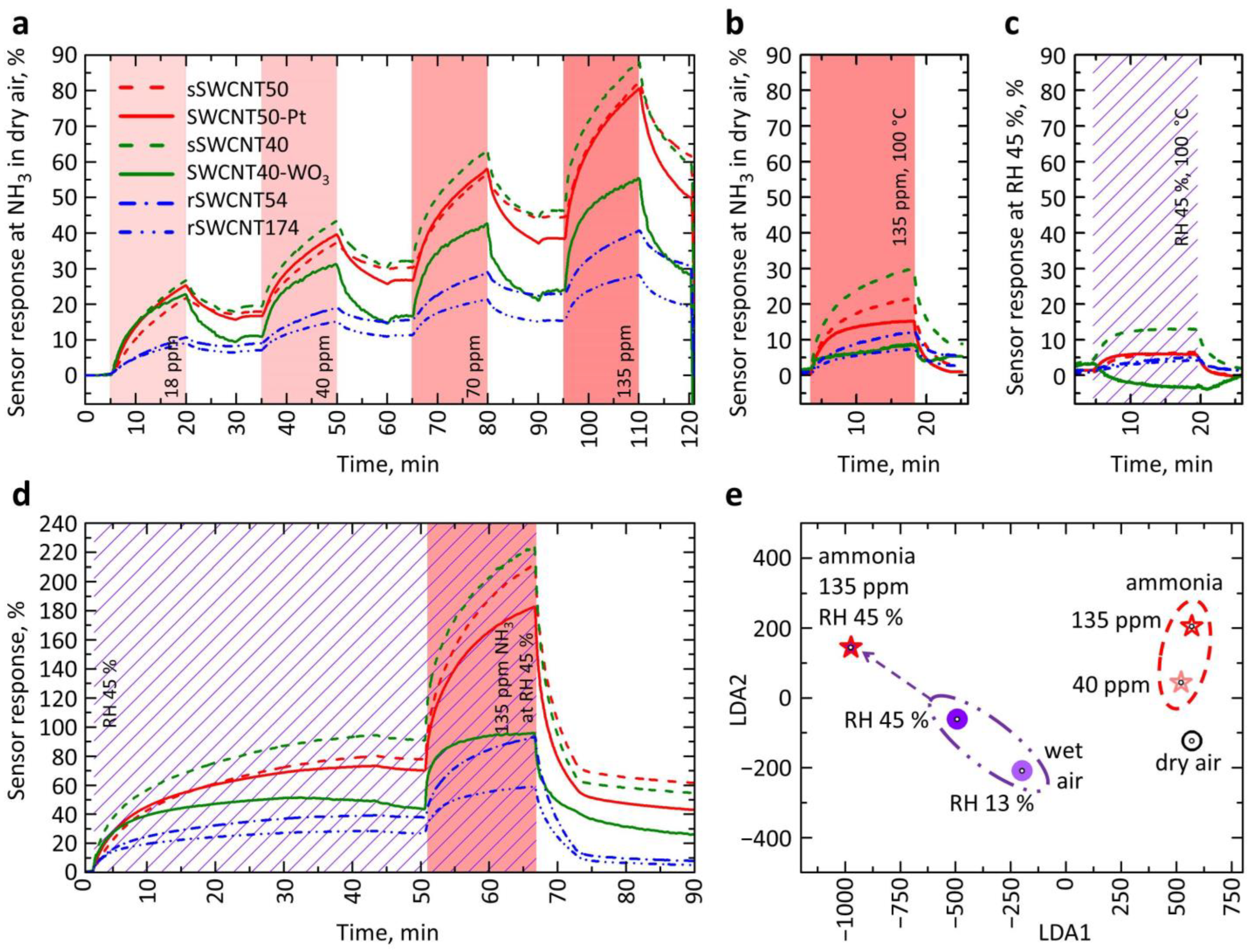
3.2. The Characterization of Gas-Sensing Performance of the Sensor Structures
3.3. The Analysis of the Gas-Selectivity of Manufactured Multisensor Array
3.4. Evaluation of Sensor Detection Limits
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Timmer, B.; Olthuis, W.; Van Den Berg, A. Ammonia sensors and their applications—A review. Sens. Actuators B Chem. 2005, 107, 666–677. [Google Scholar] [CrossRef]
- Kwak, D.; Lei, Y.; Maric, R. Ammonia gas sensors: A comprehensive review. Talanta 2019, 204, 713–730. [Google Scholar] [CrossRef] [PubMed]
- Goswami, P.; Gupta, G. Recent progress of flexible NO2 and NH3 gas sensors based on transition metal dichalcogenides for room temperature sensing. Mater. Today Chem. 2022, 23, 100726. [Google Scholar] [CrossRef]
- Kalita, A.; Hussain, S.; Malik, A.H.; Subbarao, N.V.V.; Iyer, P.K. Vapor phase sensing of ammonia at the sub-ppm level using a perylene diimide thin film device. J. Mater. Chem. C 2015, 3, 10767–10774. [Google Scholar] [CrossRef]
- Targowski, S.P.; Klucinski, W.; Babiker, S.; Nonnecke, B.J. Effect of ammonia on in vivo and in vitro immune responses. Infect. Immun. 1984, 43, 289–293. [Google Scholar] [CrossRef]
- Devos, M.; Patte, F.; Rouault, J.; Laffort, P.; Van Gemert, L.J. Standardized Human Olfactory Thresholds; Oxford University Press: Oxford, UK, 1990. [Google Scholar]
- Petrus, M.; Popa, C.; Bratu, A.M. Ammonia concentration in ambient air in a Peri-urban area using a laser photoacoustic spectroscopy detector. Materials 2022, 15, 3182. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Han, M.-F.; Jia, T.-P.; Hu, X.-R.; Zhu, H.-Q.; Tong, Z.; Lin, Y.-T.; Wang, C.; Liu, D.-Z.; Peng, Y.-Z.; et al. Emissions, measurement, and control of odor in livestock farms: A review. Sci. Total Environ. 2021, 776, 145735. [Google Scholar] [CrossRef]
- Shaalan, N.M.; Ahmed, F.; Saber, O.; Kumar, S. Gases in food production and monitoring: Recent advances in target chemiresistive gas sensors. Chemosensors 2022, 10, 338. [Google Scholar] [CrossRef]
- Tang, X.; Debliquy, M.; Lahem, D.; Yan, Y.; Raskin, J.-P. A review on functionalized graphene sensors for detection of ammonia. Sensors 2021, 21, 1443. [Google Scholar] [CrossRef]
- Bevc, S.; Mohorko, E.; Kolar, M.; Brglez, P.; Holobar, A.; Kniepeiss, D.; Podbregar, M.; Piko, N.; Hojs, N.; Knehtl, M.; et al. Measurement of breath ammonia for detection of patients with chronic kidney disease. Clin. Nephrol. 2017, 88, S14–S17. [Google Scholar] [CrossRef]
- Das, S.; Pal, S.; Mitra, M. Significance of exhaled breath test in clinical diagnosis: A special focus on the detection of diabetes mellitus. J. Med. Biol. Eng. 2016, 36, 605–624. [Google Scholar] [CrossRef] [PubMed]
- Michalski, R.; Pecyna-Utylska, P.; Kernert, J. Determination of ammonium and biogenic amines by ion chromatography. A review. J. Chromatogr. A 2021, 1651, 462319. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Maurya, S.; Pandey, K.N.; Verma, V. Metal-oxide based ammonia gas sensors: A review. Nanosci. Nanotechnol.-Asia 2021, 11, 270–289. [Google Scholar] [CrossRef]
- Chou, T.C.; Chang, C.H.; Lee, C.; Liu, W.C. Ammonia sensing characteristics of a tungsten trioxide thin-film-based sensor. IEEE Trans. Electron Devices 2018, 66, 696–701. [Google Scholar] [CrossRef]
- Büyükköse, S. Highly selective and sensitive WO3 nanoflakes based ammonia sensor. Mater. Sci. Semicond. Process. 2020, 110, 104969. [Google Scholar] [CrossRef]
- Luo, W.; Zhao, T.; Li, Y.; Wei, J.; Xu, P.; Li, X.; Wang, Y.; Zhang, W.; Elzatahry, A.A.; Alghamdi, A.; et al. A micelle fusion-aggregation assembly approach to mesoporous carbon materials with rich active sites for ultrasensitive ammonia sensing. J. Am. Chem. Soc. 2016, 138, 12586–12595. [Google Scholar] [CrossRef]
- Duong, V.; Nguyen, C.; Luong, H.; Nguyen, D.; Nguyen, H. Ultralow-detection limit ammonia gas sensors at room temperature based on MWCNT/WO3 nanocomposite and effect of humidity. Solid State Sci. 2021, 113, 106534. [Google Scholar] [CrossRef]
- Nguyet, Q.T.M.; Van Duy, N.; Manh Hung, C.; Hoa, N.D.; Van Hieu, N. Ultrasensitive NO2 gas sensors using hybrid heterojunctions of multi-walled carbon nanotubes and on-chip grown SnO2 nanowires. Appl. Phys. Lett. 2018, 112, 153110. [Google Scholar] [CrossRef]
- Chiou, J.-C.; Wu, C.-C. A wearable and wireless gas-sensing system using flexible polymer/multi-walled carbon nanotube composite films. Polymers 2017, 9, 457. [Google Scholar] [CrossRef]
- Kumar, N.; Prajesh, R. Selectivity enhancement for metal oxide (MOX) based gas sensor using thermally modulated datasets coupled with golden section optimization and chemometric techniques. Rev. Sci. Instrum. 2022, 93, 064702. [Google Scholar] [CrossRef]
- Norizan, M.N.; Abdullah, N.; Halim, N.A.; Demon, S.Z.N.; Mohamad, I.S. Heterojunctions of rGO/metal oxide nanocomposites as promising gas-sensing materials—A review. Nanomaterials 2022, 12, 2278. [Google Scholar] [CrossRef] [PubMed]
- Bannov, A.G.; Popov, M.V.; Brester, A.E.; Kurmashov, P.B. Recent advances in ammonia gas sensors based on carbon nanomaterials. Micromachines 2021, 12, 186. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xiao, S.; Du, K. Chemiresistive gas sensors based on hollow heterojunction: A review. Adv. Mater. Interfaces 2021, 8, 2002122. [Google Scholar] [CrossRef]
- Lei, G.; Lou, C.; Liu, X.; Xie, J.; Li, Z.; Zheng, W.; Zhang, J. Thin films of tungsten oxide materials for advanced gas sensors. Sens. Actuators B Chem. 2021, 341, 129996. [Google Scholar] [CrossRef]
- Ma, N.; Suematsu, K.; Yuasa, M.; Kida, T.; Shimanoe, K. Effect of water vapor on Pd-loaded SnO2 nanoparticles gas sensor. ACS Appl. Mater. Interfaces 2015, 7, 5863–5869. [Google Scholar] [CrossRef] [PubMed]
- Tischner, A.; Maier, T.; Stepper, C.; Köck, A. Ultrathin SnO2 gas sensors fabricated by spray pyrolysis for the detection of humidity and carbon monoxide. Sens. Actuators B Chem. 2008, 134, 796–802. [Google Scholar] [CrossRef]
- Qian, J.; Peng, Z.; Shen, Z.; Zhao, Z.; Zhang, G.; Fu, X. Positive impedance humidity sensors via single-component materials. Sci. Rep. 2016, 6, 25574. [Google Scholar] [CrossRef]
- Rigoni, F.; Freddi, S.; Pagliara, S.; Drera, G.; Sangaletti, L.; Suisse, J.-M.; Bouvet, M.; Malovichko, A.M.; Emelianov, A.V.; Bobrinetskiy, I.I. Humidity-enhanced sub-ppm sensitivity to ammonia of covalently functionalized single-wall carbon nanotube bundle layers. Nanotechnology 2017, 28, 255502. [Google Scholar] [CrossRef]
- Sysoev, V.V.; Strelcov, E.; Kolmakov, A. Multisensor micro-arrays based on metal oxide nanowires for electronic nose applications. In Metal Oxide Nanomaterials for Chemical Sensors; Integrated Analytical Systems; Carpenter, M., Mathur, S., Kolmakov, A., Eds.; Springer: New York, NY, USA, 2013; pp. 465–502. [Google Scholar] [CrossRef]
- Potyrailo, R.A. Multivariable sensors for ubiquitous monitoring of gases in the era of internet of things and industrial internet. Chem. Rev. 2016, 116, 11877–11923. [Google Scholar] [CrossRef]
- Jeong, S.-Y.; Kim, J.-S.; Lee, J.-H. Rational design of semiconductor-based chemiresistors and their libraries for next-generation artificial olfaction. Adv. Mater. 2020, 32, 2002075. [Google Scholar] [CrossRef]
- Gulbransen, E.A.; Andrew, K.F. Kinetics of the oxidation of pure tungsten from 500 to 1300 C. J. Electrochem. Soc. 1960, 107, 619. [Google Scholar] [CrossRef]
- Manciu, F.S.; Enriquez, J.L.; Durrer, W.G.; Yun, Y.; Ramana, C.V.; Gullapalli, S.K. Spectroscopic analysis of tungsten oxide thin films. J. Mater. Res. 2010, 25, 2401–2406. [Google Scholar] [CrossRef]
- Polikarpov, Y.A.; Romashkin, A.V.; Struchkov, N.S.; Levin, D.D. High uniform carbon nanotube thin films spray deposition on substrates with patterned structures having height difference. In Proceedings of the 2019 IEEE Conference of Russian Young Researchers in Electrical and Electronic Engineering, St. Petersburg, Russia, 28–31 January 2019; pp. 1980–1985. [Google Scholar] [CrossRef]
- Romashkin, A.V.; Polikarpov, Y.A.; Silakov, G.O.; Alexandrov, E.V. Spray deposited thin uniform NiO/Spiro-OMeTAD composite hole transport layer with top carbon nanotube electrode. J. Phys. Conf. Ser. 2021, 2086, 012097. [Google Scholar] [CrossRef]
- Frick, C.; Steiner, H.; Mazurkiewicz, A.; Riediger, U.; Rauthe, M.; Reich, T.; Gratzki, A. Central European high-resolution gridded daily data sets (HYRAS): Mean temperature and relative humidity. Meteorol. Z. 2014, 23, 15–32. [Google Scholar] [CrossRef]
- Rabchinskii, M.K.; Varezhnikov, A.S.; Sysoev, V.V.; Solomatin, M.A.; Ryzhkov, S.A.; Baidakova, M.V.; Stolyarova, D.Y.; Shnitov, V.V.; Pavlov, S.S.; Kirilenko, D.A.; et al. Hole-matrixed carbonylated graphene: Synthesis, properties, and highly-selective ammonia gas sensing. Carbon 2021, 172, 236–247. [Google Scholar] [CrossRef]
- Rigoni, F.; Tognolini, S.; Borghetti, P.; Drera, G.; Pagliara, S.; Goldoni, A.; Sangaletti, L. Enhancing the sensitivity of chemiresistor gas sensors based on pristine carbon nanotubes to detect low-ppb ammonia concentrations in the environment. Analyst 2013, 138, 7392–7399. [Google Scholar] [CrossRef]
- Kolhe, P.S.; Mutadak, P.; Maiti, N.; Sonawane, K.M. Synthesis of WO3 nanoflakes by hydrothermal route and its gas sensing application. Sens. Actuators A Phys. 2020, 304, 111877. [Google Scholar] [CrossRef]
- Diaz-Reyes, J.; Delgado-Macuil, R.J.; Dorantes-García, V.; Perez-Benitez, A.; Balderas-Lopez, J.A.; Ariza-Ortega, J.A. Physical properties characterization of WO3 films grown by hot-filament metal oxide deposition. Mater. Sci. Eng. B 2010, 174, 182–186. [Google Scholar] [CrossRef]
- Lee, C.; Robertson, C.S.; Nguyen, A.H.; Kahraman, M.; Wachsmann-Hogiu, S. Thickness of a metallic film, in addition to its roughness, plays a significant role in SERS activity. Sci. Rep. 2015, 5, 1–10. [Google Scholar] [CrossRef]
- Avrekh, M.; Monteiro, O.R.; Brown, I.G. Electrical resistivity of vacuum-arc-deposited platinum thin films. Appl. Surf. Sci. 2000, 158, 217–222. [Google Scholar] [CrossRef]
- Wang, Z.; Su, J.; Qi, H.; Pan, P.; Jiang, M. Porous nanocrystalline WO3 thin films: Fabrication, electrical and optical properties. Surf. Innov. 2021, 9, 214–221. [Google Scholar] [CrossRef]
- Sastry, D.N.; Revanasiddappa, M.; Basavaraja, C.; Suresh, T.; Raghavendra, S.C. DC conductivity studies of doped polyaniline tungsten oxide nanocomposites. Indian J. Eng. Mater. Sci. 2013, 20, 435–442. [Google Scholar]
- Abdellah, A.; Abdelhalim, A.; Horn, M.; Scarpa, G.; Lugli, P. Scalable spray deposition process for high-performance carbon nanotube gas sensors. IEEE Trans. Nanotechnol. 2013, 12, 174–181. [Google Scholar] [CrossRef]
- Penza, M.; Rossi, R.; Alvisi, M.; Cassano, G.; Signore, M.A.; Serra, E.; Giorgi, R. Pt- and Pd-nanoclusters functionalized carbon nanotubes networked films for sub-ppm gas sensors. Sens. Actuators B Chem. 2008, 135, 289–297. [Google Scholar] [CrossRef]
- Vu, T.D.; Cong, T.N.; Huu, B.L.; Duc, C.N.; Huu, L.N. Surface-modified carbon nanotubes for enhanced ammonia gas sensitivity at room temperature. J. Nanosci. Nanotechnol. 2019, 19, 7447–7451. [Google Scholar] [CrossRef]
- Abdelhalim, A.; Winkler, M.; Loghin, F.; Zeiser, C.; Lugli, P.; Abdellah, A. Highly sensitive and selective carbon nanotube-based gas sensor arrays functionalized with different metallic nanoparticles. Sens. Actuators B Chem. 2015, 220, 1288–1296. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhu, Z.; Guo, T.; Li, H.; Xue, Q. Synthesis of nanowire bundle-like WO3-W18O49 heterostructures for highly sensitive NH3 sensor application. J. Hazard. Mater. 2018, 353, 290–299. [Google Scholar] [CrossRef]
- Ani, A.; Poornesh, P.; Antony, A.; Shchetinin, I.V.; Nagaraja, K.K.; Chattopadhyay, S.; Vinayakumar, K.B. Impact of Ag on the limit of detection towards NH3-sensing in spray-coated WO3 thin-films. Sensors 2022, 22, 2033. [Google Scholar] [CrossRef]
- Sysoev, V.V.; Kiselev, I.; Trouillet, V.; Bruns, M. Enhancing the gas selectivity of single-crystal SnO2:Pt thin film chemiresistor microarray by SiO2 membrane coating. Sens. Actuators B Chem. 2013, 185, 59–69. [Google Scholar] [CrossRef]
- Sysoev, V.V.; Kiselev, I.; Frietsch, M.; Goschnick, J. Temperature gradient effect on gas discrimination power of a metal-oxide thin-film sensor microarray. Sensors 2004, 4, 37–46. [Google Scholar] [CrossRef]
| Structure | Noise, 5·σ, kΩ 1 | R0, kΩ 2 | Power Law of S(C) | LOD Estimation, Ppb |
|---|---|---|---|---|
| SWCNT40-WO3 | 6.563 | 8240 | 5.2·C0.48 | 4.8 |
| sSWCNT40 | 3.352 | 1160 | 5.1·C0.58 | 7.1 |
| SWCNT50-Pt | 0.138 | 181 | 5.1·C0.56 | 0.6 |
| sSWCNT50 | 0.752 | 1840 | 3.5·C0.64 | 0.9 |
| rSWCNT54 | 0.763 | 957 | 2.0·C0.61 | 5.1 |
| rSWCNT174 | 0.115 | 87 | 2.6·C0.49 | 2.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romashkin, A.V.; Lashkov, A.V.; Sysoev, V.V.; Struchkov, N.S.; Alexandrov, E.V.; Levin, D.D. Energy-Efficient Chemiresistive Sensor Array Based on SWCNT Networks, WO3 Nanochannels and SWCNT-Pt Heterojunctions for NH3 Detection against the Background Humidity. Chemosensors 2022, 10, 476. https://doi.org/10.3390/chemosensors10110476
Romashkin AV, Lashkov AV, Sysoev VV, Struchkov NS, Alexandrov EV, Levin DD. Energy-Efficient Chemiresistive Sensor Array Based on SWCNT Networks, WO3 Nanochannels and SWCNT-Pt Heterojunctions for NH3 Detection against the Background Humidity. Chemosensors. 2022; 10(11):476. https://doi.org/10.3390/chemosensors10110476
Chicago/Turabian StyleRomashkin, Alexey V., Andrey V. Lashkov, Victor V. Sysoev, Nikolay S. Struchkov, Evgeny V. Alexandrov, and Denis D. Levin. 2022. "Energy-Efficient Chemiresistive Sensor Array Based on SWCNT Networks, WO3 Nanochannels and SWCNT-Pt Heterojunctions for NH3 Detection against the Background Humidity" Chemosensors 10, no. 11: 476. https://doi.org/10.3390/chemosensors10110476
APA StyleRomashkin, A. V., Lashkov, A. V., Sysoev, V. V., Struchkov, N. S., Alexandrov, E. V., & Levin, D. D. (2022). Energy-Efficient Chemiresistive Sensor Array Based on SWCNT Networks, WO3 Nanochannels and SWCNT-Pt Heterojunctions for NH3 Detection against the Background Humidity. Chemosensors, 10(11), 476. https://doi.org/10.3390/chemosensors10110476









