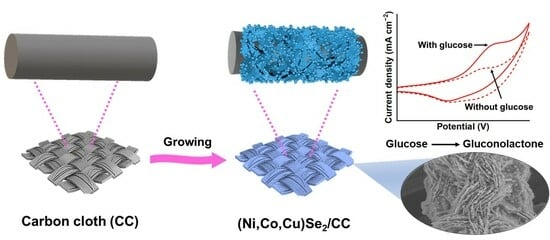
Synthesis of Quaternary (Ni, Co, Cu)Se2 Nanosheet Arrays on Carbon Cloth for Non-Enzymatic Glucose Determination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Apparatus
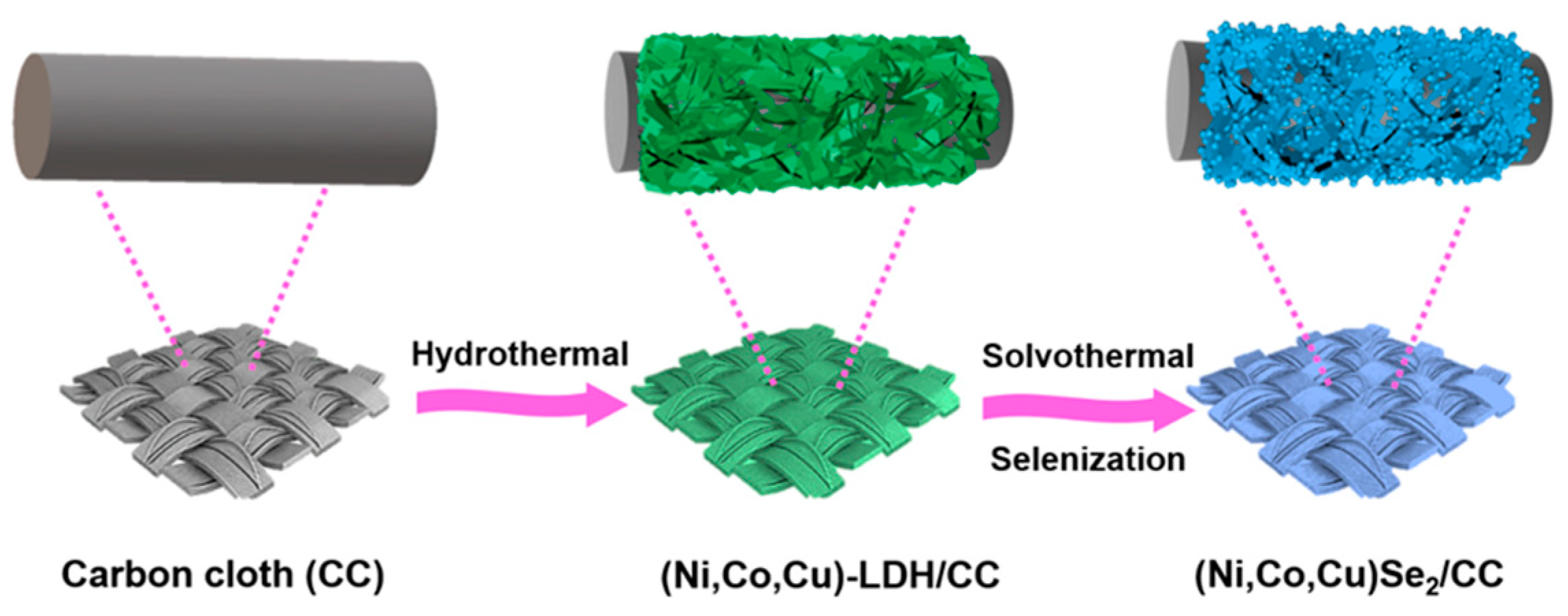
2.3. Synthesis of (Ni, Co, Cu)-LDH on CC
2.4. Synthesis of (Ni, Co, Cu)Se2 on CC
3. Results and Discussion
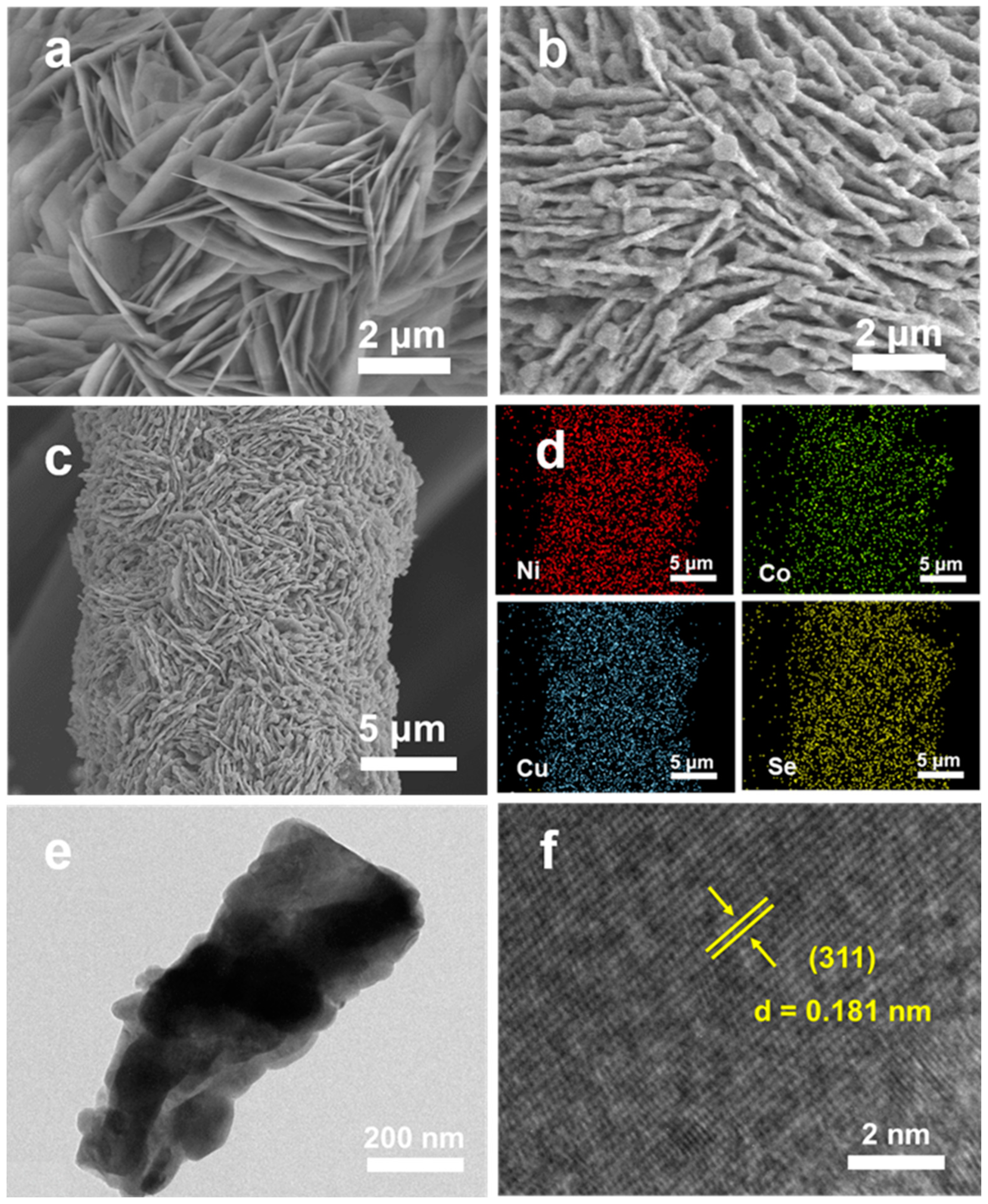
3.1. Structure and Morphology Characterization of (Ni, Co, Cu)Se2/CC
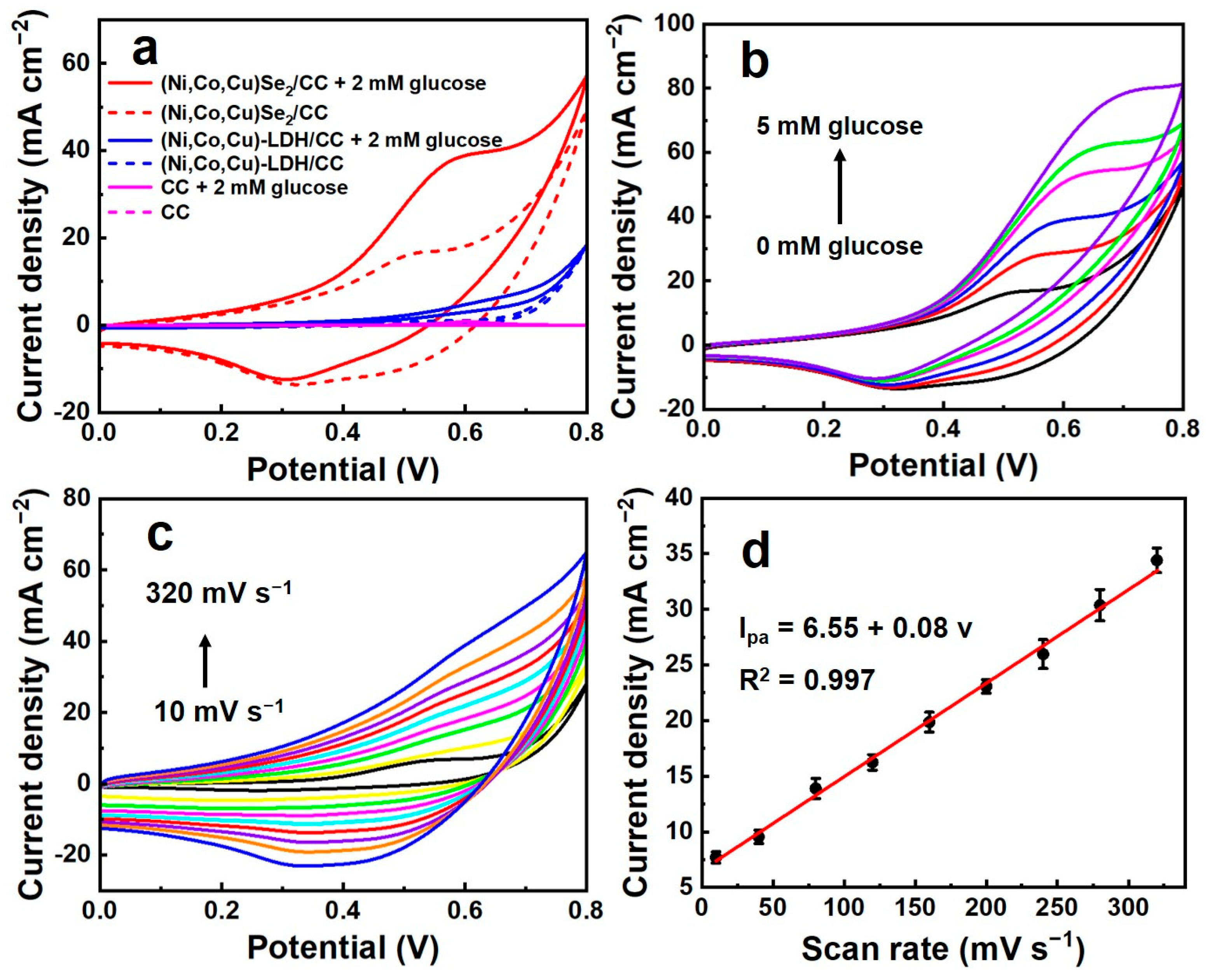
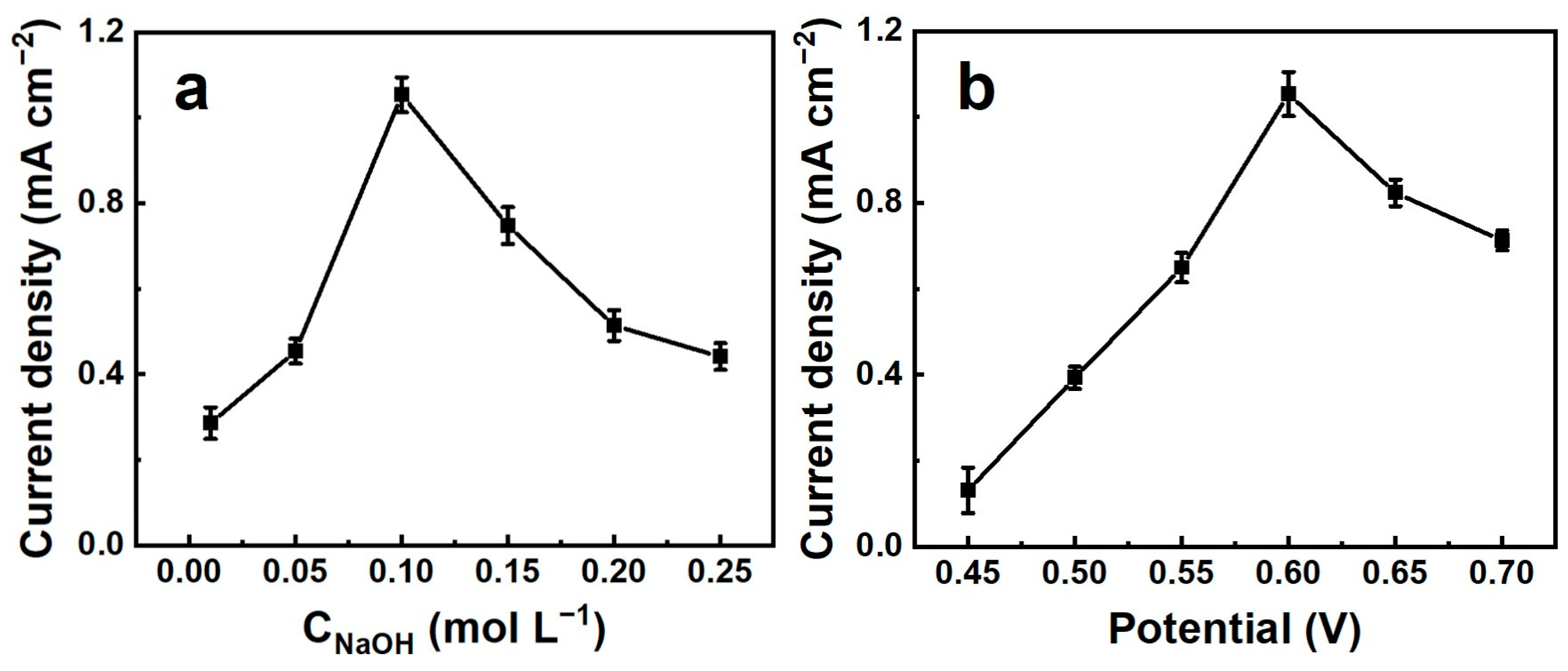
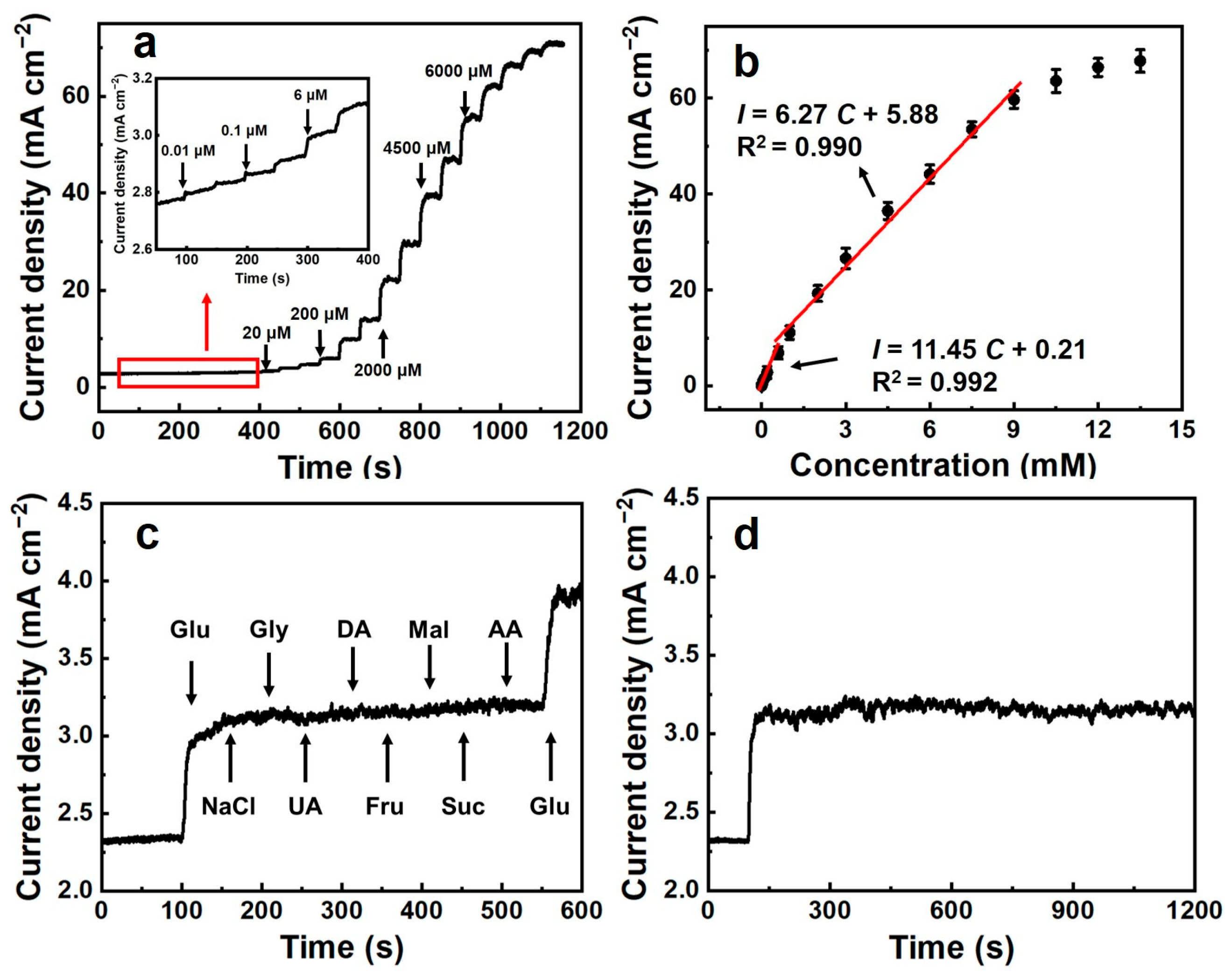
3.2. Electrochemical Performance of (Ni, Co, Cu)Se2/CC
3.3. Selectivity, Stability, Repeatability, and Reproducibility Investigation of (Ni, Co, Cu)Se2/CC
3.4. Real Sample Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rani, S.D.; Ramachandran, R.; Sheet, S.; Aziz, M.A.; Lee, Y.S.; Al-Sehemi, A.G.; Pannipara, M.; Xia, Y.; Tsai, S.Y.; Ng, F.L.; et al. NiMoO4 nanoparticles decorated carbon nanofiber membranes for the flexible and high performance glucose sensors. Sens. Actuators Chem. 2020, 312, 127886. [Google Scholar] [CrossRef]
- Fu, R.; Lu, Y.; Ding, Y.; Li, L.; Ren, Z.; Si, X.; Wu, Q. A novel non-enzymatic glucose electrochemical sensor based on CNF@Ni-Co layered double hydroxide modified glassy carbon electrode. Microchem. J. 2019, 150, 104106. [Google Scholar] [CrossRef]
- Shen, M.; Li, W.; Chen, L.; Chen, Y.; Ren, S.; Han, D. NiCo-LDH nanoflake arrays-supported Au nanoparticles on copper foam as a highly sensitive electrochemical non-enzymatic glucose sensor. Anal. Chim. Acta 2021, 1177, 338787. [Google Scholar] [CrossRef] [PubMed]
- Murugan, P.; Annamalai, J.; Atchudan, R.; Govindasamy, M.; Nallaswamy, D.; Ganapathy, D.; Reshetilov, A.; Sundramoorthy, A.K. Electrochemical sensing of glucose using glucose oxidase/PEDOT:4-sulfocalix [4]arene/MXene composite modified electrode. Micromachines 2022, 13, 304. [Google Scholar] [CrossRef] [PubMed]
- Sha, R.; Vishnu, N.; Badhulika, S. MoS2 based ultra-low-cost, flexible, non-enzymatic and non-invasive electrochemical sensor for highly selective detection of uric acid in human urine samples. Sens. Actuators Chem. 2019, 279, 53–60. [Google Scholar] [CrossRef]
- Wei, M.; Qiao, Y.X.; Zhao, H.T.; Liang, J.; Li, T.S.; Luo, Y.L.; Lu, S.Y.; Shi, X.F.; Lu, W.B.; Sun, X.P. Electrochemical non-enzymatic glucose sensors: Recent progress and perspectives. Chem. Commun. 2020, 56, 14553–14569. [Google Scholar] [CrossRef]
- Ye, Z.; Miao, R.; Miao, F.; Tao, B.; Zang, Y.; Chu, P.K. 3D nanoporous core-shell ZnO@Co3O4 electrode materials for high-performance supercapacitors and nonenzymatic glucose sensors. J. Electroanal. Chem. 2021, 903, 115766. [Google Scholar] [CrossRef]
- Shin, J.H.; Lee, M.J.; Choi, J.H.; Song, J.A.; Kim, T.H.; Oh, B.K. Electrochemical H2O2 biosensor based on horseradish peroxidase encapsulated protein nanoparticles with reduced graphene oxide-modified gold electrode. Nano Converg. 2020, 7, 39. [Google Scholar] [CrossRef]
- He, Q.G.; Wang, B.; Liang, J.; Liu, J.; Liang, B.; Li, G.L.; Long, Y.H.; Zhang, G.Y.; Liu, H.M. Research on the construction of portable electrochemical sensors for environmental compounds quality monitoring. Mater. Today Adv. 2023, 17, 100340. [Google Scholar] [CrossRef]
- Ayres, L.D.; Brooks, U.J.; Whitehead, K.; Garcia, C.D. Rapid detection of staphylococcus aureus using paper-derived electrochemical biosensors. Anal. Chem. 2022, 94, 16847–16854. [Google Scholar] [CrossRef]
- Clark, L.C.; Lyons, C. Electrode systems for continuous monitoring in cardiovascular surgery. Ann. N. Y. Acad. Sci. 1962, 102, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Naikoo, G.A.; Salim, H.; Hassan, I.U.; Awan, T.; Arshad, F.; Pedram, M.Z.; Ahmed, W.; Qurashi, A. Recent advances in non-enzymatic glucose sensors based on metal and metal oxide nanostructures for diabetes management: A review. Front. Chem. 2021, 11, 452. [Google Scholar] [CrossRef] [PubMed]
- Puttananjegowda, K.; Takshi, A.; Thomas, S. Silicon carbide nanoparticles electrospun nanofibrous enzymatic glucose sensor. Biosens. Bioelectron. 2021, 186, 113285. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Chen, D.; Chen, Y.; Dong, L. MOF Ni-BTC derived Ni/C/graphene composite for highly sensitive non-enzymatic electrochemical glucose detection. ECS J. Solid State Sci. Technol. 2021, 9, 121014. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, X.; Fu, K.; Su, Z. Fabrication of Co3O4/NiCo2O4 nanocomposite for detection of H2O2 and dopamine. Biosensors 2021, 11, 452. [Google Scholar] [CrossRef]
- Wang, D.; Cai, D.; Huang, H.; Liu, B.; Wang, L.; Liu, Y.; Li, H.; Wang, Y.; Li, Q.; Wang, T. Non-enzymatic electrochemical glucose sensor based on NiMoO4 nanorods. Nanotechnology 2015, 26, 145501. [Google Scholar] [CrossRef]
- Chen, T.W.; Ramachandran, R.; Chen, S.M.; Anushya, G.; Ramachandran, K. Graphene and perovskite-based nanocomposite for both electrochemical and gas sensor applications: An overview. Sensors 2020, 20, 6755. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, J.; Zhou, C.; Zhou, L.; Kong, Y.; Long, H.; Zhong, S. A novel self-cleaning, non-enzymatic glucose sensor working under a very low applied potential based on a Pt nanoparticle-decorated TiO2 nanotube array electrode. Electrochim. Acta 2014, 115, 269–276. [Google Scholar] [CrossRef]
- Xu, X.; Lv, H.; Sun, L.; Song, P.; Liu, B.; Chen, X. An Electrochemical non-enzymatic glucose sensor based on ultrathin PdAg single-crystalline nanowires. ChemPlusChem 2020, 85, 970–976. [Google Scholar] [CrossRef]
- Dong, M.; Hu, H.; Ding, S.; Wang, C.; Li, L. Fabrication of NiMn2O4 nanosheets on reduced graphene oxide for non-enzymatic detection of glucose. Mater. Technol. 2020, 36, 203–211. [Google Scholar] [CrossRef]
- Lin, L.; Weng, S.; Zheng, Y.; Liu, X.; Ying, S.; Chen, F.; You, D. Bimetallic PtAu alloy nanomaterials for nonenzymatic selective glucose sensing at low potential. J. Electroanal. Chem. 2020, 865, 114147. [Google Scholar] [CrossRef]
- Wang, W.; Dong, Y.; Xu, L.; Dong, W.; Niu, X.; Lei, Z. Combining bimetallic-alloy with selenium functionalized carbon to enhance electrocatalytic activity towards glucose oxidation. Electrochim. Acta 2017, 244, 16–25. [Google Scholar] [CrossRef]
- Xue, Z.; Jia, L.; Zhu, R.R.; Du, L.; Zhao, Q.H. High-performance non-enzymatic glucose electrochemical sensor constructed by transition nickel modified Ni@Cu-MOF. J. Electroanal. Chem. 2020, 858, 113783. [Google Scholar] [CrossRef]
- Li, X.; Wu, H.; Guan, C.; Elshahawy, A.M.; Dong, Y.; Pennycook, S.J.; Wang, J. (Ni,Co)Se2/NiCo-LDH core/shell structural electrode with the cactus-like (Ni,Co)Se2 core for asymmetric supercapacitors. Small 2019, 15, 1803895. [Google Scholar] [CrossRef]
- Wang, H.; Li, Y.; Deng, D.; Li, M.; Zhang, C.; Luo, L. NiO-coated CuCo2O4 nanoneedle arrays on carbon cloth for non-enzymatic glucose sensing. ACS Appl. Nano Mater. 2021, 4, 9821–9830. [Google Scholar] [CrossRef]
- Wang, H.; Wang, C.; Qing, C.; Sun, D.; Wang, B.; Qu, G.; Sun, M.; Tang, Y. Construction of carbon-nickel cobalt sulphide hetero-structured arrays on nickel foam for high performance asymmetric supercapacitors. Electrochim. Acta 2015, 174, 1104–1112. [Google Scholar] [CrossRef]
- Liu, W.; Xie, J.; Guo, Y.; Lou, S.; Gao, L.; Tang, B. Sulfurization-induced edge amorphization in copper-nickel-cobalt layered double hydroxide nanosheets promoting hydrazine electro-oxidation. J. Mater. Chem. A 2019, 7, 24437–24444. [Google Scholar] [CrossRef]
- Sakthivel, M.; Sukanya, R.; Chen, S.M.; Pandi, K.; Ho, K.C. Synthesis and characterization of bimetallic nickel-cobalt chalcogenides (NiCoSe2, NiCo2S4, and NiCo2O4) for non-enzymatic hydrogen peroxide sensor and energy storage: Electrochemical properties dependence on the metal-to-chalcogen composition. Renew. Energy 2019, 138, 139–151. [Google Scholar] [CrossRef]
- Younas, W.; Naveed, M.; Cao, C.; Khalid, S.; Rafai, S.; Wang, Z.; Wu, Y.; Yang, L. Rapid and simplistic microwave assisted method to synthesise cobalt selenide nanosheets: A prospective material for high performance hybrid supercapacitor. Appl. Surf. Sci. 2020, 505, 144618. [Google Scholar] [CrossRef]
- Song, W.; Teng, X.; Liu, Y.; Wang, J.; Niu, Y.; He, X.; Zhang, C.; Chen, Z. Rational construction of self-supported triangle-like MOF-derived hollow (Ni, Co)Se2 arrays for electrocatalysis and supercapacitors. Nanoscale 2019, 11, 6401–6409. [Google Scholar] [CrossRef]
- Singh, H.; Bernabe, J.; Chern, J.; Nath, M. Copper selenide as multifunctional non-enzymatic glucose and dopamine sensor. J. Mater. Res. 2021, 36, 1413–1424. [Google Scholar] [CrossRef]
- Ma, M.; Zhu, W.; Zhao, D.; Ma, Y.; Hu, N.; Suo, Y.; Wang, J. Surface engineering of nickel selenide nanosheets array on nickel foam: An integrated anode for glucose sensing. Sens. Actuators B Chem. 2019, 278, 110–116. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, Q.; Li, R.; Zhangsun, H.; Dong, M.; Wang, L. Surface selenylation engineering for construction of a hierarchical NiSe2/carbon nanorod: A high-performance nonenzymatic glucose sensor. ACS Appl. Mater. Interfaces 2021, 13, 22866–22873. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Chang, Y. The construct CoSe2 on carbon nanosheets as high sensitivity catalysts for electro-catalytic oxidation of glucose. Nanomaterials 2022, 12, 572. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Johnson, E.; Nath, M. Identifying high-efficiency oxygen evolution electrocatalysts from Co-Ni-Cu based selenides through combinatorial electrodeposition. J. Mater. Chem. A 2019, 7, 9877–9889. [Google Scholar] [CrossRef]
- Chen, B.; Tian, Y.; Yang, Z.; Jiang, J.; Wang, C. Construction of (Ni, Cu)Se2/reduced graphene oxide for high energy density asymmetric supercapacitor. ChemElectroChem 2017, 4, 3004–3010. [Google Scholar] [CrossRef]
- Du, Y.; Zhao, H.; Wang, W.; Yang, Y.; Wang, M.; Li, S.; Liu, Y.; Wang, L. (Ni,Co)Se@Ni(OH)2 heterojunction nanosheets as an efficient electrocatalyst for the hydrogen evolution reaction. Dalton Trans. 2021, 50, 391–397. [Google Scholar] [CrossRef]
- Sun, C.; Guo, X.; Zhang, J.; Han, G.; Gao, D.; Gao, X. Rechargeable Zn-air batteries initiated by nickel-cobalt bimetallic selenide. J. Energy Chem. 2019, 38, 34–40. [Google Scholar] [CrossRef]
- Liu, Y.L.; Yan, C.; Wang, G.G.; Li, F.; Kang, Q.; Zhang, H.Y.; Han, J.C. Selenium-rich nickel cobalt bimetallic selenides with core-shell architecture enable superior hybrid energy storage devices. Nanoscale 2020, 12, 4040–4050. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Y.; Ren, L.; Zhang, H.; Huang, Z.; Qi, X.; Wei, X.; Zhong, J. Three-dimensional-networked Ni-Co-Se nanosheet/nanowire arrays on carbon cloth: A flexible electrode for efficient hydrogen evolution. Electrochim. Acta 2016, 200, 142–151. [Google Scholar] [CrossRef]
- Fan, C.Y.; Zhang, X.H.; Shi, Y.H.; Xu, H.Y.; Zhang, J.P.; Wu, X.L. Three-dimensional hierarchical Ni3Se2 nanorod array as binder/carbon-free electrode for high-areal-capacity Na storage. Nanoscale 2018, 10, 18942–18948. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yan, D.; Lu, T.; Yao, Y.; Pan, L. An advanced CoSe embedded within porous carbon polyhedra hybrid for high performance lithium-ion and sodium-ion batteries. Chem. Eng. J. 2017, 325, 14–24. [Google Scholar] [CrossRef]
- Song, W.; Wang, K.; Jin, G.; Wang, Z.; Li, C.; Yang, X.; Chen, C. Two-Step hydrothermal synthesis of CoSe/MoSe2 as hydrogen evolution electrocatalysts in acid and alkaline electrolytes. ChemElectroChem 2019, 6, 4842–4847. [Google Scholar] [CrossRef]
- Zhong, Y.; Shi, T.; Liu, Z.; Cheng, S.; Huang, Y.; Tao, X.; Liao, G.; Tang, Z. Ultrasensitive non-enzymatic glucose sensors based on different copper oxide nanostructures by in-situ growth. Sens. Actuators Chem. 2016, 236, 326–333. [Google Scholar] [CrossRef]
- Dayakar, T.; Rao, K.V.; Bikshalu, K.; Malapati, V.; Sadasivuni, K.K. Non-enzymatic sensing of glucose using screen-printed electrode modified with novel synthesized CeO2@CuO core shell nanostructure. Biosens. Bioelectron. 2018, 111, 166–173. [Google Scholar]
- Ye, J.; Deng, D.; Wang, Y.; Luo, L.; Qian, K.; Cao, S.; Feng, X. Well-aligned Cu@C nanocubes for highly efficient nonenzymatic glucose detection in human serum. Sens. Actuators B Chem. 2020, 305, 127473. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, C.; Yang, M.; Wang, L.; Li, R.; Ma, N. Three-dimensional flexible polyurethane decorated with Ni and reduced graphene oxide for high-sensitive sensing of glucose. Mater. Chem. Phys. 2022, 278, 125679. [Google Scholar] [CrossRef]
- Luo, X.; Huang, M.; Bie, L.; He, D.; Zhang, Y.; Jiang, P. CuCo2O4 nanowire arrays non-enzymatic glucose sensing. RSC Adv. 2017, 7, 23093–23101. [Google Scholar] [CrossRef]
- Wei, C.; Zou, X.; Liu, Q.; Li, S.; Kang, C.; Xiang, W. A highly sensitive non-enzymatic glucose sensor based on CuS nanosheets modified Cu2O/CuO nanowire arrays. Electrochim. Acta 2020, 334, 135630. [Google Scholar] [CrossRef]
- Chen, D.; Pang, D.; Zhang, S.; Song, H.; Zhu, W.; Zhu, J. Synergistic coupling of NiCo2O4 nanorods onto porous Co3O4 nanosheet surface for tri-functional glucose, hydrogen-peroxide sensors and supercapacitor. Electrochim. Acta 2020, 330, 135326. [Google Scholar] [CrossRef]
- Liu, S.; Zhao, J.; Qin, L.; Liu, G.; Zhang, Q.; Li, J. Fabrication of Ni/Cu ordered bowl-like array film for the highly sensitive nonenzymatic detection of glucose. J. Mater. Sci. 2019, 55, 337–346. [Google Scholar] [CrossRef]
- Vishnu, N.; Sahatiya, P.; Kong, C.Y.; Badhulika, S. Large area, one step synthesis of NiSe2 films on cellulose paper for glucose monitoring in bio-mimicking samples for clinical diagnostics. Nanotechnology 2019, 30, 355502. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Jia, J.; Chang, Y.; Jia, M.; Wen, Z. Monodisperse copper selenide nanoparticles for ultrasensitive and selective non-enzymatic glucose biosensor. Electrochim. Acta 2019, 327, 135020. [Google Scholar] [CrossRef]
- Cui, S.; Gu, S.; Ding, Y.; Zhang, J.; Zhang, Z.; Hu, Z. Hollow mesoporous CuCo2O4 microspheres derived from metal organic framework: A novel functional materials for simultaneous H2O2 biosensing and glucose biofuel cell. Talanta 2018, 178, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Cooray, M.C.D.; Zhang, X.; Zhang, Y.; Langford, S.J.; Bond, A.; Zhang, J. Cobalt selenide nanoflake decorated reduced graphene oxide nanocomposite for efficient glucose electro-oxidation in alkaline medium. J. Mater. Chem. A 2017, 5, 19289–19296. [Google Scholar] [CrossRef]


| Modified Material | Linear Range (μM) | Detection Limit (μM) | Sensitivity (μA mM−1 cm−2) | Reference |
|---|---|---|---|---|
| CeO2@CuO | 1–89 | 0.019 | 3319.83 | [45] |
| CuCo2O4/CC | 1–930 | 0.5 | 3930 | [48] |
| CuS/Cu2O/CuO/Cu | 20–4096 | 0.89 | 4262 | [49] |
| Ni@Cu-MOF | 5–2500 | 1.67 | 1703.33 | [23] |
| Co3O4-NiCo2O4 | 1–2100 | 0.112 | 1463.13 | [50] |
| Ni/Cu | 0.5–2500 | 0.05 | 3924 | [51] |
| NiSe2/cellulose paper | 100–1000 | 24.8 | 250 | [52] |
| Cu2-xSe | up to 3375 | 50 | 536 | [53] |
| CuCo2O4 | 0.01–800 | 0.003 | 654.23 | [54] |
| CoSe–rGO a | up to 10,000 | 2.5 | 480 | [55] |
| (Ni, Co, Cu)Se2/CC | 0.01–600 600–9000 | 0.00582 | 11,450 6720 | This work |
| Serum Sample | Detected (μM) | Added (μM) | Found (μM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|---|
| 1 | 9.40 | 20 | 29.64 | 101.2 | 0.68 |
| 2 | 14.21 | 20 | 34.09 | 99.4 | 1.83 |
| 3 | 16.01 | 20 | 36.43 | 102.1 | 0.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Wang, H.; Chen, H.; Song, J.; Deng, D.; Luo, L. Synthesis of Quaternary (Ni, Co, Cu)Se2 Nanosheet Arrays on Carbon Cloth for Non-Enzymatic Glucose Determination. Chemosensors 2023, 11, 530. https://doi.org/10.3390/chemosensors11100530
Chen Y, Wang H, Chen H, Song J, Deng D, Luo L. Synthesis of Quaternary (Ni, Co, Cu)Se2 Nanosheet Arrays on Carbon Cloth for Non-Enzymatic Glucose Determination. Chemosensors. 2023; 11(10):530. https://doi.org/10.3390/chemosensors11100530
Chicago/Turabian StyleChen, Yuanyuan, Huan Wang, Huinan Chen, Jingyao Song, Dongmei Deng, and Liqiang Luo. 2023. "Synthesis of Quaternary (Ni, Co, Cu)Se2 Nanosheet Arrays on Carbon Cloth for Non-Enzymatic Glucose Determination" Chemosensors 11, no. 10: 530. https://doi.org/10.3390/chemosensors11100530