Abstract
Fipronil (FIP), a broad-spectrum phenylpyrazole insecticide, is highly toxic and threatens human health and ecological balance. Developing convenient, rapid, portable analytical technology for on-site and high-frequency testing of FIP is essential to reduce its damage. Herein, a monoclonal antibody (Clone F-3F6) against FIP, with high affinity and specificity, was produced using a novel immunogen, FIP-BSA, which was simply and directly synthesized by conjugating FIP with bovine serum albumin (BSA). Among the previously reported antibodies, F-3F6 acts more specifically against FIP. The FIP metabolites fipronil desulfinyl, fipronil sulfide, and fipronil sulfone showed lower cross-reactivity, and other pesticides were not recognized. To achieve high-frequency and on-site measurements of FIP, an evanescent wave fluorescence biosensor was built by integrating evanescent wave fluorescence technology, a functionalized fiber bioprobe, and a fluorescence-labeled F-3F6 antibody. The detection limit of FIP was 0.032 μg/L. The detection results of real milk and water samples showed that all the coefficients of variation were less than 10%, and the recovery ranged from 90 to 120%. The high reusability and stability of functionalized fiber bioprobe enables the accurate, cost-effective, high-frequency, and facile quantitative detection of FIP. This highly specific and reliable evanescent wave fluorescence biosensor will be well suited to the sensitive and high-frequency on-site analysis of only FIP in food.
1. Introduction
Fipronil (FIP), a broad-spectrum phenylpyrazole insecticide, has long been applied in agriculture, horticulture, and animal health because of its effectiveness in controlling pests [1,2,3]. This compound can disrupt the normal nerve function of insects by inhibiting the γ-aminobutyric acid type A (GABAA) reception system, which may cause excessive neuronal stimulation, severe paralysis, and even death [3,4,5]. Although FIP has a lower affinity for the native mammalian hetero-oligomeric receptor, it has a high affinity for the human receptor subunit β3, similar to GABAA receptors of insects [1,4,6,7]. The human β3 receptor is closely related to neurodevelopmental disorders, such as Angelman syndrome, autism, and epilepsy [6,8]. FIP and its metabolites are highly toxic (e.g., neurotoxicity, hepatotoxicity, and nephrotoxicity) to mammals and various aquatic species [1,7,9]. Its potential off-target harm to ecosystems and human health is of increasing concern. In line with this, many countries and organizations have placed restrictions on its use in agricultural crops and set strict maximum residue levels (MRLs) of allowable FIP in foods: 5.0 μg/kg, as set by the European Food Safety Authority, 30 μg/kg in America, and 20 μg/kg in China and Japan in accordance with the Food and Agriculture Organization [3,7,9,10,11,12]. However, human exposure may occur because of its indiscriminate use and persistence. For example, illegal FIP use in poultry farms led to the 2017 FIP egg contamination in Europe and Asia [13,14].
Traditional detection methods of FIP include gas chromatography‒mass spectrometry (GC‒MS) and liquid chromatography–tandem mass spectrometry (LC‒MS/MS) [2,15]. Although these methods are accurate and sensitive, they are time-consuming and require complicated sample handling, high testing costs, well-trained professionals, and expensive and bulky instruments, thus lacking rapid and high-frequency on-site detection ability [2]. In contrast, immunoassays are generally accurate, highly sensitive, selective, and cost-efficient. Several immunoassay methods, such as ELISAs and lateral flow immunoassays, have been developed to detect FIP [2,16,17,18]. Although ELISAs are high-throughput and relatively inexpensive methods, they require long assay times (>2 h) and bulky instruments, which limit their on-site detection ability. Lateral flow immunoassay is simple and rapid; however, it cannot achieve quantitative assays with susceptibility to matrix interference and low sensitivity [19]. Hence, it is vital to develop a convenient, rapid, portable analytical technology to achieve on-site, sensitive, and high-frequency detection of FIP in real samples.
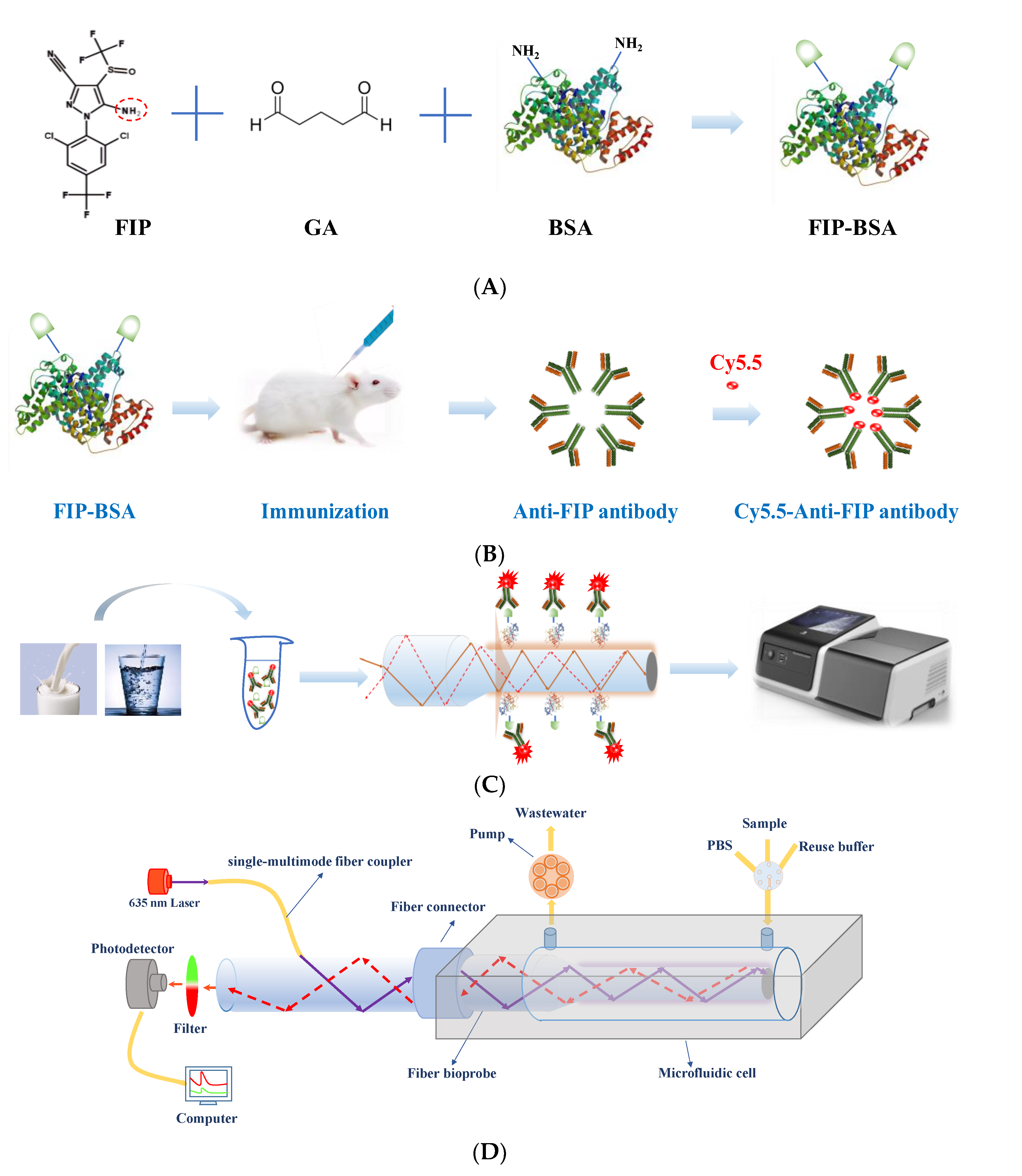
Optical biosensors have long attracted great attention for the measurement of trace targets due to their high sensitivity, rapidity, portability, and cost-effectiveness [20,21]. In this study, a portable evanescent wave fluorescence biosensor was built for rapid and sensitive on-site detection of FIP based on a highly specific monoclonal antibody (Figure 1). In this system, a single-multimode fiber coupler is used to transmit the excitation light and fluorescence, which not only simplifies the entire system due to the use of minimal optical separate elements but also improves the optical transmission efficiency and the detection sensitivity [20]. This elegant design allows the large-scale and high-frequency application of the evanescent wave fluorescence biosensor. On the other hand, antibodies are one of the most commonly used biorecognition molecules for biosensor construction because of their high specificity and sensitivity. The development of high-affinity antibodies for FIP is critical to achieve highly sensitive and specific detection [2,16]. Several anti-FIP antibodies, such as polyclonal antibodies, monoclonal antibodies, and heavy-chain-only antibodies, have been prepared based on various immunogen designs. However, the preparation of these immunogens was complicated, and the antibodies had distinct cross-reactivity for FIP metabolites, such as fipronil-sulfone, fipronil-sulfide, and fipronil-desulfinyl [2,17,18], which did not allow them to detect only FIP. To address these issues, a novel immunogen, FIP-BSA, was simply and directly synthesized by conjugating FIP with BSA using glutaraldehyde (GA) (Figure 1A). A highly specific monoclonal anti-FIP antibody, F-3F6, was prepared using the artificial antigen FIP-BSA (Figure 1B). Integrating an evanescent wave fluorescence platform and a reusable functionalized fiber bioprobe with a fluorescence-labeled anti-FIP antibody, an on-site immunoassay method was developed to detect FIP in real samples with high sensitivity, specificity, and accuracy (Figure 1C).
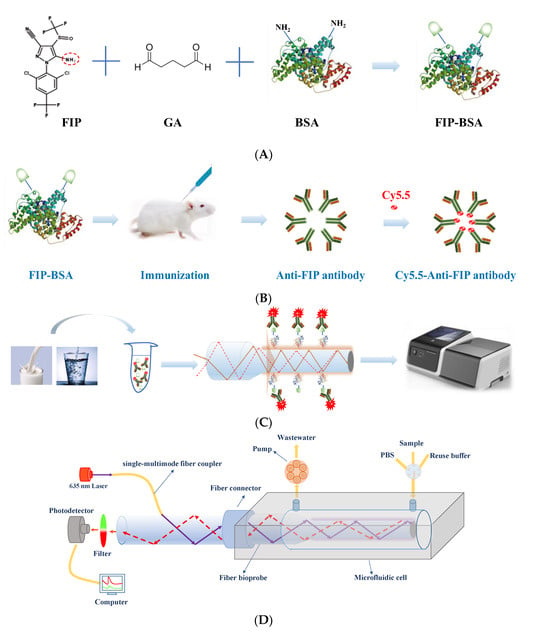
Figure 1.
Preparation of anti-FIP antibody and its application for immunoassay of FIP. (A) Synthesis of the FIP immunogen using GA to conjugate FIP with BSA. (B) Preparation of anti-FIP antibody using FIP-BSA and labeling with Cy5.5 dye. (C) FIP detection in food using an evanescent wave fluorescence detection platform based on an indirect competitive immunoassay mechanism. (D) Schematic of evanescent wave fluorescence detection platform.
2. Materials and Methods
2.1. Chemicals and Reagents
FIP was purchased from Shanghai Pesticide Research Institute Co., Ltd. (Shanghai, China). The metabolites fipronil desulfinyl, fipronil sulfide, and fipronil sulfone were purchased from Stanford Analytical Chemicals Inc. (Eugene, OR, USA). Cy5.5 was purchased from Global Life Sciences Solutions Operations UK Ltd. (Buckinghamshire, UK). 3-Mercaptopropyl-trimethoxysilane (MTS), N-(4-maleimidobutyryloxy) succinimide (GMBS), ovalbumin (OVA), bovine serum albumin (BSA), carbendazim, carbofuran, acetamiprid, carbaryl, and sodium dodecyl sulfate (SDS) were purchased from Sigma‒Aldrich (Shanghai, China). Unless specified otherwise, all other reagents were supplied by Beijing Chemical Agents (Beijing, China).
A 1.0 mg/mL FIP stock solution was prepared using methanol and stored at 4 °C. The FIP standard solutions were prepared from the stock solution by serial dilutions in phosphate buffer solution (PBS, 0.01 M, pH = 7.4). SDS solution (pH = 1.9, 0.5%) was used to regenerate the fiber bioprobe.
2.2. Synthesis of Immunogen and Coating Antigen
The immunogen FIP-BSA was prepared using the GA method (Figure 1A). Briefly, 20 mg FIP was dissolved in 3 mL DMF solution, and 10% GA was added and stirred for 10 min at room temperature. The covalent crosslinking between FIP and GA was performed through condensation of aldehyde groups of GA and amino groups of FIP to form Schiff bases. Then, 50 mg BSA was dissolved in 10 mL PBS solution, and an equal volume of borate buffer (pH = 8.6) was added. Finally, the FIP-GA solution was added dropwise to the BSA solution. The mixture was stirred overnight in an ice bath. After dialysis using PBS to guarantee purification quality, the obtained FIP-BSA was used as an immunogen and stored at −20 °C until use. The coating antigen, FIP-OVA, was prepared by a similar method by only replacing BSA with OVA (Figure S1 in Supplementary Materials).
2.3. Generation of Monoclonal Anti-FIP Antibodies
Female BALB/c mice (6 weeks old) were injected with FIP-BSA diluted with normal saline into the tail vein. The mice were immunized with a dose of 40 μg per animal every 2 weeks. After the third immunization, tail venous blood was collected by tail clipping at 7 days. The isolated serum was collected to measure the titer and inhibition ratio using indirect ELISA and indirect competitive ELISA (icELISA). Mice with high serum titers and low IC50 values (50% inhibitory concentration) were selected for cell fusion. Hybridoma cells were screened by HAT culture for approximately 10 days. The positive cells were selected by icELISA, and the supernatant of the cell culture was analyzed. The monoclonal cells were expanded and frozen in liquid nitrogen. Positive monoclonal cells (106 cells/mL) were intraperitoneally injected (1 mL per mouse) 10 days after the mice were intraperitoneally injected with paraffin oil (0.5 mL per mouse). The abdominal cavity began to accumulate fluid after 7–10 days. Monoclonal antibodies were purified from ascites using caprylic acid–ammonium sulfate precipitation. Finally, the anti-FIP antibody was labeled with Cy5.5 according to the manufacturer’s instructions (details are shown in Supporting Information).
2.4. Indirect Competitive ELISA (icELISA)
Plates were coated with FIP-OVA diluted with carbonate buffer (100 μL/well) and incubated overnight at 4 °C. Subsequently, the blocking buffer (200 μL/well) was added, and the plates were incubated for 1 h at 37 °C. Prior to sample loading, the plates were washed three times with PBST. The standard solution was diluted to concentrations of 20.0 µg/L, 10.0 µg/L, and 0.0195 µg/L and was subsequently added to the coated plate (50 μL/well). An equal volume of anti-FIP antibody diluted in PBS was added. The plate was incubated for 0.5 h at 37 °C and then washed five times with washing buffer. A 1/10,000 dilution of goat anti-rabbit IgG−HRP conjugate was introduced at a volume of 100 μL per well. The plate was incubated for 0.5 h at RT and washed five times. Substrate solution was added (100 μL/well) and left to develop color for 10 min. The reaction was stopped by the addition of 2 M H2SO4 (50 μL/well), and the absorbance was read at 450 nm.
2.5. Preparation of the Functionalized Fiber Bioprobe
The fiber bioprobe was prepared using a coating antigen as follows. The silica fiber (ϕ 600 μm, NA = 0.22) with a length of 5.5 cm was initially removed from the 3.5 cm coating layer to form the biosensing region that was tapered by the hydrofluoric acid-based tube-etching method [20]. Then, the fiber bioprobe was functionalized with FIP-OVA according to a previous study, with slight modifications (Figure S2) [22]. Briefly, the fiber bioprobe was soaked in H2SO4/H2O2 piranha solution. After rinsing using ultrapure water, the fiber bioprobe was immersed in MTS solution prepared using toluene (2%, v/v) to obtain sulfhydryl groups. After washing using dry toluene, the fiber bioprobe was added to 0.002 M GMBS ethanol solution that covalently reacted with sulfhydryl groups. The fiber nanoprobe was modified using 0.5 mg/mL FIP-OVA after the excess GMBS was washed. FIP-OVA covalently bound with the ester moiety of GMBS. Finally, the fiber bioprobe was blocked with 2 mg/mL BSA solution and stored at 4 °C before use.
2.6. Instrument: Evanescent Wave Fluorescence Detection Platform
The portable evanescent wave fluorescence detection platform (inset in Figure 1C) was used for the FIP immunoassay by integrating a fiber bioprobe with Cy5.5-anti-FIP antibody. In this biosensor, the 635 nm excitation light enters the fiber bioprobe and transmits in it via total internal reflection (Figure 1C). The evanescent wave generated on the surface of the fiber bioprobe excites the fluorescence labeled on the antibody. Part of the fluorescence couples back into the fiber bioprobe and is detected by the PD-1000 photodetector after filtration by a bandpass filter. Various solutions, including buffer, sample, and regenerated solution, were delivered with an optofluidic system operated with a mini-peristaltic pump. The controls of the optofluidic system and data acquisition and processing were automatically performed using a mini-computer.
2.7. FIP Detection Using an Evanescent Wave Fluorescence Biosensor
FIP quantitative detection was achieved based on the indirect competitive immunoassay principle. Twenty-five microliters of FIP solution of various concentrations was added to 25 µL of Cy5.5-anti-FIP antibody (0.75 µg/mL). This mixture was incubated for a certain time at RT, and the binding sites of some anti-FIP antibodies were occupied by FIP in the solution, relying on the FIP concentration. The mixture was then introduced to the microfluidic cell at 30 µL/min for 1 min. Typically, the incubation time between the Cy5.5-anti-FIP antibody and FIP-OVA was 600 s. In this process, anti-FIP antibodies with free binding sites bound with FIP-OVA on the fiber bioprobe. Increasing the FIP concentration resulted in decreasing fluorescence intensity because FIP in solution inhibited the binding of Cy5.5-anti-FIP antibodies to FIP-OVA. Finally, SDS solution was used to break the antibody–antigen association, and the active fiber bioprobe could be reused for subsequent testing.
2.8. Detection of FIP in Food
To assess the feasibility of evanescent wave fluorescence biosensors to detect FIP in real samples, we used them to detect spiked FIP concentrations in milk and water samples. The drinking water samples spiked with FIP were directly detected without any pretreatment steps. Due to the complex composition of milk, simple pretreatment was performed before detection. Briefly, a 1 mL spiked milk sample was diluted 10 times using PBS. After mixing well, it was centrifuged at 8000 rpm for 30 min at 4 °C. The supernatant was taken for testing.
3. Results
3.1. Synthesis of Immunogen and Coating Antigen
FIP cannot cause an immune response because of its small molecular weight [16]. Extensive efforts have been made in regards to immunogen preparation by introducing active functionalized groups to FIP and conjugating it to carrier proteins (e.g., thyroglobulin, BSA, or OVA), which is time-consuming and requires well-trained professionals [2,16,17]. To simplify the preparation process, we prepared the FIP-BSA immunogen by directly and covalently conjugating the amine groups of FIP and BSA using GA (Figure 1A). Previous studies demonstrated that the sensitivity of immunoassays could be greatly improved when the coating antigen was different from the immunogen [16]. Similarly, FIP was covalently conjugated with OVA to form the coating antigen FIP-OVA (Figure S2). To assess the synthesis of immunogen and coated antigen, gel electrophoresis testing of FIP-BSA, BSA, FIP-OVA, and OVA was performed. Figure S3 demonstrates that the electrophoresis bands of FIP-BSA and FIP-OVA lagged behind those of BSA and OVA, respectively. This contributed to the successful conjugation of FIP with BSA and OVA, which had a larger molecular weight than BSA and OVA, respectively. The electrophoresis bands of BSA and FIP-BSA significantly lagged behind those of OVA and FIP-OVA because the molecular weight of BSA (67 kDa) was larger than that of OVA (45 kDa). These results demonstrated that FIP-BSA and FIP-OVA were successfully synthesized.
3.2. Identification and Characteristics of the Monoclonal Antibodies
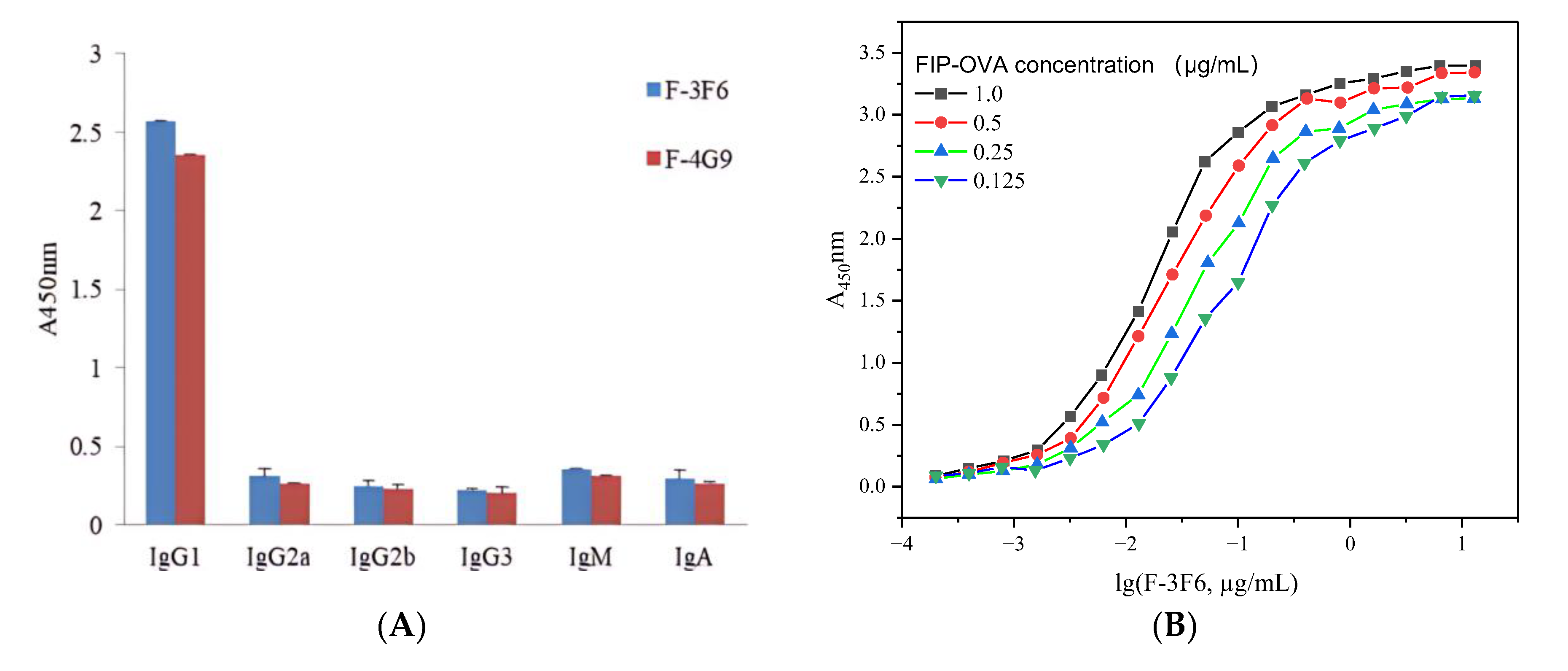
After selection with FIP–OVA and FIP, two antibody-producing clones, F-3F6 and F-4G9, were obtained. Both positive clones belonged to the IgG class because HRP-goat anti-mouse IgG was used for screening. The subclass of the two clones was determined by the SBA ClonotypingTM System/HRP kit, which showed the highest detection value for IgG1 secondary antibodies and a low detection value for IgM, IgG2a, IgG2b, IgG3, and IgA secondary antibodies, indicating that they were IgG1 antibodies (Figure 2). All the light chains of the two antibody-producing clones were κ (kappa). Furthermore, the sensitivity of the two monoclonal antibodies was tested, and F-3F6 was more sensitive to FIP than F-4G9 because the former had a larger slope and lower IC50 value than the latter (Figure S4). In order to achieve equivalent inhibition rates, a lower concentration of FIP was required for F-3F6, indicating its higher sensitivity. Then, the positive clone F-3F6 was injected into the abdominal cavity of BALB/c mice to produce many monoclonal antibodies, and ascites was collected. The indirect ELISA demonstrated that the titer of F-3F6 ascites was above 2.0 × 106, indicating that the activity and concentration of the F-3F6 ascites was high enough to harvest and apply forward (Table S1). Furthermore, the affinity constant (Ka) of F-3F6 was tested using indirect ELISA, and the Ka value was 4.6 × 1010 L/mol (Figure 2B).
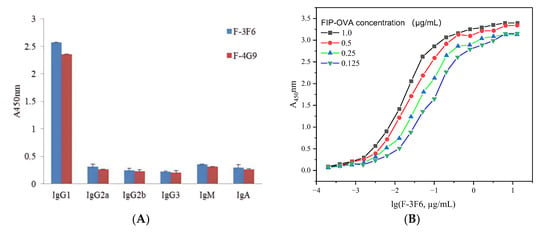
Figure 2.
Characteristics of monoclonal anti-FIP antibody. (A) Identification of the monoclonal antibodies F-3F6 and F-4G9. (B) Determination of the affinity constant of the monoclonal antibody F-3F6.
Next, the cross-reactivity of F-3F6 to several fipronil metabolites (e.g., fipronil desulfinyl, fipronil sulfide, and fipronil sulfone) and other small-molecule pesticides (e.g., carbendazim, carbofuran, acetamiprid, and carbaryl) was examined using ic-ELISA. Table 1 shows the IC50 value and molecular cross-reactivity of different substances. When the cross-reactivity of F-3F6 to FIP was defined as 100%, the cross-reactivity of F-3F6 to the FIP metabolites fipronil desulfinyl, fipronil sulfide, and fipronil sulfone was only 9.21%, 21.46, and 14.02%, respectively. However, the other pesticides, including carbendazim, carbofuran, acetamiprid, and carbaryl, were not recognized because the cross-reactivity was less than 10−3. The specificity of monoclonal antibodies raised against FIP varies according to their selected coupling location to the carrier protein. In previous studies, immunogens were synthesized by coupling FIP by introducing carboxy groups or other functionalized groups [2,17,18]. Most monoclonal and polyclonal antibodies prepared in this way did not exhibit sufficient recognition between FIP and its metabolites. The monoclonal antibody produced by the Zhou group had a cross-reactivity of approximately 100% for three FIP metabolites [23]. Vasylieva et al. designed different immunogens and coating antigens, and two antibodies showed distinct cross-reactivity of 96, 38, and 101% versus 39, 1.4, and 25% for fipronil-sulfide, fipronil-detrifluoromethylsulfonyl, and fipronil-desulfinyl, respectively [2]. Recently, Li et al. developed a broad-specificity monoclonal antibody for FIP with cross-reactivity for the FIP metabolites fipronil-sulfone, fipronil-sulfide, and fipronil-desulfinyl ranging from 44.2% to 116.8% [17]. Therefore, these antibodies were not specific enough to only recognize FIP. In our research, the immunogen was directly synthesized using GA to covalently conjugate FIP with BSA, which was significantly simpler than these methods. Meanwhile, the cross-reactivity results of F-3F6 demonstrated that it had high specificity to recognize FIP, and the immunoassay method based on F-3F6 had the potential to specifically detect FIP. The high-quality anti-FIP antibodies might contribute to the specific structure of FIP-BSA conjugates. Through directly conjugating FIP with BSA using GA, without inducing other functional groups like other literatures, the impact on haptens in preparation of the complete antigen was minimized, thus allowing the production of highly specific and sensitive antibodies.

Table 1.
The cross-reactivity of monoclonal antibody F-3F6 to FIP and its metabolites and other pesticides.
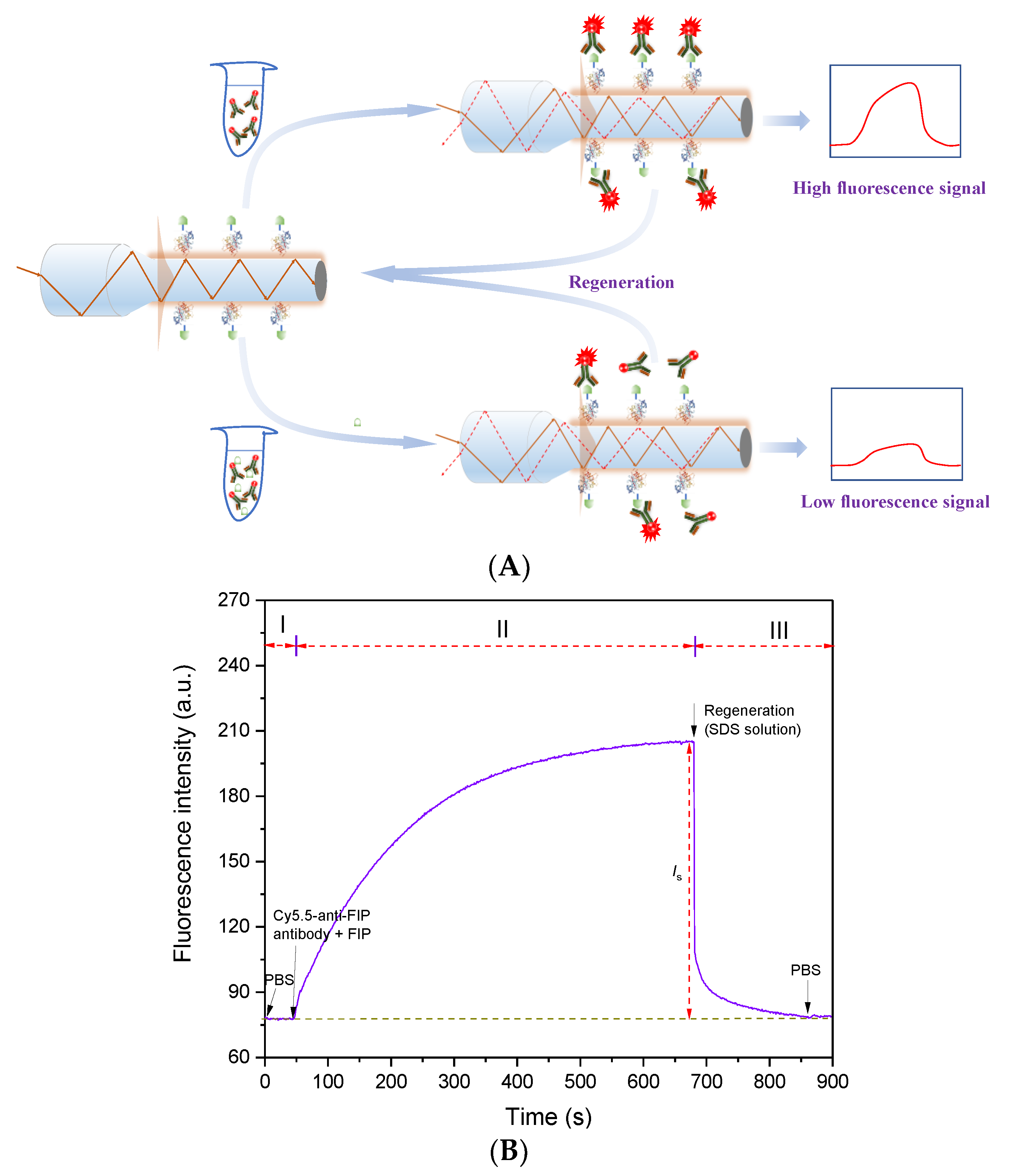
3.3. Immunoassay Mechanism of FIP Using an Evanescent Wave Fluorescence Biosensor
Using the obtained high-performance anti-FIP antibody, an evanescent wave fluorescence biosensor was built for FIP detection based on the indirect competitive immunoassay mechanism (Figure 3A). The FIP-OVA-modified fiber bioprobe was embedded in a microfluidic cell and regarded as a biorecognition element and transducer. Then, PBS buffer was added to the microfluidic cell, and the baseline was recorded (Phase I in Figure 3B). Different concentrations of FIP and a certain concentration of Cy5.5-anti-FIP antibody were mixed and prereacted. During this process, some antibodies specifically bound with FIP. After a certain time, the mixture was introduced over the fiber bioprobe surface. Part of the Cy5.5-anti-FIP antibodies with free binding sites bound with FIP-OVA on the fiber bioprobe, and the evanescent wave fluorescence biosensor detected the increasing fluorescence intensity over time (Phase II in Figure 3B). The net fluorescence signal value was calculated according to the following equation.
Is = The peak signal value of sample − The baseline value
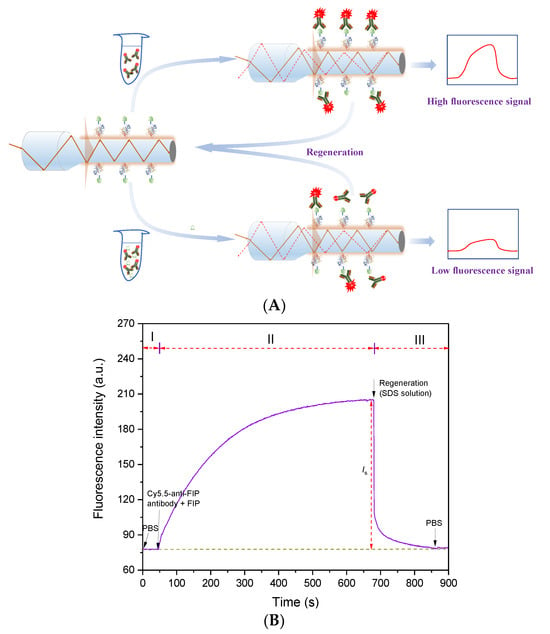
Figure 3.
Immunoassay of FIP using evanescent wave fluorescence biosensor. (A) Immunoassay mechanism of FIP. (B) Typical detection signal trace of FIP using an evanescent wave fluorescence biosensor. I, baseline ; II, binding of Cy5.5 anti-FIP antibody to FIP and FIP-OVA; III, regeneration and flushing.
Due to the increasing concentration of FIP inhibiting the Cy5.5-anti-FIP antibodies from binding with FIP-OVA on the fiber bioprobe, the higher concentration of FIP in the sample resulted in a lower detectable fluorescence signal. According to the linear relationship between fluorescence intensity and FIP concentration, the quantitative detection of FIP in samples can be achieved. Finally, to reuse the fiber bioprobe, an SDS solution was added to remove the bound Cy5.5-anti-FIP antibodies (Phase III in Figure 3B). The detection process for one test, including prereaction, immunoassay, and regeneration, took fewer than 15 min.
3.4. Optimization of Detection Conditions
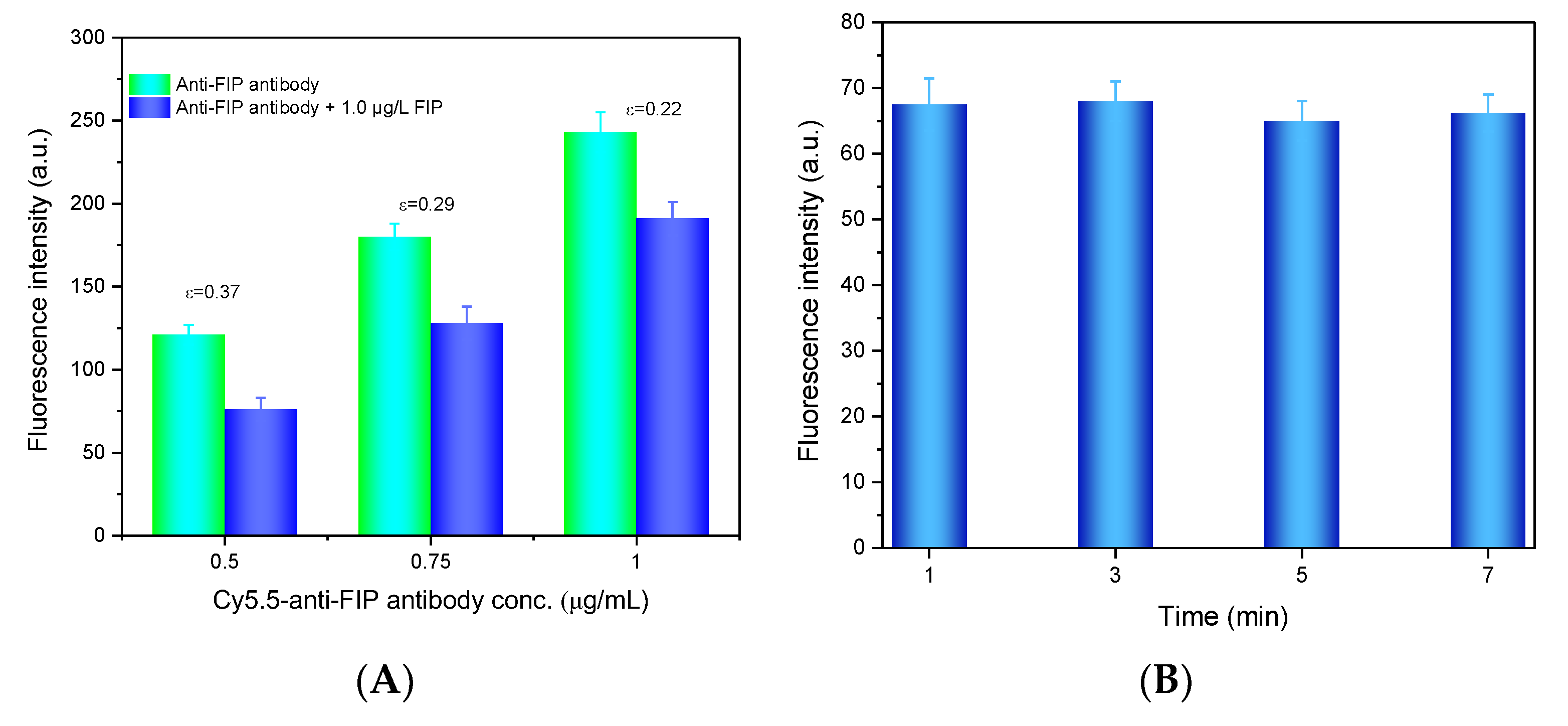
To improve the detection performance of the evanescent wave fluorescence biosensor for FIP, several conditions, including the concentration of the anti-FIP antibody, the prereaction time between the anti-FIP antibody and FIP in the sample, and the incubation time between the anti-FIP antibody and FIP-OVA on the fiber bioprobe, were optimized.
To obtain the optimal antibody concentration, a sensitivity index (ε) was defined as follows.
where Is,0 and Is,s are the net fluorescence intensity of the blank sample without FIP and the samples with FIP, respectively. The anti-FIP antibody at different concentrations (0.5 μg/mL, 0.75 μg/mL, and 1.0 μg/mL) with and without FIP was added to the microfluidic cell for testing. As shown in Figure 4A, the fluorescence intensity increased with increasing Cy5.5-anti-FIP antibody concentration because more antibodies bound to FIP-OVA on the fiber bioprobe. However, the ε value decreased with increasing antibody concentration because a lower concentration of antibody benefitted the competitive binding of FIP in the solution and led to less antibody binding with FIP-OVA. Considering the ε value and a suitable fluorescent intensity, 0.75 µg/mL Cy5.5-anti-FIP antibody was determined as the optimal concentration in the next experiments.
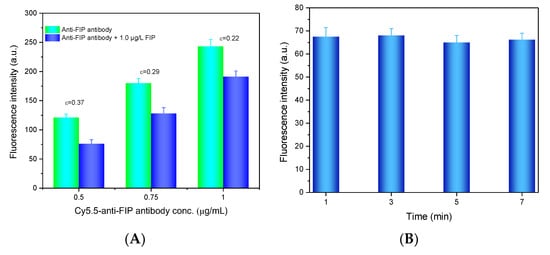
Figure 4.
Optimization of FIP detection conditions. (A) Optimization of the anti-FIP antibody concentration. The FIP concentration was 1.0 µg/L. (B) Optimization of the prereaction time for the FIP immunoassay. The anti-FIP antibody concentration was 0.75 g/mL, and the FIP concentration was 50 µg/mL.
Then, the prereaction time between the Cy5.5-anti-FIP antibody and FIP sample was optimized. Several prereaction times (1, 3, 5, and 7 min) between Cy5.5-anti-FIP antibody and FIP were evaluated. As shown in Figure 4B, prereaction time had almost no effect on the detection of the fluorescence signal. This may contribute to the high affinity constant between the anti-FIP antibody and FIP in the solution, which allowed their rapid binding. To shorten the detection time, after the sample and anti-FIP antibody were mixed, the mixture was directly introduced to the microfluidic cell for FIP detection.
Finally, the incubation time of the Cy5.5-anti-FIP antibody and FIP-OVA on the fiber bioprobe was optimized. The addition of Cy5.5-anti-FIP antibodies resulted in an increase in fluorescence intensity, which contributed to their specific binding with FIP-OVA on the fiber bioprobe surface. The fluorescence intensity increased over the incubation period and plateaued after 600 s (Figure 3B). To achieve rapid detection, the incubation time was selected as 600 s in the following experiments.
3.5. Quantitative Immunoassay of FIP
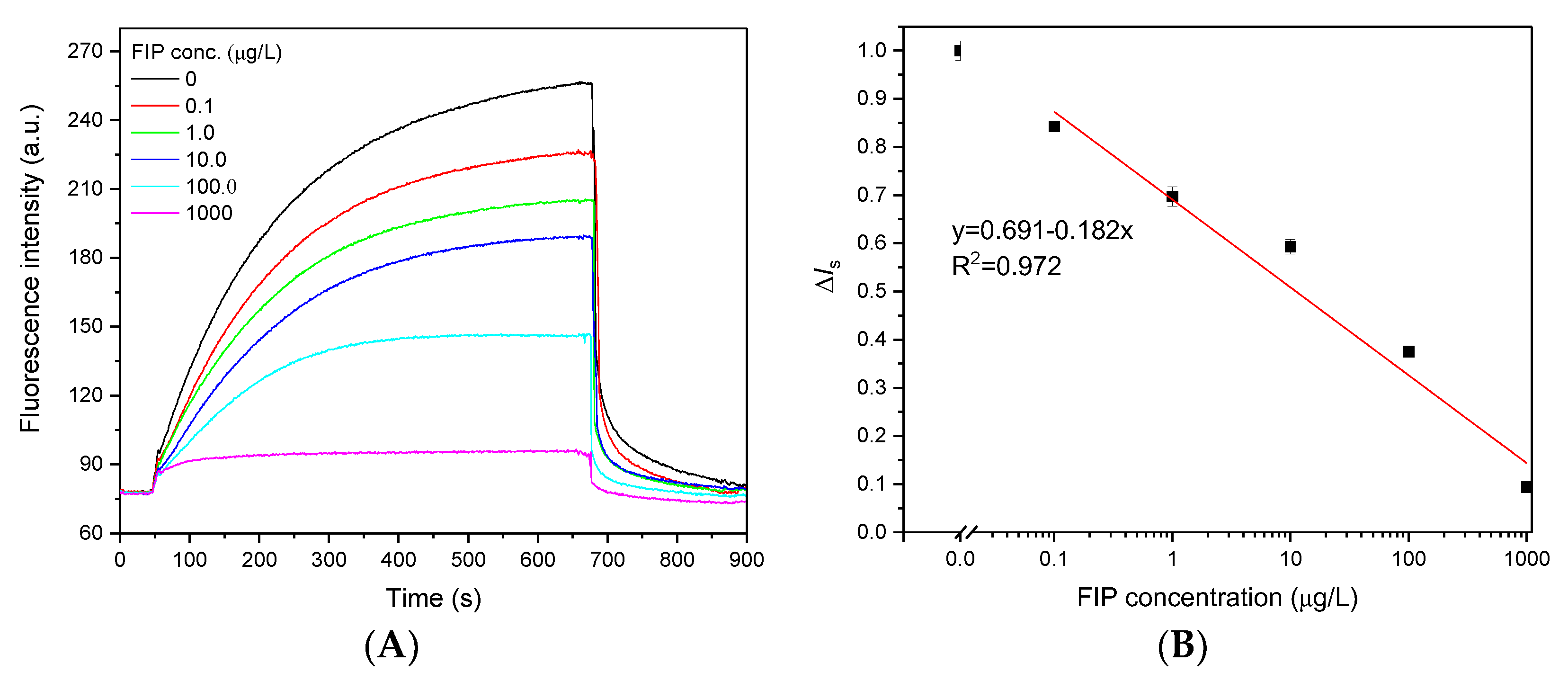
Under optimal detection conditions, quantitative analysis of FIP was performed using an evanescent wave fluorescence biosensor. Initially, 25 µL of Cy5.5-anti-FIP antibody (0.75 µg/mL) was prereacted with 25 µL of different concentrations of FIP samples; a portion of the FIP bound to the anti-FIP antibody. This mixture was directly introduced over the FIP-OVA functionalized fiber bioprobe surface, and a portion of the Cy5.5-anti-FIP antibody containing free binding sites bound to FIP-OVA. Figure 5A shows the typical fluorescence signal traces for various concentrations of FIP. Increasing the FIP concentration induced a proportional decrease in the fluorescence intensity, which contributed to less Cy5.5-anti-FIP antibody bound on the FIP-OVA-functionalized fiber bioprobe because more active sites of anti-FIP antibodies were occupied by free FIP in the samples. When the FIP concentration was 1000 µg/L, a very low fluorescence intensity was detected. These results indicated that the detected fluorescence signal originated from the binding between the Cy5.5-anti-FIP antibody and FIP-OVA on the fiber bioprobe, and the contribution of free fluorescence in the solution was negligible. The effective fluorescence intensity of the samples (Is,s) was normalized to that of the blank sample (Is,b) and calculated using Equation (3).
ΔIs = Is,b/Is,s
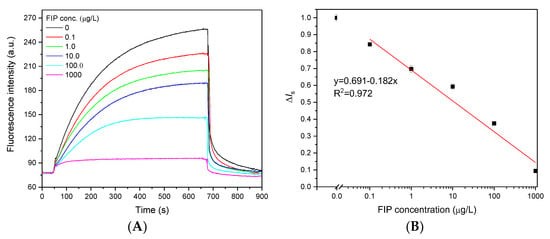
Figure 5.
Dose‒response curves for FIP detection using an evanescent wave fluorescence biosensor. (A) Typical fluorescence signal traces observed with a mixture of Cy5.5-anti-FIP antibody (0.75 μg/mL) and various concentrations of FIP. (B) Dose‒response curve of the FIP immunoassay. Error bars correspond to standard deviation (n = 3).
To plot the dose‒response curve of FIP at different concentrations, normalized signal values (ΔIs) were calculated and linearly fitted in a semilogarithmic coordinate system (Figure 4B). Based on the use of the functionalized fiber bioprobe and 3σ, the LOD of FIP was 0.032 μg/L, which was lower than that of ELISA (0.085 μg/L) (Figure S5). This LOD was also significantly lower than the MRLs set by various countries and organizations and comparable to those of other immunoassays (Table S2) [2,17,18,24]. This should contribute to the high affinity of the anti-FIP antibody and high sensitivity of the evanescent wave fluorescence platform. The linear response of FIP ranged from 0.25 to 415.0 μg/L according to the 80–20% fluorescence signal inhibition compared to the blank sample, which was far wider than that of ELISA (0.18-2.4 μg/L). The standard deviation of each FIP concentration (n = 3) was <3.25%, demonstrating the stability of the evanescent wave fluorescence biosensor for FIP detection (Figure 5B).
3.6. Detection of FIP in Milk and Water Samples
To assess its practical application ability, the proposed method was used for the detection of FIP in milk and drinking water. The spiked FIP concentrations in milk were 20.0 μg/L and 40.0 μg/L. The spiked FIP concentrations in drinking water were 1.0 μg/L and 5.0 μg/L. The milk samples were only required to remove big molecules, such as protein and fat, using the precipitation method. After a simple filter, the sample could be detected on-site by our method. However, the drinking water samples did not require any pretreatment and were directly detected. Table 2 shows that the average recoveries in all the samples varied from 90% to 120%, and the relative standard derivations were less than 10%. These results confirmed that the proposed evanescent wave fluorescent biosensor is applicable for FIP detection in real samples, and the matrix effect of the samples was negligible.

Table 2.
Recovery of the proposed methods for FIP detection in real samples.
Compared with other biosensors, our proposed method has several advantages, as follows. First, the monoclonal antibody F-3F6, obtained by immunization with the simple synthetic immunogen FIP-BSA, has high affinity and specificity for FIP, which is essential for the highly sensitive and specific detection of FIP with minimal cross-reactivity. Second, because of the limited penetration depth of evanescent waves, the detectable fluorescence signal mainly originates from Cy5.5-anti-FIP antibodies binding with FIP-OVA on the fiber bioprobe [25,26]. This minimizes the contribution of free Cy5.5-anti-FIP antibodies in the solution, and the washing process required in traditional biosensors is unnecessary. This benefits the improvement of detection sensitivity and simplification of the detection procedure. Third, the FIP-OVA-functionalized fiber bioprobe could be reused more than 100 times without significant signal loss (Figure S6) and with an RSD of 4.8%, indicating its high stability for FIP detection. It is critical to achieve the accurate, cost-effective, and facile on-site detection of targets and is also an outstanding advantage over other immunoassay methods (e.g., ELISA) [27]. Finally, the proposed method enables rapid and sensitive detection of FIP in samples with a 15 min immunoassay process, including regeneration of the fiber bioprobe, allowing it to perform high-frequency on-site detection that is important for food safety and to protect human health.
4. Conclusions
In summary, an FIP immunogen was directly synthesized through covalently conjugating FIP with BSA using GA. A monoclonal antibody, F-3F6, was obtained by immunization with FIP-BSA, which showed high affinity and specificity for FIP. Integrating an evanescent wave fluorescence biosensor and a functionalized fiber bioprobe, a rapid and sensitive on-site quantitative detection method for FIP was built, and its detection limit was 0.032 µg/L. The entire detection process only required 15 min. The high reusability and stability of the functionalized fiber bioprobe enables the accurate, cost-effective, high-frequency, and facile quantitative detection of FIP. The price of the immunosensor is not over $0.5 per testing because of good reusability of the fiber probes. The detection results of real samples showed good stability, recovery, and accuracy. This highly specific and reliable evanescent wave fluorescence biosensor will be well suited for sensitive on-site analysis to detect only FIP in food.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/chemosensors11120578/s1, Figure S1: Synthesis of coating-antigen FIP-OVA; Figure S2: Functionalization of fiber bioprobe using FIP-OVA; Figure S3: Characteristics of immunogen and coating-antigen. The SDS-PAGE image for the gel electrophoresis testing of FIP-BSA, BSA, FIP-OVA, and OVA. Marker: 170, 130, 100, 70, 55, 40, 35, 25, 15, and 10 kDa (from top to bottom); Figure S4: Sensitivity of antibodies. (A) Clone F-3F6. (B) Clone F-4G9; Figure S5: Dose–response curve of FIP using ELISA; Figure S6: The regeneration performance of the functionalized fiber bioprobe with FIP 0.75 μg/mL antibody; Table S1: Measurement of the concentration and valence of monoclonal antibody; Table S2: Comparison between other reports and this work on analytical performance of available sensors for simultaneous detection of multiple pesticides [2,17,18,19,23,24].
Author Contributions
Y.L., W.X., J.L. and E.Z. conceived this study and performed the initial experiments under the guidance of C.L. and X.Z. Y.L., W.X., J.L., E.Z., H.L., Y.Z. and J.Z. performed and analyzed the experiments on FIP detection. Y.L., W.X., and J.L. wrote the paper with guidance from C.L. and X.Z., and the manuscript was reviewed by all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Hebei Academy of Sciences, grant number 23308, and Shijiazhuang Bureau of Science and Technology, grant number 221170203A.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Review Board of Biology Institute of Hebei Academy of Sciences (protocol code SYXK (Ji):2020-002 and approved on 25 November 2020).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available on request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, X.; Martínez, M.A.; Wu, Q.; Ares, I.; Martínez-Larrañaga, M.R.; Anadón, A.; Yuan, Z. Fipronil insecticide toxicology: Oxidative stress and metabolism. Crit. Rev. Toxicol. 2016, 46, 876–899. [Google Scholar] [CrossRef] [PubMed]
- Vasylieva, N.; Ahn, K.C.; Barnych, B.; Gee, S.J.; Hammock, B.D. Development of an Immunoassay for the Detection of the Phenylpyrazole Insecticide Fipronil. Environ. Sci. Technol. 2015, 49, 10038–10047. [Google Scholar] [CrossRef] [PubMed]
- Hainzl, D.; Casida, J.E. Fipronil insecticide: Novel photochemical desulfinylation with retention of neurotoxicity. Proc. Natl. Acad. Sci. USA 1996, 93, 12764–12767. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.S.; Sharma, R.; Singh, S.K.; Singh, D.K. A comprehensive review of environmental fate and degradation of fipronil and its toxic metabolites. Environ. Res. 2021, 199, 111316. [Google Scholar] [CrossRef] [PubMed]
- Trinh, K.H.; Kadam, U.S.; Rampogu, S.; Cho, Y.; Yang, K.-A.; Kang, C.H.; Lee, K.-W.; Lee, K.O.; Chung, W.S.; Hong, J.C. Development of novel fluorescence-based and label-free noncanonical G4-quadruplex-like DNA biosensor for facile, specific, and ultrasensitive detection of fipronil. J. Hazard. Mater. 2022, 5, 127939. [Google Scholar] [CrossRef] [PubMed]
- Ratra, G.S.; Kamita, S.G.; Casida, J.E. Role of human GABA(A) receptor beta3 subunit in insecticide toxicity. Toxicol. Appl. Pharmacol. 2001, 172, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Daim, M.M.; Dessouki, A.A.; Abdel-Rahman, H.G.; Eltaysh, R.; Alkahtani, S. Hepatorenal protective effects of taurine and N-acetylcysteine against fipronilinduced injuries: The antioxidant status and apoptotic markers expression in rats. Sci. Total Environ. 2019, 650, 2063–2073. [Google Scholar] [CrossRef]
- Song, X.; Wang, X.; Liao, G.; Pan, Y.; Qian, Y.; Qiu, J. Toxic effects of fipronil and its metabolites on PC12 cell metabolism. Ecotoxic. Environ. Saf. 2021, 224, 112677. [Google Scholar] [CrossRef]
- Shimada, S.; Cutting, G.; Uhl, G.R. gamma-Aminobutyric acid A or C receptor? gamma-Aminobutyric acid rho 1 receptor RNA induces bicuculline-, barbiturate-, and benzodiazepine-insensitive gamma-aminobutyric acid responses in Xenopus oocytes. Mol. Pharmacol. 1992, 41, 683–687. [Google Scholar]
- Environmental Protection Agency (EPA), Fipronil; Tolerances for Residues, 2017, 180.517. Available online: https://www.ecfr.gov/current/title-40/chapter-I/subchapter-E/part-180/subpart-C/section-180.517#p-180.517(a) (accessed on 23 October 2023).
- GB 2763-2021; National Food Safety Standard, Maximum Residue Limits for Pesticides in Food. National Health Commission of the People's Republic of China, Ministry of Agriculture and Rural Affairs of the People's Republic of China, State Administration for Market Regulation: Beijing, China, 2021; pp. 124–125.
- Food and Agriculture Organization of the United Nations (FAO), Pesticides Use. 2019. Available online: http://www.fao.org/faostat/en/#data/RP/visualize (accessed on 23 October 2023).
- Stafford, E.G.; Tell, L.A.; Lin, Z.; Davis, J.L.; Vickroy, T.W.; Riviere, J.E.; Baynes, R.E. Consequences of fipronil exposure in egg-laying hens. J. Am. Vet. Med. Assoc. 2018, 253, 57–60. [Google Scholar] [CrossRef]
- EFSA, European Food Safety Authority; Reich, H.; Triacchini, G.A. Occurrence of residues of fipronil and other acaricides in chicken eggs and poultry muscle/fat. EFSA J. 2018, 16, e05164. [Google Scholar] [PubMed]
- Bichon, E.; Richard, C.; Le Bizec, B. Development and validation of a method for fipronil residue determination in ovine plasma using 96-well plate solid-phase extraction and gas chromatography-tandem mass spectrometry. J. Chromatogr. A 2008, 1201, 91–99. [Google Scholar] [CrossRef]
- Li, X.; Li, H.; Ma, W.; Guo, Z.; Li, X.; Song, S.; Tang, H.; Li, X.; Zhang, Q. Development of precise GC-EI-MS method to determine the residual fipronil and its metabolites in chicken egg. Food Chem. 2019, 281, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Bai, Y.; Jiang, H.; Zhang, Y.; Li, Y.; Duan, C.; Wen, K.; Yu, X.; Wang, Z. Broad-specificity antibody profiled by hapten prediction and its application in immunoassay for fipronil and major metabolites. J. Hazard. Mater. 2023, 441, 129931. [Google Scholar] [CrossRef]
- Wang, K.; Vasylieva, N.; Wan, D.; Eads, D.A.; Yang, J.; Tretten, T.; Barnych, B.; Li, J.; Li, Q.X.; Gee, S.J.; et al. Quantitative detection of fipronil and fipronil-sulfone in sera of black-tailed prairie dogs and rats after oral exposure to fipronil by camel single-domain antibody-based immunoassays. Anal. Chem. 2019, 91, 1532–1540. [Google Scholar] [CrossRef]
- Zhou, X.H.; Zhang, C.Q.; Zhang, X.; Sun, C.; Li, J.; Xiao, X.; Ouyang, Q.; Wang, Y. Determination of fipronil and its metabolites in eggs by indirect competitive ELISA and lateral-flow immunochromatographic strip. Biomed. Environ. Sci. 2020, 33, 731–734. [Google Scholar]
- Song, D.; Yang, R.; Wang, H.; Fang, S.; Liu, Y.; Long, F.; Zhu, A. Development of dual-color total internal reflection fluorescence biosensor for simultaneous quantitation of two small molecules and their affinity constants with antibodies. Biosens. Bioelectron. 2019, 126, 824–830. [Google Scholar] [CrossRef]
- Wei, Y.; Ren, Z.; Liu, C.; Jiang, T.; Wang, R.; Shi, C.; Liu, C. All-fiber biological detection microfluidic chip based on space division and wavelength division multiplexing technologies. Lab Chip 2022, 22, 4501. [Google Scholar] [CrossRef]
- Long, F.; He, M.; Zhu, A.; Shi, H. Portable optical immunosensor for highly sensitive detection of microcystin-LR in water samples. Biosens. Bioelectron. 2009, 24, 2346–2351. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, J.; Lu, Q.; Tian, J.; Kong, D.; Luo, J.; Yang, M. Development of a sensitive fluorescent immunoassay based on fluorescent nanoparticles labeling for the quantitation of fipronil in edible flowers. LWT 2023, 184, 115113. [Google Scholar] [CrossRef]
- Yao, J.; Wang, Z.; Guo, L.; Xu, X.; Liu, L.; Kuang, H.; Xu, C. Lateral flow immunoassay for the simultaneous detection of fipronil and its metabolites in food samples. Food Chem. 2021, 356, 129710. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; White, M.I. Optofluidic microsystems for chemical and biological analysis. Nat. Photon. 2011, 5, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Song, D.; Zhuo, Y.; Chen, Y.; Zhu, A.; Long, F. Simultaneous and sensitive determination of Escherichia coli O157:H7 and Salmonella Typhimurium using evanescent wave dual-color fluorescence aptasensor and fiber nanoprobe through combining the micro/nano size effect and time-resolved effect. Biosens. Bioelectron. 2021, 185, 113288. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Wang, P.; Gu, M.; Xue, J.; Wu, X. Human health risk assessment of pesticide residues in honeysuckle samples from different planting bases in China. Sci. Total Environ. 2021, 759, 142747. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).