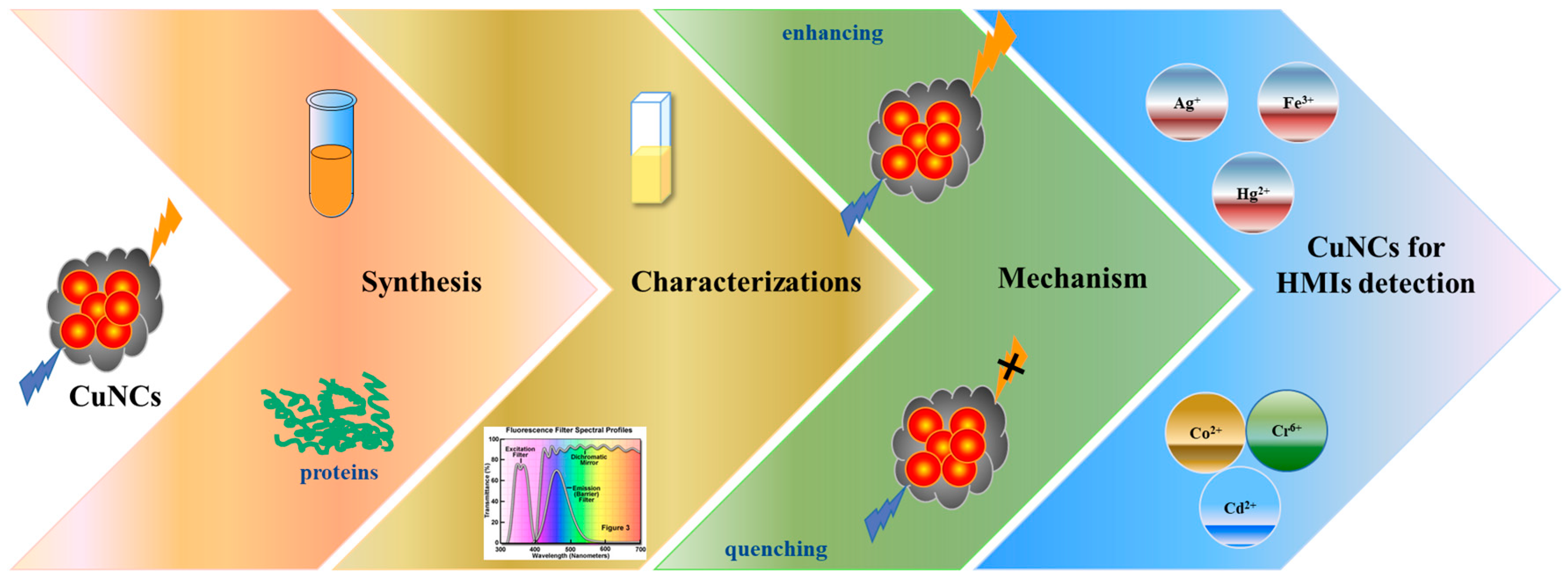
Rational Design Copper Nanocluster-Based Fluorescent Sensors towards Heavy Metal Ions: A Review
Abstract
:1. Introduction
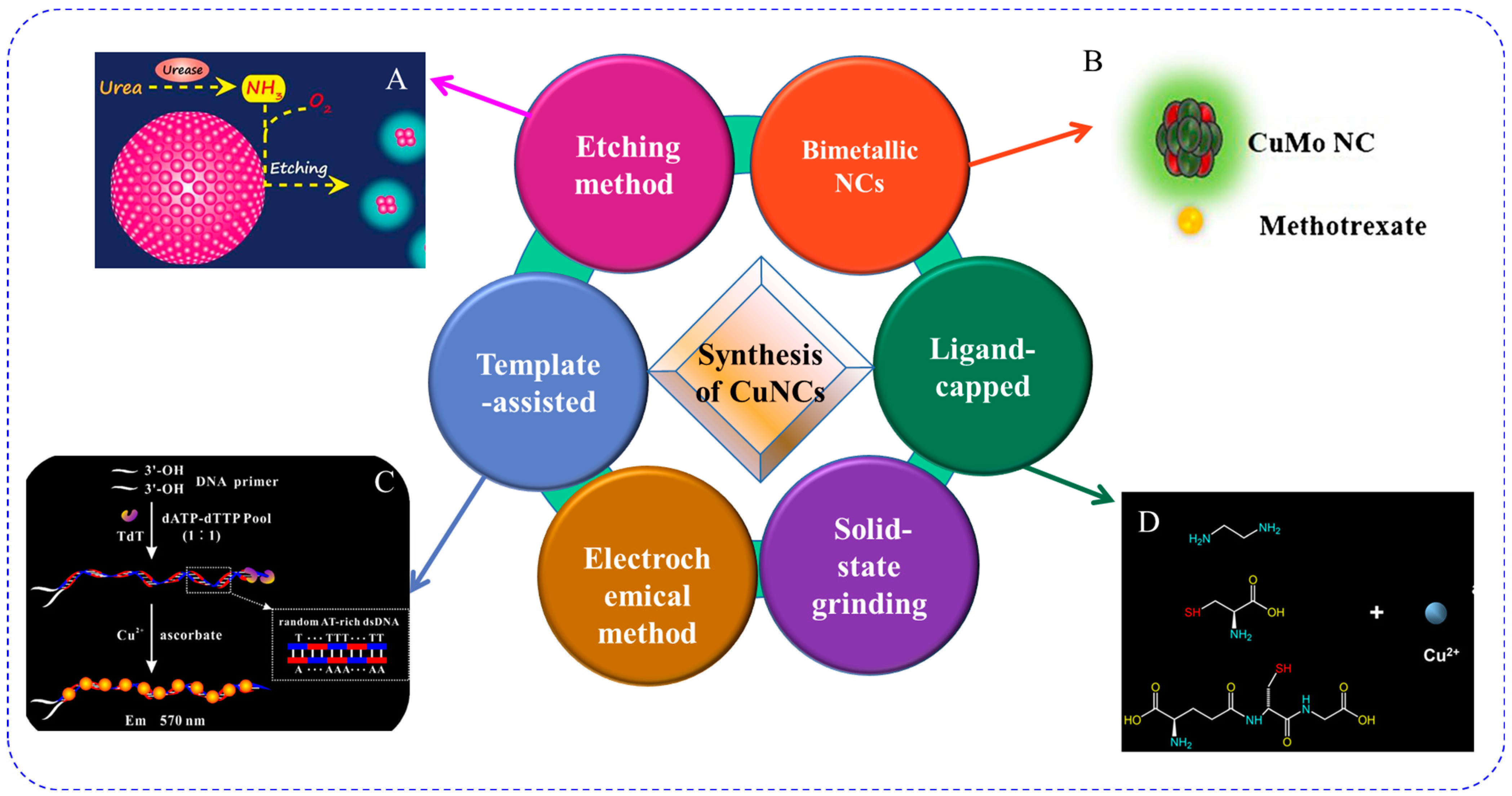
2. Synthesis of Fluorescent Copper Nanoclusters
2.1. Template-Assisted Method
2.2. Ligand-Capped Method
2.3. Etching Method
2.4. Copper-Included Bimetallic Nanoclusters
2.5. Other Methods
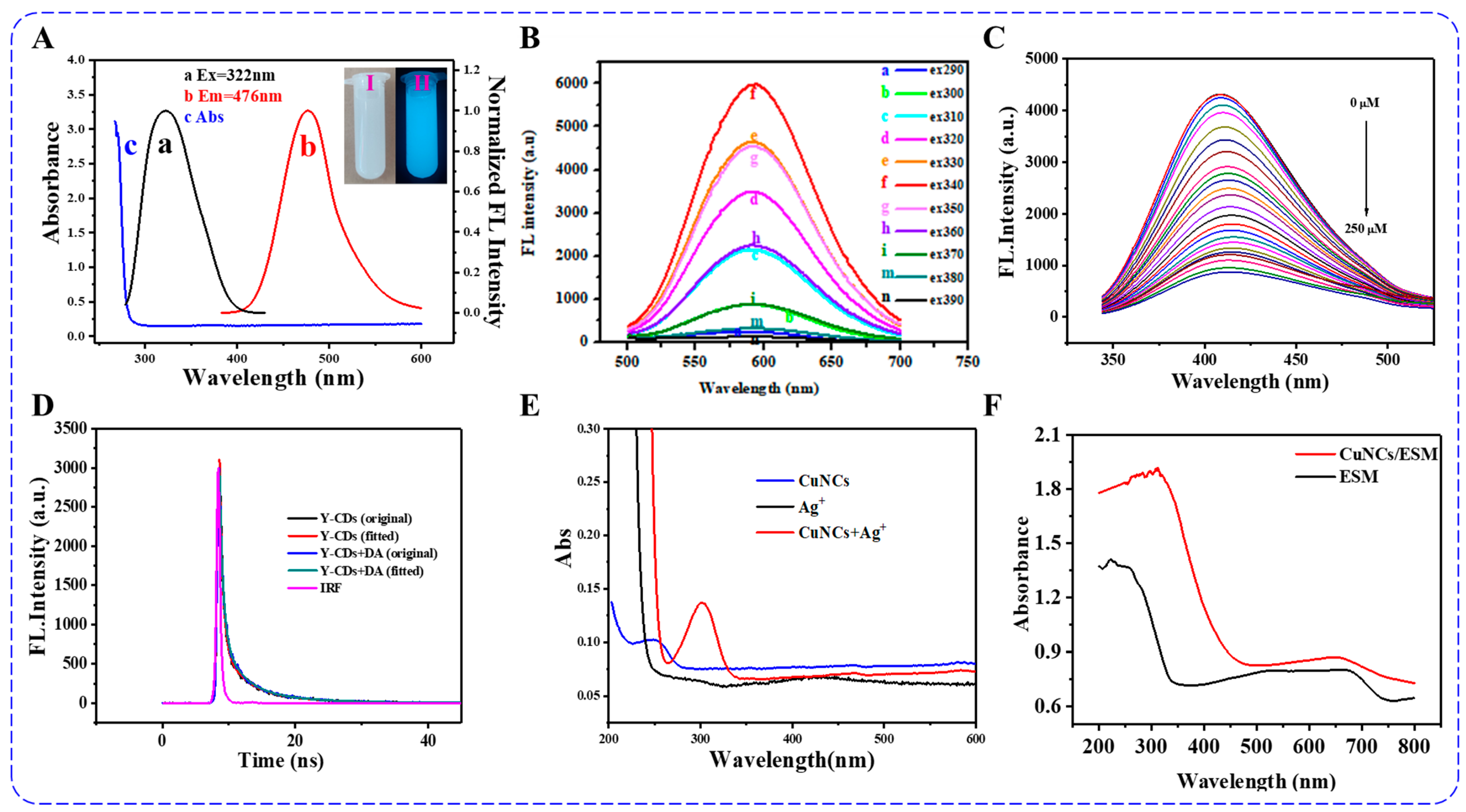
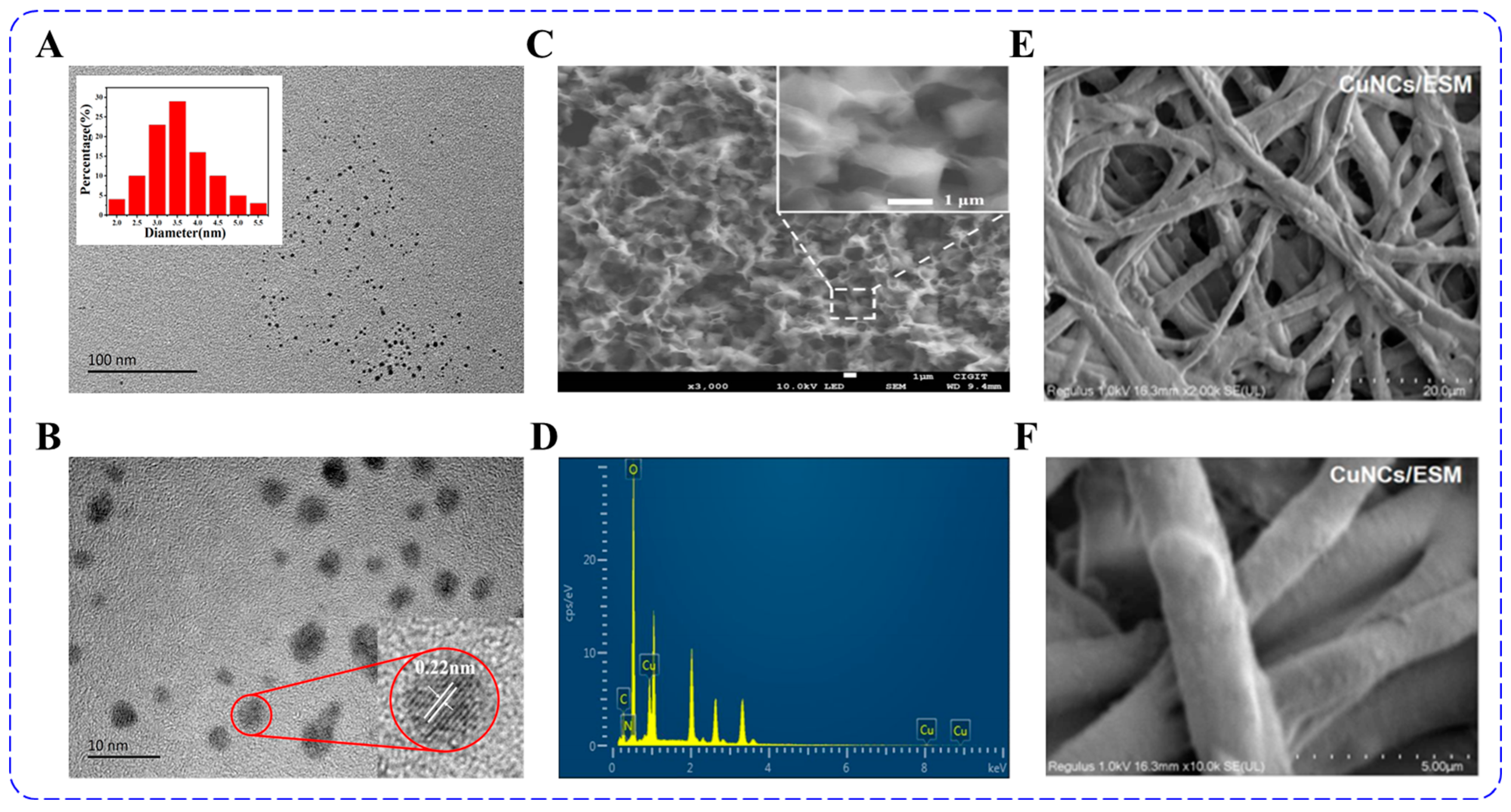
3. Characterizations of Fluorescent Copper Nanoclusters
3.1. Steady and Transient State Fluorescence
3.2. UV-Vis and UV-Vis DRS
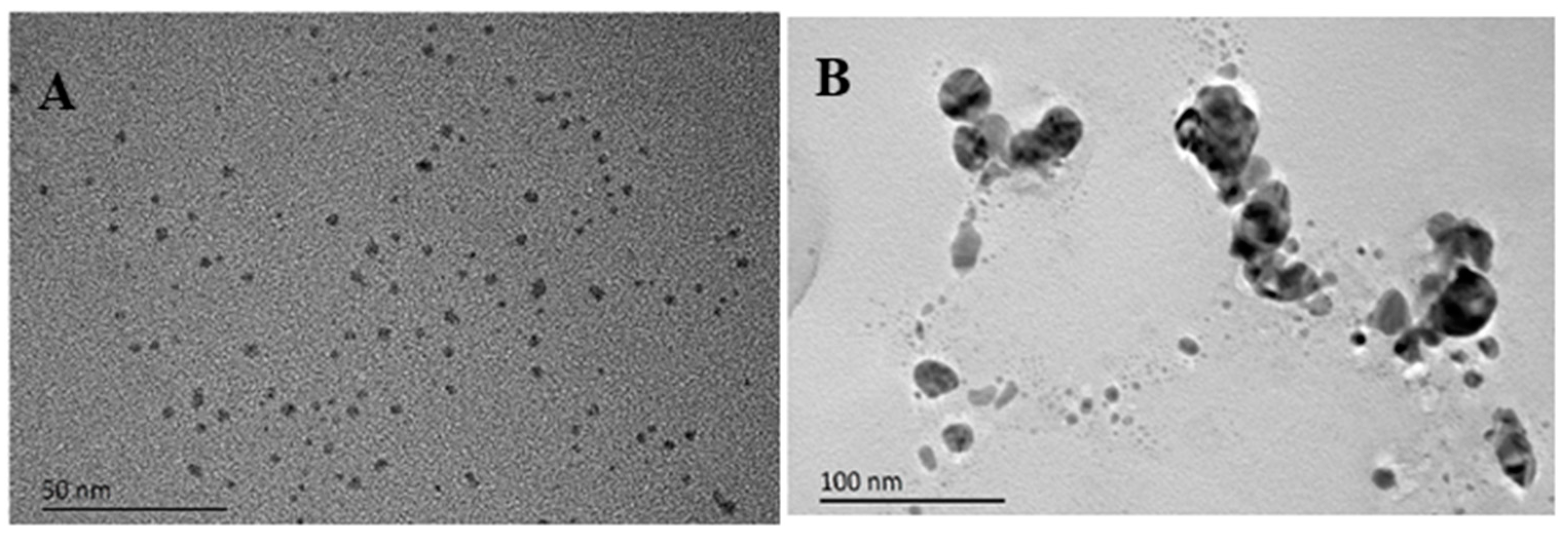
3.3. Characterization of Morphology and Size
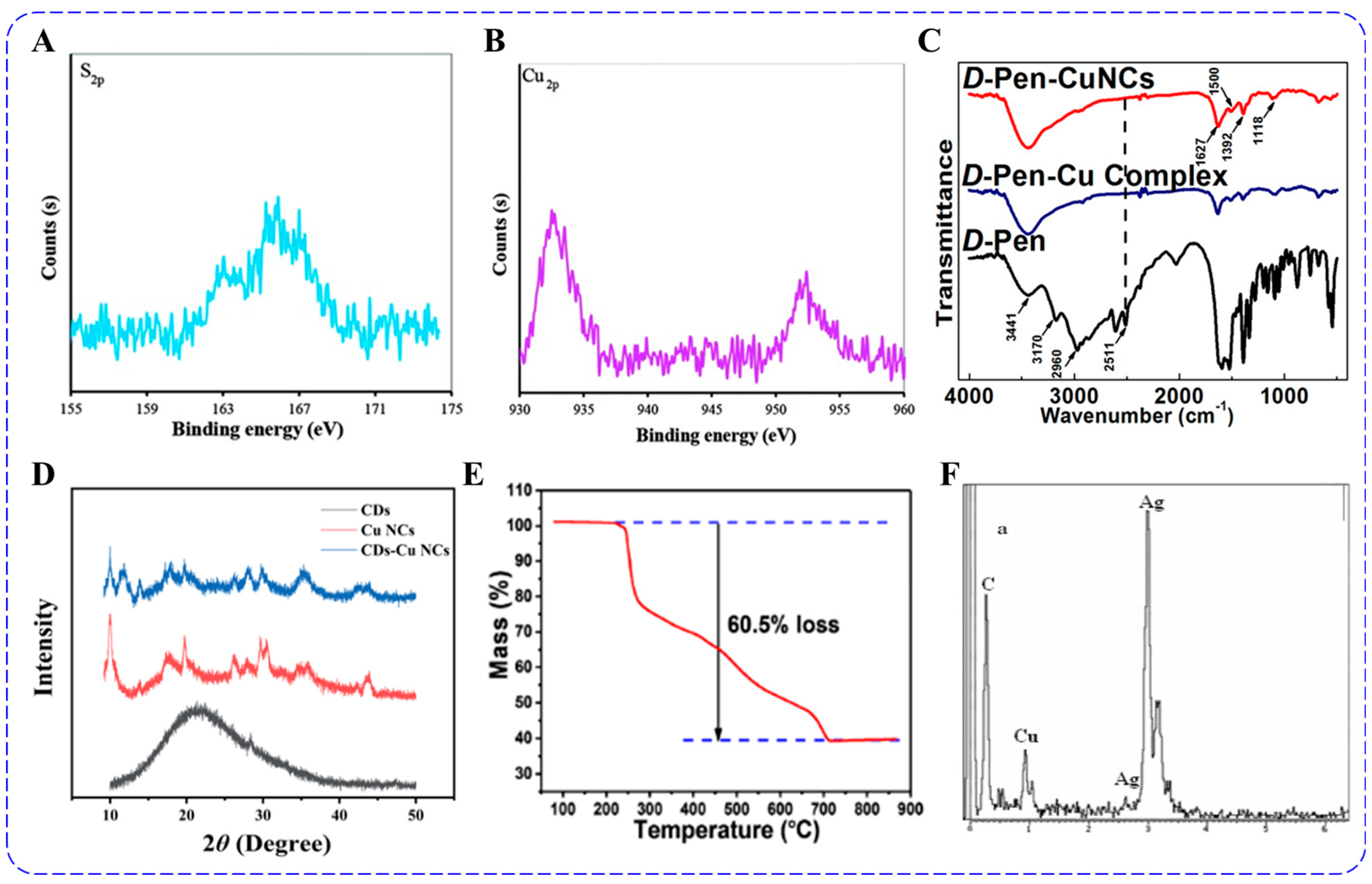
3.4. XPS and FT-IR
3.5. Others
3.6. Factors Affecting the Fluorescence of Copper Nanoclusters
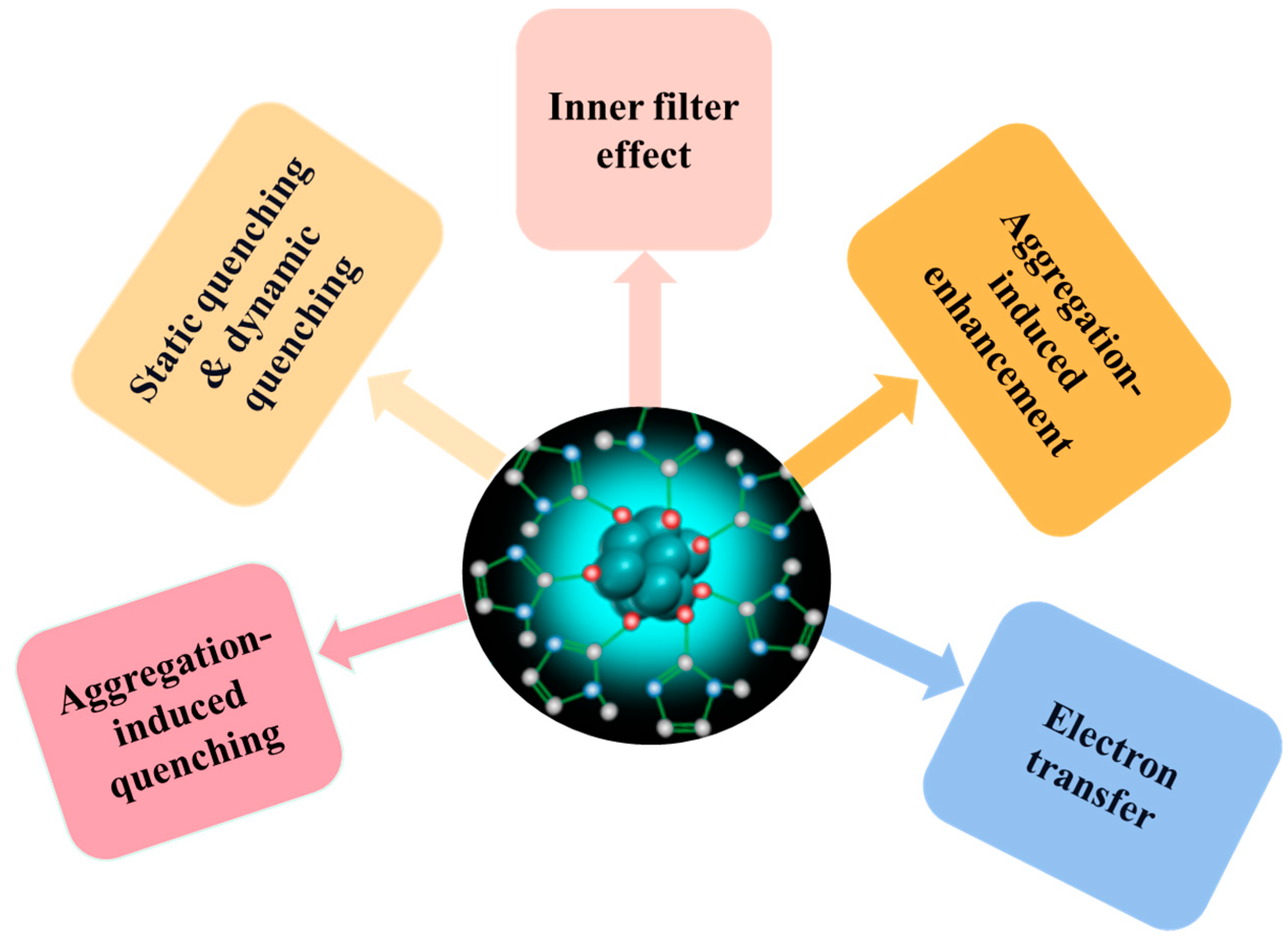
4. Mechanism of Fluorescent Sensors
4.1. Aggregation-Induced Quenching
4.2. Static Quenching & Dynamic Quenching
4.3. Inner Filter Effect
4.4. Aggregation-Induced Enhancement
4.5. Other Mechanisms
5. Copper Nanoclusters for Heavy Metal Ions Detection
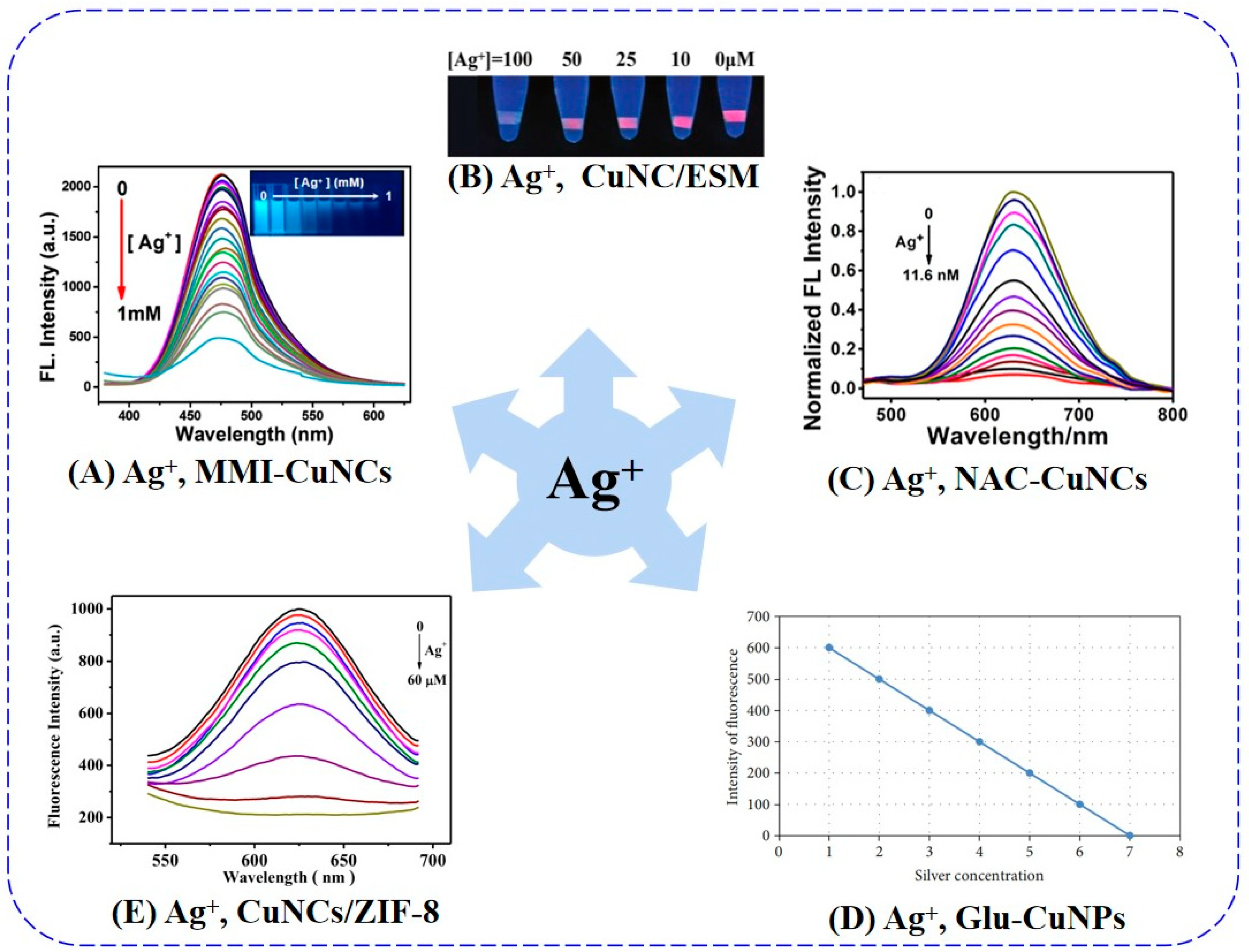
5.1. Silver Ion
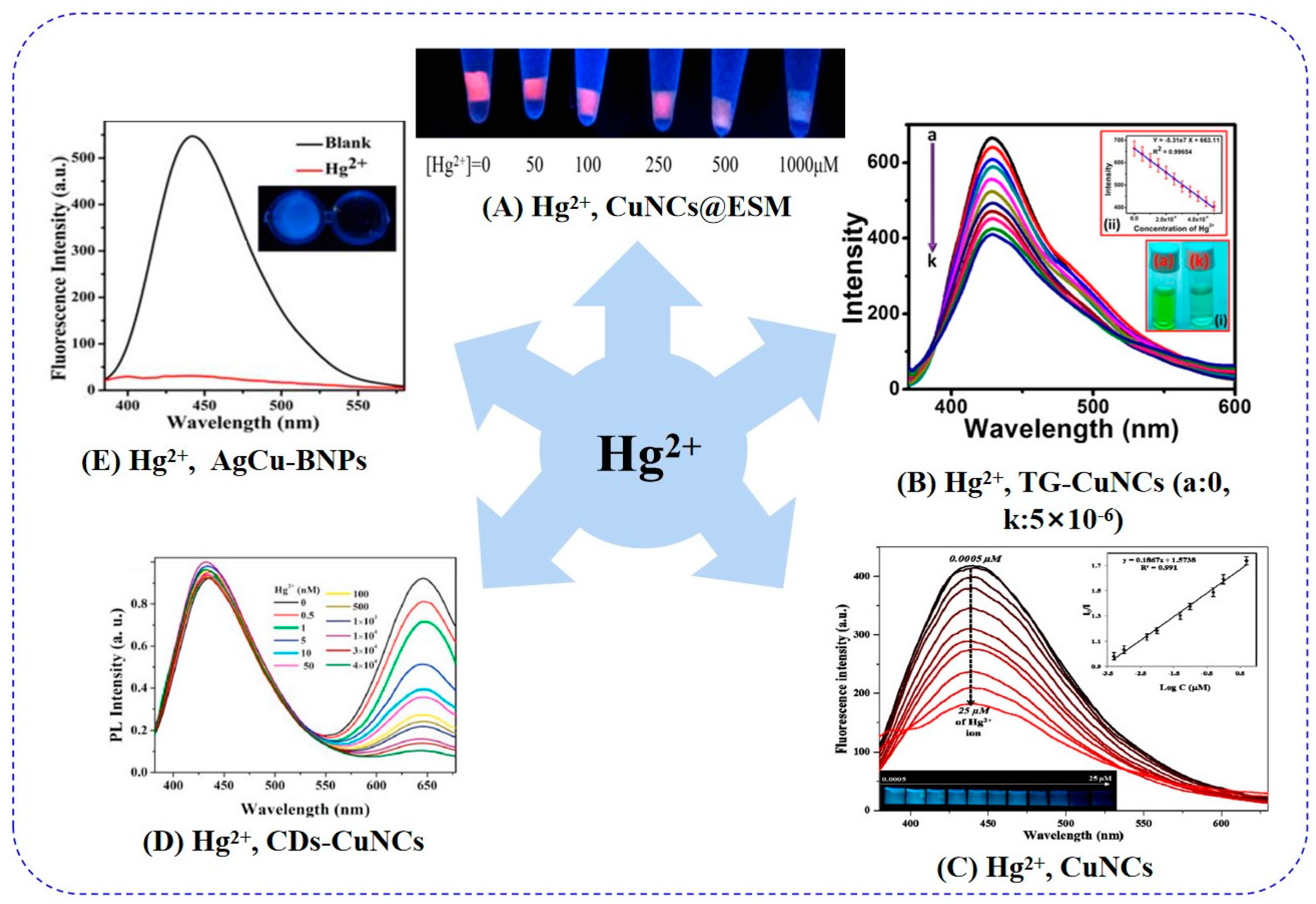
5.2. Mercury Ion
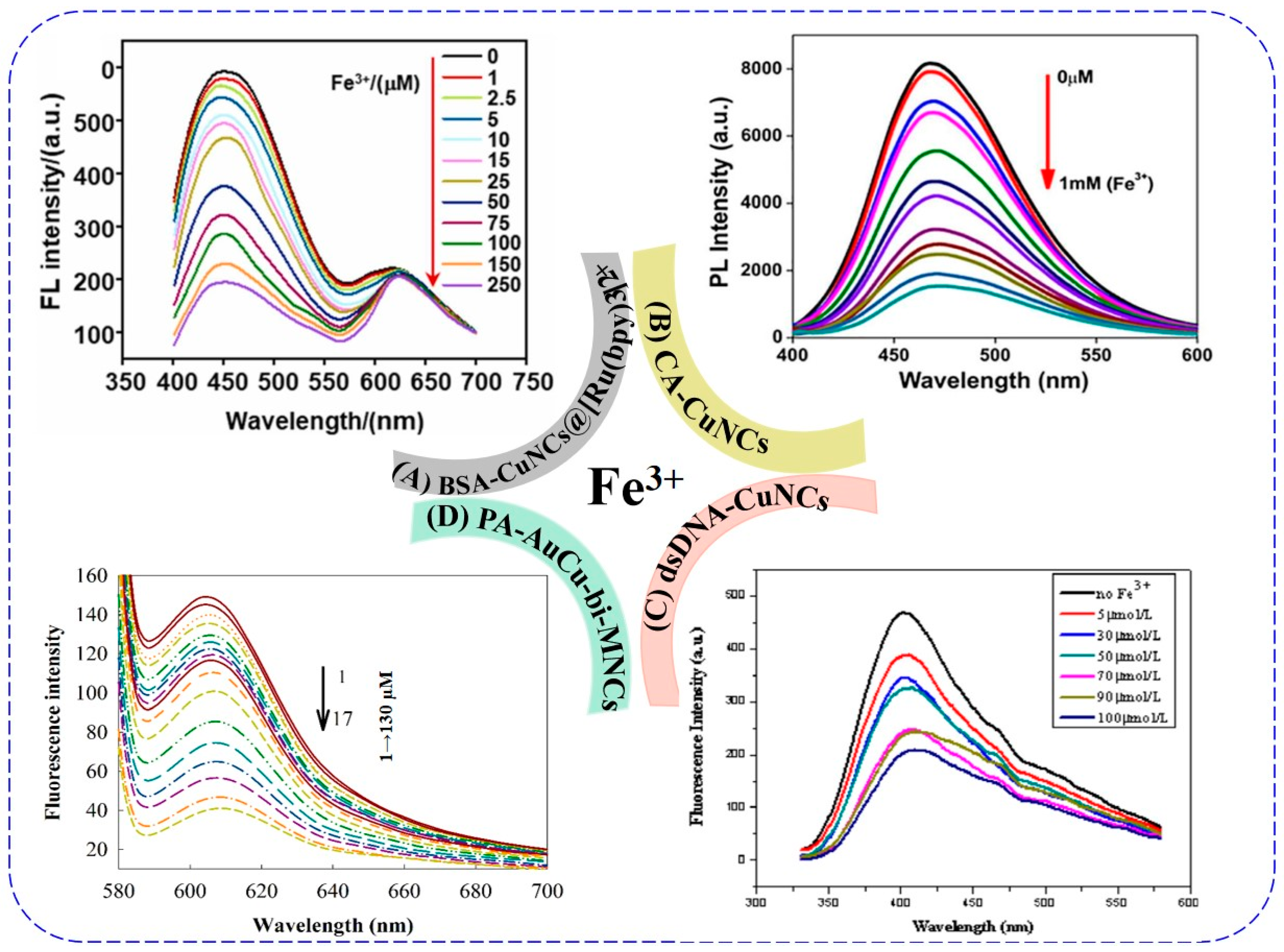
5.3. Iron Ions
5.4. Cobalt Ion
5.5. Other Ions
5.6. Selectivity Difference of CuNCs in Detecting HMIs
6. Outlook and Challenges
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gumpu, M.B.; Sethuraman, S.; Krishnan, U.M.; Rayappan, J.B.B. A review on detection of heavy metal ions in water—An electrochemical approach. Sensor Actuators B Chem. 2015, 213, 515–533. [Google Scholar] [CrossRef]
- Bansod, B.; Kumar, T.; Thakur, R.; Rana, S.; Singh, I. A review on various electrochemical techniques for heavy metal ions detection with different sensing platforms. Biosens. Bioelectron. 2017, 94, 443–455. [Google Scholar] [CrossRef]
- Nayan Kumar, H.N.; Nagaraju, D.H.; Yhobu, Z.; Shivakumar, P.; Manjunatha Kumara, K.S.; Budagumpi, S.; Praveen, B.M. Recent advances in on-site monitoring of heavy metal ions in the environment. Microchem. J. 2022, 182, 107894. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Soliman, N.K.; Moustafa, A.F. Industrial solid waste for heavy metals adsorption features and challenges; a review. J. Mater. Res. Technol. 2020, 9, 10235–10253. [Google Scholar] [CrossRef]
- Lu, S.; Hummel, M.; Chen, K.; Zhou, Y.; Kang, S.; Gu, Z. Synthesis of Au@ZIF-8 nanocomposites for enhanced electrochemical detection of dopamine. Electrochem. Commun. 2020, 114, 106715. [Google Scholar] [CrossRef]
- Nie, M.; Lu, S.; Lei, D.; Yang, C.; Zhao, Z. Rapid Synthesis of ZIF-8 Nanocrystals for Electrochemical Detection of Dopamine. J. Electrochem. Soc. 2017, 164, H952–H957. [Google Scholar] [CrossRef]
- Rotake, D.; Darji, A.D. Heavy Metal Ion Detection in Water using MEMS Based Sensor. Mater. Today Proc. 2018, 5, 1530–1536. [Google Scholar] [CrossRef]
- Xu, N.; Jin, S.; Wang, L. Metal nanoparticles-based nanoplatforms for colorimetric sensing: A review. Rev. Anal. Chem. 2021, 40, 1–11. [Google Scholar] [CrossRef]
- Lu, S.; Hummel, M.; Kang, S.; Gu, Z. Selective Voltammetric Determination of Nitrite Using Cobalt Phthalocyanine Modified on Multiwalled Carbon Nanotubes. J. Electrochem. Soc. 2020, 167, 7982. [Google Scholar] [CrossRef]
- Lu, S.; Jia, H.; Hummel, M.; Wu, Y.; Wang, K.; Qi, X.; Gu, Z. Two-dimensional conductive phthalocyanine-based metal-organic frameworks for electrochemical nitrite sensing. RSC Adv. 2021, 11, 4472–4477. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Yang, C.; Nie, M. Hydrothermal synthesized urchin-like nickel-cobalt carbonate hollow spheres for sensitive amperometric detection of nitrite. J. Alloy. Compd. 2017, 708, 780–786. [Google Scholar] [CrossRef]
- Zhu, Y.; Lu, S.; Gowri Manohari, A.; Dong, X.; Chen, F.; Xu, W.; Shi, Z.; Xu, C. Polydopamine interconnected graphene quantum dots and gold nanoparticles for enzymeless H2O2 detection. J. Electroanal. Chem. 2017, 796, 75–81. [Google Scholar] [CrossRef]
- Dong, X.; Xu, C.; Lu, S.; Wang, R.; Shi, Z.; Cui, Q.; You, T. ZIF-8 Coupling with Reduced Graphene Oxide to Enhance the Electrochemical Sensing of Dopamine. J. Electrochem. Soc. 2021, 168, 116517. [Google Scholar] [CrossRef]
- Lu, S.; Hummel, M.; Gu, Z.; Gu, Y.; Cen, Z.; Wei, L.; Zhou, Y.; Zhang, C.; Yang, C. Trash to treasure: A novel chemical route to synthesis of NiO/C for hydrogen production. Int. J. Hydrog. Energy 2019, 44, 16144–16153. [Google Scholar] [CrossRef]
- Wan, N.; Wang, T.; Tan, X.-y.; Lu, S.; Zhou, L.-l.; Huang, J.-q.; Pan, W.; Yang, Y.-m.; Shao, Z.-y. Microstructure Related Synergic Sensoring Mechanism in Graphene Oxide Humidity Sensor. J. Phys. Chem. C 2018, 122, 830–838. [Google Scholar] [CrossRef]
- Lu, S.; Hummel, M.; Wang, X.; He, W.; Pathak, R.; Dong, X.; Jia, H.; Gu, Z. Communication—In Situ Electrodeposition of Nickel Phosphide on Ni Foam for Non-Enzymatic Detection of Nitrite. J. Electrochem. Soc. 2020, 167, 146517. [Google Scholar] [CrossRef]
- Lu, S.; Wang, Y.; Xiang, H.; Lei, H.; Xu, B.B.; Xing, L.; Yu, E.H.; Liu, T.X. Mass transfer effect to electrochemical reduction of CO2: Electrode, electrocatalyst and electrolyte. J. Energy Storage 2022, 52, 104764. [Google Scholar] [CrossRef]
- Waheed, A.; Mansha, M.; Ullah, N. Nanomaterials-based electrochemical detection of heavy metals in water: Current status, challenges and future direction. Trac Trend. Anal. Chem. 2018, 105, 37–51. [Google Scholar] [CrossRef]
- Wang, Y.; Lei, H.; Lu, S.; Yang, Z.; Xu, B.B.; Xing, L.; Liu, T.X. Cu2O nano-flowers/graphene enabled scaffolding structure catalyst layer for enhanced CO2 electrochemical reduction. Appl. Catal. B 2022, 305, 121022. [Google Scholar] [CrossRef]
- Brzechwa-Chodzyńska, A.; Drożdż, W.; Harrowfield, J.; Stefankiewicz, A.R. Fluorescent sensors: A bright future for cages. Coord. Chem. Rev. 2021, 434, 213820. [Google Scholar] [CrossRef]
- Qin, D.-D.; Tang, Y.; Ma, G.; Qin, L.; Tao, C.-L.; Zhang, X.; Tang, Z. Molecular metal nanoclusters for ORR, HER and OER: Achievements, opportunities and challenges. Int. J. Hydrogen. Energ. 2021, 46, 25771–25781. [Google Scholar] [CrossRef]
- Xiao, Y.; Wu, Z.; Yao, Q.; Xie, J. Luminescent metal nanoclusters: Biosensing strategies and bioimaging applications. Agg 2021, 2, 114–132. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Y. Developing fluorescent copper nanoclusters: Synthesis, properties, and applications. Colloids Surf. B 2020, 195, 111244. [Google Scholar] [CrossRef]
- Guo, Y.; Cao, F.; Lei, X.; Mang, L.; Cheng, S.; Song, J. Fluorescent copper nanoparticles: Recent advances in synthesis and applications for sensing metal ions. Nanoscale 2016, 8, 4852–4863. [Google Scholar] [CrossRef]
- Ou, G.; Zhao, J.; Chen, P.; Xiong, C.; Dong, F.; Li, B.; Feng, X. Fabrication and application of noble metal nanoclusters as optical sensors for toxic metal ions. Anal. Bioanal. Chem. 2018, 410, 2485–2498. [Google Scholar] [CrossRef] [PubMed]
- Maity, S.; Bain, D.; Patra, A. An overview on the current understanding of the photophysical properties of metal nanoclusters and their potential applications. Nanoscale 2019, 11, 22685–22723. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Peng, Y.; Li, S.; Mu, J.; Huang, Z.; Ma, J.; Shi, Z.; Jia, Q. Metal nanocluster-based hybrid nanomaterials: Fabrication and application. Coord. Chem. Rev. 2022, 456, 214391. [Google Scholar] [CrossRef]
- Borse, S.; Jha, S.; Murthy, Z.V.P.; Kailasa, S.K. Sustainable chemistry approach for the preparation of bluish green emissive copper nanoclusters from Justicia adhatoda leaves extract: A facile analytical approach for the sensing of myoglobin and l-thyroxine. New J. Chem. 2022, 46, 15919–15928. [Google Scholar] [CrossRef]
- An, Y.; Ren, Y.; Bick, M.; Dudek, A.; Hong-Wang Waworuntu, E.; Tang, J.; Chen, J.; Chang, B. Highly fluorescent copper nanoclusters for sensing and bioimaging. Biosens. Bioelectron. 2020, 154, 112078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.-S.; Lin, Y.-F.; Nain, A.; Huang, Y.-F.; Chang, H.-T. A critical review of copper nanoclusters for monitoring of water quality. Sens. Actuators Rep. 2021, 3, 10026. [Google Scholar] [CrossRef]
- Bigdeli, A.; Ghasemi, F.; Abbasi-Moayed, S.; Shahrajabian, M.; Fahimi-Kashani, N.; Jafarinejad, S.; Farahmand Nejad, M.A.; Hormozi-Nezhad, M.R. Ratiometric fluorescent nanoprobes for visual detection: Design principles and recent advances—A review. Anal. Chim. Acta 2019, 1079, 30–58. [Google Scholar] [CrossRef]
- Dong, W.; Wang, R.; Gong, X.; Liang, W.; Dong, C. A far-red FRET fluorescent probe for ratiometric detection of l-cysteine based on carbon dots and N-acetyl-l-cysteine-capped gold nanoparticles. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 213, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.-H.; Teresa Gutierrez-Wing, M.; Choi, J.-W. Review—Recent Progress in Portable Fluorescence Sensors. J. Electrochem. Soc. 2021, 168, 017502. [Google Scholar] [CrossRef]
- Baghdasaryan, A.; Burgi, T. Copper nanoclusters: Designed synthesis, structural diversity, and multiplatform applications. Nanoscale 2021, 13, 6283–6340. [Google Scholar] [CrossRef]
- Babu Busi, K.; Palanivel, M.; Kanta Ghosh, K.; Basu Ball, W.; Gulyas, B.; Padmanabhan, P.; Chakrabortty, S. The Multifarious Applications of Copper Nanoclusters in Biosensing and Bioimaging and Their Translational Role in Early Disease Detection. Nanomaterials 2022, 12, 301. [Google Scholar] [CrossRef]
- Lu, Y.; Wei, W.; Chen, W. Copper nanoclusters: Synthesis, characterization and properties. Chinese Sci. Bull. 2012, 57, 41–47. [Google Scholar] [CrossRef] [Green Version]
- Deng, H.H.; Li, K.L.; Zhuang, Q.Q.; Peng, H.P.; Zhuang, Q.Q.; Liu, A.L.; Xia, X.H.; Chen, W. An ammonia-based etchant for attaining copper nanoclusters with green fluorescence emission. Nanoscale 2018, 10, 6467–6473. [Google Scholar] [CrossRef]
- Nerthigan, Y.; Sharma, A.K.; Pandey, S.; Wu, H.F. Cysteine capped copper/molybdenum bimetallic nanoclusters for fluorometric determination of methotrexate via the inner filter effect. Mikrochim. Acta 2019, 186, 130. [Google Scholar] [CrossRef]
- Zhou, F.; Cui, X.; Shang, A.; Lian, J.; Yang, L.; Jin, Y.; Li, B. Fluorometric determination of the activity and inhibition of terminal deoxynucleotidyl transferase via in-situ formation of copper nanoclusters using enzymatically generated DNA as template. Microchim. Acta 2017, 184, 773–779. [Google Scholar] [CrossRef]
- Jiao, M.; Li, Y.; Jia, Y.; Xu, L.; Xu, G.; Guo, Y.; Luo, X. Ligand-modulated aqueous synthesis of color-tunable copper nanoclusters for the photoluminescent assay of Hg(II). Mikrochim. Acta 2020, 187, 545. [Google Scholar] [CrossRef]
- Lettieri, M.; Palladino, P.; Scarano, S.; Minunni, M. Protein-templated copper nanoclusters for fluorimetric determination of human serum albumin. Microchimic. Acta 2021, 188, 1–9. [Google Scholar] [CrossRef]
- Tang, T.; Ouyang, J.; Hu, L.; Guo, L.; Yang, M.; Chen, X. Synthesis of peptide templated copper nanoclusters for fluorometric determination of Fe(III) in human serum. Microchimic. Acta 2016, 183, 2831–2836. [Google Scholar] [CrossRef]
- Fang, J.; Zhang, B.; Yao, Q.; Yang, Y.; Xie, J.; Yan, N. Recent advances in the synthesis and catalytic applications of ligand-protected, atomically precise metal nanoclusters. Coord. Chem. Rev. 2016, 322, 1–29. [Google Scholar] [CrossRef]
- Kardar, Z.S.; Shemirani, F.; Zadmard, R. Determination of iron(II) and iron(III) via static quenching of the fluorescence of tryptophan-protected copper nanoclusters. Mikrochim. Acta 2020, 187, 81. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.-P.; Shen, Y.-L.; Duan, G.-X.; Han, J.; Zhang, L.-P.; Lu, X. Silver nanoclusters: Synthesis, structures and photoluminescence. Mater. Chem. Front. 2020, 4, 2205–2222. [Google Scholar] [CrossRef]
- Si, H.; Shu, T.; Du, X.; Su, L.; Zhang, X. An Overview on Coinage Metal Nanocluster-Based Luminescent Biosensors via Etching Chemistry. Biosensors 2022, 12, 511. [Google Scholar] [CrossRef]
- Li, Q.; Li, Y.; Li, H.; Yan, X.; Han, G.; Chen, F.; Song, Z.; Zhang, J.; Fan, W.; Yi, C.; et al. Highly Luminescent Copper Nanoclusters Stabilized by Ascorbic Acid for the Quantitative Detection of 4-Aminoazobenzene. Nanomaterials 2020, 10, 531. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Pang, R.; Song, D.; Li, M.B. Tailoring silver nanoclusters via doping: Advances and opportunities. Nanoscale Adv. 2021, 3, 2411–2422. [Google Scholar] [CrossRef] [PubMed]
- Qing, T.; Zhang, K.; Qing, Z.; Wang, X.; Long, C.; Zhang, P.; Hu, H.; Feng, B. Recent progress in copper nanocluster-based fluorescent probing: A review. Mikrochim. Acta 2019, 186, 670. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Fang, Y.; Miao, Z.; Ma, M.; Du, X.; Takahashi, S.; Anzai, J.-i.; Chen, Q. Direct electrodeposion of reduced graphene oxide and dendritic copper nanoclusters on glassy carbon electrode for electrochemical detection of nitrite. Electrochim. Acta 2013, 107, 656–663. [Google Scholar] [CrossRef]
- Liu, X.; Shao, C.; Chen, T.; He, Z.; Du, G. Stable silver nanoclusters with aggregation-induced emission enhancement for detection of aluminum ion. Sens. Actuat B-Chem. 2019, 278, 181–189. [Google Scholar] [CrossRef]
- Zhang, C.; Shao, C.; Wang, J.; Li, Z.; Liang, M.; Wang, Y.; Liu, D.; Lu, S. Multifunctional Fluorescent Copper Nanoclusters for Ag+ Sensing, Anticounterfeiting, and Blue/White Light-Emitting Diodes. ACS Appl. Nano Mater. 2022, 5, 7449–7459. [Google Scholar] [CrossRef]
- Shao, C.; Li, C.; Zhang, C.; Ni, Z.; Liu, X.; Wang, Y. Novel synthesis of orange-red emitting copper nanoclusters stabilized by methionine as a fluorescent probe for norfloxacin sensing. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 236, 118334. [Google Scholar] [CrossRef]
- Cao, X.; Shao, C.; Zhang, C.; Liang, M.; Wang, Y.; Cheng, J.; Lu, S. Yeast powder derived carbon quantum dots for dopamine detection and living cell imaging. Anal. Methods 2022, 14, 1342–1350. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Shao, C.; Li, L.; Liu, X.; Liu, M. In situ fabrication of a luminescent copper nanocluster/eggshell membrane composite and its application in visual detection of Ag+ ions, light-emitting diodes and surface patterning. Photoch. Photobiol. Sci. 2019, 18, 2942–2951. [Google Scholar] [CrossRef]
- Hu, X.; Liu, T.; Zhuang, Y.; Wang, W.; Li, Y.; Fan, W.; Huang, Y. Recent advances in the analytical applications of copper nanoclusters. Trac-Trend Anal. Chem. 2016, 77, 66–75. [Google Scholar] [CrossRef]
- Pan, M.-C.; Lei, Y.-M.; Chai, Y.-Q.; Yuan, R.; Zhuo, Y. In situ controllable generation of copper nanoclusters confined in a poly-L-cysteine porous film with enhanced electrochemiluminescence for alkaline phosphatase detection. Anal. Chem. 2020, 92, 13581–13587. [Google Scholar] [CrossRef]
- Fang, L.; Wang, S.; Song, C.; Lu, S.; Yang, X.; Qi, X.; Liu, H. Boosting nitrate electroreduction to ammonia via in situ generated stacking faults in oxide-derived copper. Chem. Eng. J. 2022, 446, 137341. [Google Scholar] [CrossRef]
- Ruiyi, L.; Huiying, W.; Xiaoyan, Z.; Xiaoqing, L.; Xiulan, S.; Zaijun, L. D-Penicillamine and bovine serum albumin co-stabilized copper nanoclusters with remarkably enhanced fluorescence intensity and photostability for ultrasensitive detection of Ag+. New J. Chem. 2016, 40, 732–739. [Google Scholar] [CrossRef]
- Long, T.; Guo, Y.; Lin, M.; Yuan, M.; Liu, Z.; Huang, C. Optically active red-emitting Cu nanoclusters originating from complexation and redox reaction between copper (II) and D/L-penicillamine. Nanoscale 2016, 8, 9764–9770. [Google Scholar] [CrossRef]
- Lin, S.; Dong, J.; Zhang, B.; Yuan, Z.; Lu, C.; Han, P.; Xu, J.; Jia, L.; Wang, L. Synthesis of bifunctional fluorescent nanohybrids of carbon dots–copper nanoclusters via a facile method for Fe3+ and Tb3+ ratiometric detection. Anal. Methods 2021, 13, 3577–3584. [Google Scholar] [CrossRef]
- Li, G.; Luo, Y. Preparation and Characterization of Dendrimer-Templated Ag−Cu Bimetallic Nanoclusters. Inorg. Chem. 2008, 47, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Koyappayil, A.; Kim, H.T.; Lee, M.-H. An efficient and rapid synthesis route to highly fluorescent copper microspheres for the selective and sensitive excitation wavelength-dependent dual-mode sensing of NADH. Sens. Actuators B Chem. 2021, 327, 128887. [Google Scholar] [CrossRef]
- Mu, J.; Peng, Y.; Shi, Z.; Zhang, D.; Jia, Q. Copper nanocluster composites for analytical (bio)-sensing and imaging: A review. Mikrochim. Acta 2021, 188, 384. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Umar, K.; Ibrahim, M.N.M. Silver nanoparticles: Various methods of synthesis, size affecting factors and their potential applications–a review. Appl. Nanosci. 2020, 10, 1369–1378. [Google Scholar] [CrossRef]
- Pandit, S.; Kundu, S. pH-Dependent reversible emission behaviour of lysozyme coated fluorescent copper nanoclusters. J. Lumin 2020, 228, 117607. [Google Scholar] [CrossRef]
- Yang, Y.; Wei, Q.; Zou, T.; Kong, Y.; Su, L.; Ma, D.; Wang, Y. Dual-emission ratiometric fluorescent detection of dinotefuran based on sulfur-doped carbon quantum dots and copper nanocluster hybrid. Sens. Actuators B Chem. 2020, 321. [Google Scholar] [CrossRef]
- Peng, S.-K.; Yang, H.; Luo, D.; Xie, M.; Tang, W.-J.; Ning, G.-H.; Li, D. Enhancing photoluminescence efficiency of atomically precise copper(i) nanoclusters through a solvent-induced structural transformation. Inorg. Chem. Front 2022, 9, 5327–5334. [Google Scholar] [CrossRef]
- Islam, M.M.; Hu, Z.; Wang, Q.; Redshaw, C.; Feng, X. Pyrene-based aggregation-induced emission luminogens and their applications. Mater. Chem. Front. 2019, 3, 762–781. [Google Scholar] [CrossRef]
- Shao, C.; Xiong, S.; Cao, X.; Zhang, C.; Luo, T.; Liu, G. Dithiothreitol-capped red emitting copper nanoclusters as highly effective fluorescent nanoprobe for cobalt (II) ions sensing. Microchem. J. 2021, 163, 105922. [Google Scholar] [CrossRef]
- Tanwar, A.S.; Meher, N.; Adil, L.R.; Iyer, P.K. Stepwise elucidation of fluorescence based sensing mechanisms considering picric acid as a model analyte. Analyst 2020, 145, 4753–4767. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Shi, S.; Fan, C.; Jin, D. Fluorescent determination of fluazinam with polyethyleneimine-capped copper nanoclusters. Chem. Phys. Lett. 2020, 754, 137748. [Google Scholar] [CrossRef]
- Xue, R.; Geng, X.; Liang, F.; Liu, Y.; Yang, W.; Huang, Z. Natural plant compounds in synthesis and luminescence modulation of metal nanoclusters: Toward sustainable nanoprobes for sensing and bioimaging. Mater. Today Adv. 2022, 16, 100279. [Google Scholar] [CrossRef]
- Zhang, Y.; Deng, Q.; Tang, C.; Zhang, M.; Huang, Z.; Cai, Z. Fluorescent folic acid-capped copper nanoclusters for the determination of rifampicin based on inner filter effect. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023, 286, 121944. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Jiao, Y.; Gao, Z.; Yang, Y.; Li, H. N, S co-doped carbon dots for temperature probe and the detection of tetracycline based on the inner filter effect. J. Photoch. Photobio. A 2018, 367, 137–144. [Google Scholar] [CrossRef]
- Zheng, X.; Chen, Q.; Zhang, Z.; Wang, Z.; Gong, Z. An aggregation-induced emission copper nanoclusters fluorescence probe for the sensitive detection of tetracycline. Microchem. J. 2022, 180, 107570. [Google Scholar] [CrossRef]
- Sivakumar, R.; Lee, N.Y. Recent progress in smartphone-based techniques for food safety and the detection of heavy metal ions in environmental water. Chemosphere 2021, 275, 130096. [Google Scholar] [CrossRef]
- Zhang, M.; Cui, X.; Li, N. Smartphone-based mobile biosensors for the point-of-care testing of human metabolites. Mater. Today Bio. 2022, 14, 100254. [Google Scholar] [CrossRef]
- Fernandes, G.M.; Silva, W.R.; Barreto, D.N.; Lamarca, R.S.; Lima Gomes, P.C.F.; da Silveira Petruci, J.F.; Batista, A.D. Novel approaches for colorimetric measurements in analytical chemistry—A review. Anal. Chim. Acta 2020, 1135, 187–203. [Google Scholar] [CrossRef]
- Yan, Z.; Cai, Y.; Zhang, J.; Zhao, Y. Fluorescent sensor arrays for metal ions detection: A review. Measurement 2022, 187, 110355. [Google Scholar] [CrossRef]
- Fu, L.; Wang, A.; Xie, K.; Zhu, J.; Chen, F.; Wang, H.; Zhang, H.; Su, W.; Wang, Z.; Zhou, C.; et al. Electrochemical detection of silver ions by using sulfur quantum dots modified gold electrode. Sens. Actuators B Chem. 2020, 304, 127390. [Google Scholar] [CrossRef]
- Xu, G.; Wang, G.; Zhu, Y.; Chen, L.; He, X.; Wang, L.; Zhang, X. Amplified and selective detection of Ag+ ions based on electrically contacted enzymes on duplex-like DNA scaffolds. Biosens. Bioelectron. 2014, 59, 269–275. [Google Scholar] [CrossRef]
- Kang, J.; Gao, P.; Zhang, G.; Shi, L.; Zhou, Y.; Wu, J.; Shuang, S.; Zhang, Y. Rapid sonochemical synthesis of copper nanoclusters with red fluorescence for highly sensitive detection of silver ions. Microchem. J. 2022, 178, 107370. [Google Scholar] [CrossRef]
- Arthy, M.; Brindha, J.; Viswanathan, S.; Gnanasekar, A.K.; Nanammal, V.; Raju, R.; Al Obaid, S.; Almoallim, H.S.; Selva, A.; Velmurugan, P. Detection of Ag+ by Synthesizing Fluorescent Copper Nanoparticles through Ultrasensitive Free Label Approach. J. Nanomater. 2022, 2022, 8642134. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, X.; Cao, H.; Huang, Y. Modulating the size and photoluminescence of a copper nanocluster via metal-organic frameworks encapsulating strategy for fluorescence sensing. Microchem. J. 2022, 182, 107876. [Google Scholar] [CrossRef]
- Yang, D.; Zhou, T.; Tu, Y.; Yan, J. Detection of silver through amplified quenching of fluorescence from polyvinyl pyrrolidone-stabilized copper nanoclusters. Mikrochim. Acta 2021, 188, 212. [Google Scholar] [CrossRef]
- Lu, S.; Gu, Z.; Hummel, M.; Zhou, Y.; Wang, K.; Xu, B.B.; Wang, Y.; Li, Y.; Qi, X.; Liu, X. Nickel Oxide Immobilized on the Carbonized Eggshell Membrane for Electrochemical Detection of Urea. J. Electrochem. Soc. 2020, 167, 106509. [Google Scholar] [CrossRef]
- Lu, S.; Hummel, M.; Kang, S.; Pathak, R.; He, W.; Qi, X.; Gu, Z. Density Functional Theory Investigation of the NiO@Graphene Composite as a Urea Oxidation Catalyst in the Alkaline Electrolyte. ACS Omega. 2021, 6(22), 14648–14654. [Google Scholar] [CrossRef]
- Lu, S.; Hummel, M.; Gu, Z.; Wang, Y.; Wang, K.; Pathak, R.; Zhou, Y.; Jia, H.; Qi, X.; Zhao, X.; et al. Highly Efficient Urea Oxidation via Nesting Nano-Nickel Oxide in Eggshell Membrane-Derived Carbon. ACS Sustain. Chem. Eng. 2021, 9, 1703–1713. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Han, X.; Zhang, L.; Wang, X.; Chen, L. Fluorescent probe for mercury ion imaging analysis: Strategies and applications. Chem. Eng. J. 2021, 406, 127166. [Google Scholar] [CrossRef]
- Bernhoft, R.A. Mercury toxicity and treatment: A review of the literature. J. Environ. Public Health. 2012, 2012, 460508. [Google Scholar] [CrossRef]
- Li, L.; Huang, M.; Liu, X.; Sun, D.; Shao, C. In Situ Generation of Fluorescent Copper Nanoclusters Embedded in Monolithic Eggshell Membrane: Properties and Applications. Materials 2018, 11, 1913. [Google Scholar] [CrossRef] [Green Version]
- Maruthupandi, M.; Thiruppathi, D.; Vasimalai, N. One minute synthesis of green fluorescent copper nanocluster: The preparation of smartphone aided paper-based kit for on-site monitoring of nanomolar level mercury and sulfide ions in environmental samples. J. Hazard. Mater. 2020, 392, 122294. [Google Scholar] [CrossRef]
- Bhamore, J.R.; Deshmukh, B.; Haran, V.; Jha, S.; Singhal, R.K.; Lenka, N.; Kailasa, S.K.; Murthy, Z.V.P. One-step eco-friendly approach for the fabrication of synergistically engineered fluorescent copper nanoclusters: Sensing of Hg2+ ion and cellular uptake and bioimaging properties. New J. Chem. 2018, 42, 1510–1520. [Google Scholar] [CrossRef]
- Li, Z.; Pang, S.; Wang, M.; Wu, M.; Li, P.; Bai, J.; Yang, X. Dual-emission carbon dots-copper nanoclusters ratiometric photoluminescent nano-composites for highly sensitive and selective detection of Hg2+. Ceram. Int. 2021, 47, 18238–18245. [Google Scholar] [CrossRef]
- Pang, J.; Xie, R.; Chua, S.; Zou, Y.; Tang, M.; Zhang, F.; Chai, F. Preparation of fluorescent bimetallic silver/copper nanoparticles and their utility of dual-mode fluorimetric and colorimetric probe for Hg2+. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 261, 120035. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Shao, C.; Zhang, Q.; Zhang, C.; Wang, Y.; Zheng, X.; Lu, S. High-Performance Formaldehyde Sensing Using Paper-Based Fluorescent Copper Nanoclusters. IEEE Sens. J. 2023, 23, 2076–2084. [Google Scholar] [CrossRef]
- Chiang, T.H.; Hsiao, H.H. Single drop analysis of mercury ions by rational design of peptide coated gold nanoparticles integrated with MALDI-MS measurement. Talanta 2023, 253, 123913. [Google Scholar] [CrossRef]
- Zhang, C.; Liang, M.; Shao, C.; Li, Z.; Cao, X.; Wang, Y.; Wu, Y.; Lu, S. Visual Detection and Sensing of Mercury Ions and Glutathione Using Fluorescent Copper Nanoclusters. ACS Appl. Bio Mater. 2023. [Google Scholar] [CrossRef] [PubMed]
- Shojaeifard, Z.; Heidari, N.; Hemmateenejad, B. Bimetallic AuCu nanoclusters-based florescent chemosensor for sensitive detection of Fe3+ in environmental and biological systems. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 209, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Kalaiyarasan, G.; Joseph, J.; Kumar, P. Phosphorus-Doped Carbon Quantum Dots as Fluorometric Probes for Iron Detection. ACS Omega 2020, 5, 22278–22288. [Google Scholar] [CrossRef]
- Wang, Y.; Jian, J.; Sun, B.; Wei, Y.; Pan, D.; Cao, J.; Shen, Y. Engineering of onsite point-of-care testing of Fe3+ with visual ratiometric fluorescent signals of copper nanoclusters-driven portable smartphone. Sensor Actuators B-Chem. 2022, 370, 132413. [Google Scholar] [CrossRef]
- Tai, Y.-T.; Simon, T.; Chu, Y.-Y.; Ko, F.-H. One-pot synthesis of copper nanoconjugate materials as luminescent sensor for Fe3+ and I− detection in human urine sample. Sens. Bio-Sens. Res. 2020, 27, 100319. [Google Scholar] [CrossRef]
- Wuri, H.; Ai, J.; Ga, L. Template method synthesis of highly fluorescent duplex oligonucleotide copper nanomaterials for Fe3+ sensing. Mater. Res. Express 2020, 7, 125001. [Google Scholar] [CrossRef]
- Singh, R.; Majhi, S.; Sharma, K.; Ali, M.; Sharma, S.; Choudhary, D.; Tripathi, C.S.P.; Guin, D. BSA stabilized copper nanoclusters as a highly sensitive and selective probe for fluorescence sensing of Fe3+ ions. Chem. Phys. Lett 2022, 787. [Google Scholar] [CrossRef]
- Ke, B.; Ma, L.; Kang, T.; He, W.; Gou, X.; Gong, D.; Du, L.; Li, M. In Vivo Bioluminescence Imaging of Cobalt Accumulation in a Mouse Model. Anal. Chem. 2018, 90, 4946–4950. [Google Scholar] [CrossRef]
- Zhao, R.X.; Liu, A.Y.; Wen, Q.L.; Wu, B.C.; Wang, J.; Hu, Y.L.; Pu, Z.F.; Ling, J.; Cao, Q. Glutathione stabilized green-emission gold nanoclusters for selective detection of cobalt ion. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 254, 119628. [Google Scholar] [CrossRef] [PubMed]
- Han, B.-Y.; Hu, X.-X.; Xu, J.; He, G.-H. Lysozyme-Directed Synthesis of Yellow-Emitting Copper Nanoclusters for Cobalt Ions (Co2+) Sensing. J. Nanosci. Nanotechno. 2018, 18, 7933–7938. [Google Scholar] [CrossRef]
- Huang, M.; Tong, C. Silicon nanoparticles / gold nanoparticles composite as a fluorescence probe for sensitive and selective detection of Co2+ and vitamin B12 based on the selective aggregation and inner filter effect. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 268, 120706. [Google Scholar] [CrossRef]
- Lin, Y.S.; Chiu, T.C.; Hu, C.C. Fluorescence-tunable copper nanoclusters and their application in hexavalent chromium sensing. RSC Adv. 2019, 9, 9228–9234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, B.; Xiang, R.; Hou, X.; Yu, M.; Peng, T.; Li, Y.; He, G. One-step rapid synthesis of single thymine-templated fluorescent copper nanoclusters for “turn on” detection of Mn2+. Anal. Methods 2017, 9, 2590–2595. [Google Scholar] [CrossRef]
- Bai, H.; Tu, Z.; Liu, Y.; Tai, Q.; Guo, Z.; Liu, S. Dual-emission carbon dots-stabilized copper nanoclusters for ratiometric and visual detection of Cr2O72− ions and Cd2+ ions. J. Hazard Mater. 2020, 386, 121654. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, T.; Bilal, M.; Nabeel, F.; Iqbal, H.M.N.; Li, C.; Zhou, Y. Fluorescent sensor based models for the detection of environmentally-related toxic heavy metals. Sci. Total. Environ. 2018, 615, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Nain, A.; Tseng, Y.-T.; Lin, Y.-S.; Wei, S.-C.; Mandal, R.P.; Unnikrishnan, B.; Huang, C.-C.; Tseng, F.-G.; Chang, H.-T. Tuning the photoluminescence of metal nanoclusters for selective detection of multiple heavy metal ions. Sens. Actuators B Chem. 2020, 321. [Google Scholar] [CrossRef]
- Benavides, J.; Quijada-Garrido, I.; Garcia, O. The synthesis of switch-off fluorescent water-stable copper nanocluster Hg2+ sensors via a simple one-pot approach by an in situ metal reduction strategy in the presence of a thiolated polymer ligand template. Nanoscale 2020, 12, 944–955. [Google Scholar] [CrossRef] [Green Version]

| Type of CuNCs | λex/λem (nm) | Read Out | Sensing Mechanism | Reaction Time | Limit of Detection | Published Time | Reference Value | Ref. |
|---|---|---|---|---|---|---|---|---|
| MMI-CuNCs | 322/476 | Turn-off | AIQ, Static quenching | 30 min | 6.7 nM | 2022 | 1.2 pM 2021 [87] | [53] |
| CuNC/ESM | 360/623 | Turn-off | high-affinity metallophilic interactions | — | — | 2019 | [56] | |
| NAC-CuNCs | 340/630 | Turn-off | dynamic quenching | 2 min | 7.76 × 10−11 M | 2022 | [84] | |
| Glc-CuNPs | 472/542 | Turn-off | AIQ | 30 min | — | 2022 | [85] | |
| CuNCs/ZIF-8 | 360/627 | Turn-off | the formation of Ag-S bonds | 3 min | 0.33 μM | 2022 | [86] |
| Type of CuNCs | λex/λem (nm) | Read Out | Sensing Mechanism | Reaction Time | Limit of Detection | Published Time | Reference Value | Ref. |
|---|---|---|---|---|---|---|---|---|
| CuNCs@ESM | — | Turn-off | high-affinity metallophilic interactions | 1 h (Hg2+: 500 µM) | — | 2018 | 0.19 pmol/μL 2023 [99] | [93] |
| TG-CuNCs | 350/430 | Turn-off | static and dynamic quenching | 3 min | 1.7 nM | 2020 | [94] | |
| CuNCs | 365/440 | Turn-off | AIQ | 22 min | 0.12 nM | 2018 | [95] | |
| CD-CuNCs | dual- emission | — | strong affinity | — | 0.31 nM | 2021 | [96] | |
| AgCu-BNPs | 350/442 | Turn-off | IFE, static and dynamic quenching | — | 9 nM | 2021 | [97] |
| Type of CuNCs | λex/λem (nm) | Read Out | Sensing Mechanism | Reaction Time | Limit of Detection | Published Time | Reference Value | Ref. |
|---|---|---|---|---|---|---|---|---|
| BSA-CuNCs@ [Ru(bpy)3]2+ | Dual -emission | — | AIQ | 3 min | 0.086 μM | 2022 | 10 nM 2022 [106] | [103] |
| CA-CuNCs | 385/467 | Turn-off | electron transfer, AIQ | — | 423 nM | 2020 | [104] | |
| dsDNA-CuNCs | 312/400 | Turn-off | AIQ | 1 h | 5 μM | 2020 | [105] | |
| PA-AuCu-bi-MNCs | 275/605 | Turn-off | IFE | 5 min | 0.1 µM | 2019 | [101] |
| Metal Ions | Type of CuNCs | λex/λem (nm) | Read Out | Sensing Mechanism | Reaction Time | Limit of Detection | Published Time | Reference Value | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Co2+ | DTT-CuNCs | 382/627 | Turn-off | AIQ | 30 min | 25 nM | 2021 | 60 nM 2022 [110] | [71] |
| GSH-AuNCs | 412/500 | Turn-off | Static quenching | 15 min | 0.124 μM (Co2+: 2.0–50.0 μM) | 2021 | [108] | ||
| Lys-CuNCs | 334/596 | Turn-off | — | — | 2.4 nM | 2018 | [109] | ||
| Cr6+ | bi-ligand CuNCs | 330/411 (Cu NC-2 a) | Turn-off | IFE | — | 0.03 mM | — | — | [111] |
| Mn2+ | CuNCs@T b | 354/561 | Turn-on | AIE | 40 min | 10 μM | — | — | [112] |
| Cd2+ | GSH@CDs-CuNCs | dual- emission | — | AIE (750 nm) | 15 min | 0.6 μmol·L−1 | — | — | [113] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, L.; Liang, M.; Hummel, M.; Shao, C.; Lu, S. Rational Design Copper Nanocluster-Based Fluorescent Sensors towards Heavy Metal Ions: A Review. Chemosensors 2023, 11, 159. https://doi.org/10.3390/chemosensors11030159
Yuan L, Liang M, Hummel M, Shao C, Lu S. Rational Design Copper Nanocluster-Based Fluorescent Sensors towards Heavy Metal Ions: A Review. Chemosensors. 2023; 11(3):159. https://doi.org/10.3390/chemosensors11030159
Chicago/Turabian StyleYuan, Lili, Mengna Liang, Matthew Hummel, Congying Shao, and Shun Lu. 2023. "Rational Design Copper Nanocluster-Based Fluorescent Sensors towards Heavy Metal Ions: A Review" Chemosensors 11, no. 3: 159. https://doi.org/10.3390/chemosensors11030159





