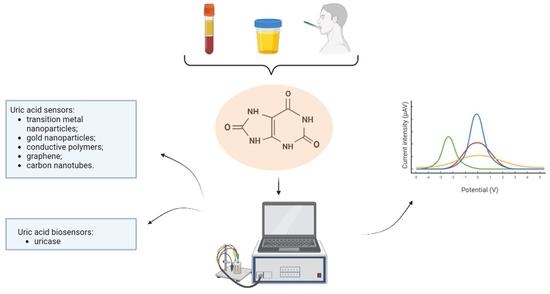
New Trends in Uric Acid Electroanalysis
Abstract
1. Introduction
2. Methodology
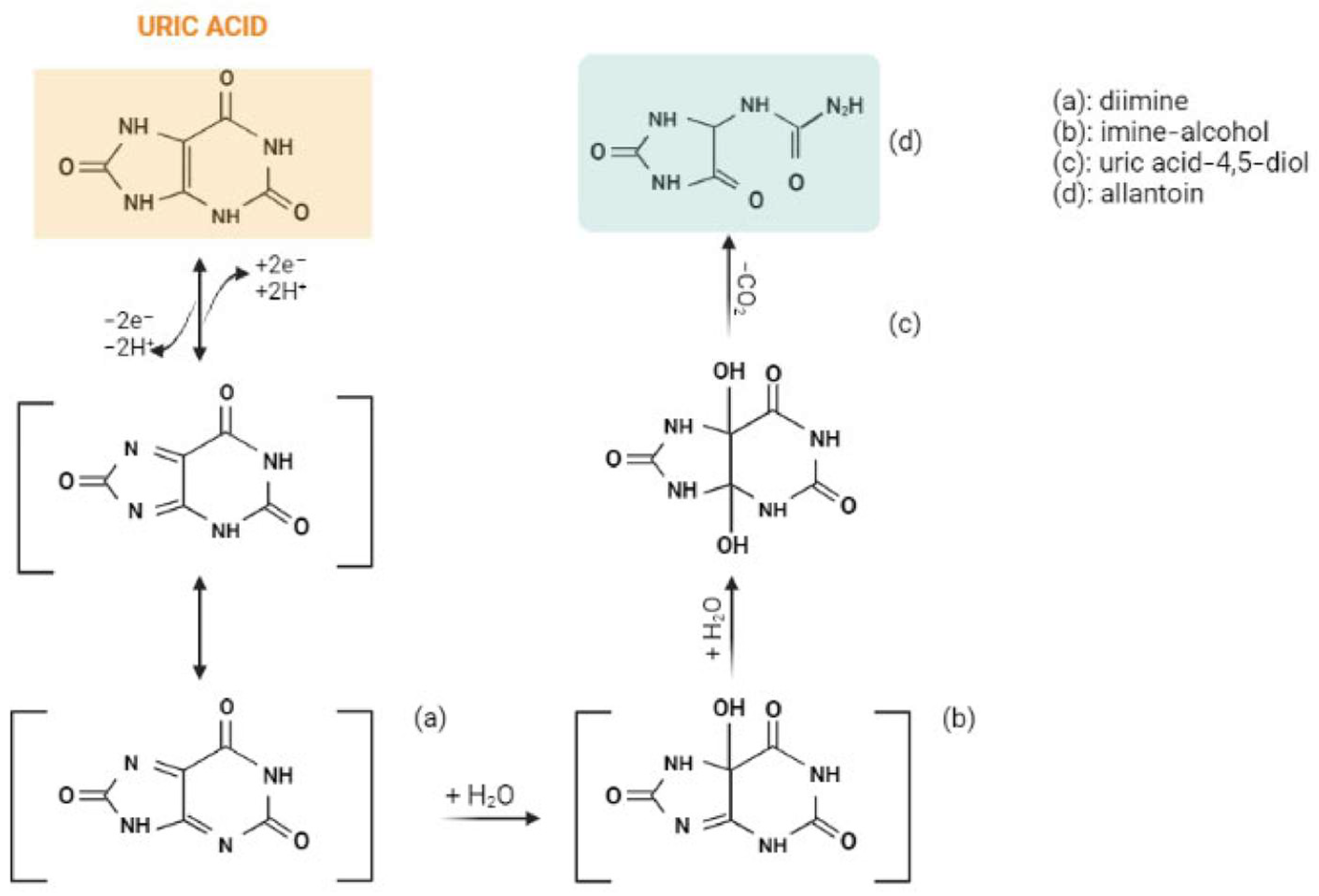
3. Results and Discussion
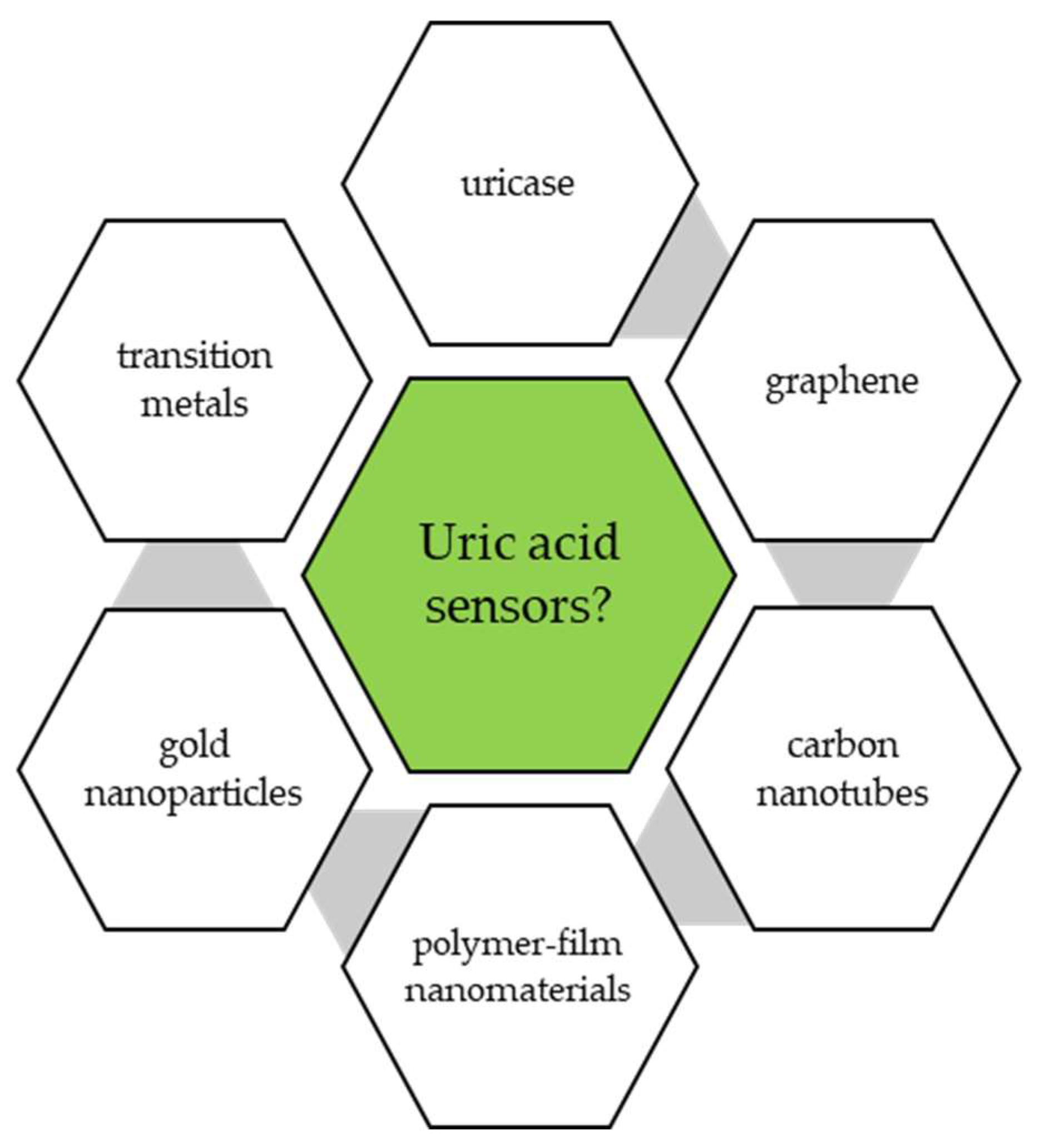
3.1. Sensors for Uric Acid Electroanalysis
3.1.1. Transition Metal Nanoparticles for Uric Acid Detection
- Glassy carbon electrode coated with titanium dioxide nanoparticles.
- Palladium nanoparticles/reduced graphite oxide nanocomposites.
- A glassy carbon electrode coated with copper oxide.
| Electrode | Technique | pH | Interference | Biological Sample; Relative Recovery (RR) | UA Linear Range (μM) | UA LOD (μM) | Ref. |
|---|---|---|---|---|---|---|---|
| GCE/MC–GO–Fe3O4 1 | CV, DPV | 7.0 | UA, AA, DA, G, sucrose, L-Cys, citric acid, Fe2+, Cl−, Na+, NO3− | Human urine RR > 96% | 0.5–140 | 0.17 | [37] |
| TiO2 NPs/GCE 2 | DPV | 7.0 | UA | Human urine RR: 97–99.6% | 1–9 | 0.764 | [39] |
| PdNPs/rGO/GCE 3 | DPV | 7.2 | UA, AA, DA | Human serum RR: 96.6–108.5% | 0.3–1400 | 16.67 | [42] |
| SnO2/chitosan/GCE 4 | DPV | UA, AA, DA | Human urine RR: 97.4% | 3–200 | 1 | [50] | |
| CuO/GCE 5 | CV | 7.4 | UA, UR, lactic acid, ethanol, G, K+, Na+ | Human urine RR: 95–104% | 0.001–351,000 | 0.6 | [40] |
| RuON-GCE 6 | DPV | 7.0 | UA, E | Human urine RR: 98–101.6% | 3.0–56.6; 56.6–758.6 | 0.47 | [51] |
| MoS2 NSA/CNFs 7 | CV, DPV | 7.0 | UA, levodopa | Human urine RR: 99.7–102.6% | 1–60 | 1 | [52] |
| CuO nano-rice/GCE 8 | CV, DPV | 7.0 | UA, AA, DA, G, fructose, galactose, lactose, Na+, Cl−, K+, Ca2+, Br−, CO23−, NH4+, NO2−, NO3−, SO42−, SO32− | Human urine RR: 98.6–102.6% | 1–60 | 1.2 | [53] |
| Fe3O4@CNT-N/GCE 9 | SWV | 2.5 | UA, AA, DA | - | 25–85 | 0.47 | [22] |
| ZnO NWAs/GF/GCE 10 | DPV | 7.4 | UA, AA, DA | Human serum | 0–40 | 0.001 | [24] |
3.1.2. Gold-Coated Electrodes
- Au nanorod-decorated graphene oxide (GO/AuNR) glassy carbon electrode (GCE).
- Gold Nanoparticle-Decorated Polypyrole/Graphene Oxide Nanosheets.
- ITO-rGO-AuNPs electrode for uric acid detection.
- Poly(diallyldimethylammonium chloride)-functionalized reduced graphene oxide and polyoxometalates-doped Au nanoparticle sensor
- A sensor based on reduced graphene oxide functionalized by poly(amido-amine), multi-walled carbon nanotubes and Au nanoparticles.
- Nafion-based electrode modified with Azure A-coated carbon nanotubes coated with gold nanoparticles.
| Electrode | Technique | pH | Interference | Biological Sample. Relative Recovery (RR) | UA Linear Range (μM) | UA LOD (μM) | Ref. |
|---|---|---|---|---|---|---|---|
| GO/AuNR/GCE 1 | DPV | - | UA, AA, DA, G, UR, Mg2+ | Human urine | 10–90 | 0.4 | [27] |
| AuNPs@GO/PPy/CFP 2 | DPV | 7.0 | UA, AA, DA | Human urine RR: 96.8–109% | 2–360 | 1.68 | [30] |
| AuNPs-GO/Au-IDA 3 | CV | 7.0 | UA, AA, DA, G, E | Human urine | 2–1050 | 0.62 | [28] |
| GCE-PErGO-AuNP 4 | CV, DPV | 7.4 | UA, AA, DA | Human urine | 20–260 | 20 | [31] |
| AuRGO/GCE 5 | DPV | 7.0 | UA, AA, DA | Human serum RR: 97.5–102% | 88–53 | 1.8 | [32] |
| Au@Pd-RGO/GCE 6 | DPV | 7.0 | UA, AA, DA | Human urine RR: 97.1–102.5% | 0.02–500; 0.1–350 | 0.005; 0.02 | [33] |
| PEI/[P2W16V2-Au/PDDA-rGO]8 7 | DPV | 7.0 | UA, AA, DA, NaCl, KCl, NH4Cl, L-Cys, L-Glu, CA, UR, G | Human urine RR: 95.2–103.1% | 0.25–1500 | 0.08 | [34] |
| rGO-PAMAM-CNT-Au 8 | DPV | 4.0 | UA, AA, DA | - | 1–114 | 0.33 | [35] |
| Naf/AuNPs/AzA/MWCNTs 9 | DPV | 7.0 | UA, AA, DA, Trp, Na+, K+, Ca2+, Mg2+, G, citric acid, tartaric acid | Human urine RR: 99.7–103% | 0.5–50 | 0.28 | [36] |
| ITO-rGO-AuNPs 10 | LSV | 8.0 | UA, AA, Cl, Na+, Ca2+ NH4+ | Human urine, milk | 10–500 | 3.6 | [65] |
| EGFET-AuE 11 | - | 7.0 | UA, AA, G, bilirubin, hemoglobin | Human urine, serum | 1–1000 | 0.5 | [16] |
3.1.3. Chemically Modified Electrodes
- A glassy carbon electrode modified with electrochemically reduced graphene oxide (ErGO) and poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) (PEDOT:PSS).
- Poly(2-(N-morpholine)ethane sulfonic acid)/RGO-modified electrode.
- Zeolite Imidazolate Framework-11 modified electrode.
- Screen-printed carbon electrode equipped with vertically-ordered mesoporous silica-nanochannel film.
| Electrode | Technique | pH | Interference | Biological Sample; Relative Recovery (RR) | UA Linear Range (μM) | UA LOD (μM) | Ref. |
|---|---|---|---|---|---|---|---|
| ZIF-11/GCE 1 | DP-ASV | 7.0 | UA, AA, G, sodium benzoate, saccharine, XA, hypoxanthine, KCl, Na2CO3, Na2SO4, CaCO3 | Human urine RR: 94.5–104.4% | 50–540 | 0.48 | [14] |
| NgB/CPE 2 | CV, DPV | 7.0 | UA, AA, DA | Human urine RR: 99.4–100.4% | 12.5–750 | 5 | [74] |
| ErGO/PEDOT:PSS/GCE 3 | DPV | - | UA, DA | Human urine RR: 96.8–109% | 10–100 | 1.08 | [76] |
| PMES/RGO/GCE 4 | CV | 7.0 | UA, AA, DA, L-Cys, L-Lys, L-Tyr, G | Human urine RR: 103.35% | 0.1–100 | 0.056 | [75] |
| NG/GCE 5 | DPV | 6.0 | UA, AA, DA | - | 0.1–20 | 0.045 | [87] |
| MC/GCE 6 | CV, DPV | 1.0 | UA, AA, DA | Synthetic urine RR: 101% | 10–150 | 1.7 | [88] |
| BDG-based electrode 7 | SWV | 2.25 | UA | Human urine RR: 95% RR: 95.2–103.1% | 8–1000 | 7.7 | [89] |
| PMB-ERGO/GCE 8 | SWV | 3.0 | UA, XA | Human urine RR: 97.8% | 0.08–400 | 0.03 | [15] |
| PEDOT-nf/PGE and Ox-PEDOT-nf/PGE 9 | CV | 2.0 | UA | Human urine, serum RR: 104–107% | 0.1–20 | 0.0013 | [90] |
| MWNTs/MGF/GCE 10 | DPV | 7.3 | UA, AA, DA, Trp, Na+, K+, Ca2+, Mg2+, G | - | 5–100; 300–10,000 | 0.93 | [10] |
| GCE/tosyl-CNPsE 11 | CV | 2.0 | UA, AA | Human urine RR: 106% | 0.1–100 | 0.2 | [91] |
| CTAB/GO/MWNTs/GCE 12 | DPV | 7.0 | UA, AA, DA, NO2− | Human urine RR: 99–115% | 3–600 | 1 | [92] |
| EGNWsE 13 | DPV | 7.4 | UA, AA, DA | - | 2.6–200 | 0.000033 | [93] |
| GEF/CFE 14 | DPV | 7.0 | UA, AA, DA | Human urine, serum | 3.98–371 | 2 | [94] |
| Trp-GR/GCE 15 | DPV | 7.0 | UA, AA, DA | Human urine RR: 97.3–99.9% Human serum RR: 92.6–98.7% | 10–1000 | 1.24 | [95] |
| NH2-VMSF/ErGO/SPCE 16 | DPV | 5.0 | UA, AA, DA, G, UR, Na+, K+, Ca2+, Mg2+ | Human whole blood RR: 99.0–107.0% | 0.5–180 | 0.129 | [86] |
3.2. Biosensors for Detection of Uric Acid
- Zinc tetraaminophthalocyanine-functionalized graphene nanosheets/GCE with uricase.
- The ferrocene-conjugated uricase biosensor on a nafion polymer membrane.
- Uricase-thionine-single-walled carbon nanotube-modified electrode
| Electrode | Technique | pH | Interference | Biological Sample. Relative Recovery (RR) | UA Linear Range (μM) | UA LOD (μM) | Ref. |
|---|---|---|---|---|---|---|---|
| UOx/CNT/CMC 1 | CV | 7.4 | UA, AA, UR | Human urine, serum RR: 96.3% | 20–5000 | 2.8 | [101] |
| RGO/AuNP hybrid film 2 | Amperometry | 7.6 | UA, AA, DA | - | - | 1 | [55] |
| UOx-Th-SWNTs/GC 3 | - | - | UA, AA, 3,4-dihydroxyphenylacetic acid, 4-acetamidophenol | HEK 293A cells RR: 100.9–101.4% | 2–2000 | 0.5 | [96] |
| UOx/PBG/CNT/CFE and UOx PTH/CNT/CFE 4 | Amperometry | 7.0 | UA, AA, G, citric acid, creatinine, NH4+, phenol, UR | Human urine RR: 95–105% | 2–100 | 0.6 | [97] |
| UOx/rGO/ZnPc-NH2/GCE 5 | - | - | UA | Human urine RR: 92.5–97.6% | 0.5–100 | 0.15 | [103] |
| MP/SWCNT/SPE 6 | CV | 7.4 | UA, AA, DA | Human urine | 0.001–0.20 | 0.83 | [98] |
| UOx/AuNP/c-MWCNT/Au 7 | CV | 7.5 | UA, AA, G, chol, UR, pyruvate, bilirubin, CuSO4, KCl, FAD, NaCl, ZnSO4, NADH, CaCl2, EDTA, NEM, riboflavin, MnCl2, FM | Human serum RR: 95–97% | 5–800 | 5 | [102] |
| UOx- PANI-PB-PtE 8 | CV | 7.2 | UA, AA, UR, G | Human serum | 10–160 | 2.6 | [99] |
| UOx-PANI-MWCNT/ITO 9 | CV, DPV | - | UA | Human serum | 10–1000 | 10 | [100] |
| UOx/Nafion/ZnO-NFs/Au 10 | Amperometry | 7.4 | UA, AA, UR, G | - | 0.5–1500 | 0.5 | [104] |
| Naf/UOx/Fc/GCE 11 | DPV, Amperometry | 7.4 | UA, AA, DA, UR, G, XA | Human serum RR: 95% | 0.5–50; 25–600 | 0.23 | [23] |
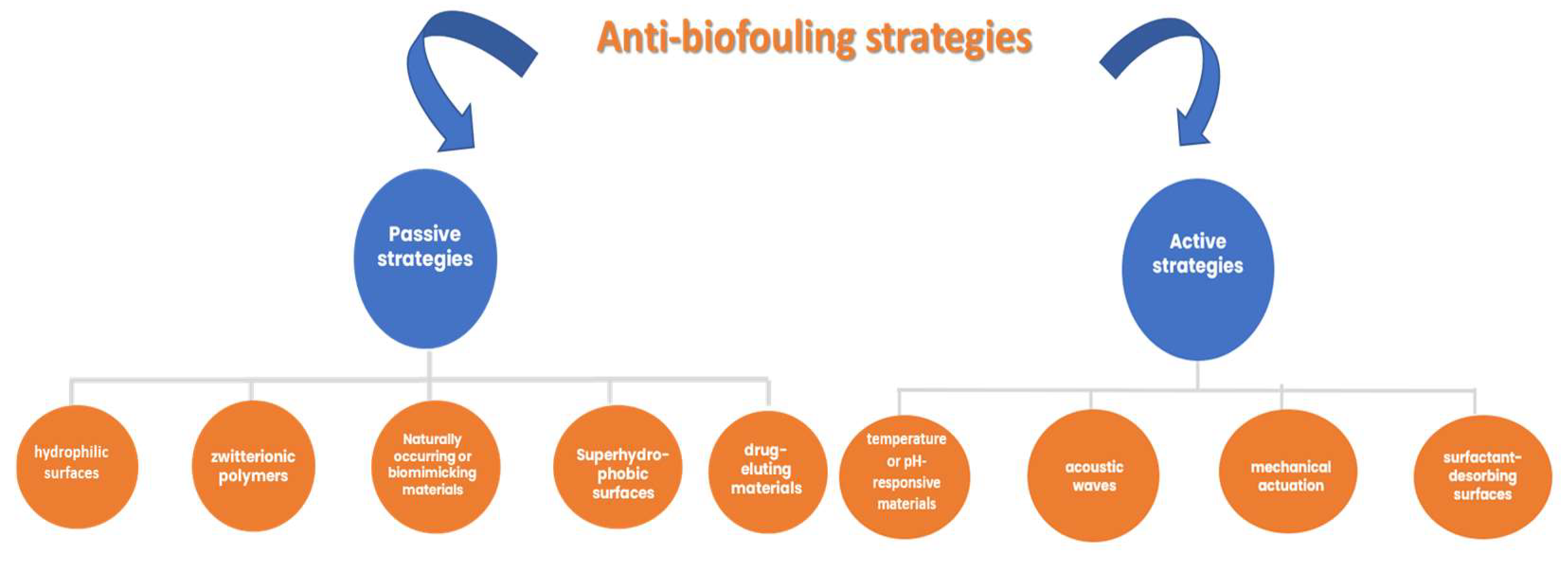
4. Challenges and Perspectives of Uric Acid Electrochemical Detection
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Islam, M.N.; Ahmed, I.; Anik, M.I.; Ferdous, M.S.; Khan, M.S. Developing Paper Based Diagnostic Technique to Detect Uric Acid in Urine. Front. Chem. 2018, 6, 496. [Google Scholar] [CrossRef]
- Benn, C.L.; Dua, P.; Gurrell, R.; Loudon, P.; Pike, A.; Storer, R.I.; Vangjeli, C. Physiology of Hyperuricemia and Urate-Lowering Treatments. Front. Med. 2018, 5, 160. [Google Scholar] [CrossRef]
- Chong, D.P. Theoretical Study of Uric Acid and its Ions in Aqueous Solution. J. Theor. Comput. Sci. 2013, 1. [Google Scholar] [CrossRef]
- Desideri, G.; Castaldo, G.; Lombardi, A.; Mussap, M.; Testa, A.; Pontremoli, R.; Punzi, L.; Borghi, C. Is it time to revise the normal range of serum uric acid levels? Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 1295–1306. [Google Scholar]
- de Lassichere, C.; Latapie, L.; Evrard, D.; Gros, P. New Insight into the EC’ Mechanism of Uric Acid Regeneration in the Presence of Ascorbic Acid on a Poly(3,4-ethylenedioxithiophene) Modified Gold Electrode. Electroanalysis 2018, 30, 1653. [Google Scholar] [CrossRef]
- Sajid, M.; Nazal, K.M.; Mansha, M.; Alsharaa, A.; Jillani, S.M.S.; Basheer, C. Chemically modified electrodes for electrochemical detection of dopamine in the presence of uric acid and ascorbic acid: A review. Trends Anal. Chem. 2016, 76, 15–29. [Google Scholar] [CrossRef]
- Lupu, S.; Lete, C.; Balaure, P.C.; Caval, D.I.; Mihailciuc, C.; Lakard, B.; Hihn, J.Y.; Del Campo, F.J. Development of amperometric biosensors based on nanostructured tyrosinase-conducting polymer, composite Electrodes. Sensors 2013, 13, 6759–6774. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.-P.-P.; Sulaiman Rahman, H. Antioxidant and Oxidative Stress: A Mutual Interplay in Age-Related Diseases. Front. Pharmacol. 2018, 9, 1162. [Google Scholar] [CrossRef] [PubMed]
- Picillo, M.; Santangelo, G.; Moccia Erro, R.; Amboni, M.; Prestipino, E.; Longo, K.; Vitale, C.; Spina, E.; Orefice, G.; Barone, P.; et al. Serum uric acid is associated with apathy in early, drug naive Parkinson’s disease. J. Neural Transm. 2015, 2015, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, Y.; Ye, D.; Luo, J.; Su, B.; Zhang, S.; Kong, J. An electrochemical sensor for simultaneous determination of ascorbic acid, dopamine, uric acid and tryptophan based on MWNTs bridged mesocellular graphenefoam nanocomposite. Talanta 2014, 127, 255–261. [Google Scholar] [CrossRef]
- Elhag, S.; Ibupoto, Z.H.; Nur, O.; Willander, M. Incorporating β-Cyclodextrin with ZnO nanorods: A potentiometric strategy for selectivity and detection of dopamine. Sensors 2014, 14, 1654–1664. [Google Scholar] [CrossRef]
- Kumar, S.; Vicente-Beckett, V. Glassy carbon electrodes modified with multiwalled carbon nanotubes for the determination of ascorbic acid by square-wave voltammetry. Beilstein J. Nanotechnol. 2012, 3, 388–396. [Google Scholar] [CrossRef]
- Levite, M. Dopamine and Tcells: Dopamine receptors and potent effects on T cells, dopamine production in T cells, and abnormalities in the dopaminergic system in T cells in autoimmune, neurological and psychiatric diseases. Acta Physiol. 2016, 216, 42–89. [Google Scholar] [CrossRef]
- Thanh, T.S.; Qui, P.T.; Thanh Tu, N.T.; Toan, T.T.T.; Hoa, T.T.B.; Son, L.V.T.; Nguyen, D.M.; Tuyen, T.N.; Khieu, D.Q. Electrochemical Determination of Uric Acid in Urine by Using Zeolite Imidazolate Framework-11 Modified Electrode. J. Nanomater. 2021, 2021, 9914062. [Google Scholar] [CrossRef]
- Liu, G.; Wei, M.; Luo, Y.; Sun, D.; Shao, S. Simultaneous determination of uric acid and xanthine using a poly(methylene blue) and electrochemically reduced graphene oxide composite film modified electrode. J. Anal. Methods Chem. 2014, 2014, 984314. [Google Scholar] [CrossRef]
- Guan, W.; Duan, X.; Reed, M.A. Highly specific and sensitive non-enzymatic determination of uric acid in serum and urine by extended gate field effect transistor sensors. Biosens. Bioelectron. 2014, 51, 225–231. [Google Scholar] [CrossRef]
- Tadese, A.; Subramanian, P.A.; Woldu, A.; Pal, R. Electrochemical determination and comparison of ascorbic acid in freshly prepared and bottled fruit juices: A cyclic voltammetric study. J. Chem. Pharm. Res. 2014, 6, 880–888. [Google Scholar]
- Carr, A.C.; Shaw, G.M.; Fowler, A.A.; Natarajan, R. Ascorbate-dependent vasopressor synthesis: A rationale for vitamin C administration in severe sepsis and septic shock? Crit. Care 2015, 19, 418. [Google Scholar] [CrossRef]
- Ide, K.; Yamada, H.; Umegaki, K.; Mizuno, K.; Kawakami, N.; Hagiwara, Y.; Matsumoto, M.; Yoshida, H.; Kim, K.; Shiosaki, E.; et al. Lymphocyte vitamin C levels as potential biomarker for progression of Parkinson’s disease. Nutrition 2015, 31, 406–408. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Wang, B.; Meng, F.; Cheng, Y.; Zhu, C. Microwave-assisted preparation of N-doped carbon dots as a biosensor for electrochemical dopamine detection. J. Colloid Interface Sci. 2015, 452, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Chairam, S.; Sriraska, W.; Amatatongchai, M.; Somsook, E. Electrocatalytic oxidation of ascorbic acid using a poly (aniline-co-m-ferrocenylaniline) modified glassy Carbon Electrode. Sensors 2014, 11, 10166–10179. [Google Scholar] [CrossRef]
- Fernandes, D.M.; Costa, M.; Pereira, C.; Bachiller-Baeza, B.; Rodriguez-Ramos, I.; Guerrero-Ruiz, A.; Freire, C. Novel electrochemical sensor based on N-doped carbon nanotubes and Fe3O4 nanoparticles: Simultaneous voltammetric determination of ascorbic acid, dopamine and uric acid. J. Colloid Interface Sci. 2014, 432, 207–213. [Google Scholar] [CrossRef]
- Ghoh, T.; Sarkar, P.; Turner, A.P.F. A novel third generation uric acid biosensor using uricase, electro-activated with ferrocene on a Nafion coated glassy carbon electrode. Bioelectrochemistry 2015, 102, 1–9. [Google Scholar] [CrossRef]
- Yue, H.Y.; Huang, S.; Chang, J.; Heo, C.; Yao, F.; Adhikari, S.; Gunes, F.; Liu, L.C.; Lee, T.H.; Oh, E.S.; et al. ZnO Nanowire arrays on 3D hierachical graphene foam: Biomarker detection of Parkinson’s disease. ACS Chem. Neurosci. 2014, 8, 1639–1646. [Google Scholar] [CrossRef]
- Wu, Y.; Deng, P.; Tian, Y.; Feng, J.; Xiao, J.; Li, J.; Liu, J.; Li, G.; He, Q. Simultaneous and sensitive determination of ascorbic acid, dopamine and uric acid via an electrochemical sensor based on PVP-graphene composite. J. Nanobiotechnol. 2020, 18, 112. [Google Scholar] [CrossRef]
- Vrabelj, T.; Finšgar, M. Recent Progress in Non-Enzymatic Electroanalytical Detection of Pesticides Based on the Use of Functional Nanomaterials as Electrode Modifiers. Biosensors 2022, 12, 263. [Google Scholar] [CrossRef] [PubMed]
- Safitri, H.; Wahyuni, W.T.; Rohaeti, E.; Khalil, M.; Marken, F. Optimization of uric acid detection with Au nanorod-decorated graphene oxide (GO/AuNR) using response surface methodology. RSC Adv. 2022, 12, 25269–25278. [Google Scholar] [CrossRef] [PubMed]
- Abellán-Llobregat, A.; Vidal, L.; Rodríguez-Amaro, R.; Berenguer-Murcia, A.; Canals, A.; Morallón, E. Au-IDA Microelectrodes Modified with Au-Doped Graphene Oxide for the Simultaneous Determination of Uric Acid and Ascorbic Acid in Urine Samples. Electrochim. Acta 2017, 227, 275–284. [Google Scholar] [CrossRef]
- Schlesinger, O.; Lital, A. Encapsulation of Microorganisms, Enzymes, and Redox Mediators in Graphene Oxide and Reduced Graphene Oxide. Methods Enzymol. 2018, 609, 197–219. [Google Scholar] [PubMed]
- Tan, C.; Zhao, J.; Sun, P.; Zheng, W.; Cui, G. Gold Nanoparticle Decorated Polypyrrole/Graphene Oxide Nanosheets as a Modified Electrode for Simultaneous Determination of Ascorbic Acid, Dopamine and Uric Acid. New. J. Chem. 2020, 12, 4916–4926. [Google Scholar] [CrossRef]
- Imran, H.; Palinci, N.M.; Venkataraman, D. Facile and Green Synthesis of Graphene Oxide by Electrical Exfoliation of Pencil Graphite and Gold Nanoparticle for Non-Enzymatic Simultaneous Sensing of Ascorbic Acid, Dopamine and Uric Acid. RSC Adv. 2015, 78, 63513–63520. [Google Scholar] [CrossRef]
- Wang, C.; Du, J.; Wang, H.; Zou, C.; Jiang, F.; Yang, P.; Du, Y. A Facile Electrochemical Sensor Based on Reduced Graphene Oxide and Au Nanoplates Modified Glassy Carbon Electrode for Simultaneous Detection of Ascorbic Acid, Dopamine and Uric Acid. Sens. Actuators B Chem. 2014, 204, 302–309. [Google Scholar] [CrossRef]
- Jiang, J.; Du, X. Sensitive Electrochemical Sensors for Simultaneous Determination of Ascorbic Acid, Dopamine, and Uric Acid Based on Au@Pd-Reduced Graphene Oxide Nanocomposites. Nanoscale 2014, 19, 11303–11309. [Google Scholar] [CrossRef]
- Bai, Z.; Zhou, C.; Xu, H.; Wang, G.; Pang, H.; Ma, H. Polyoxometalates-Doped Au Nanoparticles and Reduced Graphene Oxide: A New Material for the Detection of Uric Acid in Urine. Sens. Actuators B Chem. 2017, 243, 361–371. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, W.; Zhong, X.; Chai, Y.; Yuan, R. Simultaneous Determination of Dopamine, Ascorbic Acid and Uric Acid Using a Multi-Walled Carbon Nanotube and Reduced Graphene Oxide Hybrid Functionalized by PAMAM and Au Nanoparticles. Anal. Methods 2015, 4, 1471–1477. [Google Scholar] [CrossRef]
- Filik, H.; Avan, A.A.; Aydar, S. Simultaneous Detection of Ascorbic Acid, Dopamine, Uric Acid and Tryptophan with Azure A-Interlinked Multi-Walled Carbon Nanotube/Gold Nanoparticles Composite Modified Electrode. Arab. J. Chem. 2016, 3, 471–480. [Google Scholar] [CrossRef]
- Sohouli, E.; Khosrowshahi, E.M.; Radi, P.; Naghian, E.; Rahimi-Nasrabadi, M.; Ahmadi, F. Electrochemical sensor based on modified methylcellulose by graphene oxide and Fe3O4 nanoparticles: Application in the analysis of uric acid content in urine. J. Electroanal. Chem. 2020, 877, 114503. [Google Scholar] [CrossRef]
- Liao, C.; Li, Y.; Tjong, S.C. Graphene Nanomaterials: Synthesis, Biocompatibility, and Cytotoxicity. Int. J. Mol. Sci. 2018, 19, 3564. [Google Scholar] [CrossRef]
- Rajeswari, B.; Reddy, K.V.N.S.; Devi, A.S.; Madhavi, G.; Reddy, I.R.V.S. Determination of Uric Acid Using TiO2 Nanoparticles Modified Glassy Carbon Electrode. Biointerface Res. Appl. Chem. 2022, 12, 6058–6065. [Google Scholar]
- Buledi, J.; Ameen, S.; Memon, S.; Fatima, A.; Solangi, A.; Mallah, A.; Karimi, F.; Malakmohammadi, S.; Agarwal, S.; Gupta, V. An Improved Non-Enzymatic Electrochemical Sensor Amplified with CuO Nanostructures for Sensitive Determination of Uric Acid. Open. Chem. 2021, 19, 481–491. [Google Scholar] [CrossRef]
- Dubey, R.S.; Krishnamurthy, K.V.; Singh, S. Experimental Studies of TiO2 Nanoparticles Synthesized by Sol-Gel and Solvothermal Routes for DSSCs Application. Results Phys. 2019, 14, 102390. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, Y.; Xu, Z.; Wang, S.; Chen, B.; Zhang, D.; Fang, Y. Simultaneous Detection of Ascorbic Acid, Dopamine, and Uric Acid Using a Novel Electrochemical Sensor Based on Palladium Nanoparticles/Reduced Graphene Oxide Nanocomposite. Int. J. Anal. Chem. 2020, 2020, 8812443. [Google Scholar] [CrossRef] [PubMed]
- Manna, S.; Kumar, S.; Sharma, A.; Sahoo, S.; Dey, M.K.; Mishra, P.K.; Satpati, A.K. rGO/ReO3 nano composite modified electrode for the ultra-sensitive determination of dopamine and uric acid. Biosens. Bioelectron. X 2022, 11, 100156. [Google Scholar] [CrossRef]
- Yin, F.Z.; Wu, L.; Yang, H.G.; Su, Y.H. Recent Progress in Biomedical Applications of Titanium Dioxide. Phys. Chem. Chem. Phys. 2013, 14, 4844. [Google Scholar] [CrossRef] [PubMed]
- Sousa, S.V.; Corniciuc, C.; Teixeira, M.R. The effect of TiO2 nanoparticles removal on drinking water quality produced by conventional treatment C/F/S. Water Res. 2017, 109, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, W.M.M.; Rastogi, T.; Kümmerer, K. Application of Titanium Dioxide Nanoparticles as a Photocatalyst for the Removal of Micropollutants Such as Pharmaceuticals from Water. Curr. Opin. Green. Sustain. Chem. 2017, 6, 1–10. [Google Scholar] [CrossRef]
- Shetti, N.P.; Nayak, D.S.; Malode, S.J.; Kulkarni, R.M. Electrochemical Sensor Based upon Ruthenium Doped TiO2 Nanoparticles for the Determination of Flufenamic Acid. J. Electrochem. Soc. 2017, 164, 3036–3042. [Google Scholar] [CrossRef]
- Wang, H.-M.; Wang, C.-C.; Wang, A.-J.; Zhang, L.; Luo, X.; Yuan, P.-X.; Feng, J.-J. Green synthesis of Pd nanocones as a novel and effective electrochemiluminescence illuminant for highly sensitive detection of dopamine. Sens. Actuators B Chem. 2019, 281, 588–594. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, K.; Xu, D.; Yang, G.; Huang, H.; Nie, F.; Liu, C.; Yang, S. CuO Nanostructures: Synthesis, Characterization, Growth Mechanisms, Fundamental Properties, and Applications. Prog. Mater. Sci. 2014, 60, 208–337. [Google Scholar] [CrossRef]
- Sun, D.; Zhao, Q.; Tan, F.; Wang, X.; Gao, J. Simultaneous Detection of Dopamine, Uric Acid, and Ascorbic Acid Using SnO2 Nanoparticles/Multi-Walled Carbon Nanotubes/Carbon Paste Electrode. Anal. Methods 2012, 10, 3283. [Google Scholar] [CrossRef]
- Zare, H.R.; Ghanbari, Z.; Nasirizadeh, N.; Benvidi, A. Simultaneous Determination of Adrenaline, Uric Acid, and Cysteine Using Bifunctional Electrocatalyst of Ruthenium Oxide Nanoparticles. Comptes Rendus Chim. 2013, 3, 287–295. [Google Scholar] [CrossRef]
- Wang, W.Q.; Yue, H.Y.; Yu, Z.M.; Huang, S.; Song, S.S.; Gao, X.; Guan, E.H.; Zhang, H.J.; Wang, Z. Synthesis and Application of MoS2 Nanosheet Arrays/Carbon Nanofibers for Simultaneous Electrochemical Determination of Levodopa and Uric Acid. IEEE Sens. J. 2019, 15, 5988–5994. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Sudha, V.; Kumar, S.M.S.; Thangamuthu, R. Simultaneous Determination of Dopamine and Uric Acid Using Copper Oxide Nano-Rice Modified Electrode. J. Alloys Compd. 2018, 748, 338–347. [Google Scholar] [CrossRef]
- Wu, B.; Yeasmin, S.; Liu, Y.; Cheng, L.-Y. Sensitive and selective electrochemical sensor for serotonin detection based on ferrocene-gold nanoparticles decorated multiwall carbon nanotubes. Sens. Actuators B Chem. 2022, 354, 131216. [Google Scholar] [CrossRef]
- Zhang, P.; Huang, Y.; Lu, X.; Zhang, S.; Li, J.; Wei, G.; Su, Z. One-Step Synthesis of Large-Scale Graphene Film Doped with Gold Nanoparticles at Liquid–Air Interface for Electrochemistry and Raman Detection Applications. Langmuir. 2014, 30, 8980–8989. [Google Scholar] [CrossRef] [PubMed]
- Marlinda, A.R.; Sagadevan, S.; Yusoff, N.; Pandikumar, A.; Huang, N.M.; Akbarzadeh, O.; Johan, M.R. Gold Nanorods-Coated Reduced Graphene Oxide as a Modified Electrode for the Electrochemical Sensory Detection of NADH. J. Alloys Compd. 2020, 842, 156552. [Google Scholar] [CrossRef]
- Tukimin, N.; Abdullah, J.; Sulaiman, Y. Development of a PrGO-Modified Electrode for Uric Acid Determination in the Presence of Ascorbic Acid by an Electrochemical Technique. Sensors 2017, 17, 1539. [Google Scholar] [CrossRef]
- Akhavan, O.; Bijanzad, K.; Mirsepah, A. Synthesis of Graphene from Natural and Industrial Carbonaceous Wastes. RSC Adv. 2014, 39, 20441–20448. [Google Scholar] [CrossRef]
- Nikoobakht, B.; El-Sayed, M.A. Preparation and Growth Mechanism of Gold Nanorods (NRs) Using Seed-Mediated Growth Method. Chem. Mater. 2003, 10, 1957–1962. [Google Scholar] [CrossRef]
- Putra, B.R.; Nisa, U.; Heryanto, R.; Rohaeti, E.; Khalil, M.; Izzataddini, A.; Wahyuni, W.T. A Facile Electrochemical Sensor Based on a Composite of Electrochemically Reduced Graphene Oxide and a PEDOT:PSS Modified Glassy Carbon Electrode for Uric Acid Detection. Anal. Sci. 2022, 1, 157–166. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, J.; Jia, Z.; Huo, D.; Liu, Q.; Zhong, D.; Hu, Y.; Yang, M.; Bian, M.; Hou, C. In-Situ Growth of Gold Nanoparticles on a 3D-Network Consisting of a MoS2/RGO Nanocomposite for Simultaneous Voltammetric Determination of Ascorbic Acid, Dopamine and Uric Acid. Microchim. Acta 2019, 2, 92. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Xu, C.; Zhao, D.; Zhou, J. Facile Fabrication of Hierarchical Nanoporous AuAg Alloy and Its Highly Sensitive Detection towards Dopamine and Uric Acid. Sens. Actuators B Chem. 2016, 225, 241–248. [Google Scholar] [CrossRef]
- Li, W.; Ma, L.; Wu, B.; Zhang, Y.; Li, Z. A Chemically Reduced Graphene Oxide–Au Nanocage Composite for the Electrochemical Detection of Dopamine and Uric Acid. Anal. Methods 2017, 25, 3819–3824. [Google Scholar] [CrossRef]
- Mahalakshmi, S.; Sridevi, V. In Situ Electrodeposited Gold Nanoparticles on Polyaniline-Modified Electrode Surface for the Detection of Dopamine in Presence of Ascorbic Acid and Uric Acid. Electrocatalysis 2021, 12, 415–435. [Google Scholar] [CrossRef]
- Mazzara, F.; Patella, B.; Ganci, F.; O’Riordan, A.; Aiello, G.; Torino, C.; Vilasi, A.; Sunseri, C.; Inguanta, R. Flexible Electrode Based on Gold Nanoparticles and Reduced Graphene Oxide for Uric Acid Detection Using Linear Sweep Voltammetry. Chem. Eng. Trans. 2021, 87, 421–426. [Google Scholar]
- Liu, K.; Zhang, J.; Yang, G.; Wang, C.; Zhu, J.-J. Direct Electrochemistry and Electrocatalysis of Hemoglobin Based on Poly(Diallyldimethylammonium Chloride) Functionalized Graphene Sheets/Room Temperature Ionic Liquid Composite Film. Electrochem. Commun. 2010, 3, 402–405. [Google Scholar] [CrossRef]
- Xu, Z.; Gao, N.; Dong, S. Preparation and Layer-by-Layer Self-Assembly of Positively Charged Multiwall Carbon Nanotubes. Talanta 2006, 3, 753–758. [Google Scholar] [CrossRef]
- Shi, X.; Wang, S.; Meshinchi, S.; Van Antwerp, M.E.; Bi, X.; Lee, I.; Baker, J.R., Jr. Dendrimer-Entrapped Gold Nanoparticles as a Platform for Cancer-Cell Targeting and Imaging. Small 2007, 7, 1245–1252. [Google Scholar] [CrossRef]
- Antoni, P.; Hed, Y.; Nordberg, A.; Nyström, D.; von Holst, H.; Hult, A.; Malkoch, M. Bifunctional Dendrimers: From Robust Synthesis and Accelerated One-Pot Postfunctionalization Strategy to Potential Applications. Angew. Chem. Int. Ed. 2009, 12, 2126–2130. [Google Scholar] [CrossRef]
- Peng, X.; Pan, Q.; Garry, L.R. Bimetallic Dendrimer-Encapsulated Nanoparticles as Catalysts: A Review of the Research Advances. Chem. Soc. Rev. 2008, 8, 1619. [Google Scholar] [CrossRef]
- Frasconi, M.; Tortolini, C.; Botrè, F.; Mazzei, F. Multifunctional au nanoparticle dendrimer-based surface plasmon resonance biosensor and its application for improved insulin detection. Anal. Chem. 2010, 82, 7335–7342. [Google Scholar] [CrossRef] [PubMed]
- Duraisamy, R.; Kiruthiga, P.M.; Yilma, B.; Berekute, A.K. Adsorption of Azure B dye on Rice husk activated carbon: Equilibrium, Kinetic and Thermodynamic Studies. Int. J. Water Res. 2015, 5, 18–28. [Google Scholar]
- Li, N.; Yuan, R.; Chai, Y.; Chen, S.; An, H.; Li, W. New Antibody Immobilization Strategy Based on Gold Nanoparticles and Azure I/Multi-Walled Carbon Nanotube Composite Membranes for an Amperometric Enzyme Immunosensor. J. Phys. Chem. C 2007, 111, 8443–8450. [Google Scholar] [CrossRef]
- Chitravathi, S.; Kumara Swamy, B.E.; Mamatha, G.P.; Sherigara, B.S. Electrochemical Behavior of Poly (Naphthol Green B)-Film Modified Carbon Paste Electrode and Its Application for the Determination of Dopamine and Uric Acid. J. Electroanal. Chem. 2012, 667, 66–75. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, N.; Zhang, L.; Wang, H.; Shi, H.; Liu, Q. Simultaneous voltammetric detection of dopamine, ascorbic acid and uric acid using a poly(2-(N-morpholine)ethane sulfonic acid)/RGO modified electrode. RSC Adv. 2018, 8, 5280–5285. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Yu, J.; Tian, Q.; Shi, J.; Zhang, M.; Chen, S.; Zang, L. Application of PEDOT:PSS and Its Composites in Electrochemical and Electronic Chemosensors. Chemosensors 2021, 9, 79. [Google Scholar] [CrossRef]
- Ma, X.; Luo, M.; Gao, W.; Yuan, J.; An, Q.; Zhang, M.; Hu, Z.; Gao, J.; Wang, J.; Zou, Y.; et al. Achieving 14.11% Efficiency of Ternary Polymer Solar Cells by Simultaneously Optimizing Photon Harvesting and Exciton Distribution. J. Mater. Chem. A 2019, 7, 7843–7851. [Google Scholar] [CrossRef]
- Anand, V.K.; Bukke, A.; Bhatt, K.; Kumar, S.; Sharma, S.; Goyal, R.; Virdi, G.S. Highly Sensitive and Reusable Cu+2/Polyaniline/Reduced Graphene Oxide Nanocomposite Ink-Based Non-Enzymatic Glucose Sensor. Appl. Phys. A 2020, 7, 500. [Google Scholar] [CrossRef]
- Suvina, V.; Murali Krishna, S.; Nagaraju, D.H.; Melo, J.S.; Geetha Balakrishna, R. Polypyrrole-Reduced Graphene Oxide Nanocomposite Hydrogels: A Promising Electrode Material for the Simultaneous Detection of Multiple Heavy Metal Ions. Mater. Lett. 2018, 232, 209–212. [Google Scholar] [CrossRef]
- Singh, M.; Sahu, A.; Mahata, S.; Shukla, P.; Rai, A.; Rai, V.K. Efficient Electrocatalytic Oxidation of P-Phenylenediamine Using a Novel PANI/ZnO Anchored Bio-Reduced Graphene Oxide Nanocomposite. New. J. Chem. 2019, 17, 6500–6506. [Google Scholar] [CrossRef]
- Ma, K.; Yang, L.; Liu, J.; Liu, J. Electrochemical Sensor Nanoarchitectonics for Sensitive Detection of Uric Acid in Human Whole Blood Based on Screen-Printed Carbon Electrode Equipped with Vertically-Ordered Mesoporous Silica-Nanochannel Film. Nanomaterials 2022, 12, 1157. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; He, Y.; Klausen, L.H.; Yan, N.; Liu, J.; Chen, F.; Song, W.; Dong, M.; Zhang, Y. Growing vertical aligned mesoporous silica thin film on nanoporous substrate for enhanced degradation, drug delivery and bioactivity. Bioact. Mater. 2021, 6, 1452–1463. [Google Scholar] [CrossRef] [PubMed]
- Saadaoui, M.; Fernández, I.; Luna, G.; Díez, P.; Campuzano, S.; Raouafi, N.; Sánchez, A.; Pingarrón, J.M.; Villalonga, R. Label-free electrochemical genosensor based on mesoporous silica thin film. Anal. Bioanal. Chem. 2016, 408, 7321–7327. [Google Scholar] [CrossRef] [PubMed]
- Paul, G.; Verma, S.; Jalil, O.; Thakur, D.; Pandey, C.M.; Kumar, D. PEDOT PSS-grafted graphene oxide-titanium dioxide nanohybrid-based conducting paper for glucose detection. Polym. Adv. Technol. 2021, 32, 1774–1782. [Google Scholar] [CrossRef]
- Promsuwan, K.; Soleh, A.; Saisahas, K.; Saichanapan, J.; Kanatharana, P.; Thavarungkul, P.; Guo, C.; Li, C.M.; Limbut, W. Discrimination of Dopamine by an Electrode Modified with Negatively Charged Manganese Dioxide Nanoparticles Decorated on a Poly(3,4 Ethylenedioxythiophene)/Reduced Graphene Oxide Composite. J. Colloid Interface Sci. 2021, 597, 314–324. [Google Scholar] [CrossRef]
- Sayyad, P.W.; Sontakke, K.S.; Farooqui, A.A.; Shirsat, S.M.; Tsai, M.-L.; ShirsaT, M.D. A Novel Three-Dimensional Electrochemical Cd(II) Biosensor Based on l-Glutathione Capped Poly(3,4-Ethylenedioxythiophene):Polystyrene Sulfonate/Carboxylated Multiwall CNT Network. J. Sci. Adv. Mater. Devices 2022, 7, 100504. [Google Scholar] [CrossRef]
- Sheng, Z.-H.; Zheng, X.-Q.; Xu, J.-Y.; Bao, W.-J.; Wang, F.-B.; Xia, X.-H. Electrochemical sensor based on nitrogen doped graphene: Simultaneous determination of ascorbic acid, dopamine and uric acid. Biosens. Bioelectron. 2012, 34, 125–131. [Google Scholar] [CrossRef]
- Rattanaumpa, T.; Maensiri, S.; Ngamchuea, K. Microporous carbon in the selective electro-oxidation of molecular biomarkers: Uric acid, ascorbic acid, and dopamine. RSC Adv. 2022, 12, 18709–18721. [Google Scholar] [CrossRef]
- Cinkova, K.; Kianičkova, K.; Stanković, D.M.; Vojs, M.; Marton, M.; Švorc, Ľ. The doping level of boron-doped diamond electrodes affects the voltammetric sensing of uric acid. Anal. Methods 2018, 10, 991–996. [Google Scholar] [CrossRef]
- Ozcan, A.; Ilkbas, S. Preparation of poly(3,4-ethylenedioxythiophene) nanofibers modified pencil graphite electrode and investigation of over-oxidation conditions for the selective and sensitive determination of uric acid in body fluids. Anal. Chim. Acta 2015, 891, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Amiri, M.; Imanzadeh, H.; Banaei, A. Carbon nanoparticles with tosyl functional group for distinguishing voltammetric peaks of ascorbic acid and uric acid. Mater. Sci. Eng. 2015, C47, 189–195. [Google Scholar] [CrossRef]
- Yang, Y.J.; Li, W. CTAB functionalized graphene oxide/multiwalled carbon nanotube composite modified electrode for the simultaneous determination of ascorbic acid, dopamine, uric acid and nitrite. Biosens. Bioelectron. 2014, 56, 300–306. [Google Scholar] [CrossRef]
- Roy, P.K.; Ganguly, A.; Yang, W.K.; Wu, C.T.; Hwang, J.S.; Tai, Y.; Chen, K.H.; Chen, L.C.; Chattopadhyay, S. Edge promoted ultrasensitive electrochemical detection of organic bio-molecules on epitaxial graphene nanowalls. Biosens. Bioelectron. 2015, 70, 137–144. [Google Scholar]
- Du, J.; Yue, R.; Ren, F.; Yao, Z.; Jiang, F.; Yang, P.; Du, Y. Novelgraphene flowersmodified carbon fibers for simultaneous determination of ascorbic acid, dopamine and uric acid. Biosens. Bioelectron. 2014, 53, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Lian, Q.; He, Z.; He, Q.; Luo, A.; Yan, K.; Zhang, D.; Lu, X.; Zhou, X. Simultaneous determination of ascorbic acid, dopamine and uric acid based on tryptophan functionalized graphene. Anal. Chim. Acta 2014, 823, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Wang, Q.; Jin, J.; Wu, P.; Wang, H.; Yu, S.; Zhang, H.; Cai, C. Low-potential detection of endogenous and physiological uric acid at uricase − thionine − single-walled carbon nanotube modified electrodes. Anal. Chem. 2010, 82, 2448–2455. [Google Scholar] [CrossRef]
- Ghica, M.E.; Brett, C.M.A. Poly(brilliant green) and poly(thionine) modified carbon nanotube coated carbon film electrodes for glucose and uric acid biosensors. Talanta 2014, 130, 198–206. [Google Scholar] [CrossRef]
- Herrastia, Z.; Martíneza, F.; Baldrich, E. Detection of uric acid at reversibly nanostructured thin-film microelectrodes. Sens. Actuators 2016, B234, 667–673. [Google Scholar] [CrossRef]
- Thakur, B.; Sawant, S.N. Polyaniline/Prussian-Blue-Based Amperometric Biosensor for Detection of Uric Acid. ChemPlusChem 2012, 2, 166–174. [Google Scholar] [CrossRef]
- Arora, K.; Choudhary, M.; Malhotra, B.D. Enhancing performance of uricase using multiwalled carbon nanotube doped polyaniline. Appl. Biochem. Biotechnol. 2014, 174, 1174–1187. [Google Scholar] [CrossRef]
- Fukuda, T.; Muguruma, H.; Iwasa, H.; Tanaka, T.; Hiratsuka, A.; Shimizu, T.; Tsuji, K.; Kishimoto, T. Electrochemical Determination of Uric Acid in Urine and Serum with Uricase/Carbon Nanotube/Carboxymethylcellulose Electrode. Anal. Biochem. 2020, 590, 113533. [Google Scholar] [CrossRef]
- Rawal, R.; Chawla, S.; Chauhan, N.; Dahiya, T.; Pundir, C.S. Construction of amperometric uric acid biosensor based on uricase immobilized on PBNPs/cMWCNT/PANI/Au composite. Int. J. Biol. Macromol. 2012, 50, 112–118. [Google Scholar] [CrossRef]
- Shi, Y.-M.; Mei, L.; Zhang, J.-X.; Hu, K.; Zhang, X.; Li, Z.-Z.; Miao, M.-S.; Li, X.-M. Synthesis of Zinc Tetraaminophthalocyanine Functionalized Graphene Nanosheets as an Enhanced Material for Sensitive Electrochemical Determination of Uric Acid. Electroanalysis 2020, 7, 1507–1515. [Google Scholar] [CrossRef]
- Usman, A.S.M.; Ibupoto, Z.H.; Kashif, M.; Hashim, U.; Willander, M. A potentiometric indirect uric acid sensor based on ZnO nanoflakes and immobilized uricase. Sensors 2012, 12, 2787–2797. [Google Scholar]
- Hwang, C.; Lee, W.J.; Kim, S.D.; Park, S.; Kim, J.H. Recent Advances in Biosensor Technologies for Point-of-Care Urinalysis. Biosensors 2022, 12, 1020. [Google Scholar] [CrossRef] [PubMed]
- Irkham, I.; Ibrahim, A.U.; Pwavodi, P.C.; Al-Turjman, F.; Hartati, Y.W. Smart Graphene-Based Electrochemical Nanobiosensor for Clinical Diagnosis: Review. Sensors 2023, 23, 2240. [Google Scholar] [CrossRef] [PubMed]
- Balogun, S.A.; Fayemi, O.E. Recent Advances in the Use of CoPc-MWCNTs Nanocomposites as Electrochemical Sensing Materials. Biosensors 2022, 12, 850. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, Z.; Liang, Q.; Zhou, M.; Xu, S.; Li, Z.; Sun, D. Tetra-substituted cobalt (II) phthalocyanine/multi-walled carbon nanotubes as new efficient catalyst for the selective oxidation of styrene using tert-butyl hydroperoxide. Fuller. Nanotub. Carbon. Nanostruct. 2020, 28, 799–807. [Google Scholar] [CrossRef]
- Şavk, A.; Özdil, B.; Demirkan, B.; Nas, M.S.; Calimli, M.H.; Alma, M.H.; Asiri, A.M.; Şen, F. Multiwalled carbon nanotube-based nanosensor for ultrasensitive detection of uric acid, dopamine, and ascorbic acid. Mater. Sci. Eng. C 2019, 99, 248–254. [Google Scholar] [CrossRef]
- Wu, B.; Yeasmin, S.; Liu, Y.; Cheng, L.J. Ferrocene Functionalized Gold Nanoparticles on Carbon Nanotube Electrodes for Portable Dopamine Sensor. In Proceedings of the Electrochemical Society Meeting Abstracts 239, Digital Meeting, 30 May–3 June 2021; Volume 55, p. 1345. [Google Scholar]
- Musarraf, H.M.; Asiri, A.M.; Uddin, J.; Marwani, H.M.; Rahman, M.M. Development of a L-cysteine Sensor Based on Thallium Oxide Coupled Multi-walled Carbon Nanotube Nanocomposites with Electrochemical Approach. Chem. Asian J. 2022, 17, e202101117. [Google Scholar] [CrossRef]
- Knežević, S.; Ognjanović, M.; Stanković, V.; Zlatanova, M.; Nešić, A.; Gavrović-Jankulović, M.; Stanković, D. La(OH)3 Multi-Walled Carbon Nanotube/Carbon Paste-Based Sensing Approach for the Detection of Uric Acid—A Product of Environmentally Stressed Cells. Biosensors 2022, 12, 705. [Google Scholar] [CrossRef] [PubMed]
- Negrea, S.; Andelescu, A.A.; Ilies, B.; Motoc, S.; Cretu, C.; Cseh, L.; Rastei, M.; Donnio, B.; Szerb, E.I.; Manea, F. Design of Nanostructured Hybrid Electrodes Based on a Liquid Crystalline Zn(II) Coordination Complex-Carbon Nanotubes Composition for the Specific Electrochemical Sensing of Uric Acid. Nanomater. 2022, 12, 4215. [Google Scholar] [CrossRef] [PubMed]
- Choukairi, M.; Bouchta, D.; Bounab, L.; González-Romero, E.; Achache, M.; Draoui, K.; Chaouket, F.; Raissouni, I.; Gharous, M. A Carbon Paste Electrode Modified by Bentonite and l-Cysteine for Simultaneous Determination of Ascorbic and Uric Acids: Application in Biological Fluids. ChemistryOpen 2023, 12, e202200201. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lee, H. Anti-Biofouling Strategies for Long-Term Continuous Use of Implantable Biosensors. Chemosensors 2020, 8, 66. [Google Scholar] [CrossRef]
- Menon, S.; Sam, S.; Keerthi, K.; Kumar, K.G. Carbon Nanomaterial-Based Sensors: Emerging Trends, Markets, and Concerns. Carbon. Nanomater.-Based Sens. 2022, 2022, 347–379. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chelmea, L.; Badea, M.; Scarneciu, I.; Moga, M.A.; Dima, L.; Restani, P.; Murdaca, C.; Ciurescu, D.; Gaman, L.E. New Trends in Uric Acid Electroanalysis. Chemosensors 2023, 11, 341. https://doi.org/10.3390/chemosensors11060341
Chelmea L, Badea M, Scarneciu I, Moga MA, Dima L, Restani P, Murdaca C, Ciurescu D, Gaman LE. New Trends in Uric Acid Electroanalysis. Chemosensors. 2023; 11(6):341. https://doi.org/10.3390/chemosensors11060341
Chicago/Turabian StyleChelmea, Ligia, Mihaela Badea, Ioan Scarneciu, Marius Alexandru Moga, Lorena Dima, Patrizia Restani, Cecilia Murdaca, Daniel Ciurescu, and Laura Elena Gaman. 2023. "New Trends in Uric Acid Electroanalysis" Chemosensors 11, no. 6: 341. https://doi.org/10.3390/chemosensors11060341
APA StyleChelmea, L., Badea, M., Scarneciu, I., Moga, M. A., Dima, L., Restani, P., Murdaca, C., Ciurescu, D., & Gaman, L. E. (2023). New Trends in Uric Acid Electroanalysis. Chemosensors, 11(6), 341. https://doi.org/10.3390/chemosensors11060341