Surface-Enhance Raman Spectroscopy Detection of Thiabendazole in Frozen Food Products: The Case of Blueberries and Their Extracts
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
2.2. Silver Colloid Synthesis
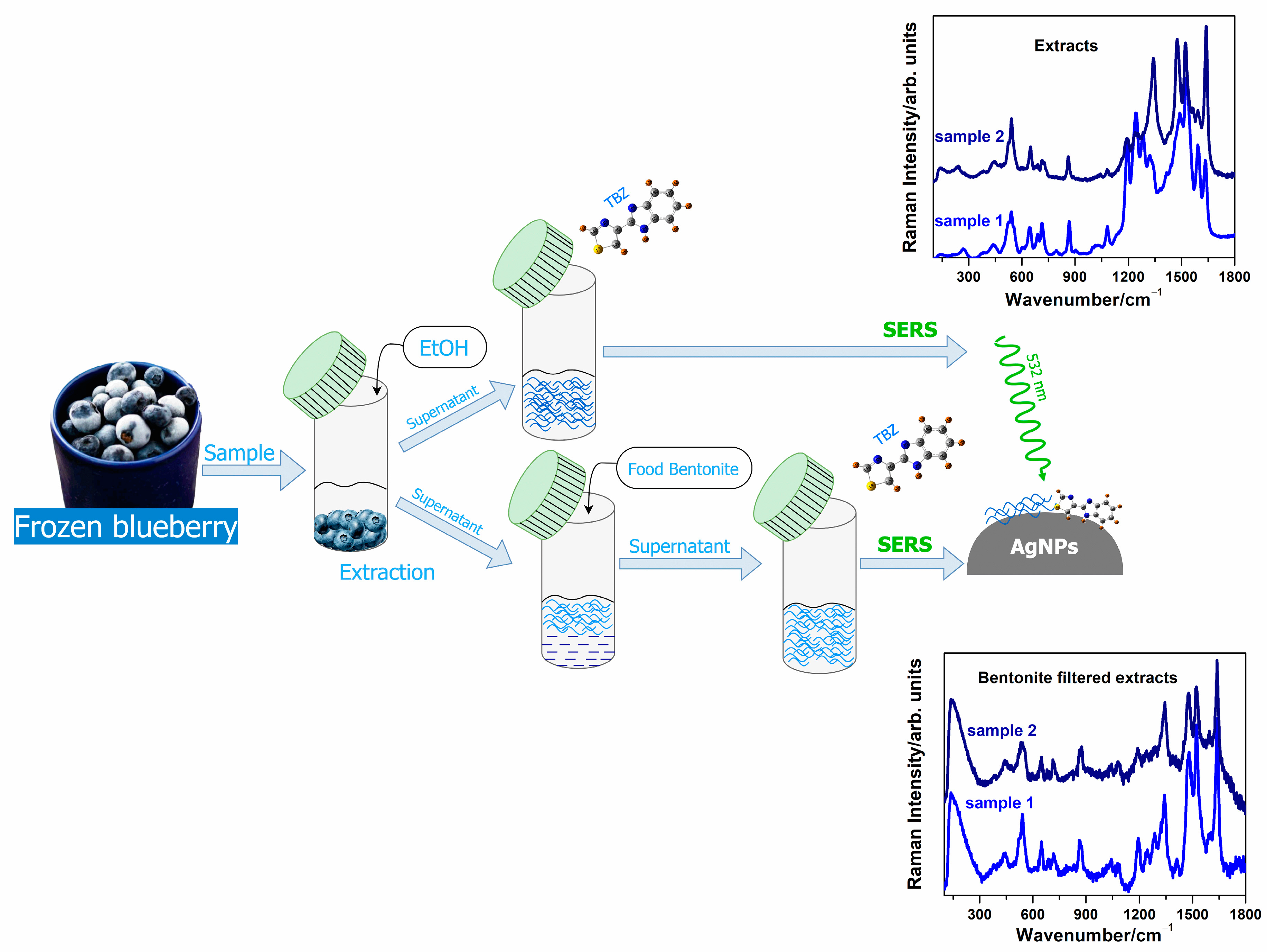
2.3. Sample Preparation and Analysis
2.3.1. Concentration Dependence SERS Spectra of TBZ
2.3.2. SERS Spectra of the Frozen Blueberry Fruit Extracts and TBZ-Treated Extracts
2.3.3. SERS Spectra of the Bentonite-Filtered Extracts
2.4. Instrumentation
3. Results
3.1. SERS Spectra of the Frozen Blueberry Fruit Extracts
3.2. The Concentration Dependence SERS Spectra of TBZ
3.3. SERS Spectra of the TBZ-Treated Frozen Blueberry Fruit Extracts (Multiplexed SERS)
3.4. SERS Spectra of Bentonite-Filtered Extracts
3.5. SERS Spectra of the Bentonite-Filtered Extracts Treated with TBZ
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, M.L.; Gao, Y.; Han, X.X.; Zhao, B. Detection of Pesticide Residues in Food Using Surface-Enhanced Raman Spectroscopy: A Review. J. Agric. Food Chem. 2017, 65, 6719–6726. [Google Scholar] [CrossRef]
- Veneziano, A.; Vacca, G.; Arana, S.; De Simone, F.; Rastrelli, L. Determination of Carbendazim, Thiabendazole and Thiophanate-Methyl in Banana (Musa Acuminata) Samples Imported to Italy. Food Chem. 2004, 87, 383–386. [Google Scholar] [CrossRef]
- Castillo, L.E.; Ruepert, C.; Solis, E. Pesticide Residues in the Aquatic Environment of Banana Plantation: Areas in the North Atlantic Zone of Costa Rica. Environ. Toxicol. Chem. 2000, 19, 1942–1950. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (Eu) 2021/1807. Off. J. Eur. Union 2021, 51, 1–37. [Google Scholar]
- Environmental Protection Agency. Thiabendazole; Tolerances for Residues; Environmental Protection Agency: Chicago, IL, USA, 2022; pp. 552–553. [Google Scholar]
- Wolfgang Bödeker Pesticide Atlas-Health. Available online: https://eu.boell.org/en/PesticideAtlas-health (accessed on 18 October 2022).
- European Council. Regulamentul (CE) NR. 1333/2008 al Parlamentului European și al Consiliului din 16 Decembrie 2008 Privind Aditivii Alimentari; European Council: Brussels, Belgium, 2010; Volume 40, pp. 1–89.
- Zheng, D.; Hu, X.; Fu, X.; Xia, Z.; Zhou, Y.; Peng, L.; Yu, Q.; Peng, X. Flowerlike Ni-NiO Composite as Magnetic Solid-Phase Extraction Sorbent for Analysis of Carbendazim and Thiabendazole in Edible Vegetable Oils by Liquid Chromatography-Mass Spectrometry. Food Chem. 2022, 374, 131761. [Google Scholar] [CrossRef]
- Almutairi, M.; Alsaleem, T.; Al Herbish, H.; Al Sayari, A.A.; Alowaifeer, A.M. LC-MS/MS and GC-MS/MS Analysis of Pesticide Residues in Ecuadorian and Filipino Cavendish Bananas Imported into Saudi Arabia. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2021, 38, 1376–1385. [Google Scholar] [CrossRef]
- Choi, S.I.; Han, X.; Lee, S.J.; Men, X.; Oh, G.; Lee, D.S.; Lee, O.H. Validation of an Analytical Method for the Determination of Thiabendazole in Various Food Matrices. Separations 2022, 9, 135. [Google Scholar] [CrossRef]
- Fares, N.V.; Hassan, Y.A.A.; Hussein, L.A.; Ayad, M.F. Determination of Fungicides’ Residues and Their Degradation Kinetics in Orange Tree Fruits Using Liquid Chromatography—Tandem Mass Spectrometry Coupled with QuEChERS Method. Microchem. J. 2021, 168, 106376. [Google Scholar] [CrossRef]
- Calero, M.; Fernández, R.; García, M.; Juan-borrás, M.; Escriche, I.; Arnau, A.; Montoya, Á.; Jiménez, Y. High Fundamental Frequency (HFF) Monolithic Quartz Crystal Microbalance with Dissipation Array for the Simultaneous Detection of Pesticides and Antibiotics in Complex Food. Biosensors 2022, 12, 433. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Wang, Y.; Jiang, L.; Zhang, H.; Zhao, Y.; Liu, P.; Liu, J.; Hammock, B.D.; Zhang, C. A Polydopamine-Coated Gold Nanoparticles Quenching Quantum Dots-Based Dual-Readout Lateral Flow Immunoassay for Sensitive Detection of Carbendazim in Agriproducts. Biosensors 2022, 12, 83. [Google Scholar] [CrossRef]
- Budetić, M.; Samardžić, M.; Bubnjar, K.; Dandić, A.; Živković, P.; Széchenyi, A.; Kiss, L. A New Sensor for Direct Potentiometric Determination of Thiabendazole in Fruit Peels Using the Gran Method. Food Chem. 2022, 392, 133290. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; He, L. Recent Advance in SERS Techniques for Food Safety and Quality Analysis: A Brief Review. Curr. Opin. Food Sci. 2019, 28, 82–87. [Google Scholar] [CrossRef]
- Nie, P.; Dong, T.; Xiao, S.; Lin, L.; He, Y.; Qu, F. Quantitative Determination of Thiabendazole in Soil Extracts by Surface-Enhanced Raman Spectroscopy. Molecules 2018, 23, 1949. [Google Scholar] [CrossRef]
- Tao, M.; Fang, H.; Feng, X.; He, Y.; Liu, X.; Shi, Y.; Wei, Y.; Hong, Z. Rapid Trace Detection of Pesticide Residues on Tomato by Surface-Enhanced Raman Spectroscopy and Flexible Tapes. J. Food Qual. 2022, 2022, 6947775. [Google Scholar] [CrossRef]
- Wang, C.M.; Roy, P.K.; Juluri, B.K.; Chattopadhyay, S. A SERS Tattoo for In Situ, Ex Situ, and Multiplexed Detection of Toxic Food Additives. Sens. Actuators B Chem. 2018, 261, 218–225. [Google Scholar] [CrossRef]
- Lin, L.; Dong, T.; Nie, P.; Qu, F.; He, Y.; Chu, B.; Xiao, S. Rapid Determination of Thiabendazole Pesticides in Rape by Surface Enhanced Raman Spectroscopy. Sensors 2018, 18, 1082. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Sun, D.W.; Pu, H.; Wei, Q. Rapid Nondestructive Detection of Mixed Pesticides Residues on Fruit Surface Using SERS Combined with Self-Modeling Mixture Analysis Method. Talanta 2020, 217, 120998. [Google Scholar] [CrossRef]
- “0Bentonite”, Lexico, Lexico UK English Dictionary, Oxford University Press., Archived from the original on 27 April 2021. Available online: https://www.dictionary.com/browse/bentonite (accessed on 26 June 2023).
- Stiufiuc, R.; Iacovita, C.; Lucaciu, C.M.; Stiufiuc, G.; Dutu, A.G.; Braescu, C.; Leopold, N. SERS-Active Silver Colloids Prepared by Reduction of Silver Nitrate with Short-Chain Polyethylene Glycol. Nanoscale Res. Lett. 2013, 8, 47. [Google Scholar] [CrossRef]
- Information, B.; Labelling, O. Brief Information about the Organic Labeling According to Reg. (EC) 834/07 (Including Reg. (EC) 271/10), NOP and JAS; CERES: Boston, MA, USA, 2022; pp. 6–8. [Google Scholar]
- Jacquet, F.; Jeuffroy, M.H.; Jouan, J.; Le Cadre, E.; Litrico, I.; Malausa, T.; Reboud, X.; Huyghe, C. Pesticide-Free Agriculture as a New Paradigm for Research. Agron. Sustain. Dev. 2022, 42, 8. [Google Scholar] [CrossRef]
- Zaffino, C.; Russo, B.; Bruni, S. Surface-Enhanced Raman Scattering (SERS) Study of Anthocyanidins. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 149, 41–47. [Google Scholar] [CrossRef]
- Kim, M.S.; Kim, M.K.; Lee, C.J.; Jung, Y.M.; Lee, M.S. Surface-Enhanced Raman Spectroscopy of Benzimidazolic Fungicides: Benzimidazole and Thiabendazole. Bull. Korean Chem. Soc. 2009, 30, 2930–2934. [Google Scholar] [CrossRef]
- Müller, C.; David, L.; Chiş, V.; Pînzaru, S.C. Detection of Thiabendazole Applied on Citrus Fruits and Bananas Using Surface Enhanced Raman Scattering. Food Chem. 2014, 145, 814–820. [Google Scholar] [CrossRef]
- Brezestean, I.A.; Tosa, N.; Falamas, A.; Cuibus, D.; Muntean, C.M.; Bende, A.; Cozar, B.; Berghian-Grosan, C.; Farcău, C. Silver Nanoparticle Films Obtained by Convective Self-Assembly for Surface-Enhanced Raman Spectroscopy Analyses of the Pesticides Thiabendazole and Endosulfan. Front. Chem. 2022, 10, 915337. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, M.; Graves, P.R.; Hill, I.R.; Robinson, J. Raman Spectroscopy of Pyridine Adsorbed on Roughened β Palladium Hydride Electrodes. Chem. Phys. Lett. 1983, 95, 322–324. [Google Scholar] [CrossRef]
- Xia, J.; Wang, D.; Liang, P.; Zhang, D.; Du, X.; Ni, D.; Yu, Z. Vibrational (FT-IR, Raman) Analysis of Tea Catechins Based on Both Theoretical Calculations and Experiments. Biophys. Chem. 2020, 256, 106282. [Google Scholar] [CrossRef]
- Huang, C.C.; Chen, W. A SERS Method with Attomolar Sensitivity: A Case Study with the Flavonoid Catechin. Microchim. Acta 2018, 185, 120. [Google Scholar] [CrossRef]
- El-Nahhal, Y.Z. Development of Controlled Release Formulations of Thiabendazole. J. Agric. Chem. Environ. 2014, 3, 1–8. [Google Scholar] [CrossRef]
- Ahmed Said, A.E.A.; Goda, M.N. Superior Competitive Adsorption Capacity of Natural Bentonite in the Efficient Removal of Basic Dyes from Aqueous Solutions. ChemistrySelect 2021, 6, 2790–2803. [Google Scholar] [CrossRef]
- PubChem. Thiabendazole. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Thiabendazole#section=Henry%27s-Law-Constant (accessed on 26 June 2023).
- Chen, J.; Huang, Y.; Kannan, P.; Zhang, L.; Lin, Z.; Zhang, J.; Chen, T.; Guo, L. Flexible and Adhesive Surface Enhance Raman Scattering Active Tape for Rapid Detection of Pesticide Residues in Fruits and Vegetables. Anal. Chem. 2016, 88, 2149–2155. [Google Scholar] [CrossRef]
- Liu, B.; Han, G.; Zhang, Z.; Liu, R.; Jiang, C.; Wang, S.; Han, M.Y. Shell Thickness-Dependent Raman Enhancement for Rapid Identification and Detection of Pesticide Residues at Fruit Peels. Anal. Chem. 2012, 84, 255–261. [Google Scholar] [CrossRef]
- Zhou, N.; Meng, G.; Huang, Z.; Ke, Y.; Zhou, Q.; Hu, X. A Flexible Transparent Ag-NC@PE Film as a Cut-and-Paste SERS Substrate for Rapid: In Situ Detection of Organic Pollutants. Analyst 2016, 141, 5864–5869. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Cai, W.; Yang, L.; Liu, J. Highly Uniform and Optical Visualization of SERS Substrate for Pesticide Analysis Based on Au Nanoparticles Grafted on Dendritic α-Fe 2O3. Nanoscale 2013, 5, 11193–11199. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Sun, Y.; Ni, P.; Lu, W.; Jiang, S.; Wang, Y.; Li, Z.; Li, Z. Three-Dimensional TiO2 Supported Silver Nanoparticles as Sensitive and UV-Cleanable Substrate for Surface Enhanced Raman Scattering. Sens. Actuators B Chem. 2017, 242, 260–268. [Google Scholar] [CrossRef]
- Yang, J.K.; Kang, H.; Lee, H.; Jo, A.; Jeong, S.; Jeon, S.J.; Kim, H.I.; Lee, H.Y.; Jeong, D.H.; Kim, J.H.; et al. Single-Step and Rapid Growth of Silver Nanoshells as SERS-Active Nanostructures for Label-Free Detection of Pesticides. ACS Appl. Mater. Interfaces 2014, 6, 12541–12549. [Google Scholar] [CrossRef]
- Tang, X.; Dong, R.; Yang, L.; Liu, J. Fabrication of Au Nanorod-Coated Fe3O4 Microspheres as SERS Substrate for Pesticide Analysis by near-Infrared Excitation. J. Raman Spectrosc. 2015, 46, 470–475. [Google Scholar] [CrossRef]
- Kumar, S.; Goel, P.; Singh, J.P. Flexible and Robust SERS Active Substrates for Conformal Rapid Detection of Pesticide Residues from Fruits. Sens. Actuators B Chem. 2017, 241, 577–583. [Google Scholar] [CrossRef]
- Shiohara, A.; Langer, J.; Polavarapu, L.; Liz-Marzán, L.M. Solution Processed Polydimethylsiloxane/Gold Nanostar Flexible Substrates for Plasmonic Sensing. Nanoscale 2014, 6, 9817–9823. [Google Scholar] [CrossRef]
- He, L.; Chen, T.; Labuza, T.P. Recovery and Quantitative Detection of Thiabendazole on Apples Using a Surface Swab Capture Method Followed by Surface-Enhanced Raman Spectroscopy. Food Chem. 2014, 148, 42–46. [Google Scholar] [CrossRef]
- Liou, P.; Nayigiziki, F.X.; Kong, F.; Mustapha, A.; Lin, M. Cellulose Nanofibers Coated with Silver Nanoparticles as a SERS Platform for Detection of Pesticides in Apples. Carbohydr. Polym. 2017, 157, 643–650. [Google Scholar] [CrossRef]
- Moldovan, R.; Milenko, K.; Vereshchagina, E.; Iacob, B.C.; Schneider, K.; Farcău, C.; Bodoki, E. EC-SERS Detection of Thiabendazole in Apple Juice Using Activated Screen-Printed Electrodes. Food Chem. 2023, 405. [Google Scholar] [CrossRef]
- Tian, L.; Su, M.; Yu, F.; Xu, Y.; Li, X.; Li, L.; Liu, H.; Tan, W. Liquid-State Quantitative SERS Analyzer on Self-Ordered Metal Liquid-like Plasmonic Arrays. Nat. Commun. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Alsammarraie, F.K.; Lin, M.; Mustapha, A.; Lin, H.; Chen, X.; Chen, Y.; Wang, H.; Huang, M. Rapid Determination of Thiabendazole in Juice by SERS Coupled with Novel Gold Nanosubstrates. Food Chem. 2018, 259, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Wijaya, W.; Pang, S.; Labuza, T.P.; He, L. Rapid Detection of Acetamiprid in Foods Using Surface-Enhanced Raman Spectroscopy (SERS). J. Food Sci. 2014, 79, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Lai, K.; Rasco, B.A.; Huang, Y. Determination of Carbaryl Pesticide in Fuji Apples Using Surface-Enhanced Raman Spectroscopy Coupled with Multivariate Analysis. Lwt 2015, 60, 352–357. [Google Scholar] [CrossRef]
- Xu, Q.; Guo, X.; Xu, L.; Ying, Y.; Wu, Y.; Wen, Y.; Yang, H. Template-Free Synthesis of SERS-Active Gold Nanopopcorn for Rapid Detection of Chlorpyrifos Residues. Sens. Actuators B Chem. 2017, 241, 1008–1013. [Google Scholar] [CrossRef]
- Li, C.; Yang, C.; Xu, S.; Zhang, C.; Li, Z.; Liu, X.; Jiang, S.; Huo, Y.; Liu, A.; Man, B. Ag2O@Ag Core-Shell Structure on PMMA as Low-Cost and Ultra-Sensitive Flexible Surface-Enhanced Raman Scattering Substrate. J. Alloys Compd. 2017, 695, 1677–1684. [Google Scholar] [CrossRef]
- Zhai, C.; Li, Y.Y.; Peng, Y.K.; Xu, T.F. Detection of Chlorpyrifos in Apples Using Gold Nanoparticles Based on Surface Enhanced Raman Spectroscopy. Int. J. Agric. Biol. Eng. 2015, 8, 113–120. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Müller Molnár, C.; Berghian-Groșan, C.; Măgdaș, D.A.; Cîntă Pînzaru, S. Surface-Enhance Raman Spectroscopy Detection of Thiabendazole in Frozen Food Products: The Case of Blueberries and Their Extracts. Chemosensors 2023, 11, 505. https://doi.org/10.3390/chemosensors11090505
Müller Molnár C, Berghian-Groșan C, Măgdaș DA, Cîntă Pînzaru S. Surface-Enhance Raman Spectroscopy Detection of Thiabendazole in Frozen Food Products: The Case of Blueberries and Their Extracts. Chemosensors. 2023; 11(9):505. https://doi.org/10.3390/chemosensors11090505
Chicago/Turabian StyleMüller Molnár, Csilla, Camelia Berghian-Groșan, Dana Alina Măgdaș, and Simona Cîntă Pînzaru. 2023. "Surface-Enhance Raman Spectroscopy Detection of Thiabendazole in Frozen Food Products: The Case of Blueberries and Their Extracts" Chemosensors 11, no. 9: 505. https://doi.org/10.3390/chemosensors11090505
APA StyleMüller Molnár, C., Berghian-Groșan, C., Măgdaș, D. A., & Cîntă Pînzaru, S. (2023). Surface-Enhance Raman Spectroscopy Detection of Thiabendazole in Frozen Food Products: The Case of Blueberries and Their Extracts. Chemosensors, 11(9), 505. https://doi.org/10.3390/chemosensors11090505








