A Review on Metal Oxide Semiconductor-Based Chemo-Resistive Ethylene Sensors for Agricultural Applications
Abstract
:1. Introduction
2. Materials and Fabrications
2.1. SnO2
2.1.1. CVD
2.1.2. USP
2.1.3. Sputtering Method
2.2. ZnO
2.2.1. Wet Chemical Method
2.2.2. Electrochemical Deposition
2.2.3. Sputtering Method
2.3. Other MOSs
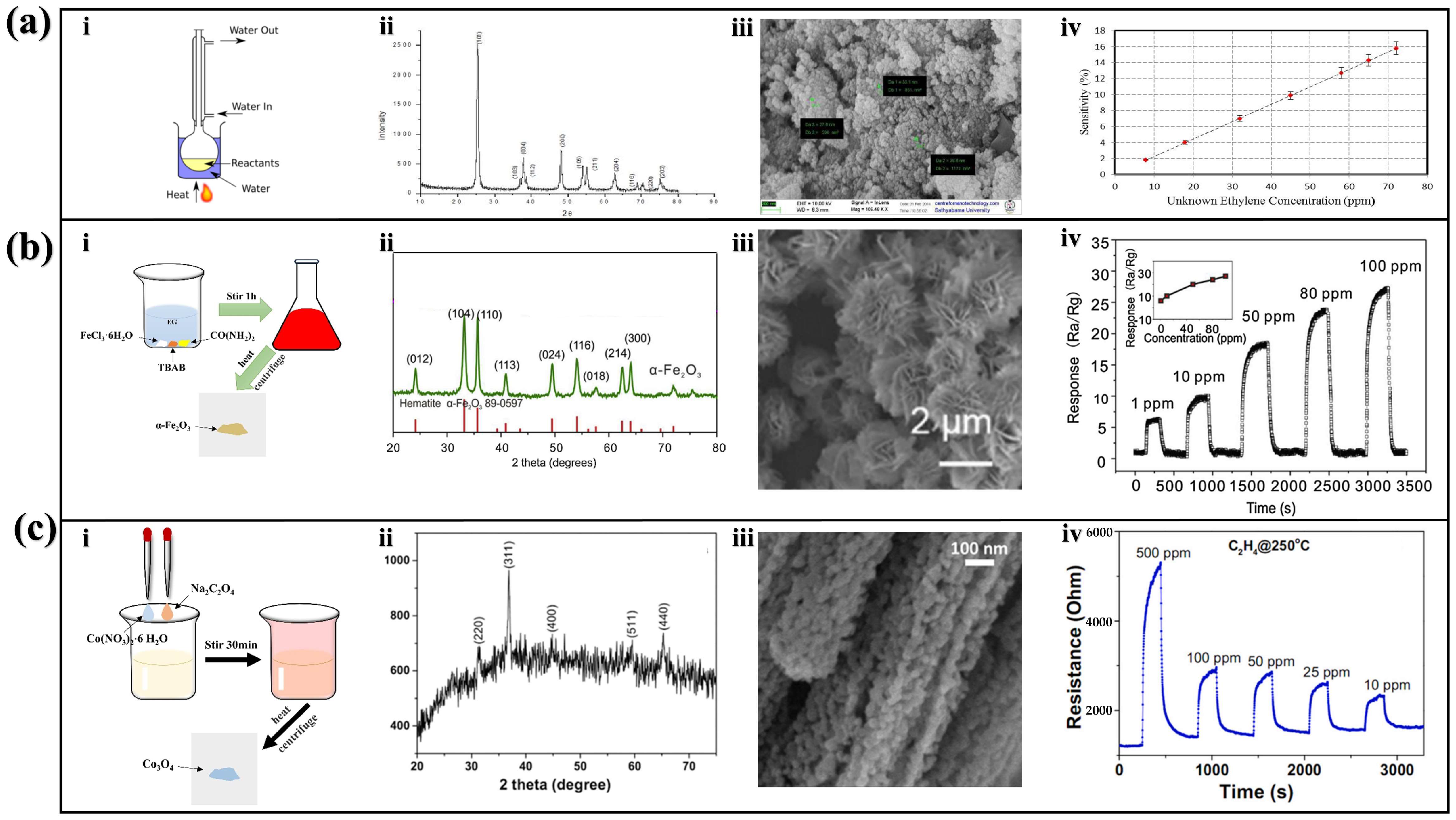
2.3.1. TiO2 Fabricated by Reflux Method
2.3.2. Fe2O3 Fabricated by Wet Chemical Method
2.3.3. Co3O4 Fabricated by Hydrothermal Method
3. Applications in Agriculture
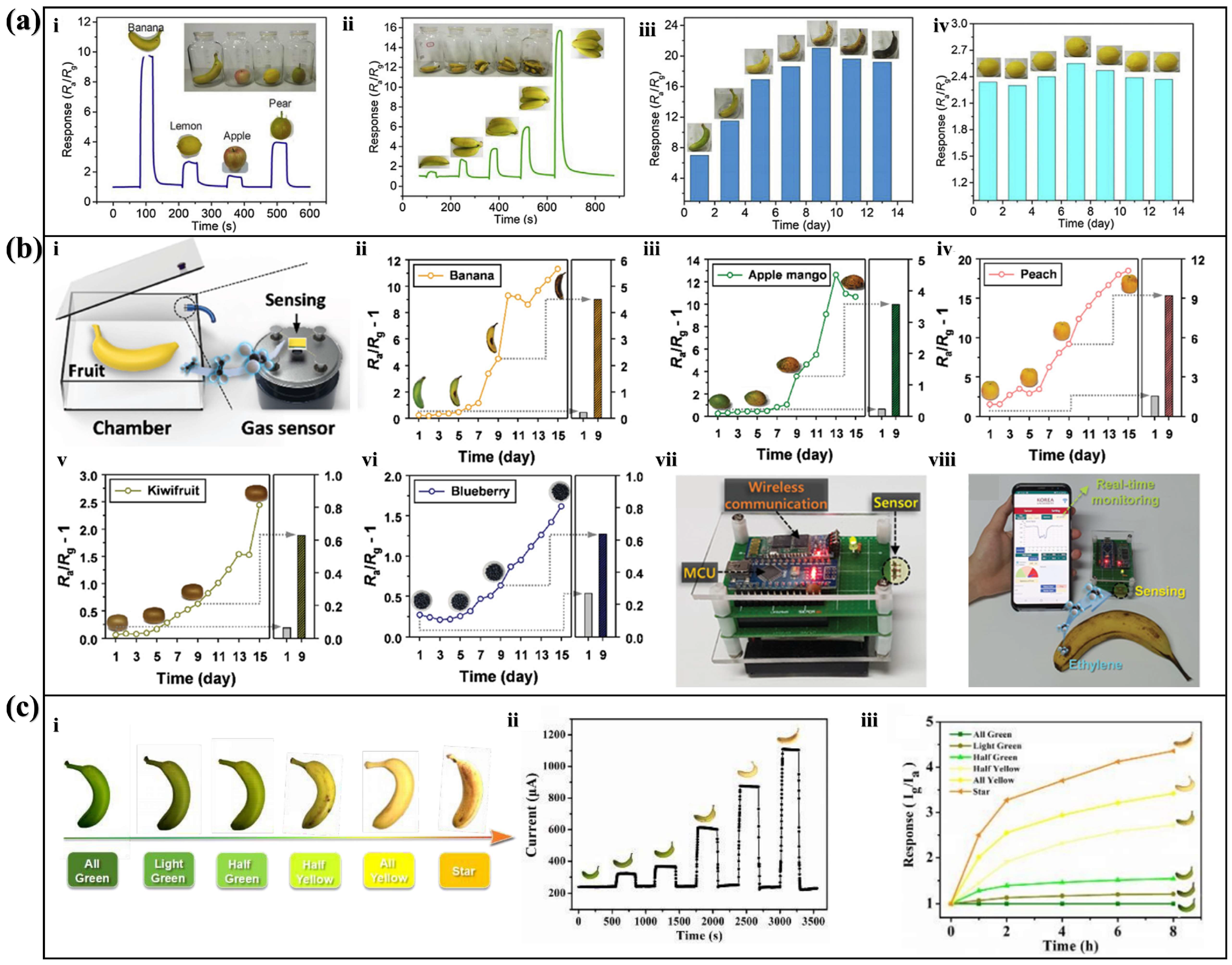
3.1. Pd-Loaded SnO2 Sensor for Fruit Maturity Detection
3.2. Cr2O3-Tailored SnO2 Sensor for Fruit Maturity Detection
3.3. ZnO Nanosheet Sensor for Banana Maturity Detection
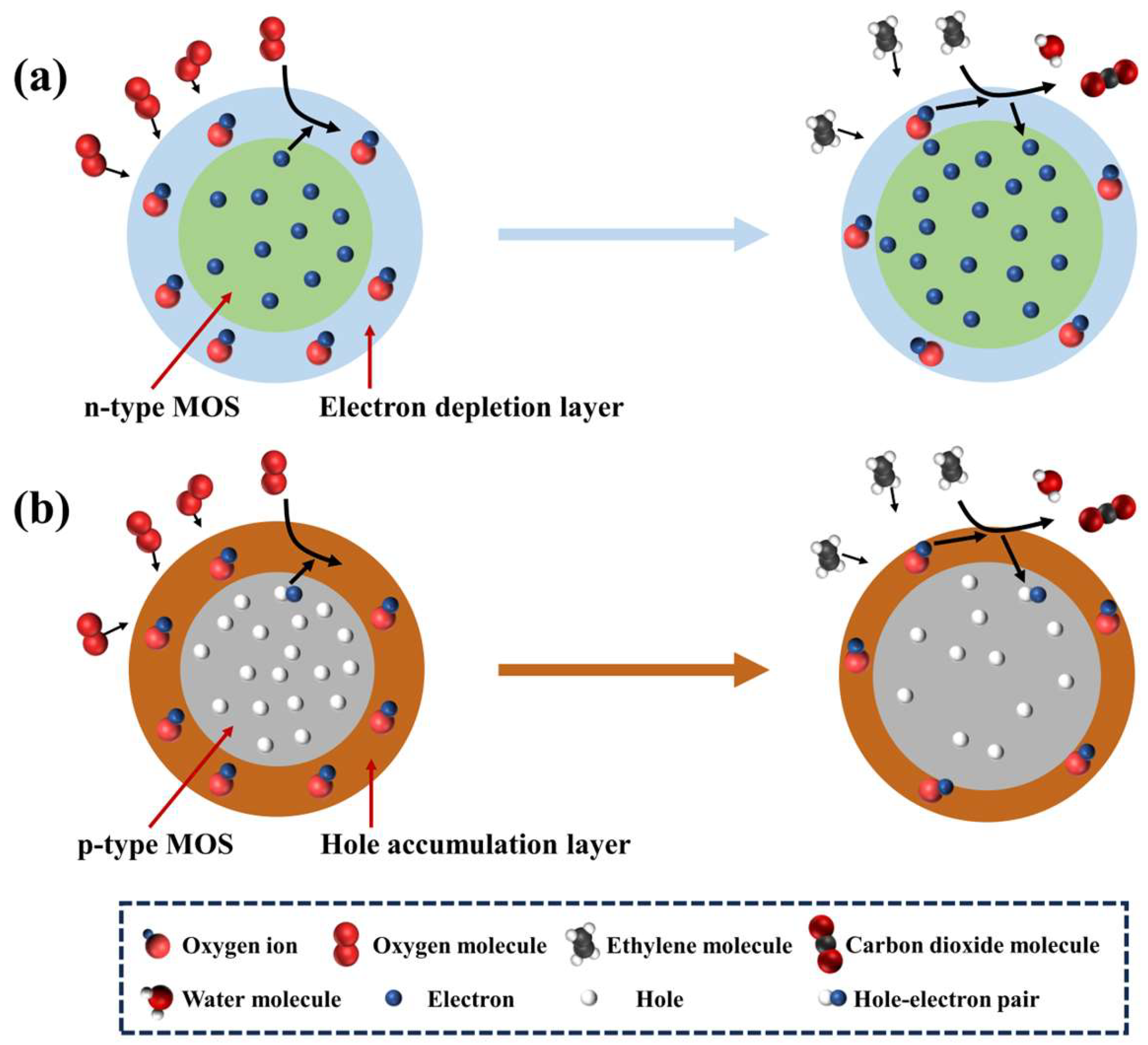
4. Sensing Mechanisms
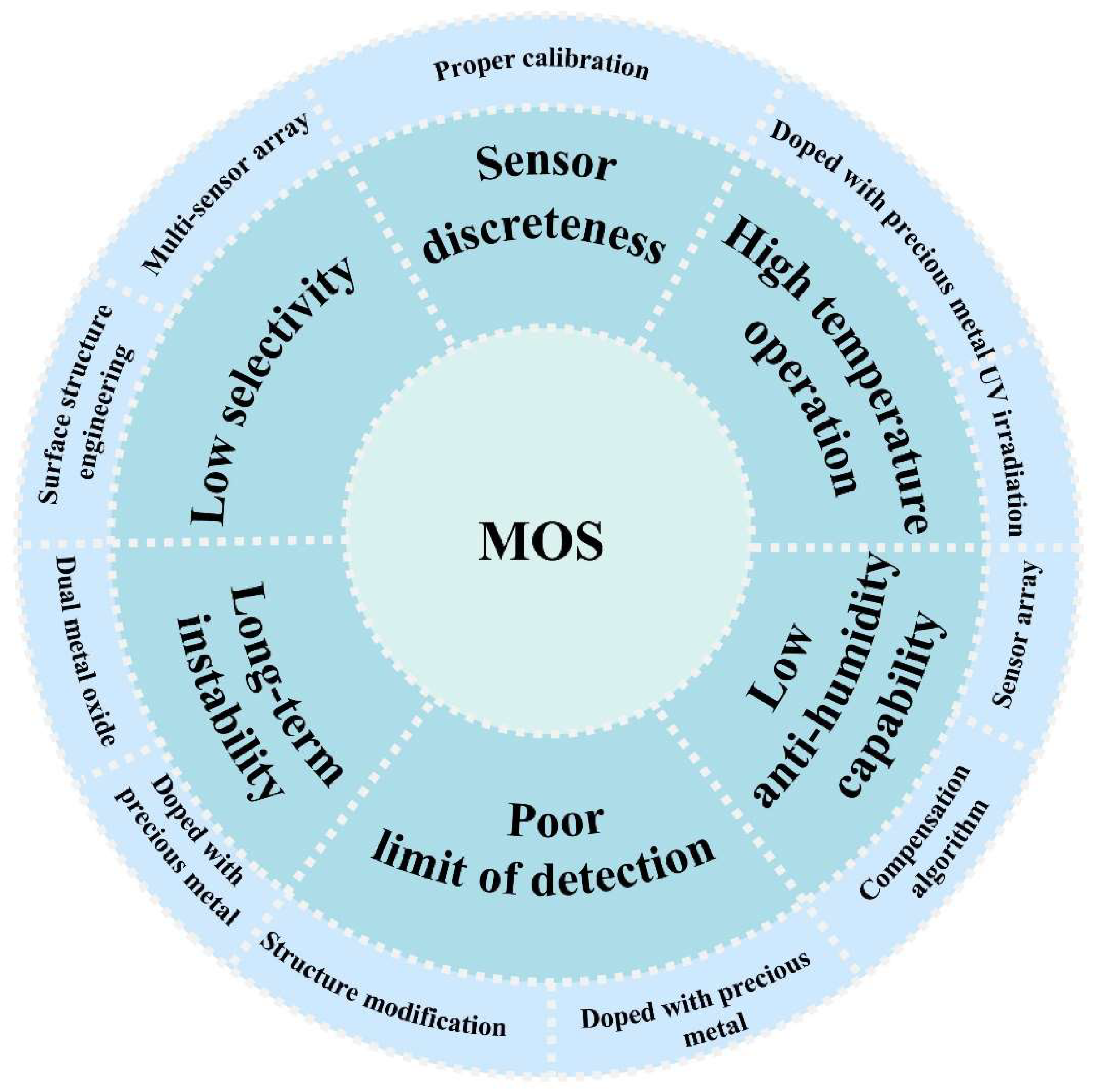
5. Challenges and Perspectives
- Low selectivity. MOS-based chemo-resistive gas sensors are susceptible to multiple gases, such as ethane mentioned in Section 2.2.3, which interfere with the detection of ethylene. In particular, the complex composition of gases in agricultural environments, such as water vapor, ethanol, ammonia, and nitrous oxide, makes accurate detection of ethylene a huge challenge. In order to eliminate the interference of non-target gas, we can perform surface structure engineering on the gas-sensitive layer, such as coating microchannels to block interfering gas molecules from reaching the sensing layer [89]. In addition, utilizing the multi-sensor array is also an effective way to combine the response result of multiple sensors to identify ethylene concentrations [90].
- Poor limit of detection. The limit of detection refers to the lowest concentration of ethylene that the sensor can reliably detect and measure. In the field of agriculture, trace amounts of ethylene can have a large impact on the growth and ripeness of fruits and plants. In addition, the ethylene content in agriculture is usually at the ppb level. It is important to optimize the limit of detection of ethylene sensors [91]. A possible solution is structure modification, such as creating a porous structure to increase the surface area, which can effectively reduce the limit of detection. In addition, doping with precious metals is also an effective strategy [61].
- High-temperature operation. Due to the inherent nature of ethylene, ethylene sensors typically need to operate at high temperatures (such as 250 °C [70], 300 °C [49], and 350 °C [71]). The high temperature can easily cause damage to the surface of the detection object, limiting its application on plant surfaces and other scenes [72]. Also, the high temperature of the sensor puts higher requirements on the stability of the sensor. Moreover, considering the long working time of the ethylene sensor, the high working temperature also greatly increases the energy consumption. To solve this problem, doping with noble metals is an effective strategy, which acts as catalysts that can effectively reduce the activation energy of ethylene molecules and significantly reduce the working temperature. In addition, UV irradiation can energize the sensors, which can also lower the operating temperature [50].
- Low anti-humidity capability. Water vapor decreases the sensitivity of the sensor to ethylene gas because water molecules compete with ethylene molecules for oxygen species and reduce the available surface area for the adsorption of ethylene molecules on the MOx surface. Therefore, an increase in humidity decreases the sensitivity of the sensor to ethylene gas. By using a temperature and humidity compensation algorithm, the sensor output can be corrected based on the temperature and humidity information of the environment, thus reducing the effect of humidity on sensor sensitivity. Additionally, it is possible to use the humidity sensor in conjunction with a target gas sensor [92].
- Long-term instability. In the process of measuring ethylene, in reality, the concentration is often too low. Because the sensors at this stage are still not very sensitive to low concentrations of ethylene, the results will be unstable when measuring. Possible solutions to this issue are doping with precious metals or using bimetallic oxide-based sensors. Precious metals can act as catalysts. Bimetallic oxides increase the specific surface area of the material and thus provide more active sites. These improved methods make the sensor show high stability, even at a low-concentration detection [93].
- Sensor discreteness. In practice, people find out that even the same series of sensors, due to small differences in the manufacturing process, can lead to slightly different performance between devices. The sensor’s working environment and long-term use or aging may also cause the performance of the sensor to change. The sensitivity, response time and other characteristics of the sensor may change with the change of these factors, resulting in the response difference of the sensor under the same conditions. This challenge may (at least partially) be overcome by proper calibration.
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saltveit, M.E. Effect of ethylene on quality of fresh fruits and vegetables. Postharvest Biol. Technol. 1999, 15, 279–292. [Google Scholar] [CrossRef]
- Barry, C.S.; Giovannoni, J.J. Ethylene and fruit ripening. J. Plant Growth Regul. 2007, 26, 143–159. [Google Scholar] [CrossRef]
- Pratt, H.K.; Goeschl, J. Physiological roles of ethylene in plants. Annu. Rev. Plant Physiol. 1969, 20, 541–584. [Google Scholar] [CrossRef]
- Bapat, V.A.; Trivedi, P.K.; Ghosh, A.; Sane, V.A.; Ganapathi, T.R.; Nath, P. Ripening of fleshy fruit: Molecular insight and the role of ethylene. Biotechnol. Adv. 2010, 28, 94–107. [Google Scholar] [CrossRef]
- Bleecker, A.B.; Kende, H. Ethylene: A gaseous signal molecule in plants. Annu. Rev. Cell Dev. Biol. 2000, 16, 1–18. [Google Scholar] [CrossRef]
- Bailén, G.; Guillén, F.; Castillo, S.; Serrano, M.; Valero, D.; Martínez-Romero, D. Use of activated carbon inside modified atmosphere packages to maintain tomato fruit quality during cold storage. J. Agric. Food Chem. 2006, 54, 2229–2235. [Google Scholar] [CrossRef]
- Warton, M.; Wills, R.; Ku, V. Ethylene levels associated with fruit and vegetables during marketing. Aust. J. Exp. Agric. 2000, 40, 465–470. [Google Scholar] [CrossRef]
- Kader, A.A. A perspective on postharvest horticulture (1978–2003). HortScience 2003, 38, 1004–1008. [Google Scholar] [CrossRef]
- Hu, B.; Sun, D.-W.; Pu, H.; Wei, Q. Recent advances in detecting and regulating ethylene concentrations for shelf-life extension and maturity control of fruit: A review. Trends Food Sci. Technol. 2019, 91, 66–82. [Google Scholar] [CrossRef]
- Wang, Y.; Diao, P.; Kong, L.; Yu, R.; Zhang, M.; Zuo, T.; Fan, Y.; Niu, Y.; Yan, F.; Wuriyanghan, H. Ethylene enhances seed germination and seedling growth under salinity by reducing oxidative stress and promoting chlorophyll content via ETR2 pathway. Front. Plant Sci. 2020, 11, 1066. [Google Scholar] [CrossRef]
- Swarup, R.; Perry, P.; Hagenbeek, D.; Van Der Straeten, D.; Beemster, G.T.; Sandberg, G.; Bhalerao, R.; Ljung, K.; Bennett, M.J. Ethylene upregulates auxin biosynthesis in Arabidopsis seedlings to enhance inhibition of root cell elongation. Plant Cell 2007, 19, 2186–2196. [Google Scholar] [CrossRef]
- Iqbal, N.; Khan, N.A.; Ferrante, A.; Trivellini, A.; Francini, A.; Khan, M. Ethylene role in plant growth, development and senescence: Interaction with other phytohormones. Front. Plant Sci. 2017, 8, 475. [Google Scholar] [CrossRef]
- Lelièvre, J.M.; Latchè, A.; Jones, B.; Bouzayen, M.; Pech, J.C. Ethylene and fruit ripening. Physiol. Plant. 1997, 101, 727–739. [Google Scholar] [CrossRef]
- Matilla, A.J. Ethylene in seed formation and germination. Seed Sci. Res. 2000, 10, 111–126. [Google Scholar] [CrossRef]
- Ecker, J.R.; Davis, R.W. Plant defense genes are regulated by ethylene. Proc. Natl. Acad. Sci. USA 1987, 84, 5202–5206. [Google Scholar] [CrossRef]
- Adams, D.; Yang, S. Ethylene biosynthesis: Identification of 1-aminocyclopropane-1-carboxylic acid as an intermediate in the conversion of methionine to ethylene. Proc. Natl. Acad. Sci. USA 1979, 76, 170–174. [Google Scholar] [CrossRef]
- Keller, N.; Ducamp, M.-N.; Robert, D.; Keller, V. Ethylene removal and fresh product storage: A challenge at the frontiers of chemistry. Toward an approach by photocatalytic oxidation. Chem. Rev. 2013, 113, 5029–5070. [Google Scholar] [CrossRef] [PubMed]
- Jedermann, R.; Behrens, C.; Westphal, D.; Lang, W. Applying autonomous sensor systems in logistics—Combining sensor networks, RFIDs and software agents. Sens. Actuators A Phys. 2006, 132, 370–375. [Google Scholar] [CrossRef]
- Cristescu, S.M.; Mandon, J.; Arslanov, D.; De Pessemier, J.; Hermans, C.; Harren, F.J. Current methods for detecting ethylene in plants. Ann. Bot. 2013, 111, 347–360. [Google Scholar] [CrossRef]
- Caprioli, F.; Quercia, L. Ethylene detection methods in post-harvest technology: A review. Sens. Actuators B Chem. 2014, 203, 187–196. [Google Scholar] [CrossRef]
- McDonagh, C.; Burke, C.S.; MacCraith, B.D. Optical chemical sensors. Chem. Rev. 2008, 108, 400–422. [Google Scholar] [CrossRef]
- Manne, J.; Jäger, W.; Tulip, J. Sensitive detection of ammonia and ethylene with a pulsed quantum cascade laser using intra and interpulse spectroscopic techniques. Appl. Phys. B 2009, 94, 337–344. [Google Scholar] [CrossRef]
- Scotoni, M.; Rossi, A.; Bassi, D.; Buffa, R.; Iannotta, S.; Boschetti, A. Simultaneous detection of ammonia, methane and ethylene at 1.63 μm with diode laser photoacoustic spectroscopy. Appl. Phys. B 2006, 82, 495–500. [Google Scholar] [CrossRef]
- Kathirvelan, J.; Vijayaraghavan, R. Review on sensitive and selective ethylene detection methods for fruit ripening application. Sens. Rev. 2020, 40, 421–435. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, Z.; Yu, S.; Mi, Q.; Pan, Q. Diversiform metal oxide-based hybrid nanostructures for gas sensing with versatile prospects. Coord. Chem. Rev. 2020, 413, 213272. [Google Scholar] [CrossRef]
- Simon, I.; Bârsan, N.; Bauer, M.; Weimar, U. Micromachined metal oxide gas sensors: Opportunities to improve sensor performance. Sens. Actuators B Chem. 2001, 73, 1–26. [Google Scholar] [CrossRef]
- Hagleitner, C.; Hierlemann, A.; Lange, D.; Kummer, A.; Kerness, N.; Brand, O.; Baltes, H. Smart single-chip gas sensor microsystem. Nature 2001, 414, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Esser, B.; Schnorr, J.M.; Swager, T.M. Selective detection of ethylene gas using carbon nanotube-based devices: Utility in determination of fruit ripeness. Angew. Chem. Int. Ed. 2012, 51, 5752–5756. [Google Scholar] [CrossRef]
- Kathirvelan, J.; Vijayaraghavan, R.; Thomas, A. Ethylene detection using TiO2–WO3 composite sensor for fruit ripening applications. Sens. Rev. 2017, 37, 147–154. [Google Scholar] [CrossRef]
- Zanolli, Z.; Leghrib, R.; Felten, A.; Pireaux, J.-J.; Llobet, E.; Charlier, J.-C. Gas sensing with Au-decorated carbon nanotubes. ACS Nano 2011, 5, 4592–4599. [Google Scholar] [CrossRef]
- Garg, N.; Deep, A.; Sharma, A.L. Metal-organic frameworks based nanostructure platforms for chemo-resistive sensing of gases. Coord. Chem. Rev. 2021, 445, 214073. [Google Scholar] [CrossRef]
- Maekawa, T.; Tamaki, J.; Miura, N.; Yamazoe, N. Sensing behavior of CuO-loaded SnO2 element for H2S detection. Chem. Lett. 1991, 20, 575–578. [Google Scholar] [CrossRef]
- Ishihara, T.; Shiokawa, K.; Eguchi, K.; Arai, H. Selective detection of nitrogen monoxide by the mixed oxide of Cr2O3–Nb2O5. Chem. Lett. 1988, 17, 997–1000. [Google Scholar] [CrossRef]
- Ishihara, T.; Shiokawa, K.; Egeuchi, K. H, Arai, The mixed oxide A1203-V205 as a semiconductor gas sensor for NO and NO2. Sens. Actuators 1989, 19, 259–265. [Google Scholar] [CrossRef]
- Ishihara, T.; Matsubara, S. Capacitive type gas sensors. J. Electroceram. 1998, 2, 215–228. [Google Scholar] [CrossRef]
- Bruce, J.; Bosnick, K.; Heidari, E.K. Pd-decorated ZnO nanoflowers as a promising gas sensor for the detection of meat spoilage. Sens. Actuators B Chem. 2022, 355, 131316. [Google Scholar] [CrossRef]
- Tonezzer, M. Single nanowire gas sensor able to distinguish fish and meat and evaluate their degree of freshness. Chemosensors 2021, 9, 249. [Google Scholar] [CrossRef]
- Jang, J.S.; Jung, H.J.; Chong, S.; Kim, D.H.; Kim, J.; Kim, S.O.; Kim, I.D. 2D materials decorated with ultrathin and porous graphene oxide for high stability and selective surface activity. Adv. Mater. 2020, 32, 2002723. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.-S.; Winter, L.R.; Kim, C.; Fortner, J.D.; Elimelech, M. Selective and sensitive environmental gas sensors enabled by membrane overlayers. Trends Chem. 2021, 3, 547–560. [Google Scholar] [CrossRef]
- Ivec, M.; Leitner, R.; Waldner, R.; Gostner, J.; Überall, F. The effect of sensor temperature and MOx layer thickness on the sensitivity of SnO2-and WO3-based chemiresistive sensors to ethylene gas. In Smart Sensors, Actuators, and MEMS VII; and Cyber Physical Systems; SPIE: Bellingham, WA, USA, 2015; pp. 270–277. [Google Scholar]
- Bose, A.C.; Kalpana, D.; Thangadurai, P.; Ramasamy, S. Synthesis and characterization of nanocrystalline SnO2 and fabrication of lithium cell using nano-SnO2. J. Power Sources 2002, 107, 138–141. [Google Scholar] [CrossRef]
- Banerjee, R.; Das, D. Properties of tin oxide films prepared by reactive electron beam evaporation. Thin Solid Films 1987, 149, 291. [Google Scholar] [CrossRef]
- Liu, Y.; Zha, S.; Liu, M. Novel Nanostructured Electrodes for Solid Oxide Fuel Cells Fabricated by Combustion Chemical Vapor Deposition (CVD). Adv. Mater. 2004, 16, 256–260. [Google Scholar] [CrossRef]
- Liu, Y.; Zha, S.; Liu, M. Nanocomposite Electrodes Fabricated by a Particle-Solution Spraying Process for Low-Temperature SOFCs. Chem. Mater. 2004, 16, 3502–3506. [Google Scholar] [CrossRef]
- Akhir, M.A.; Mohamed, K.; Rezan, S.A.; Arafat, M.; Haseeb, A.; Uda, M.; Nuradibah, M. Ethylene gas sensing properties of tin oxide nanowires synthesized via CVD method. In Proceedings of the IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2018; p. 012038. [Google Scholar]
- Akhir, M.A.M.; Mohamed, K.; Rezan, S.A.; Lee, H.L.; Izah, S.S.M. An assessment of chemical vapor deposition synthesis of SnO2 nanowires by statistical design. Key Eng. Mater. 2016, 701, 52–56. [Google Scholar] [CrossRef]
- Nguyen, T.K.; Yu, S.H.; Yan, J.; Chua, D.H. SnO2-anchored carbon fibers chemical vapor deposition (CVD) synthesis: Effects of growth parameters on morphologies and electrochemical behaviors. J. Mater. Sci. 2020, 55, 15588–15601. [Google Scholar] [CrossRef]
- Sun, J.; Sun, P.; Zhang, D.; Xu, J.; Liang, X.; Liu, F.; Lu, G. Growth of SnO2 nanowire arrays by ultrasonic spray pyrolysis and their gas sensing performance. RSC Adv. 2014, 4, 43429–43435. [Google Scholar] [CrossRef]
- Jadsadapattarakul, D.; Thanachayanont, C.; Nukeaw, J.; Sooknoi, T. Improved selectivity, response time and recovery time by [0 1 0] highly preferred-orientation silicalite-1 layer coated on SnO2 thin film sensor for selective ethylene gas detection. Sens. Actuators B Chem. 2010, 144, 73–80. [Google Scholar] [CrossRef]
- Ahn, H.; Noh, J.H.; Kim, S.-B.; Overfelt, R.A.; Yoon, Y.S.; Kim, D.-J. Effect of annealing and argon-to-oxygen ratio on sputtered SnO2 thin film sensor for ethylene gas detection. Mater. Chem. Phys. 2010, 124, 563–568. [Google Scholar] [CrossRef]
- Ardekani, S.R.; Aghdam, A.S.R.; Nazari, M.; Bayat, A.; Yazdani, E.; Saievar-Iranizad, E. A comprehensive review on ultrasonic spray pyrolysis technique: Mechanism, main parameters and applications in condensed matter. J. Anal. Appl. Pyrolysis 2019, 141, 104631. [Google Scholar] [CrossRef]
- Jamkhande, P.G.; Ghule, N.W.; Bamer, A.H.; Kalaskar, M.G. Metal nanoparticles synthesis: An overview on methods of preparation, advantages and disadvantages, and applications. J. Drug Deliv. Sci. Technol. 2019, 53, 101174. [Google Scholar] [CrossRef]
- Abid, N.; Khan, A.M.; Shujait, S.; Chaudhary, K.; Ikram, M.; Imran, M.; Haider, J.; Khan, M.; Khan, Q.; Maqbool, M. Synthesis of nanomaterials using various top-down and bottom-up approaches, influencing factors, advantages, and disadvantages: A review. Adv. Colloid Interface Sci. 2022, 300, 102597. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Yu, F.; Zhang, L.; Wang, W.; Chen, L.; Li, Y. Review of ZnO-based nanomaterials in gas sensors. Solid State Ion. 2021, 360, 115544. [Google Scholar] [CrossRef]
- Wang, Z.L. Splendid one-dimensional nanostructures of zinc oxide: A new nanomaterial family for nanotechnology. ACS Nano 2008, 2, 1987–1992. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Jiang, Z.-Y.; Kuang, Q.; Xie, Z.-X.; Huang, R.-B.; Zheng, L.-S. Shape-controlled fabrication of porous ZnO architectures and their photocatalytic properties. J. Solid State Chem. 2009, 182, 115–121. [Google Scholar] [CrossRef]
- Cui, J.; Jiang, J.; Shi, L.; Zhao, F.; Wang, D.; Lin, Y.; Xie, T. The role of Ni doping on photoelectric gas-sensing properties of ZnO nanofibers to HCHO at room-temperature. RSC Adv. 2016, 6, 78257–78263. [Google Scholar] [CrossRef]
- Zhu, L.; Zeng, W. Room-temperature gas sensing of ZnO-based gas sensor: A review. Sens. Actuators A Phys. 2017, 267, 242–261. [Google Scholar] [CrossRef]
- Chen, J.; Ma, Q.; Wu, X.-J.; Li, L.; Liu, J.; Zhang, H. Wet-chemical synthesis and applications of semiconductor nanomaterial-based epitaxial heterostructures. Nano-Micro Lett. 2019, 11, 1–28. [Google Scholar] [CrossRef]
- Jin, Z.; Wang, D.-C.; Xie, W.-J.; Ding, Y.; Li, J. Highly Sensitive Ethylene Sensors Based on Ultrafine Pd Nanoparticles-Decorated Porous ZnO Nanosheets and Their Application in Fruit Ripeness Detection. Processes 2023, 11, 1686. [Google Scholar] [CrossRef]
- Sholehah, A.; Karmala, K.; Huda, N.; Utari, L.; Septiani, N.L.W.; Yuliarto, B. Structural effect of ZnO-Ag chemoresistive sensor on flexible substrate for ethylene gas detection. Sens. Actuators A Phys. 2021, 331, 112934. [Google Scholar] [CrossRef]
- Aldosary, A.F.; Shar, M.A.; AlQahtani, H.R. High-sensitivity detection of ethane and ethylene using gamma-irradiated ZnO chemiresistors. Meas. Sens. 2022, 24, 100600. [Google Scholar] [CrossRef]
- Liu, L.; Mandler, D. Using nanomaterials as building blocks for electrochemical deposition: A mini review. Electrochem. Commun. 2020, 120, 106830. [Google Scholar] [CrossRef]
- Sholehah, A.; Faroz, D.F.; Huda, N.; Utari, L.; Septiani, N.L.W.; Yuliarto, B. Synthesis of ZnO flakes on flexible substrate and its application on ethylene sensing at room temperature. Chemosensors 2019, 8, 2. [Google Scholar] [CrossRef]
- Tesfamichael, T.; Cetin, C.; Piloto, C.; Arita, M.; Bell, J. The effect of pressure and W-doping on the properties of ZnO thin films for NO2 gas sensing. Appl. Surf. Sci. 2015, 357, 728–734. [Google Scholar] [CrossRef]
- Li, B.; Li, M.; Meng, F.; Liu, J. Highly sensitive ethylene sensors using Pd nanoparticles and rGO modified flower-like hierarchical porous α-Fe2O3. Sens. Actuators B Chem. 2019, 290, 396–405. [Google Scholar] [CrossRef]
- Ma, C.Y.; Mu, Z.; Li, J.J.; Jin, Y.G.; Cheng, J.; Lu, G.Q.; Hao, Z.P.; Qiao, S.Z. Mesoporous Co3O4 and Au/Co3O4 catalysts for low-temperature oxidation of trace ethylene. J. Am. Chem. Soc. 2010, 132, 2608–2613. [Google Scholar] [CrossRef] [PubMed]
- Gholami, M.; Bahar, M.; Azim-Araghi, M. The preparation of TiO2 nanoparticles and investigation of its electrical properties as CO2 gas sensor at room temperature. Chem. Phys. Lett. 2012, 48, 9626–9628. [Google Scholar]
- Le Dang, T.T.; Do, T.N.T.; Tonezzer, M.; Tran, V.D.N.; Chu, T.X.; Chu, M.H.; Nguyen, D.H. Eco-friendly facile synthesis of Co3O4–Pt nanorods for ethylene detection towards fruit quality monitoring. Sens. Actuators A Phys. 2023, 362, 114607. [Google Scholar] [CrossRef]
- Zhao, Q.; Duan, Z.; Yuan, Z.; Li, X.; Si, W.; Liu, B.; Zhang, Y.; Jiang, Y.; Tai, H. High performance ethylene sensor based on palladium-loaded tin oxide: Application in fruit quality detection. Chin. Chem. Lett. 2020, 31, 2045–2049. [Google Scholar] [CrossRef]
- Jeong, S.Y.; Moon, Y.K.; Kim, T.H.; Park, S.W.; Kim, K.B.; Kang, Y.C.; Lee, J.H. A new strategy for detecting plant hormone ethylene using oxide semiconductor chemiresistors: Exceptional gas selectivity and response tailored by nanoscale Cr2O3 catalytic overlayer. Adv. Sci. 2020, 7, 1903093. [Google Scholar] [CrossRef]
- Wang, L.-P.; Jin, Z.; Luo, T.; Ding, Y.; Liu, J.-H.; Wang, X.-F.; Li, M.-Q. The detection of ethylene using porous ZnO nanosheets: Utility in the determination of fruit ripeness. New J. Chem. 2019, 43, 3619–3624. [Google Scholar] [CrossRef]
- Eom, T.H.; Cho, S.H.; Suh, J.M.; Kim, T.; Yang, J.W.; Lee, T.H.; Jun, S.E.; Kim, S.J.; Lee, J.; Hong, S.H. Visible Light Driven Ultrasensitive and Selective NO2 Detection in Tin Oxide Nanoparticles with Sulfur Doping Assisted by l-Cysteine. Small 2022, 18, 2106613. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Hasani, A.; Kim, Y.; Park, S.Y.; Lee, M.G.; Sohn, W.; Nguyen, T.P.; Choi, K.S.; Kim, S.Y.; Jang, H.W. NO2 sensing properties of porous Au-incorporated tungsten oxide thin films prepared by solution process. Sens. Actuators B Chem. 2019, 286, 512–520. [Google Scholar] [CrossRef]
- Kim, Y.; Kwon, K.C.; Kang, S.; Kim, C.; Kim, T.H.; Hong, S.-P.; Park, S.Y.; Suh, J.M.; Choi, M.-J.; Han, S. Two-dimensional NbS2 gas sensors for selective and reversible NO2 detection at room temperature. ACS Sens. 2019, 4, 2395–2402. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-W.; Jeong, S.-Y.; Moon, Y.K.; Kim, K.; Yoon, J.-W.; Lee, J.-H. Highly selective and sensitive detection of breath isoprene by tailored gas reforming: A synergistic combination of macroporous WO3 spheres and Au catalysts. ACS Appl. Mater. Interfaces 2022, 14, 11587–11596. [Google Scholar] [CrossRef] [PubMed]
- Bulemo, P.M.; Kim, I.-D. Recent advances in ABO3 perovskites: Their gas-sensing performance as resistive-type gas sensors. J. Korean Ceram. Soc. 2020, 57, 24–39. [Google Scholar] [CrossRef]
- Kim, T.-H.; Yoon, J.-W.; Lee, J.-H.; Kim, T.-H.; Yoon, J.-W.; Lee, J.-H. A volatile organic compound sensor using porous Co3O4 spheres. J. Korean Ceram. Soc. 2016, 53, 134–138. [Google Scholar] [CrossRef]
- Andrysiewicz, W.; Krzeminski, J.; Skarżynski, K.; Marszalek, K.; Sloma, M.; Rydosz, A. Flexible gas sensor printed on a polymer substrate for sub-ppm acetone detection. Electron. Mater. Lett. 2020, 16, 146–155. [Google Scholar] [CrossRef]
- Cho, S.H.; Suh, J.M.; Eom, T.H.; Kim, T.; Jang, H.W. Colorimetric sensors for toxic and hazardous gas detection: A review. Electron. Mater. Lett. 2021, 17, 1–17. [Google Scholar] [CrossRef]
- Eom, T.H.; Cho, S.H.; Suh, J.M.; Kim, T.; Lee, T.H.; Jun, S.E.; Yang, J.W.; Lee, J.; Hong, S.-H.; Jang, H.W. Substantially improved room temperature NO2 sensing in 2-dimensional SnS2 nanoflowers enabled by visible light illumination. J. Mater. Chem. A 2021, 9, 11168–11178. [Google Scholar] [CrossRef]
- Lee, C.W.; Suh, J.M.; Choi, S.; Jun, S.E.; Lee, T.H.; Yang, J.W.; Lee, S.A.; Lee, B.R.; Yoo, D.; Kim, S.Y. Surface-tailored graphene channels. Npj 2D Mater. Appl. 2021, 5, 39. [Google Scholar] [CrossRef]
- Wang, C.; Yin, L.; Zhang, L.; Xiang, D.; Gao, R. Metal oxide gas sensors: Sensitivity and influencing factors. Sensors 2010, 10, 2088–2106. [Google Scholar] [CrossRef]
- Barsan, N.; Koziej, D.; Weimar, U. Metal oxide-based gas sensor research: How to? Sens. Actuators B Chem. 2007, 121, 18–35. [Google Scholar] [CrossRef]
- Matsunaga, N.; Sakai, G.; Shimanoe, K.; Yamazoe, N. Diffusion equation-based study of thin film semiconductor gas sensor-response transient. Sens. Actuators B Chem. 2002, 83, 216–221. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, Y.; Kim, T.; Eom, T.H.; Kim, S.Y.; Jang, H.W. Chemoresistive materials for electronic nose: Progress, perspectives, and challenges. InfoMat 2019, 1, 289–316. [Google Scholar] [CrossRef]
- Awang, Z. Gas sensors: A review. Sens. Transducers 2014, 168, 61–75. [Google Scholar]
- Presti, D.L.; Di Tocco, J.; Massaroni, C.; Cimini, S.; De Gara, L.; Singh, S.; Raucci, A.; Manganiello, G.; Woo, S.L.; Schena, E. Current understanding, challenges and perspective on portable systems applied to plant monitoring and precision agriculture. Biosens. Bioelectron. 2022, 222, 115005. [Google Scholar] [CrossRef] [PubMed]
- Wusiman, M.; Taghipour, F. Methods and mechanisms of gas sensor selectivity. Crit. Rev. Solid State Mater. Sci. 2022, 47, 416–435. [Google Scholar] [CrossRef]
- Leangtanom, P.; Wisitsoraat, A.; Chanlek, N.; Phanichphant, S.; Kruefu, V. Highly sensitive and selective ethylene gas sensors based on CeOx-SnO2 nanocomposites prepared by a Co-precipitation method. Mater. Chem. Phys. 2020, 254, 123540. [Google Scholar] [CrossRef]
- Giberti, A.; Carotta, M.C.; Guidi, V.; Malagù, C.; Martinelli, G.; Piga, M.; Vendemiati, B. Monitoring of ethylene for agro-alimentary applications and compensation of humidity effects. Sens. Actuators B Chem. 2004, 103, 272–276. [Google Scholar] [CrossRef]
- Krivec, M.; Mc Gunnigle, G.; Abram, A.; Maier, D.; Waldner, R.; Gostner, J.M.; Überall, F.; Leitner, R. Quantitative ethylene measurements with MOx chemiresistive sensors at different relative air humidities. Sensors 2015, 15, 28088–28098. [Google Scholar] [CrossRef]
- Chen, X.; Wreyford, R.; Nasiri, N. Recent Advances in Ethylene Gas Detection. Materials 2022, 15, 5813. [Google Scholar] [CrossRef]



| Fabrication Methods | Advantages | Disadvantages | Applicable MOSs | Used in Agriculture | Refs. |
|---|---|---|---|---|---|
| CVD | High controllability | Limited material applicability | SnO2 | Yes | [43,44] |
| USP | Stable coatings | Difficult operation | SnO2 | No | [48,51] |
| Sputtering method | Repeatability | High cost | SnO2, ZnO | No | [52,53] |
| Wet chemical method | Complex structure | Limited production | ZnO, Fe2O3 | Yes | [59] |
| Electrochemical deposition | Low-temperature conditions | Doping impurity | ZnO | Yes | [63] |
| Reflux method | Uniform particle | Long reaction time | TiO2 | No | [29] |
| Hydrothermal method | High crystallinity | Complicated process | Co3O4 | No | [66] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, K.; Cai, Y.; Wang, Z.; Zhang, Z.; Xian, J.; Zhang, C. A Review on Metal Oxide Semiconductor-Based Chemo-Resistive Ethylene Sensors for Agricultural Applications. Chemosensors 2024, 12, 13. https://doi.org/10.3390/chemosensors12010013
Hu K, Cai Y, Wang Z, Zhang Z, Xian J, Zhang C. A Review on Metal Oxide Semiconductor-Based Chemo-Resistive Ethylene Sensors for Agricultural Applications. Chemosensors. 2024; 12(1):13. https://doi.org/10.3390/chemosensors12010013
Chicago/Turabian StyleHu, Kongcan, Yahan Cai, Ziru Wang, Zhengwei Zhang, Jieyu Xian, and Cheng Zhang. 2024. "A Review on Metal Oxide Semiconductor-Based Chemo-Resistive Ethylene Sensors for Agricultural Applications" Chemosensors 12, no. 1: 13. https://doi.org/10.3390/chemosensors12010013
APA StyleHu, K., Cai, Y., Wang, Z., Zhang, Z., Xian, J., & Zhang, C. (2024). A Review on Metal Oxide Semiconductor-Based Chemo-Resistive Ethylene Sensors for Agricultural Applications. Chemosensors, 12(1), 13. https://doi.org/10.3390/chemosensors12010013







