Ferrocene-Containing Gallic Acid-Derivative Modified Carbon-Nanotube Electrodes for the Fast Simultaneous and Selective Determination of Cytostatics from Aqueous Solutions
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Testing of Voltammetric Methods
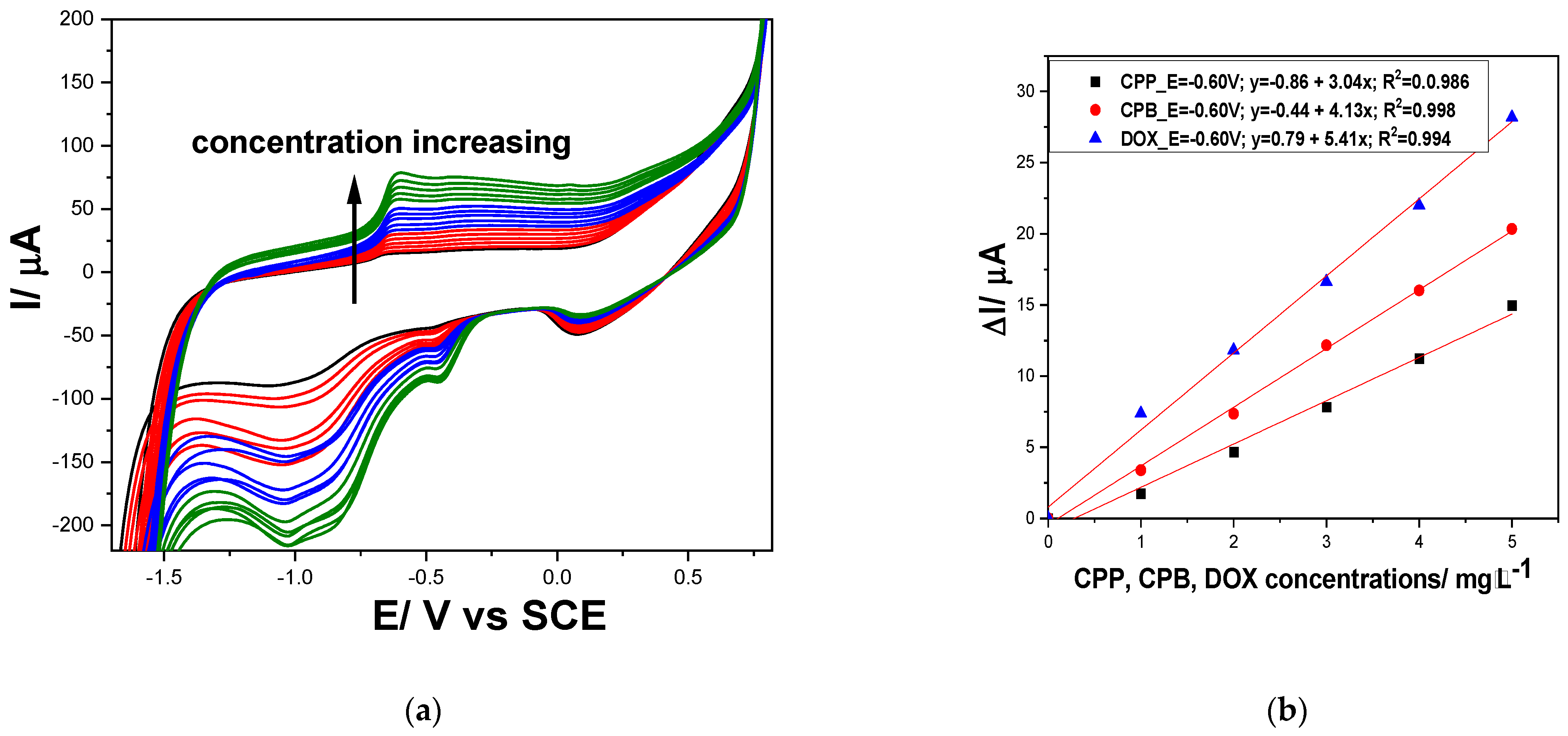
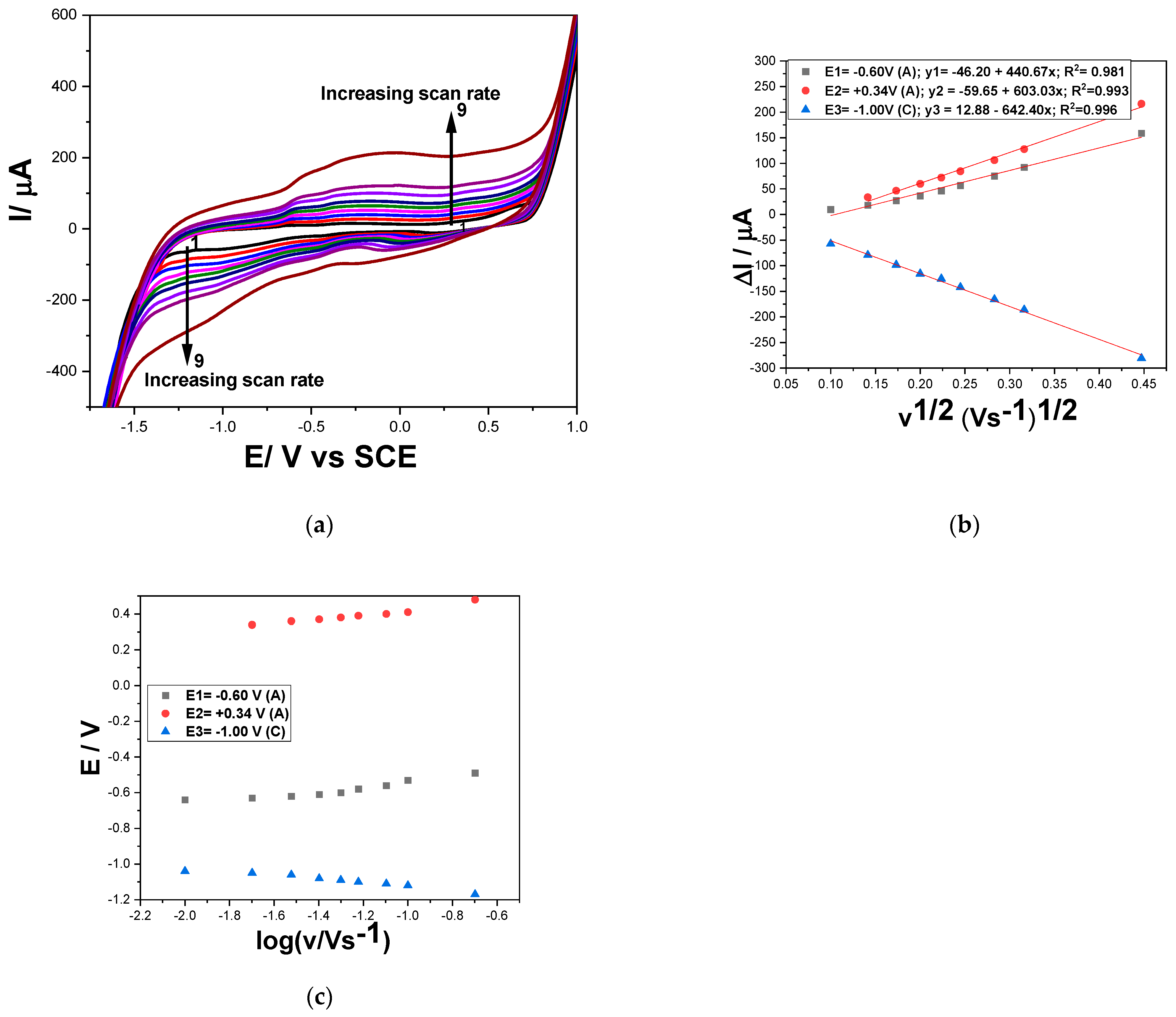
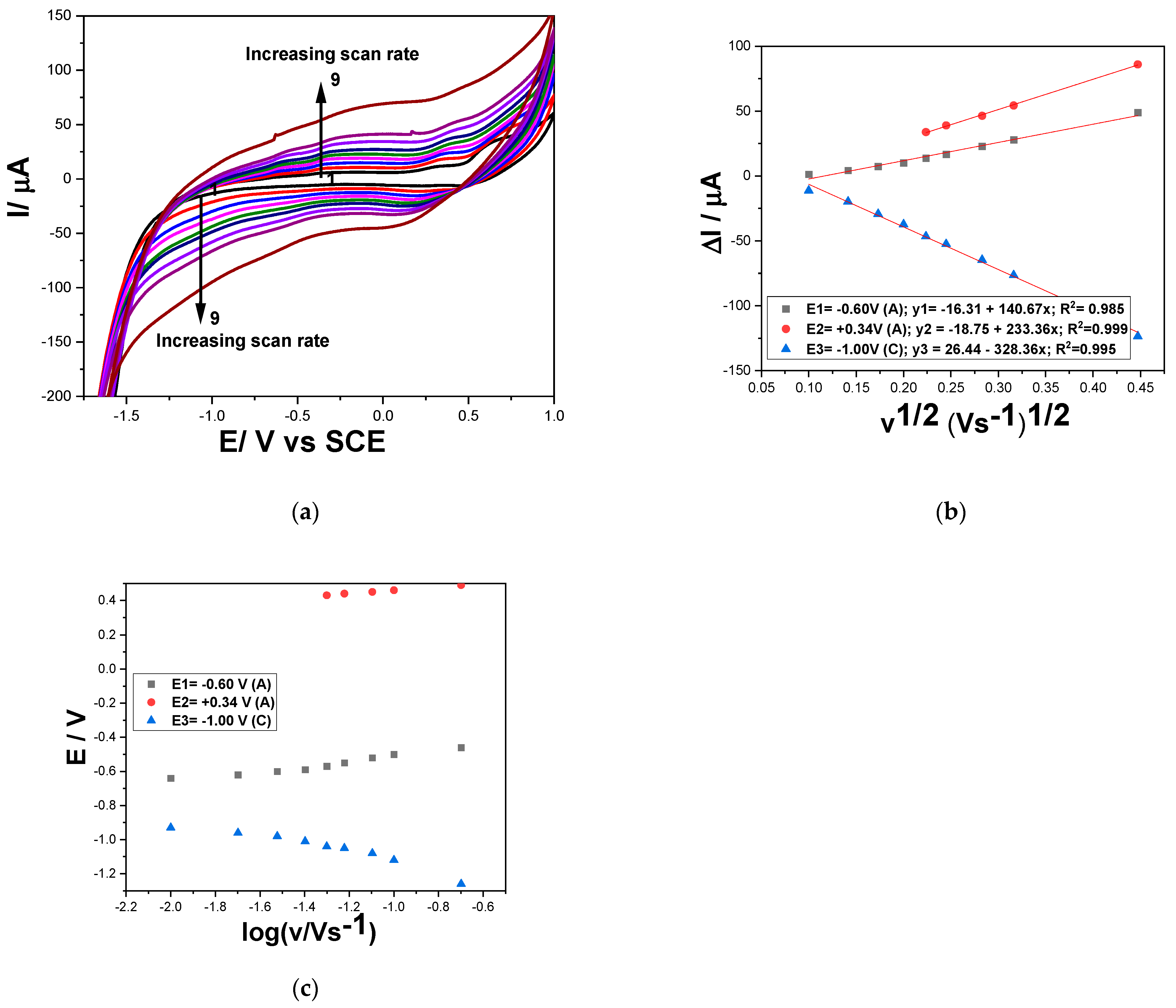
3.1.1. Cyclic Voltammetry (CV)
ΔIc = −642.40 v1/2 + 12.88 (E = −1.00 V)
ΔIc = −328.36 v1/2 + 26.44 (E = −1.00 V)
3.1.2. Simultaneous Determination of Cytostatics/Selective Determination of DOX Using Differential-Pulsed and Square-Wave Voltammetry Techniques
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Patel, M.; Kumar, R.; Kishor, K.; Mlsna, T.; Pittman, C.U.; Mohan, D. Pharmaceuticals of Emerging Concern in Aquatic Systems: Chemistry, Occurrence, Effects, and Removal Methods. Chem. Rev. 2019, 119, 3510–3673. [Google Scholar] [CrossRef] [PubMed]
- Jureczko, M.; Kalka, J. Cytostatic pharmaceuticals as water contaminants. Eur. J. Pharmacol. 2020, 866, 172816. [Google Scholar] [CrossRef] [PubMed]
- Waleng, N.J.; Nomngongo, P.N. Occurrence of Pharmaceuticals in the Environmental Waters: African and Asian Perspectives. Environ. Chem. Ecotoxicol. 2022, 4, 50–66. [Google Scholar] [CrossRef]
- Hughes, S.R.; Kay, P.; Brown, L.E. Global Synthesis and Critical Evaluation of Pharmaceutical Data Sets Collected from River Systems. Environ. Sci. Technol. 2013, 47, 661–677. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Pineros, M.; Znaor, A.; Bray, F. Cancer Statistics for the Year 2020: An Overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chang, V.W.C.; Giannis, A.; Wang, J.Y. Removal of Cytostatic Drugs from Aquatic Environment: A Review. Sci. Total Environ. 2013, 445–446, 281–298. [Google Scholar] [CrossRef]
- Gouveia, T.I.A.; Alves, A.; Santos, M.S.F. New Insights on Cytostatic Drug Risk Assessment in Aquatic Environments Based on Measured Concentrations in Surface Waters. Environ. Int. 2019, 133, 105236. [Google Scholar] [CrossRef] [PubMed]
- Feier, B.; Florea, A.; Cristea, C.; Sandulescu, R. Electrochemical Detection and Removal of Pharmaceuticals in Waste Waters. Curr. Opin. Electrochem. 2018, 11, 1–11. [Google Scholar] [CrossRef]
- Huang, B.; Xiao, L.; Dong, H.; Zhang, X.; Gan, W.; Mahboob, S.; Al-Ghanim, K.A.; Yuan, Q.; Li, Y. Electrochemical Sensing Platform Based on Molecularly Imprinted Polymer Decorated N,S Co-doped Activated Graphene for Ultrasensitive and Selective Determination of Cyclophosphamide. Talanta 2017, 164, 601–607. [Google Scholar] [CrossRef]
- Madrakian, T.; Ghasemi, H.; Haghshenasa, E.; Afkhamia, A. Preparation of a ZnO Nanoparticles/Multiwalled Carbon Nanotubes/Carbon Paste Electrode as a Sensitive Tool for Capecitabine Determination in Real Samples. RSC Adv. 2016, 6, 33851–33856. [Google Scholar] [CrossRef]
- Booker, V.; Halsall, C.; Llewellyn, N.; Johnson, A.; Williams, R. Prioritising Anticancer Drugs for Environmental Monitoring and Risk Assessment Purposes. Sci. Total Environ. 2014, 473–474, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Canela, C.; Cortes-Francisco, N.; Ventura, F.; Caixach, J.; Lacorte, S. Liquid Chromatography Coupled to Tandem Mass Spectrometry and High-Resolution Mass Spectrometry as Analytical Tools to Characterize Multi-class Cytostatic Compounds. J. Chromatogr. A 2013, 1276, 78–94. [Google Scholar] [CrossRef] [PubMed]
- Santana-Viera, S.; Hernandez-Arencibia, P.; Sosa-Ferrera, Z.; Santana-Rodriguez, J.J. Simultaneous and Systematic Analysis of Cytostatic Drugs in Wastewater Samples by Ultra-High Performance Liquid Chromatography Tandem Mass Spectrometry. J. Chromatogr. B 2019, 1110–1111, 124–132. [Google Scholar] [CrossRef]
- Omanovic, D.; Garnier, C.; Gibbon–Walsh, K.; Pizeta, I. Electroanalysis in Environmental Monitoring: Tracking Trace Metals—A Mini Review. Electrochem. Commun. 2015, 61, 78–83. [Google Scholar] [CrossRef]
- Uslu, B.; Ozkan, S. Electroanalytical Application of Carbon-Based Electrodes to the Pharmaceuticals. Anal. Lett. 2007, 40, 817–853. [Google Scholar] [CrossRef]
- Boumya, W.; Taoufik, N.; Achak, M.; Barka, N. Chemically Modified Carbon-Based Electrodes for the Determination of Paracetamol in Drugs and Biological Samples. J. Pharm. Anal. 2021, 11, 138–154. [Google Scholar] [CrossRef]
- Huseinov, A.; Nawarathne, C.P.; Alvarez, N.T. Chemically Bonded Carbon Nanotube Film to a Nanostructured Gold Electrode for Electrochemical Sensing of Hydrogen Peroxide. ACS Appl. Nano Mater. 2023, 6, 20082–20088. [Google Scholar] [CrossRef]
- Nardi, N.; Baumgarten, L.G.; Dreyer, J.P.; Santana, E.R.; Winiarski, J.P.; Cruz Vieira, I. Nanocomposite Based on Green Synthesis of Gold Nanoparticles Decorated with Functionalized Multi-Walled Carbon Nanotubes for the Electrochemical Determination of Hydroxychloroquine. J. Pharm. Biomed. Anal. 2023, 236, 115681. [Google Scholar] [CrossRef]
- Motoc, S.; Manea, F.; Orha, C.; Pop, A. Enhanced Electrochemical Response of Diclofenac at a Fullerene–Carbon Nanofiber Paste Electrode. Sensors 2019, 19, 1332. [Google Scholar] [CrossRef]
- Ilies, S.; Schinteie, B.; Pop, A.; Negrea, S.; Cretu, C.; Szerb, E.I.; Manea, F. Graphene Quantum Dots and Cu(I) Liquid Crystal for Advanced Electrochemical Detection of Doxorubicine in Aqueous Solutions. Nanomaterials 2021, 11, 2788. [Google Scholar] [CrossRef]
- Motoc, S.; Manea, F.; Baciu, A.; Orha, C.; Pop, A. Electrochemical Method for Ease Determination of sodium Diclofenac Trace Levels in Water Using Graphene—Multi-Walled Carbon Nanotubes Paste Electrode. Int. J. Environ. Res. Public Health 2022, 19, 29. [Google Scholar] [CrossRef]
- Motoc, S.; Manea, F.; Baciu, A.; Vasilie, S.; Pop, A. Highly Sensitive and Simultaneous Electrochemical Determinations of Non-Steroidal Anti-Inflammatory Drugs in Water Using Nanostructured Carbon-Based Paste Electrodes. Sci. Total Environ. 2022, 846, 1574128. [Google Scholar] [CrossRef]
- Saleh Ahammad, A.J.; Lee, J.J.; Rahman, M.A. Electrochemical Sensors Based on Carbon Nanotubes. Sensors 2009, 9, 2289–2319. [Google Scholar] [CrossRef]
- Beitollahi, H.; Khalilzadeh, M.A.; Tajik, S.; Safaei, M.; Zhang, K.; Jang, H.W.; Shokouhimehr, M. Recent Advances in Applications of Voltammetric Sensors Modified with Ferrocene and Its Derivatives. ACS Omega 2020, 5, 2049–2059. [Google Scholar] [CrossRef]
- Rauf, U.; Shabir, G.; Bukhari, S.; Albericio, F.; Saeed, A. Contemporary Developments in Ferrocene Chemistry: Physical, Chemical, Biological and Industrial Aspects. Molecules 2023, 28, 5765. [Google Scholar] [CrossRef] [PubMed]
- Popa, E.; Andelescu, A.A.; Ilies, S.; Visan, A.; Cretu, C.; Scarpelli, F.; Crispini, A.; Manea, F.; Szerb, E.I. Hetero-Bimetallic Ferrocene-Containing Zinc(II)-Terpyridyl-Based Metallomesogen: Structural and Electrochemical Characterization. Materials 2023, 16, 1946. [Google Scholar] [CrossRef]
- Kahkeshani, N.; Farzaei, F.; Fotouhi, M.; Alavi, S.S.; Bahramsoltani, R.; Naseri, R.; Momtaz, S.; Abbasabadi, Z.; Rahimi, R.; Farzaei, M.H.; et al. Pharmacological Effects of Gallic Acid in Health and Diseases: A Mechanistic Review. Iran. J. Basic Med. Sci. 2019, 22, 225–237. [Google Scholar] [CrossRef]
- Badea, M.; di Modugno, F.; Floroian, L.; Tit, D.M.; Restani, P.; Bungau, S.; Iovan, C.; Badea, G.E.; Aleya, L. Electrochemical Strategies for Gallic Acid Detection: Potential for Application in Clinical, Food or Environmental Analyses. Sci. Total Environ. 2019, 672, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Wung, J.; Lu, J.; Larson, D.D.; Olsen, K. Voltammetric Sensor for Uranium Based on the Propyl Gallate Modified Carbon Paste Electrode. Electroanalysis 1995, 7, 247–250. [Google Scholar] [CrossRef]
- Ganesh, H.V.S.; Noroozifar, M.; Kerman, K. Epigallocatechin Gallate-Modified Graphite Paste Electrode for Simultaneous Detection of Redox-Active Biomolecules. Sensors 2018, 18, 23. [Google Scholar] [CrossRef]
- Singh, T.A.; Sharma, V.; Thakur, N.; Tejwan, N.; Sharma, A.; Das, J. Selective and Sensitive Electrochemical Detection of Doxorubicin via a Novel Magnesium Oxide/Carbon Dot Nanocomposite Based Sensor. Inorg. Chem. Commun. 2023, 150, 110527. [Google Scholar] [CrossRef]
- Rus, I.; Tertis, M.; Barbalata, C.; Porfire, A.; Tomuta, I.; Sandulescu, R.; Cristea, C. An Electrochemical Strategy for the Simultaneous Detection of Doxorubicin and Simvastatin for Their Potential Use in the Treatment of Cancer. Biosensors 2021, 11, 15. [Google Scholar] [CrossRef]
- Hajian, R.; Tayebi, Z.; Shams, N. Fabrication of an Electrochemical Sensor for Determination of Doxorubicin in Human Plasma and its Interaction with DNA. J. Pharm. Anal. 2017, 7, 27–33. [Google Scholar] [CrossRef]
- Materon, E.M.; Wong, A.; Fatibello-Filho, O.; Faria, R.C. Development of a Simple Electrochemical Sensor for the Simultaneous Detection of Anticancer Drugs. J. Electroanal. Chem. 2018, 827, 64–72. [Google Scholar] [CrossRef]
- Shamsadin-Azad, Z.; Taher, M.A.; Beitollahi, H. Metal Organic Framework-235/Graphene Oxide Nanocomposite Modified Electrode as an Electrochemical Sensor for the Voltammetric Determination of Doxorubicin in Presence of Dacarbazine. Microchem. J. 2024, 196, 109580. [Google Scholar] [CrossRef]
- Yang, M.; Sun, Z.; Jin, H.; Gui, R. Sulfur Nanoparticle-Encapsulated MOF and Boron Nanosheet-Ferrocene Complex Modified Electrode Platform for Ratiometric Electrochemical Sensing of Adriamycin and Real-Time Monitoring of Drug Release. Microchem. J. 2022, 177, 107319. [Google Scholar] [CrossRef]
- Jemelkova, Z.; Zima, J.; Barek, J. Voltammetric and amperometric determination of doxorubicin using carbon paste electrodes. Collect. Czechoslov. Chem. Commun. 2009, 74, 1503–1515. [Google Scholar] [CrossRef]
- Skalova, S.; Langmaier, J.; Barek, J.; Vyskocil, V.; Navratil, T. Doxorubicin determination using two novel voltammetric approaches: A comparative study. Electrochim. Acta 2020, 330, 135180. [Google Scholar] [CrossRef]
- Zhang, Q.; Shan, X.; Fu, Y.; Liu, P.; Li, X.; Liu, B.; Zhang, L.; Li, D. Electrochemical Determination of the Anticancer Drug Capecitabine Based on a Graphene-Gold Nanocomposite Modified Glassy Carbon Electrode. Int. J. Electrochem. Sci. 2017, 12, 10773–10782. [Google Scholar] [CrossRef]
- Eshaghi, Z.; Moeipour, F. Carbon Nanotube/Polyurethane Modified Hollow Fiber-Pencil Graphite Electrode for In Situ Concentration and Electrochemical Quantification of Anticancer Drugs Capecitabine and Erlotinib. Eng. Life Sci. 2019, 19, 302–314. [Google Scholar] [CrossRef] [PubMed]
- Sinha, P.; Doi, S.; Sharma, D.K. Electrochemical Behaviour and Adsorptive Stripping Voltammetric Determination of Cyclophosphamide. Chem. Sci. Trans. 2018, 7, 229–239. [Google Scholar] [CrossRef][Green Version]
- Baj-Rossi, C.; Micheli, G.D.; Carrara, S. Electrochemical Detection of Anti-Breast-Cancer Agents in Human Serum by Cytochrome P450-Coated Carbon Nanotubes. Sensors 2012, 12, 6520–6537. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Vulugundam, G.; Kondaiah, P.; Bhattacharya, S. Co-liposomes of Redox-Active Alkyl-Ferrocene Modified Low MW Branched PEI and DOPE for Efficacious Gene Delivery in Serum. J. Mater. Chem. B 2015, 3, 2318–2330. [Google Scholar] [CrossRef]
- Venkataraman, N.V.; Bhagyalakshmi, S.; Vasudevan, S.; Seshadri, R. Conformation and Orientation of Alkyl Chains in the Layered Organic–Inorganic Hybrids: (CnH2n+1NH3)2PbI4 (n = 12,16,18). Phys. Chem. Chem. Phys. 2002, 4, 4533–4538. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Paul, S. Conducting Polypyrrole Modified with Ferrocene for Applications in Carbon Monoxide Sensors. Sens. Actuators B 2007, 125, 60–65. [Google Scholar] [CrossRef]
- Beijnen, J.H.; Wiese, G.; Underberg, W.J. Aspects of the chemical stability of doxorubicin and seven other anthracyclines in acidic solution. Pharm. Weekbl. 1985, 7, 109–116. [Google Scholar] [CrossRef]
- Ljoncheva, M.; Kosjek, T.; Isidori, M.; Heath, E. 5-Fluorouracil and its prodrug capecitabine: Occurrence, fate and effect in environment. In Fate and Effect of Anticancer Drugs in the Environment; Isidori, M., Kosjek, T., Filipic, M., Eds.; Springer: Cham, Switzerland, 2020; pp. 331–375. [Google Scholar]
- Dumitru, R.; Negrea, S.; Ianculescu, A.; Păcurariu, C.; Vasile, B.; Surdu, A.; Manea, F. Lanthanum Ferrite Ceramic Powders: Synthesis, Characterization and Electrochemical Detection Application. Materials 2020, 13, 2061. [Google Scholar] [CrossRef]
- Rodrigues, H.; Lima, S.; da Silva, J.S.; de Oliveira Farias, E.A.; Sousa Teixeira, P.R.; Eiras, C.; Cunha Nunes, L.C. Electrochemical Sensors and Biosensors for the Analysis of Antineoplastic Drugs. Biosens. Bioelectron. 2018, 108, 27–37. [Google Scholar] [CrossRef]
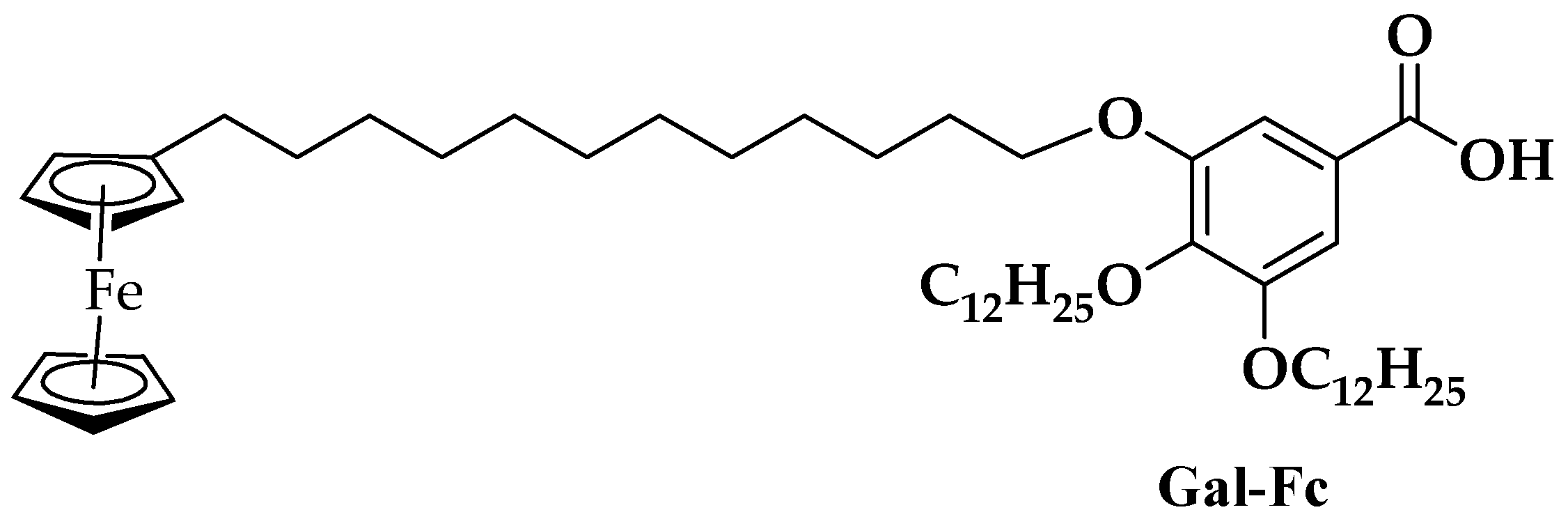
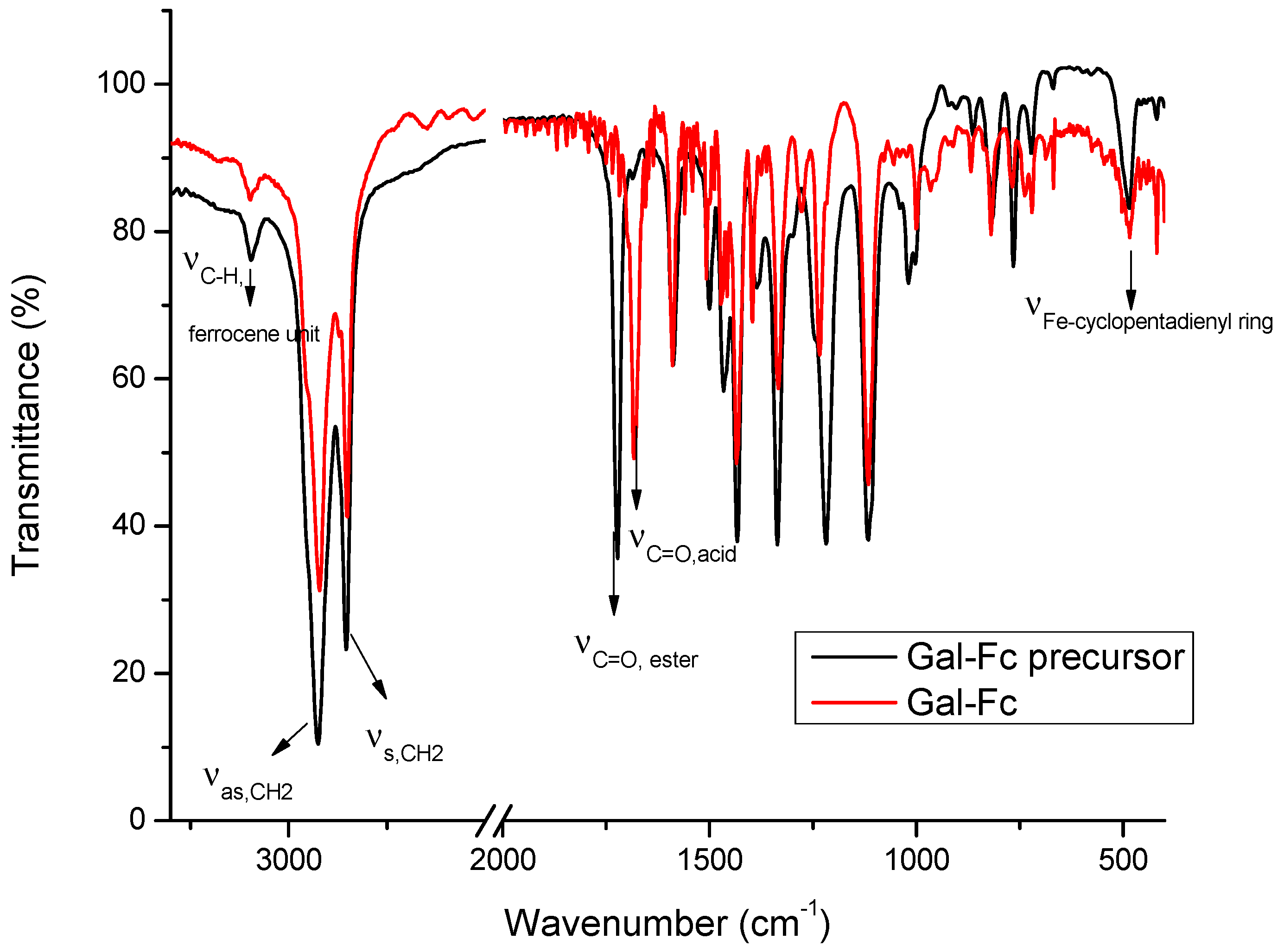
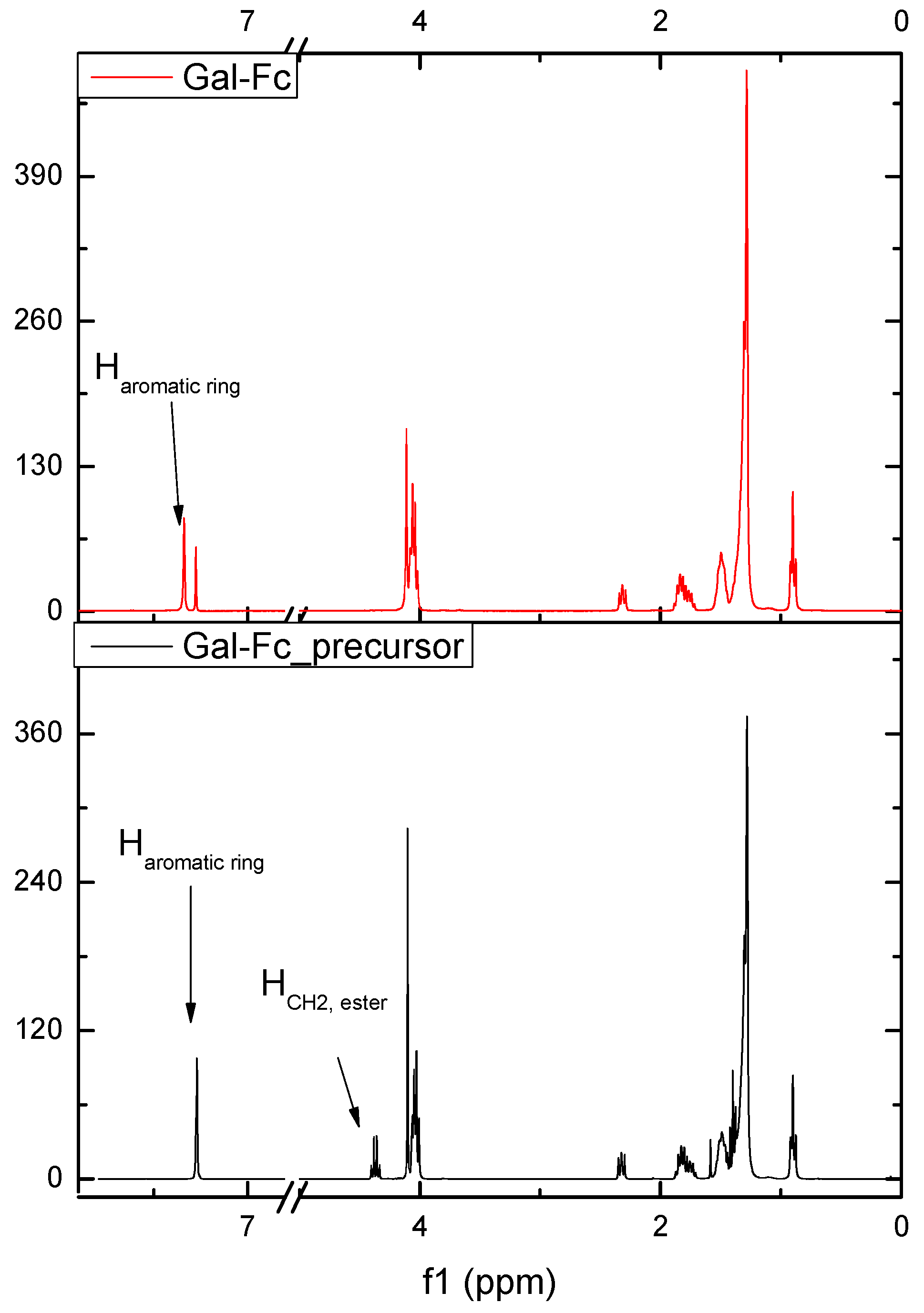
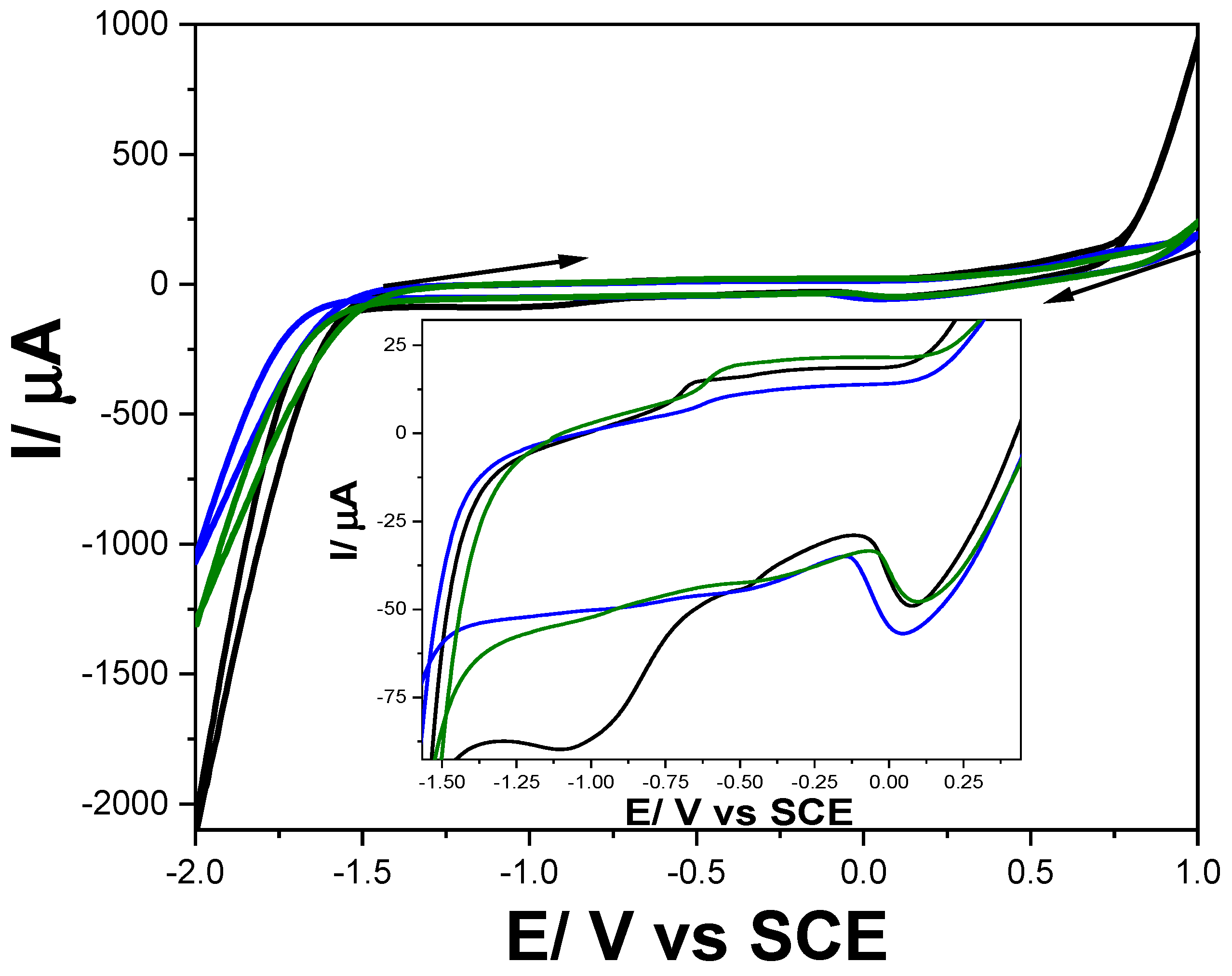



| pH | DOX | CPB | CPP | |||
|---|---|---|---|---|---|---|
| E/V vs. SCE | ΔI/ µA/mg·L−1 | E/V vs. SCE | ΔI/µA/mg·L−1 | E/V vs. SCE | ΔI/µA/mg·L−1 | |
| 3 | −0.46 | 0.51 | −0.46 | - | −0.46 | - |
| 5 | −0.46 | 4.05 | −0.46 | 3.25 | −0.50 | 2.76 |
| 12 | −0.60 | 5.41 | −0.60 | 4.13 | −0.60 | 3.04 |
| Technique | DOX | CPB | CPP | |||
|---|---|---|---|---|---|---|
| E/V vs. SCE | ΔI/ µA/mg·L−1 | E/V vs. SCE | ΔI/µA/mg·L−1 | E/V vs. SCE | ΔI/µA/mg·L−1 | |
| DPV | −0.20 | 73.71 | −0.20 | 5.00 | −0.20 | 6.55 |
| SWV | −0.60 | 311.9 | −0.60 | 22.90 | −0.60 | 30.25 |
| Electrode | Analyte | LOD | Method | Reference |
|---|---|---|---|---|
| CDs-5.0/MgO/SPCE a | DOX | 90 nM | CV | [31] |
| Pt/MWCNTs b | DOX | 3.7 nM | CV | [32] |
| graphite-based disposable SPE c | DOX/Simvastatin * | 180 nM | CA | [33] |
| 2.78 µM | LSV | |||
| CuNPs-CB-Nafion/GCE d | DOX/ Methotrexate * | 24 nM | SWV | [34] |
| MOF-235/GO nanocomposite modified CPE e | DOX | 5 nM | CV | [35] |
| SNPs@MOF/BNSs-Fc/GCE f | DOX | 2 nM | SWV | [36] |
| p-AgSAE g | DOX | 0.84 µM | DPCSV | [38] |
| ZnO/MWCNTs/CPE h | CPB | 30 nM | DPV | [10] |
| AuNPs/SGNF-modified GCE i | CPB | 17 nM | DPV | [39] |
| MWCNT-PUFIX/HF-PGE j | CPB/Erlotinib * | 0.110 µM | DPV | [40] |
| GCE k | CPP | 1.1 µM | CV | [41] |
| Current work | DOX/CPB/CPP * | 1.13/30/32 nM | SWV | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Motoc, S.; Andelescu, A.; Visan, A.; Baciu, A.; Szerb, E.I.; Manea, F. Ferrocene-Containing Gallic Acid-Derivative Modified Carbon-Nanotube Electrodes for the Fast Simultaneous and Selective Determination of Cytostatics from Aqueous Solutions. Chemosensors 2024, 12, 15. https://doi.org/10.3390/chemosensors12010015
Motoc S, Andelescu A, Visan A, Baciu A, Szerb EI, Manea F. Ferrocene-Containing Gallic Acid-Derivative Modified Carbon-Nanotube Electrodes for the Fast Simultaneous and Selective Determination of Cytostatics from Aqueous Solutions. Chemosensors. 2024; 12(1):15. https://doi.org/10.3390/chemosensors12010015
Chicago/Turabian StyleMotoc (m. Ilies), Sorina, Adelina Andelescu, Alexandru Visan, Anamaria Baciu, Elisabeta I. Szerb, and Florica Manea. 2024. "Ferrocene-Containing Gallic Acid-Derivative Modified Carbon-Nanotube Electrodes for the Fast Simultaneous and Selective Determination of Cytostatics from Aqueous Solutions" Chemosensors 12, no. 1: 15. https://doi.org/10.3390/chemosensors12010015
APA StyleMotoc, S., Andelescu, A., Visan, A., Baciu, A., Szerb, E. I., & Manea, F. (2024). Ferrocene-Containing Gallic Acid-Derivative Modified Carbon-Nanotube Electrodes for the Fast Simultaneous and Selective Determination of Cytostatics from Aqueous Solutions. Chemosensors, 12(1), 15. https://doi.org/10.3390/chemosensors12010015



.png)





