Determination of Phenethyl Isothiocyanate, Erucin, Iberverin, and Erucin Nitrile Concentrations in Healthy and Pieris rapae-Infected Broccoli Tissues Using Gas Chromatography-Mass Spectrometry
Abstract
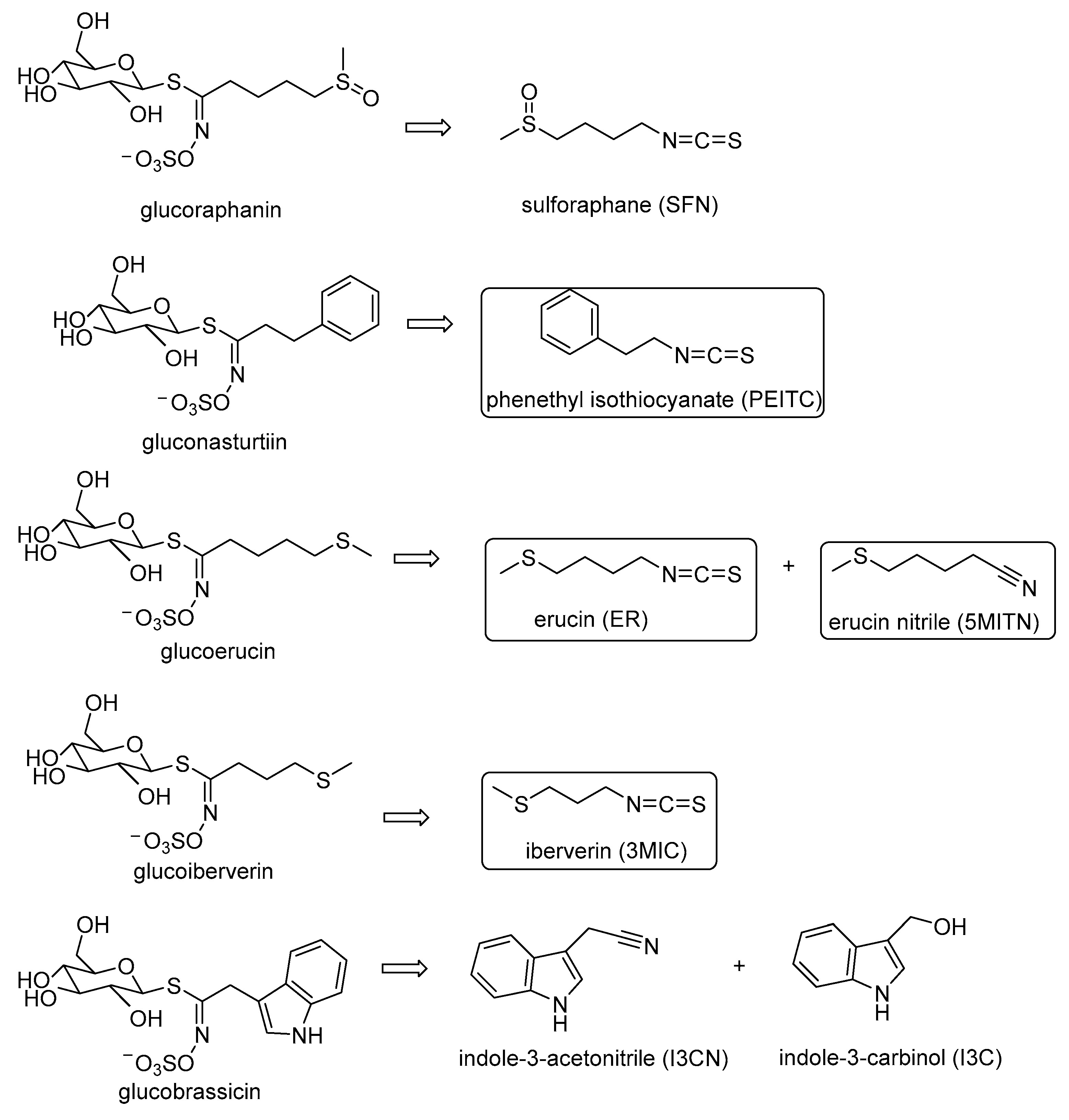
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Standard Solutions
2.3. Sampling
2.4. GLS Hydrolysis and Extraction Conditions
2.5. Analysis of Glucosinolate Hydrolysis Products
2.5.1. GC-MS Method
2.5.2. Validation, Linearity, and Detection Limits
3. Results
3.1. Broccoli Plants
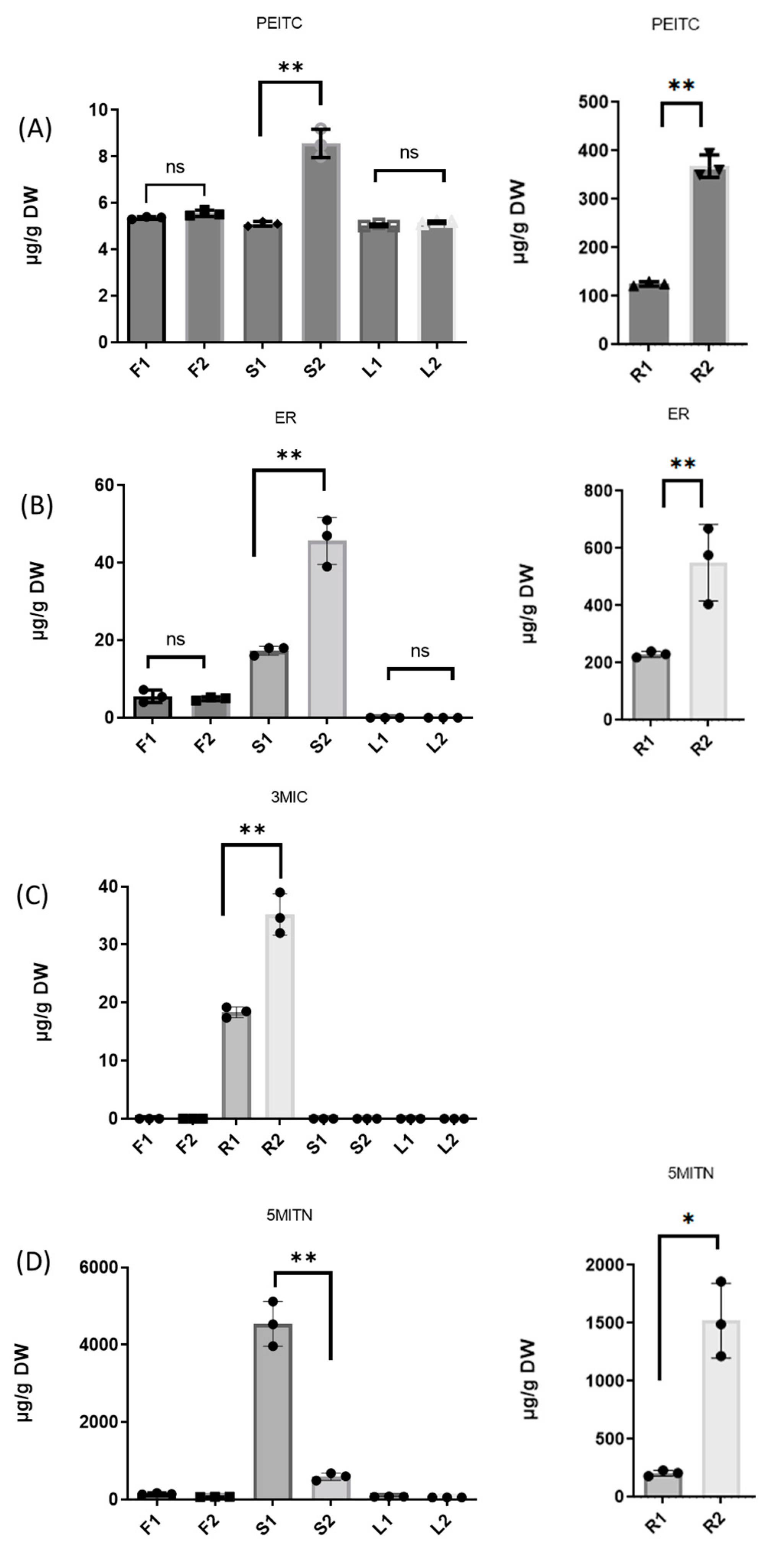
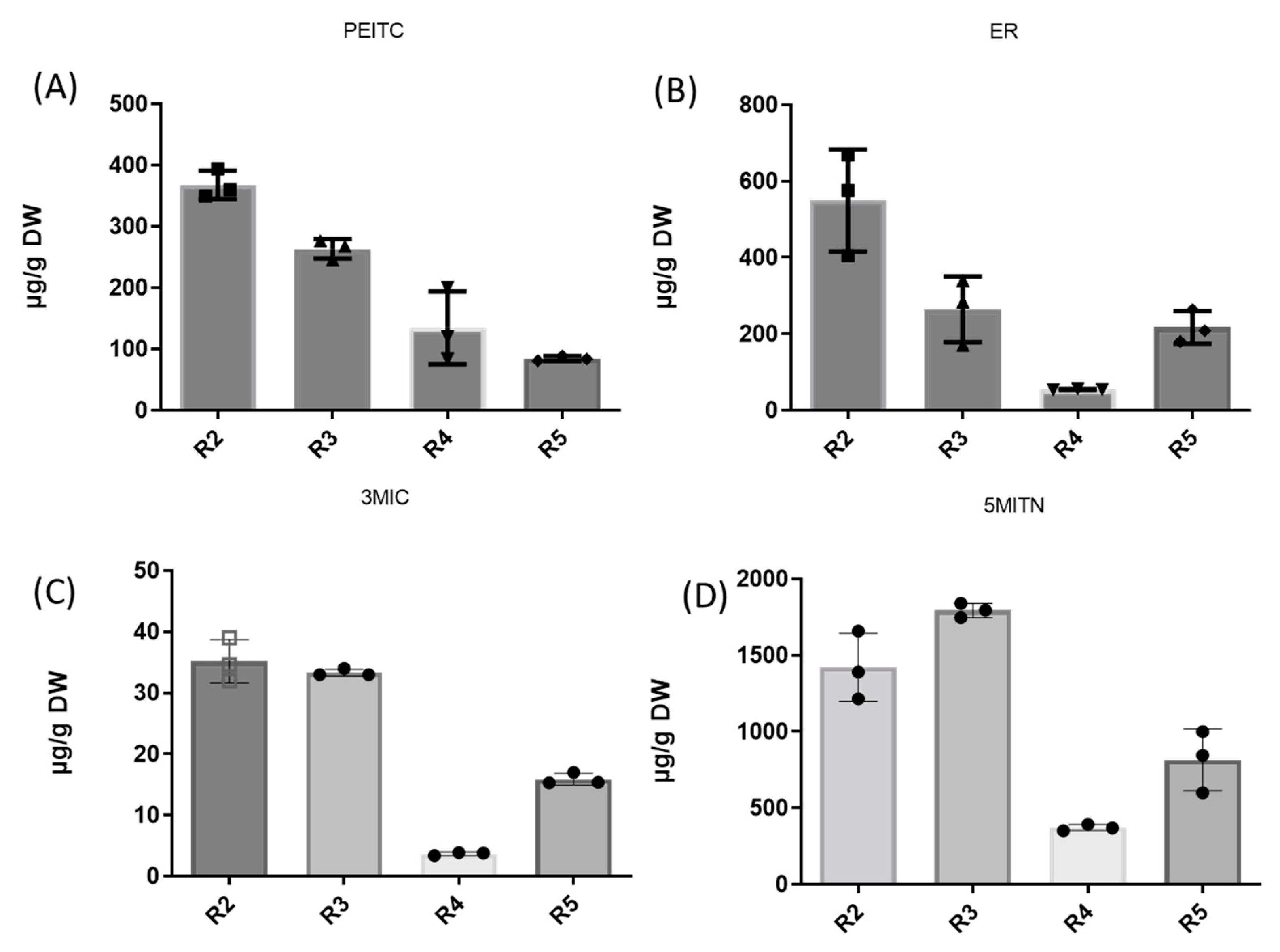
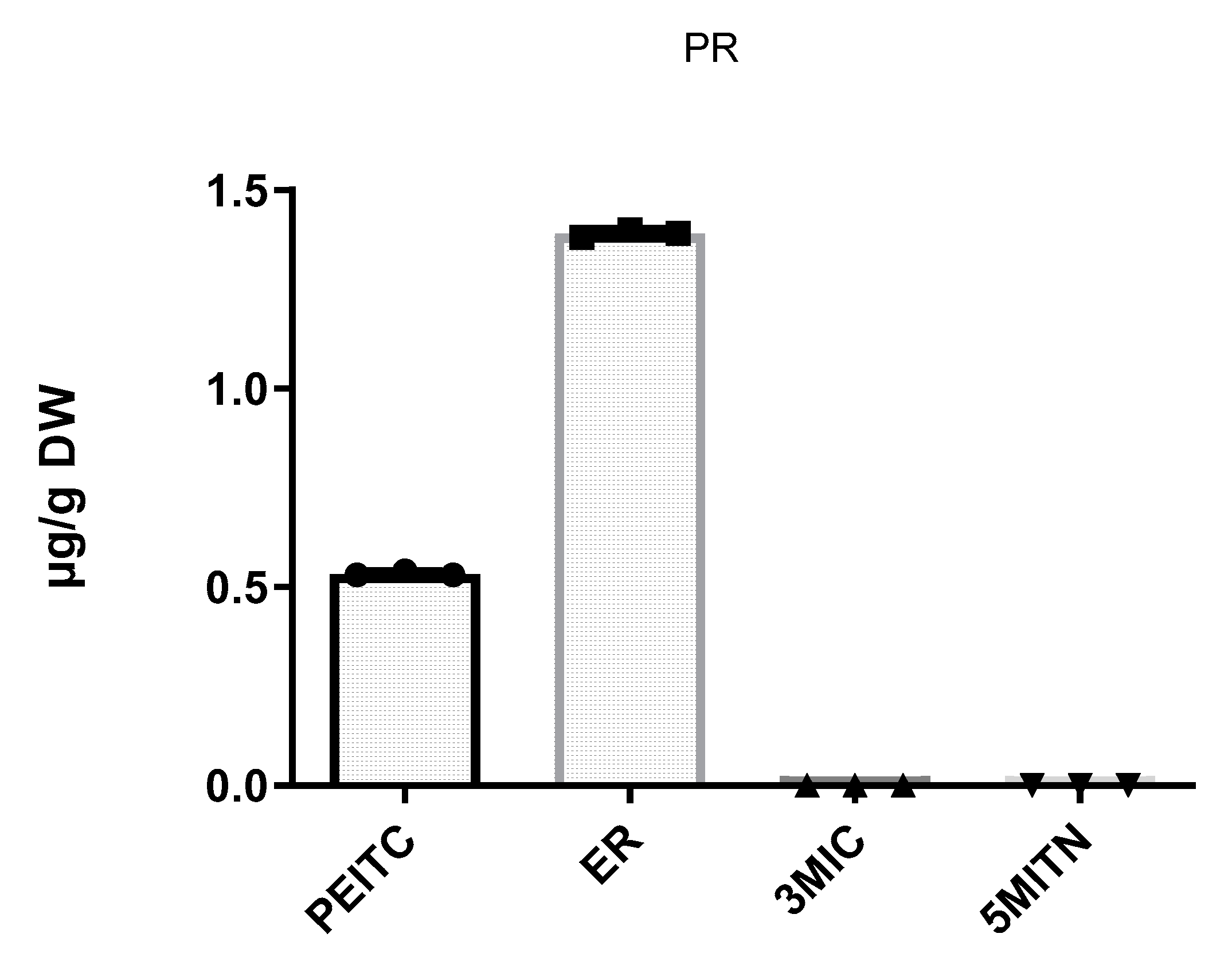
3.2. Extraction of Isothiocyanates PEITC, ER, 3MIC, and Nitrile 5MITN from Broccoli Samples, and Determination via GC-MS
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shen, Y.; Wang, J.; Shaw, R.K.; Yu, H.; Sheng, X.; Zhao, Z.; Li, S.; Gu, H. Development of GBTS and KASP panels for genetic diversity, population structure, and fingerprinting of a large collection of broccoli (Brassica oleracea L. var. italica) in China. Front. Plant Sci. 2021, 12, 655254. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.P.T.; Stewart, J.; Lopez, M.; Ioannou, I.; Allais, F. Glucosinolates: Natural occurrence, biosynthesis, accessibility, isolation, structures, and biological activities. Molecules 2020, 25, 4537. [Google Scholar] [CrossRef]
- Li, H.; Xia, Y.; Liu, H.-Y.; Guo, H.; He, X.-Q.; Liu, Y.; Wu, D.-T.; Mai, Y.-H.; Li, H.-B.; Zou, L.; et al. Nutritional values, beneficial effects, and food applications of broccoli (Brassica oleracea var. italica Plenck). Trends Food Sci. Technol. 2022, 119, 288–308. [Google Scholar] [CrossRef]
- Wittstock, U.; Kurzbach, E.; Herfurth, A.-M.; Stauber, E. Glucosinolate breakdown. Adv. Bot. Res. 2016, 80, 125–169. [Google Scholar]
- Ilahy, R.; Tlili, I.; Pek, Z.; Montefusco, A.; Siddiqui, M.W.; Homa, F.; Hdider, C.; R’Him, T.; Lajos, H.; Lenucci, M.S. Pre- and post-harvest factors affecting glucosinolate content in broccoli. Front. Nutr. 2020, 7, 147. [Google Scholar] [CrossRef] [PubMed]
- Palliyaguru, D.L.; Yuan, J.M.; Kensler, T.W.; Fahey, J.W. Isothiocyanates: Translating the power of plants to people. Mol. Nutr. Food Res. 2018, 62, 1700965. [Google Scholar] [CrossRef] [PubMed]
- Eagles, S.K.; Gross, A.S.; McLachlan, A.J. The effects of cruciferous vegetable-enriched diets on drug metabolism: A systematic review and meta-analysis of dietary intervention trials in humans. Clin. Pharmacol. Therap. 2020, 108, 212–227. [Google Scholar] [CrossRef] [PubMed]
- Radošević, K.; Srček, V.G.; Bubalo, M.C.; Brnčić, S.R.; Takács, K.; Redovniković, I.R. Assessment of glucosinolates, antioxidative and antiproliferative activity of broccoli and collard extracts. J. Food Comp. 2017, 61, 59–66. [Google Scholar] [CrossRef]
- Kaiser, A.E.; Baniasadi, M.; Giansiracusa, D.; Giansiracusa, M.; Garcia, M.; Fryda, Z.; Wong, T.L.; Bishayee, A. Sulforaphane: A broccoli bioactive phytocompound with cancer preventive potential. Cancers 2021, 13, 4796. [Google Scholar] [CrossRef]
- Zhang, Y.; Talalay, P.; Cho, C.G.; Posner, G.H. A major inducer of anticarcinogenic protective enzymes from broccoli: Isolation and elucidation of structure. Proc. Natl. Acad. Sci. USA 1992, 89, 2399–2403. [Google Scholar] [CrossRef]
- Ernst Insa, M.A.; Palani, K.; Esatbeyoglu, T.; Schwarz, K.; Rimbach, G. Synthesis and Nrf2-inducing activity of the isothiocyanates iberverin, iberin and cheirolin. Pharmacol. Res. 2013, 70, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Melchini, A.; Traka, M.H.; Catania, S.; Miceli, N.; Taviano, M.F.; Maimone, P.; Francisco, M.; Mithen, R.F.; Costa, C. Antiproliferative activity of the dietary isothiocyanate erucin, a bioactive compound from cruciferous vegetables, on human prostate cancer cells. Nutr. Cancer 2013, 65, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Manici, L.M.; Lazzeri, L.; Baruzzi, G.; Leoni, O.; Galletti, S.; Palmieri, S. Suppressive activity of some glucosinolate enzyme degradation products on Pythium irregulare and Rhizoctonia solani in sterile soil. Pest Manag. Sci. 2000, 56, 921–926. [Google Scholar] [CrossRef]
- Crowley, E.; Rowan, N.J.; Faller, D.; Friel, A.M. Natural and synthetic isothiocyanates possess anticancer potential against liver and prostate cancer in vitro. Anticancer Res. 2019, 39, 3469–3485. [Google Scholar] [CrossRef] [PubMed]
- Chuang, W.-T.; Yun-Ta, L.; Huang, C.-S.; Lo, C.-W.; Yao, H.-T.; Chen, H.-W.; Lii, C.-K. Benzyl isothiocyanate and phenethyl isothiocyanate inhibit adipogenesis and hepatosteatosis in mice with obesity induced by a high-fat diet. J. Agric. Food Chem. 2019, 67, 7136–7146. [Google Scholar] [CrossRef] [PubMed]
- Neubauer, C.; Heitmann, B.; Müller, C. Biofumigation potential of Brassicaceae cultivars to Verticillium dahliae. Eur. J. Plant Pathol. 2014, 140, 341–352. [Google Scholar] [CrossRef]
- Hooker, W.J.; Walker, J.C.; Smith, F.G. Toxicity of beta-phenethyl isothiocyanate to certain fungi. Am. J. Bot. 1943, 30, 632–637. [Google Scholar]
- Wilson, A.E.; Bergaentzlé, M.; Bindler, F.; Marchioni, E.; Lintz, A.; Ennahar, S. In vitro efficacies of various isothiocyanates from cruciferous vegetables as antimicrobial agents against foodborne pathogens and spoilage bacteria. Food Control 2013, 30, 318–324. [Google Scholar] [CrossRef]
- Baena, R.; Salinas, H. Cancer chemoprevention by dietary phytochemicals: Epidemiological evidence. Maturitas 2016, 94, 13–19. [Google Scholar] [CrossRef]
- Belitz, H.-D.; Grosch, W.; Schieberle, P. Food Chemistry, 4th ed.; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Ghawi, S.K.; Methven, L.; Niranjan, K. The potential to intensify sulforaphane formation in cooked broccoli (Brassica oleracea var. italica) using mustard seeds (Sinapis alba). Food Chem. 2013, 138, 1734–1741. [Google Scholar] [CrossRef]
- Torres-Contreras, A.M.; Nair, V.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Stability of bioactive compounds in broccoli as affected by cutting styles and storage time. Molecules 2017, 22, 636. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Pei, Y.; Qi, A.; Jingnan, R.; Gang, F.; Zelan, W.; Xixiang, L.; Siyi, P. Identification of glucosinolates and volatile odor compounds in microwaved radish (Raphanus sativus L.) seeds and the corresponding oils by UPLC-IMS-QTOF-MS and GC × GC-qMS analysis. Food Res. Int. 2023, 169, 112873. [Google Scholar]
- Hanschen, F.S.; Schreiner, M. Isothiocyanates, nitriles, and epithionitriles from glucosinolates are affected by genotype and developmental stage in Brassica oleracea varieties. Front. Plant Sci. 2017, 8, 1095. [Google Scholar] [CrossRef] [PubMed]
- Aires, A.; Rosa, E.; Carvalho, R. Effect of nitrogen and sulfur fertilization on glucosinolates in the leaves and roots of broccoli sprouts (Brassica oleracea var. italica). J. Sci. Food Agric. 2006, 86, 1512–1516. [Google Scholar] [CrossRef]
- Zhang, Y.; Makaza, N.; Jiang, C.; Wu, Y.; Nishanbaev, S.Z.; Zou, L.; Sun, J.; Song, X.; Wu, Y. Supplementation of cooked broccoli with exogenous moringa myrosinase enhanced isothiocyanate formation. Food Chem. 2022, 395, 133651. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.G.; Lim, S.; Kim, J.; Lee, E.J. The mechanism of deterioration of the glucosinolate-myrosynase system in radish roots during cold storage after harvest. Food Chem. 2017, 233, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Krishna Moorthy, P.N.; Prasannakumar, N.R.; Mani, M.; Saroja, S.; Ranganath, H.R. Pests and their management in cruciferous vegetables (cabbage, cauliflower, knol khol, broccoli, radish, turnip, beet root). In Trends in Horticultural Entomology; Mani, M., Ed.; Spinger: Berlin/Heidelberg, Germany, 2022; pp. 997–1011. [Google Scholar]
- Renwick, J.A.A.; Radke, C.D.; Sachdev-Gupta, K.; Städler, E. Leaf surface chemicals stimulating oviposition by Pieris rapae (Lepidoptera: Pieridae) on cabbage. Chemoecology 1992, 3, 33–38. [Google Scholar] [CrossRef]
- Van Loon, J.J.A.; Blaakmeer, A.; Griepink, F.C.; Van Beek, T.A.; Schoonhoven, L.M.; De Groot, A. Leaf surface compound from Brassica oleracea (Cruciferae) induces oviposition by Pieris brassicae (Lepidoptera: Pieridae). Chemoecology 1992, 3, 39–44. [Google Scholar] [CrossRef]
- Huang, X.; Renwick, J.A.A. Relative activities of glucosinolates as oviposition stimulants for Pieris rapae and P. napi oleracea. J. Chem. Ecol. 1994, 20, 1025–1037. [Google Scholar] [CrossRef]
- Miles, C.I.; del Campo, M.L.; Renwick, J.A. Behavioral and chemosensory responses to a host recognition cue by larvae of Pieris rapae. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2005, 191, 147–155. [Google Scholar] [CrossRef]
- Stadler, E.; Renwick, J.; Radke, C.D.; Sachdev-Gupta, K. Tarsal contact chemoreceptor response to glucosinolates and cardenolides mediating oviposition in Pieris rape. Physiol. Entomol. 1995, 20, 175–187. [Google Scholar] [CrossRef]
- Badenes-Pérez, F.R. Plant glucosinolate content and host-plant preference and suitability in the small white butterfly (Lepidoptera: Pieridae) and comparison with another specialist lepidopteran. Plants 2023, 12, 2148. [Google Scholar] [CrossRef] [PubMed]
- Kokotou, M.G.; Revelou, P.K.; Pappas, C.; Constantinou-Kokotou, V. High resolution mass spectrometry studies of sulforaphane and indole-3-carbinol in broccoli. Food Chem. 2017, 237, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Ciska, E.; Drabinska, N.; Honke, J.; Narwojsz, A. Boiled Brussels sprouts: A rich source of glucosinolates and the corresponding nitriles. J. Funct Foods 2015, 19, 91–99. [Google Scholar] [CrossRef]
- Karanikolopoulou, S.; Revelou, P.-K.; Xagoraris, M.; Kokotou, M.G.; Constantinou-Kokotou, V. Current Methods for the extraction and analysis of isothiocyanates and indoles in cruciferous vegetables. Analytica 2021, 2, 93–120. [Google Scholar] [CrossRef]
- Arora, R.; Sharma, D.; Kumar, R.; Singh, B.; Vig, A.P.; Arora, S. Evaluating extraction conditions of glucosinolate hydrolytic products from seeds of Eruca sativa (Mill.) Thell. using GC-MS. J. Food Sci. 2014, 79, C1964–C1969. [Google Scholar] [CrossRef] [PubMed]
- Mukerjee, A.K.; Ashare, R. Isothiocyanates in the chemistry of heterocycles. Chem. Rev. 1991, 91, 1–24. [Google Scholar] [CrossRef]
- FAOSTAT. Food and Agriculture Organization Corporate Statistical Database. 2021. Available online: http://www.fao.org/faostat/en/#data/QC/visualize (accessed on 5 October 2023).
- Wittstock, U.; Agerbirk, N.; Stauber, E.J.; Olsen, C.E.; Hippler, M.; Mitchell-Olds, T.; Gershenzon, J.; Vogel, H. Successful herbivore attack due to metabolic diversion of a plant chemical defense. Proc. Natl. Acad. Sci. USA 2004, 101, 4859–4864. [Google Scholar] [CrossRef]
- Kaur, S.; Mahesh Kumar Samota, M.K.; Choudhary, M.; Choudhary, M.; Pandey, A.K.; Sharma, A.; Thakur, J. How do plants defend themselves against pathogens-Biochemical mechanisms and genetic interventions. Physiol. Mol. Biol. Plants 2022, 28, 485–504. [Google Scholar] [CrossRef]
- Itoh, Y.; Okumura, Y.; Fujii, T.; Ishikawa, Y.; Omura, H. Effects of mating on host selection by female small white butterflies Pieris rapae (Lepidoptera: Pieridae). J. Comp. Phys. A 2018, 204, 245–255. [Google Scholar] [CrossRef]
- Lund, M.; Brainard, D.C.; Szendrei, Z. Cue hierarchy for host plant selection in Pieris rapae. Entomol. Exp. Appl. 2019, 167, 330–340. [Google Scholar] [CrossRef]
- Yang, J.; Guo, H.; Jiang, N.-J.; Tang, R.; Li, G.-C.; Huang, L.-Q.; Van Loon, J.J.; Wang, C.-Z. Identification of a gustatory receptor tuned to sinigrin in the cabbage butterfly Pieris rapae. PLoS Genet. 2021, 17, e1009527. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Hernández, M.C.; Medina, S.; Gil-Izquierdo, A.; Martínez-Ballesta, C.; Moreno, D.A. Broccoli isothiocyanates content and in vitro availability according to variety and origin. Mac. J. Chem. Chem. Eng. 2013, 32, 251–264. [Google Scholar] [CrossRef]
- Brown, P.D.; Morra, M.J. Control of soil-borne plant pests using glucosinolate-containing plants. Adv. Agron. 1997, 61, 167–231. [Google Scholar]
- Rosa, E.A.S.; Heaney, R.K.; Fenwick, G.R.; Portas, C.A.M. Glucosinolates in Crop Plants; Horticultural Reviews; Wiley & Sons, Inc.: Hoboken, NJ, USA, 1997. [Google Scholar]
- Burow, M.; Losansky, A.; Muller, R.; Plock, A.; Kliebenstein, D.J.; Wittstock, U. The genetic basis of constitutive and herbivore-induced ESP-independent nitrile formation in arabidopsis. Plant Physiol. 2009, 149, 561–574. [Google Scholar] [CrossRef]
- Eugui, D.; Velasco, P.; Abril-Urías, P.; Escobar, C.; Gómez-Torres, O.; Caballero, S.; Poveda, J. Glucosinolate-extracts from residues of conventional and organic cultivated broccoli leaves (Brassica oleracea var. italica) as potential industrially-scalable efficient biopesticides against fungi, oomycetes and plant parasitic nematodes. Ind. Crops Prod. 2003, 200, 116841. [Google Scholar] [CrossRef]
- Szymandera-Buszka, K.; Piechocka, J.; Zaremba, A.; Przeor, M.; Jędrusek-Golinska, A. Pumpkin, cauliflower and broccoli as new carriers of thiamine compounds for food fortification. Foods 2021, 10, 578. [Google Scholar] [CrossRef]
- Shi, M.; Ying, D.-Y.; Hlaing, M.M.; Ye, J.-H.; Sanguansri, L.; Augustin, M.A. Development of broccoli by-products as carriers for delivering EGCG. Food Chem. 2019, 301, 125301. [Google Scholar] [CrossRef]
- Krupa-Kozak, U.; Drabinska, N.; Rosell, C.M.; Fadda, C.; Anders, A.; Jelinski, T.; Ostaszyk, A. Broccoli leaf powder as an attractive by-product ingredient: Effect on batter behaviour, technological properties and sensory quality of gluten-free mini sponge cake. Int. J. Food Sci. Technol. 2019, 54, 1121–1129. [Google Scholar] [CrossRef]
- Krupa-Kozak, U.; Drabinska, N.; Bączek, N.; Simkova, K.; Starowicz, M.; Jelinski, T. Application of broccoli leaf powder in gluten-free bread: An innovative approach to improve its bioactive potential and technological quality. Foods 2021, 10, 819. [Google Scholar] [CrossRef]
| Mean Value ± SD μg/g DW | ||||
|---|---|---|---|---|
| Broccoli Tissue | PEITC | ER | 3MIC | 5MITN |
| F1 | 5.3 ± 0.06 | 5.1 ± 1.86 | n.d | 137.2 ± 5.73 |
| F2 | 5.6 ± 0.14 | 5.0 ± 0.49 | n.d. | 72.6 ± 9.92 |
| R1 | 126.7 ± 4.83 | 230.5 ± 10.14 | 18.5 ± 1.07 | 208.3 ± 27.98 |
| R2 | 367.6 ± 23.43 | 576.3 ± 148.51 | 34.6 ± 3.74 | 1491.1 ± 331.55 |
| S1 | 5.1 ± 0.02 | 18.0 ± 1.37 | n.d. | 4534.7 ± 578.98 |
| S2 | 8.7 ± 0.73 | 47.4 ± 7.62 | n.d. | 604.1 ± 99.25 |
| L1 | 5.0 ± 0.01 | n.d. | n.d. | 75.5 ± 3.94 |
| L2 | 5.2 ± 0.03 | n.d. | n.d. | 56.8 ± 1.17 |
| Mean Value ± SD µg/g DW | ||||
|---|---|---|---|---|
| Broccoli Root | PEITC | ER | 3MIC | 5MITN |
| R2 | 367.6 ± 23.43 | 576.3 ± 148.51 | 34.6 ± 3.74 | 1491.1 ± 331.55 |
| R3 | 272.8 ± 112.53 | 284.0 ± 108.71 | 33.1 ± 0.66 | 1795.1 ± 65.60 |
| R4 | 134.3 ± 46.40 | 54.7 ± 1.67 | 3.9 ± 0.08 | 371.3 ± 21.03 |
| R5 | 86.5 ± 2.43 | 209.0 ± 71.68 | 15.4 ± 2.52 | 846.2 ± 270.02 |
| Mean ± SD | 215 ± 90.21 | 280.75 ± 120.54 | 21.75 ± 8.76 | 1125.75 ± 540.11 |
| Mean Value ± SD μg/g DW | ||||
|---|---|---|---|---|
| PEITC | ER | 3MIC | 5MITN | |
| Pieris rapae | 0.5 ± 0.00 | 1.4 ± 0.02 | n.d. | n.d. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mesimeri, I.-D.; Revelou, P.-K.; Constantinou-Kokotou, V.; Kokotou, M.G. Determination of Phenethyl Isothiocyanate, Erucin, Iberverin, and Erucin Nitrile Concentrations in Healthy and Pieris rapae-Infected Broccoli Tissues Using Gas Chromatography-Mass Spectrometry. Chemosensors 2024, 12, 16. https://doi.org/10.3390/chemosensors12010016
Mesimeri I-D, Revelou P-K, Constantinou-Kokotou V, Kokotou MG. Determination of Phenethyl Isothiocyanate, Erucin, Iberverin, and Erucin Nitrile Concentrations in Healthy and Pieris rapae-Infected Broccoli Tissues Using Gas Chromatography-Mass Spectrometry. Chemosensors. 2024; 12(1):16. https://doi.org/10.3390/chemosensors12010016
Chicago/Turabian StyleMesimeri, Irene-Dimitra, Panagiota-Kyriaki Revelou, Violetta Constantinou-Kokotou, and Maroula G. Kokotou. 2024. "Determination of Phenethyl Isothiocyanate, Erucin, Iberverin, and Erucin Nitrile Concentrations in Healthy and Pieris rapae-Infected Broccoli Tissues Using Gas Chromatography-Mass Spectrometry" Chemosensors 12, no. 1: 16. https://doi.org/10.3390/chemosensors12010016
APA StyleMesimeri, I.-D., Revelou, P.-K., Constantinou-Kokotou, V., & Kokotou, M. G. (2024). Determination of Phenethyl Isothiocyanate, Erucin, Iberverin, and Erucin Nitrile Concentrations in Healthy and Pieris rapae-Infected Broccoli Tissues Using Gas Chromatography-Mass Spectrometry. Chemosensors, 12(1), 16. https://doi.org/10.3390/chemosensors12010016










