Bipyridyl Ruthenium-Decorated Ni-MOFs on Carbon Nanotubes for Electrocatalytic Oxidation and Sensing of Glucose
Abstract
1. Introduction
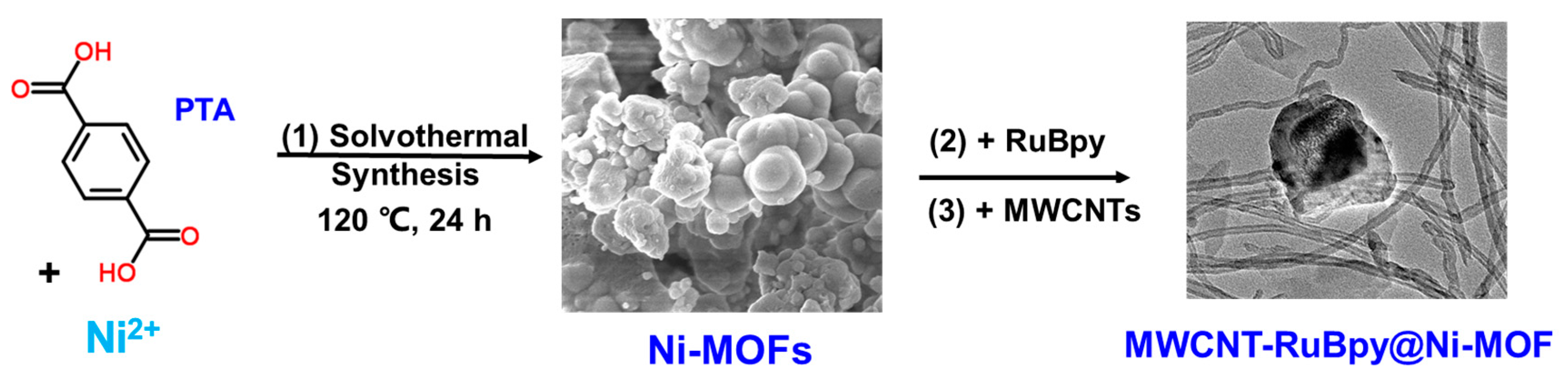
2. Materials and Methods
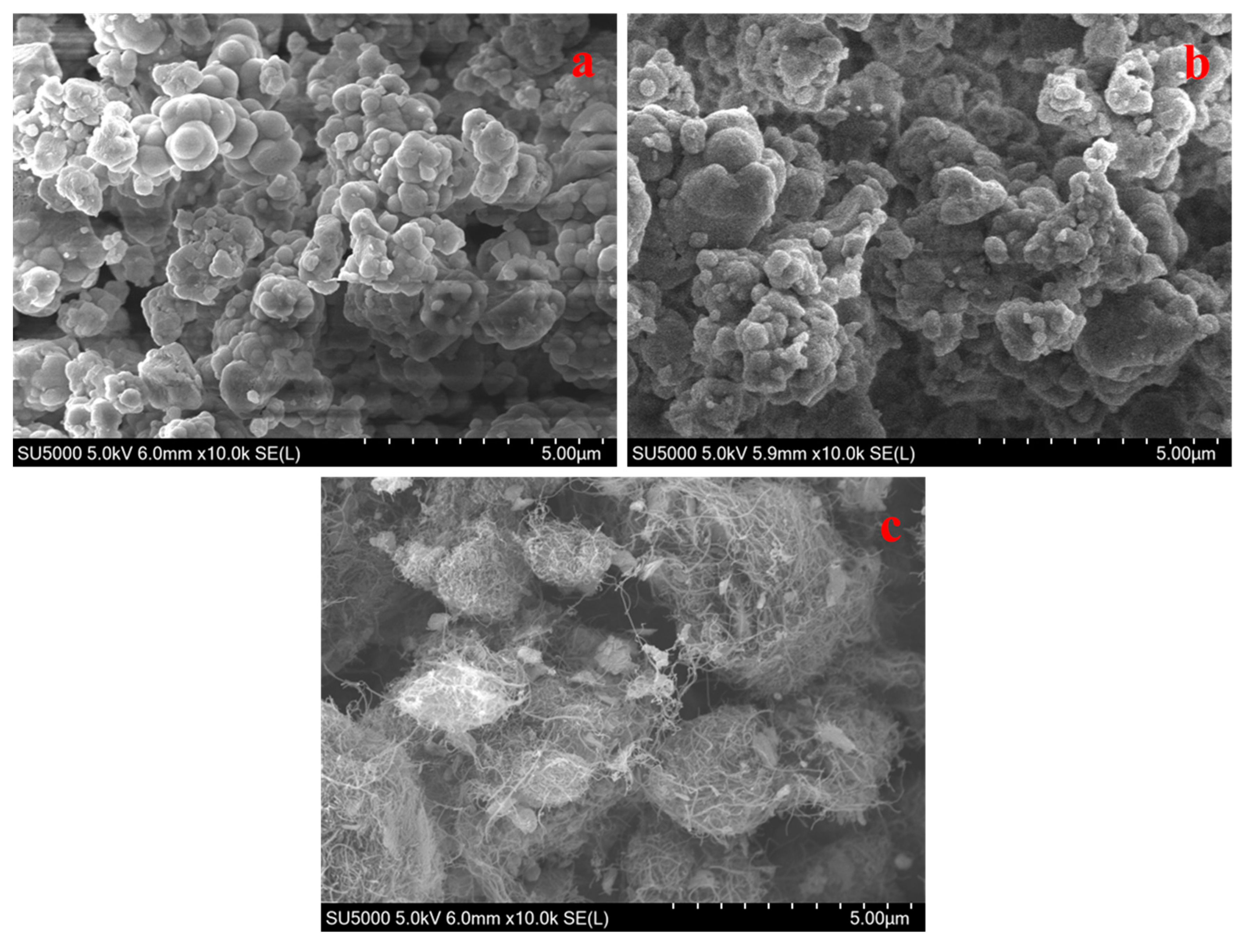
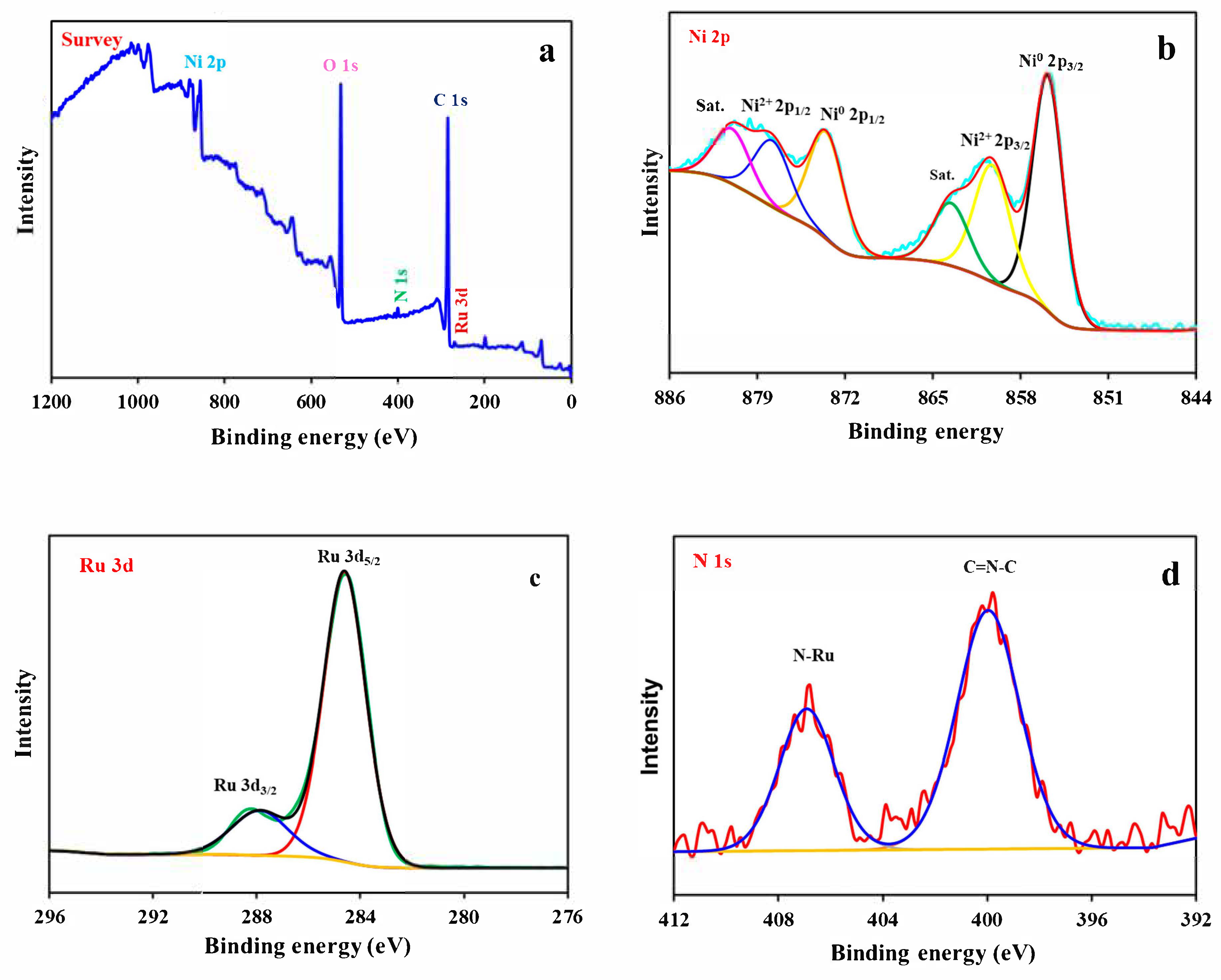
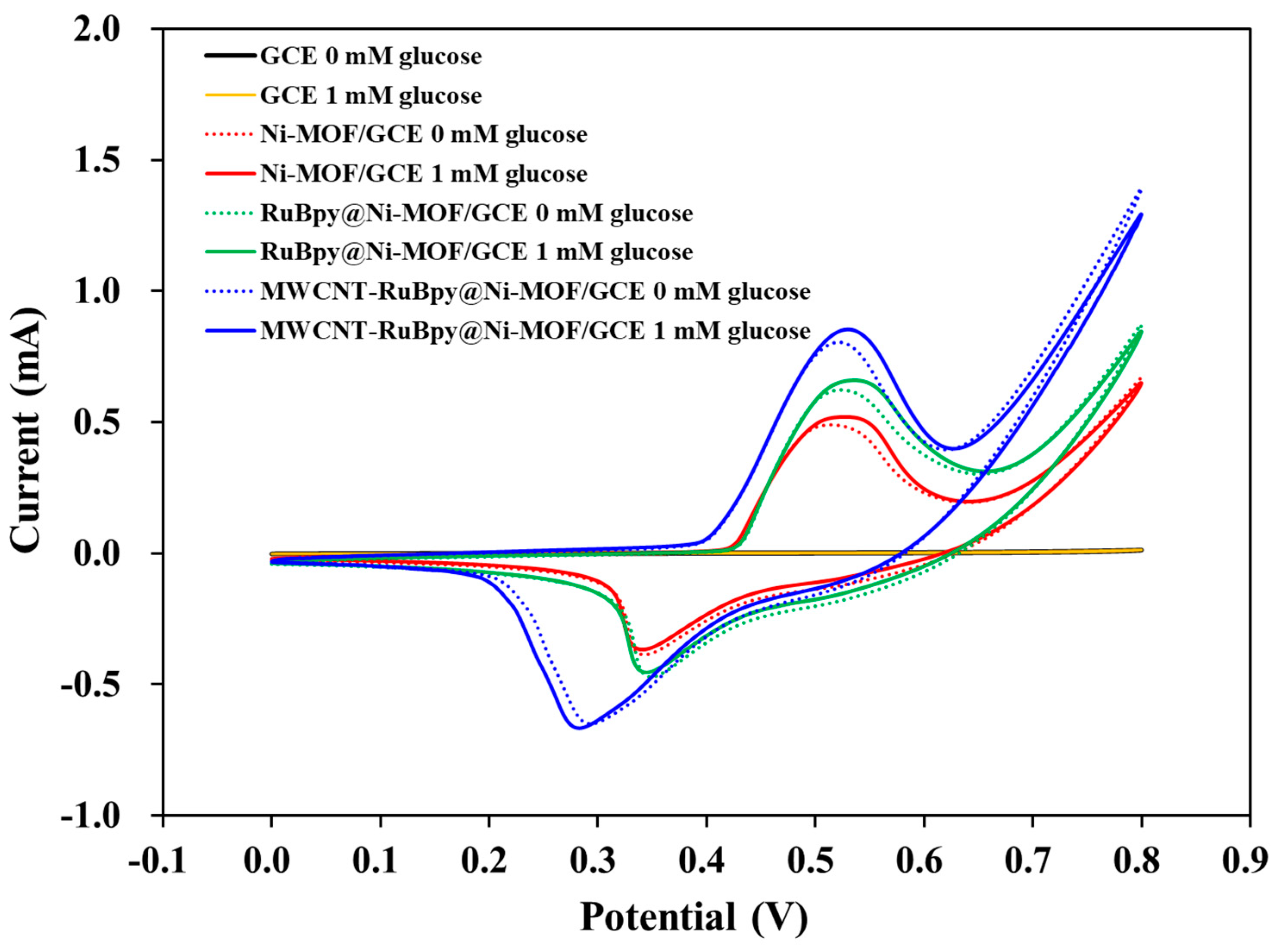
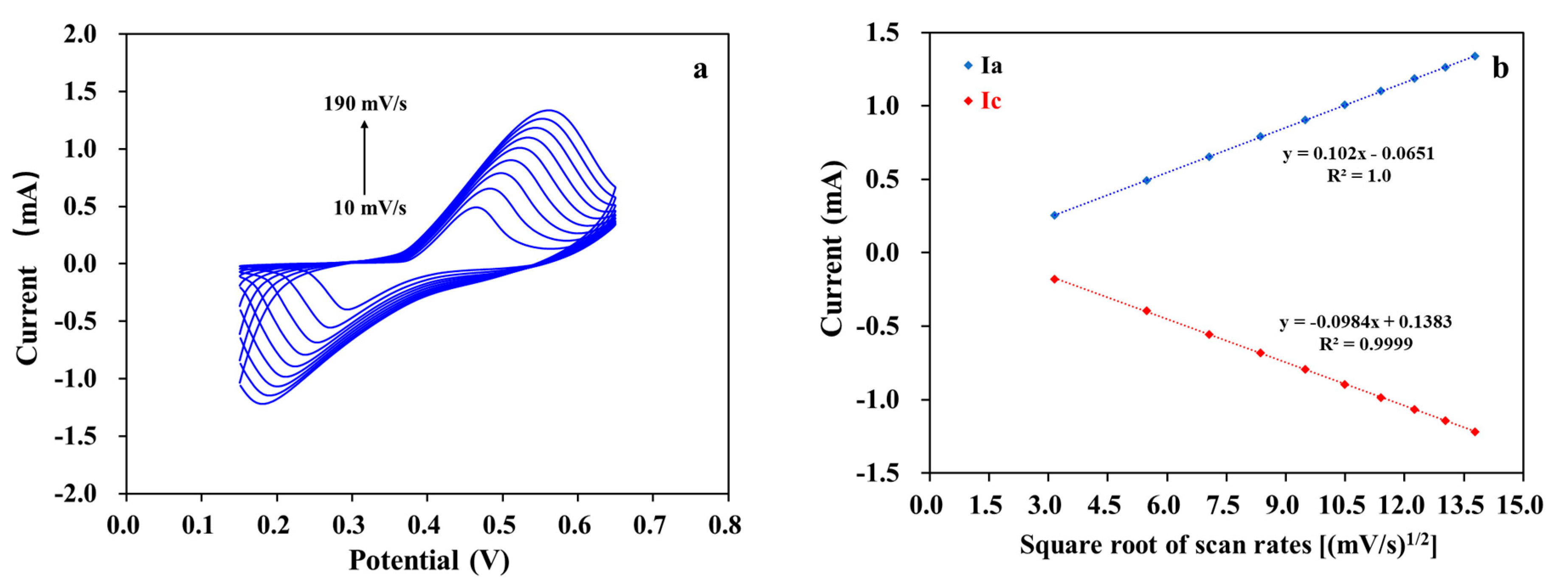
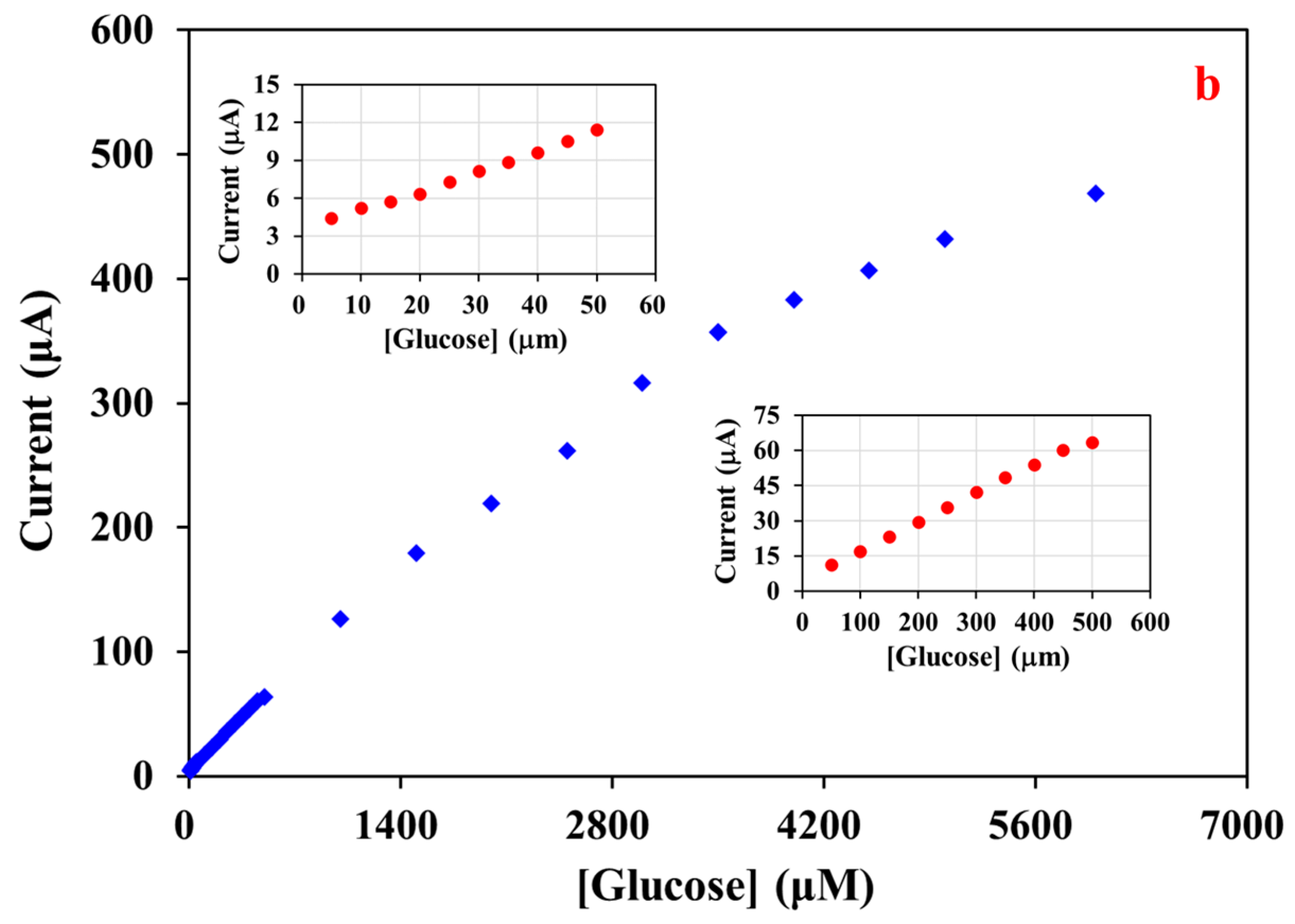
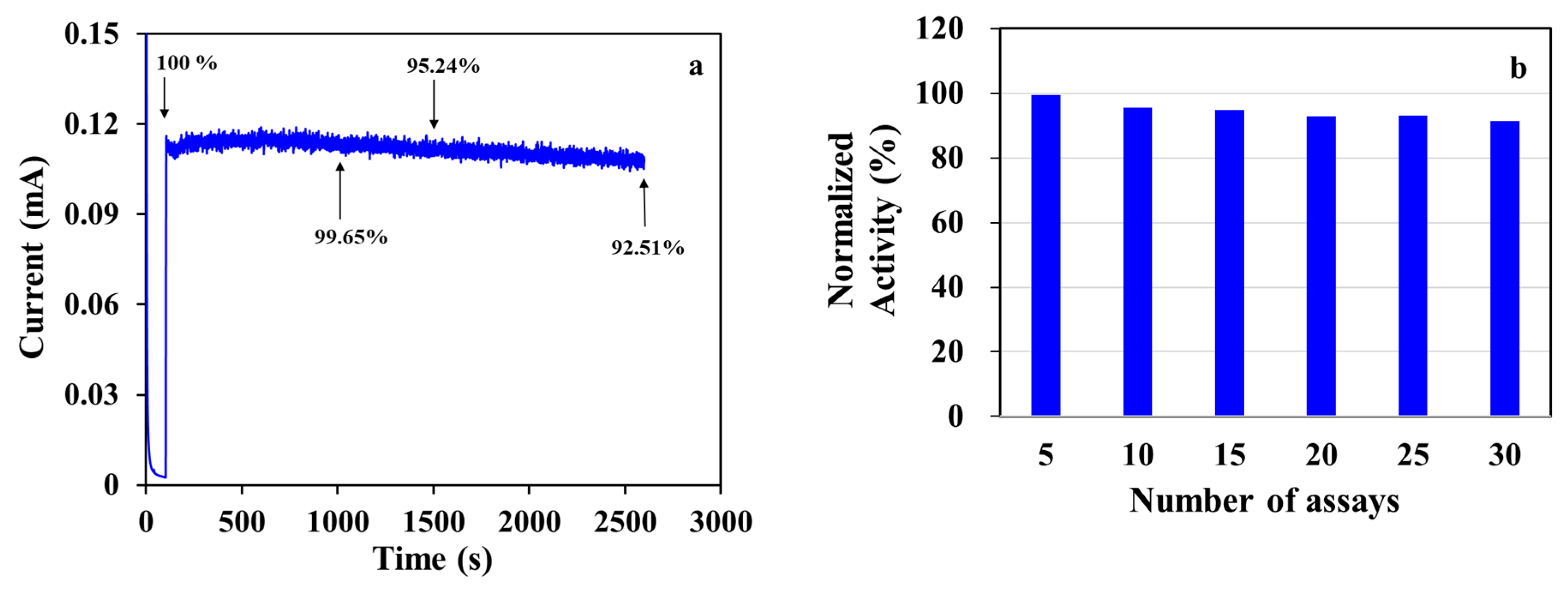
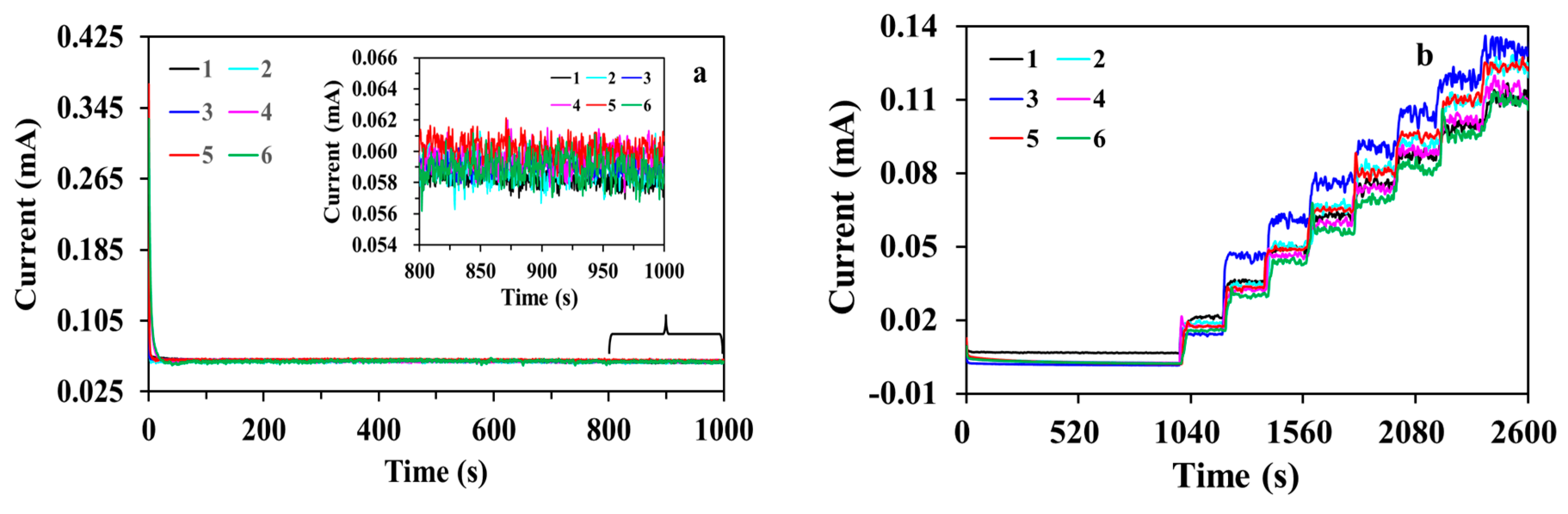
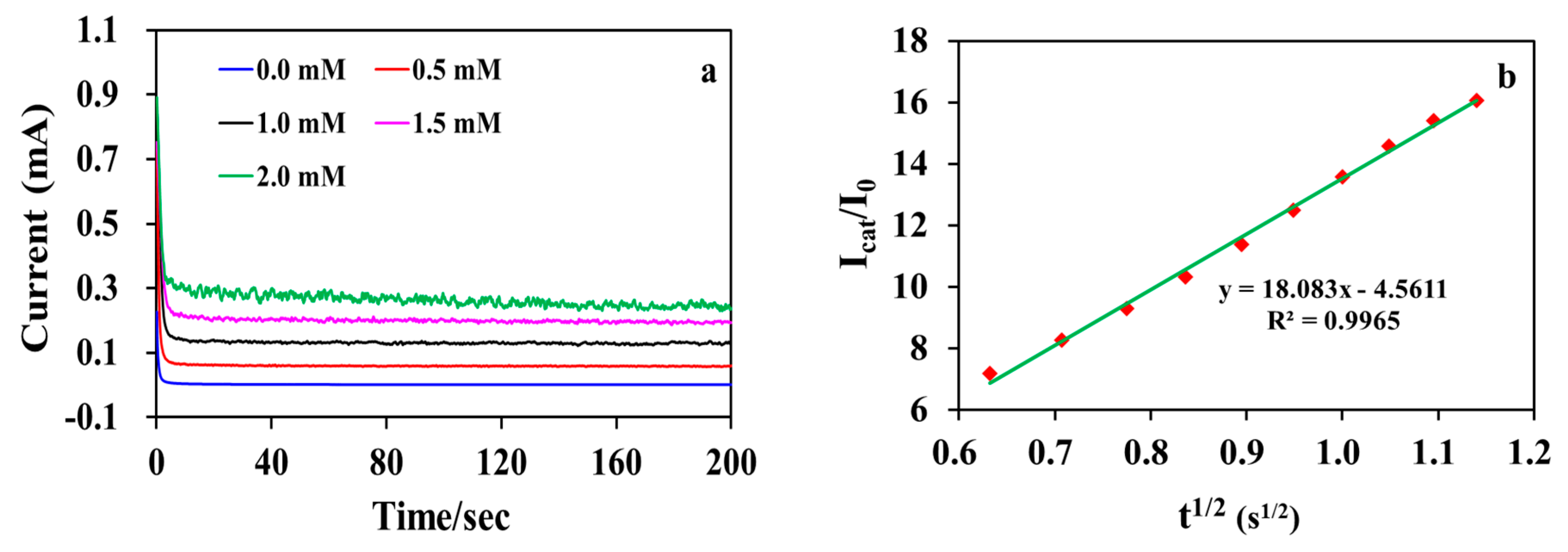
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lopa, N.S.; Rahman, M.; Ahmed, F.; Sutradhar, S.C.; Ryu, T.; Kim, W. A Ni-based redox-active metal-organic framework for sensitive and non-enzymatic detection of glucose. J. Electroanal. Chem. 2018, 822, 43–49. [Google Scholar] [CrossRef]
- Chen, C.; Zhong, Y.; Cheng, S.; Huanga, Y.; Li, T.; Shi, T.; Liao, G.; Tang, Z. In Situ Fabrication of Porous Nanostructures Derived from Bimetal-Organic Frameworks for Highly Sensitive Non-Enzymatic Glucose Sensors. J. Electrochem. Soc. 2020, 167, 027531. [Google Scholar] [CrossRef]
- Wang, F.; Chen, X.; Chen, L.; Yang, J.; Wang, Q. High-performance non-enzymatic glucose sensor by hierarchical flower-like nickel(II)-based MOF/carbon nanotubes composite. Mater. Sci. Eng. C 2019, 96, 41–50. [Google Scholar] [CrossRef]
- Zheng, W.; Liu, Y.; Yang, P.; Chen, Y.; Tao, J.; Hu, J.; Zhao, P. Carbon nanohorns enhanced electrochemical properties of Cu-based metal organic framework for ultrasensitive serum glucose sensing. J. Electroanal. Chem. 2020, 862, 114018. [Google Scholar] [CrossRef]
- Wang, H.-B.; Zhang, H.-D.; Chen, Y.; Li, Y.; Gan, T. H2O2-mediated fluorescence quenching of double-stranded DNA templated copper nanoparticles for label-free and sensitive detection of glucose. RSC Adv. 2015, 5, 77906–77912. [Google Scholar] [CrossRef]
- Wang, H.-B.; Chen, Y.; Li, N.; Liu, Y.-M. A fluorescent glucose bioassay based on the hydrogen peroxide-induced decomposition of a quencher system composed of MnO2 nanosheets and copper nanoclusters. Microchim. Acta 2017, 184, 515–523. [Google Scholar] [CrossRef]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; Van Duyne, R.P.; Hupp, J.T. Metal–Organic Framework Materials as Chemical Sensors. Chem. Rev. 2012, 112, 1105–1125. [Google Scholar] [CrossRef]
- Lustig, W.P.; Mukherjee, S.; Rudd, N.D.; Desai, A.V.; Li, J.; Ghosh, S.K. Metal–organic frameworks: Functional luminescent and photonic materials for sensing applications. Chem. Soc. Rev. 2017, 46, 3242–3285. [Google Scholar] [CrossRef]
- Yang, Q.; Xu, Q.; Jiang, H.-L. Metal–organic frameworks meet metal nanoparticles: Synergistic effect for enhanced catalysis. Chem. Soc. Rev. 2017, 46, 4774–4808. [Google Scholar] [CrossRef]
- Jiao, L.; Wang, Y.; Jiang, H.-L.; Xu, Q. Metal–Organic Frameworks as Platforms for Catalytic Applications. Adv. Mater. 2018, 30, 1703663. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Li, Z.; Garcia, H. Catalysis and photocatalysis by metal organic frameworks. Chem. Soc. Rev. 2018, 47, 8134–8172. [Google Scholar] [CrossRef]
- McKeithan, C.R.; Mayers, J.M.; Wojtas, L.; Larsen, R.W. Photophysical studies of Ru(II)tris(2,2′-bipyridine) encapsulated within the ZnHKUST-1 metal organic framework. Inorg. Chim. Acta 2018, 483, 1–5. [Google Scholar] [CrossRef]
- Jin, X.; Li, G.; Xu, T.; Su, L.; Yan, D.; Zhang, X. Ruthenium-based Conjugated Polymer and Metal-organic Framework Nanocomposites for Glucose Sensing. Electroanalysis 2021, 33, 1902–1910. [Google Scholar] [CrossRef]
- Chen, J.; Yin, H.; Zhou, J.; Wang, L.; Gong, J.; Ji, Z.; Nie, Q. Efficient Nonenzymatic Sensors Based on Ni-MOF Microspheres Decorated with Au Nanoparticles for Glucose Detection. J. Electron. Mater. 2020, 49, 4754–4763. [Google Scholar] [CrossRef]
- Abbasi, A.R.; Yousefshahi, M.; Daasbjerg, K. Non-enzymatic Electroanalytical Sensing of Glucose Based on Nano Nickel-Coordination Polymers-Modified Glassy Carbon Electrode. J. Inorg. Organomet. Polym. Mater. 2020, 30, 2027–2038. [Google Scholar] [CrossRef]
- Li, L.-X.; He, S.; Zeng, S.; Chen, W.-T.; Ye, J.-W.; Zhou, H.-L.; Huang, X.-C. Equipping carbon dots in a defect-containing MOF via self-carbonization for explosive sensing. J. Mater. Chem. C 2022, 11, 321–328. [Google Scholar] [CrossRef]
- Elizbit Liaqat, U.; Hussain, Z.; Baig, M.M.; Khan, M.A.; Arif, D. Preparation of porous ZIF-67 network interconnected by MWCNTs and decorated with Ag nanoparticles for improved non-enzymatic electrochemical glucose sensing. J. Korean Ceram. Soc. 2021, 58, 598–605. [Google Scholar] [CrossRef]
- Wen, Y.; Meng, W.; Li, C.; Dai, L.; He, Z.; Wang, L.; Li, M.; Zhu, J. Enhanced glucose sensing based on a novel composite CoII-MOF/Acb modified electrode. Dalton Trans. 2018, 47, 3872–3879. [Google Scholar] [CrossRef]
- Li, W.-H.; Lv, J.; Li, Q.; Xie, J.; Ogiwara, N.; Huang, Y.; Jiang, H.; Kitagawa, H.; Xu, G.; Wang, Y. Conductive metal–organic framework nanowire arrays for electrocatalytic oxygen evolution. J. Mater. Chem. A 2019, 7, 10431–10438. [Google Scholar] [CrossRef]
- Lennon, S.J.; Robinson, F.P.A. The experimental determination of potential-pH diagrams for the Ni-H2O and low alloy steel-H2O systems. Corros. Sci. 1986, 26, 995–1007. [Google Scholar] [CrossRef]
- Camasso, N.M.; Sanford, M.S. Design, synthesis, and carbon-heteroatom coupling reactions of organometallic nickel(IV) complexes. Science 2015, 347, 1218–1220. [Google Scholar] [CrossRef]
- Leem, Y.J.; Cho, K.; Oh, K.H.; Han, S.-H.; Nam, K.M.; Chang, J. A self-assembled Ni(cyclam)-BTC network on ITO for an oxygen evolution catalyst in alkaline solution. Chem. Commun. 2017, 53, 3454–3457. [Google Scholar] [CrossRef]
- Rahmanifar, M.S.; Hesari, H.; Noori, A.; Masoomi, M.Y.; Morsali, A.; Mousavi, M.F. A dual Ni/Co-MOF-reduced graphene oxide nanocomposite as a high performance supercapacitor electrode material. Electrochim. Acta 2018, 275, 76–86. [Google Scholar] [CrossRef]
- Gao, M.; Sheng, W.; Zhuang, Z.; Fang, Q.; Gu, S.; Jiang, J.; Yan, Y. Efficient Water Oxidation Using Nanostructured α-Nickel-Hydroxide as an Electrocatalyst. J. Am. Chem. Soc. 2014, 136, 7077–7084. [Google Scholar] [CrossRef] [PubMed]
- Bediako, D.K.; Lassalle-Kaiser, B.; Surendranath, Y.; Yano, J.; Yachandra, V.K.; Nocera, D.G. Structure–Activity Correlations in a Nickel–Borate Oxygen Evolution Catalyst. J. Am. Chem. Soc. 2012, 134, 6801–6809. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Zheng, S.; Li, X.; Zhang, G.; Guo, X.; Xue, H.; Pang, H. Facile synthesis of ultrathin Ni-MOF nanobelts for high-efficiency determination of glucose in human serum. J. Mater. Chem. B 2017, 5, 5234–5239. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Liu, Q.; Lu, S.; Chen, G.; Gao, S.; Lu, W.; Sun, X. High-performance non-enzymatic glucose detection: Using a conductive Ni-MOF as an electrocatalyst. J. Mater. Chem. B 2020, 8, 5411–5415. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Bard, A.J. Electrogenerated chemiluminescent emission from an organized (L-B) monolayer of a tris(2,2′-bipyridine)ruthenium(2+)-based surfactant on semiconductor and metal electrodes. J. Phys. Chem. 1988, 92, 5566–5569. [Google Scholar] [CrossRef]
- Miller, C.J.; McCord, P.; Bard, A.J. Study of Langmuir monolayers of ruthenium complexes and their aggregation by electrogenerated chemiluminescence. Langmuir 1991, 7, 2781–2787. [Google Scholar] [CrossRef]
- Zholudov, Y.; Snizhko, D.; Kukoba, A.; Bilash, H.; Rozhitskii, M. Aqueous electrochemiluminescence of polycyclic aromatic hydrocarbons immobilized into Langmuir–Blodgett film at the electrode. Electrochim. Acta 2008, 54, 360–363. [Google Scholar] [CrossRef]
- Sato, Y.; Uosaki, K. Electrochemical and electrogenerated chemiluminescence properties of tris(2,2′-bipyridine)ruthenium(II)-tridecanethiol derivative on ITO and gold electrodes. J. Electroanal. Chem. 1995, 384, 57–66. [Google Scholar] [CrossRef]
- Obeng, Y.S.; Bard, A.J. Electrogenerated chemiluminescence. 53. Electrochemistry and emission from adsorbed monolayers of a tris(bipyridyl)ruthenium(II)-based surfactant on gold and tin oxide electrodes. Langmuir 1991, 7, 195–201. [Google Scholar] [CrossRef]
- Collinson, M.M.; Novak, B.; Martin, S.A.; Taussig, J.S. Electrochemiluminescence of Ruthenium(II) Tris(bipyridine) Encapsulated in Sol−Gel Glasses. Anal. Chem. 2000, 72, 2914–2918. [Google Scholar] [CrossRef]
- Sykora, M.; Meyer, T.J. Electrogenerated Chemiluminescence in SiO2 Sol−Gel Polymer Composites. Chem. Mater. 1999, 11, 1186–1189. [Google Scholar] [CrossRef]
- Matsui, K.; Momose, F. Luminescence Properties of Tris(2,2′-bipyridine)ruthenium(II) in Sol−Gel Systems of SiO2. Chem. Mater. 1997, 9, 2588–2591. [Google Scholar] [CrossRef]
- Choi, H.N.; Cho, S.-H.; Lee, W.-Y. Electrogenerated Chemiluminescence from Tris(2,2′-bipyridyl)ruthenium(II) Immobilized in Titania−Perfluorosulfonated Ionomer Composite Films. Anal. Chem. 2003, 75, 4250–4256. [Google Scholar] [CrossRef]
- Lin, Z.; Chen, G. Determination of carbamates in nature water based on the enhancement of electrochemiluminescent of Ru(bpy)32+ at the multi-wall carbon nanotube-modified electrode. Talanta 2006, 70, 111–115. [Google Scholar] [CrossRef]
- Hun, X.; Zhang, Z. Electrogenerated chemiluminescence sensor for metoclopramide determination based on Ru(bpy)32+-doped silica nanoparticles dispersed in Nafion on glassy carbon electrode. J. Pharm. Biomed. Anal. 2008, 47, 670–676. [Google Scholar] [CrossRef]
- Martin, A.F.; Nieman, T.A. Chemiluminescence biosensors using tris(2,2′-bipyridyl)ruthenium(II) and dehydrogenases immobilized in cation exchange polymers. Biosens. Bioelectron. 1997, 12, 479–489. [Google Scholar] [CrossRef]
- Zhao, C.-Z.; Egashira, N.; Kurauchi, Y.; Ohga, K. Electrochemiluminescence Sensor Having a Pt Electrode Coated with a Ru(bpy)32+-Modified Chitosan/Silica Gel Membrane. Anal. Sci. 1998, 14, 439–441. [Google Scholar] [CrossRef][Green Version]
- Wang, H.; Xu, G.; Dong, S. Electrochemiluminescence of tris(2,2′-bipyridine)ruthenium(II) immobilized in poly(p-styrenesulfonate)–silica–Triton X-100 composite thin-films. Analyst 2001, 126, 1095–1099. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, F.; Dong, S. Layer-by-layer assembly of functional silica and Au nanoparticles for fabricating electrogenerated chemiluminescence sensor. Electrochim. Acta 2008, 53, 6423–6427. [Google Scholar] [CrossRef]
- Lee, J.-K.; Lee, S.-H.; Kim, M.; Kim, H.; Kim, D.-H.; Lee, W.-Y. Organosilicate thin film containing Ru(bpy)32+ for an electrogenerated chemiluminescence (ECL) sensor. Chem. Commun. 2003, 1602–1603. [Google Scholar] [CrossRef]
- Li, J.; Xu, Y.; Wei, H.; Huo, T.; Wang, E. Electrochemiluminescence Sensor Based on Partial Sulfonation of Polystyrene with Carbon Nanotubes. Anal. Chem. 2007, 79, 5439–5443. [Google Scholar] [CrossRef]
- Guo, Z.; Dong, S. Electrogenerated Chemiluminescence from Ru(Bpy)32+ Ion-Exchanged in Carbon Nanotube/Perfluorosulfonated Ionomer Composite Films. Anal. Chem. 2004, 76, 2683–2688. [Google Scholar] [CrossRef]
- Kang, C.H.; Choi, Y.-B.; Kim, H.-H.; Choi, H.N.; Lee, W.-Y. Electrogenerated Chemiluminescence Sensor Based on a Self-Assembled Monolayer of Ruthenium(II)-bis(2,2′-bipyridyl)(aminopropyl imidazole) on Gold Deposited Screen Printed Electrode. Electroanalysis 2011, 23, 2131–2138. [Google Scholar] [CrossRef]
- Xu, X.; Li, H.; Xu, Z. Multifunctional luminescent switch based on a porous PL-MOF for sensitivity recognition of HCl, trace water and lead ion. Chem. Eng. J. 2022, 436, 135028. [Google Scholar] [CrossRef]
- Ando, Y.; Zhao, X.; Shimoyama, H.; Sakai, G.; Kaneto, K. Physical properties of multiwalled carbon nanotubes. Int. J. Inorg. Mater. 1999, 1, 77–82. [Google Scholar] [CrossRef]
- Wei, B.Q.; Vajtai, R.; Ajayan, P.M. Reliability and current carrying capacity of carbon nanotubes. Appl. Phys. Lett. 2001, 79, 1172–1174. [Google Scholar] [CrossRef]
- Zeraati, M.; Alizadeh, V.; Kazemzadeh, P.; Safinejad, M.; Kazemian, H.; Sargazi, G. A new nickel metal organic framework (Ni-MOF) porous nanostructure as a potential novel electrochemical sensor for detecting glucose. J. Porous Mater. 2022, 29, 257–267. [Google Scholar] [CrossRef]
- Ma, X.; Tang, K.-l.; Yang, M.; Shi, W.; Zhao, W. Metal–organic framework-derived yolk–shell hollow Ni/NiO@C microspheres for bifunctional non-enzymatic glucose and hydrogen peroxide biosensors. J. Mater. Sci. 2021, 56, 442–456. [Google Scholar] [CrossRef]
- Morgan, D.J. Resolving ruthenium: XPS studies of common ruthenium materials. Surf. Interface Anal. 2015, 47, 1072–1079. [Google Scholar] [CrossRef]
- Al-Hinaai, M.; Khudaish, E.A. Electrochemical Construction of a Polymer-Metal Complex Surface Network for Selective Determination of Dopamine in Blood Serum. Anal. Lett. 2022, 55, 1249–1268. [Google Scholar] [CrossRef]
- Ren, Z.; Mao, H.; Luo, H.; Liu, Y. Glucose sensor based on porous Ni by using a graphene bottom layer combined with a Ni middle layer. Carbon 2019, 149, 609–617. [Google Scholar] [CrossRef]
- Cao, M.; Wang, H.; Ji, S.; Zhao, Q.; Pollet, B.G.; Wang, R. Hollow core-shell structured Cu2O@Cu1.8S spheres as novel electrode for enzyme free glucose sensing. Mater. Sci. Eng. C 2019, 95, 174–182. [Google Scholar] [CrossRef]
- Li, G.; Chen, D.; Chen, Y.; Dong, L. MOF Ni-BTC Derived Ni/C/Graphene Composite for Highly Sensitive Non-Enzymatic Electrochemical Glucose Detection. ECS J. Solid State Sci. Technol. 2020, 9, 121014. [Google Scholar] [CrossRef]
- Jia, H.; Shang, N.; Feng, Y.; Ye, H.; Zhao, J.; Wang, H.; Wang, C.; Zhang, Y. Facile preparation of Ni nanoparticle embedded on mesoporous carbon nanorods for non-enzymatic glucose detection. J. Colloid Interface Sci. 2021, 583, 310–320. [Google Scholar] [CrossRef]
- Chen, J.; Yin, H.; Zhou, J.; Wang, L.; Ji, Z.; Zheng, Y.; Nie, Q. Hybrid Ni3N-nitrogen-doped carbon microspheres (Ni3N@C) in situ derived from Ni-MOFs as sensitive non-enzymatic glucose sensors. Mater. Technol. 2021, 36, 286–295. [Google Scholar] [CrossRef]
- Li, G.; Xie, G.; Chen, D.; Gong, C.; Chen, X.; Zhang, Q.; Pang, B.; Zhang, Y.; Li, C.; Hu, J.; et al. Facile synthesis of bamboo-like Ni3S2@NCNT as efficient and stable electrocatalysts for non-enzymatic glucose detection. Appl. Surf. Sci. 2022, 585, 152683. [Google Scholar] [CrossRef]
- Asadian, E.; Shahrokhian, S.; Iraji Zad, A. Highly sensitive nonenzymetic glucose sensing platform based on MOF-derived NiCo LDH nanosheets/graphene nanoribbons composite. J. Electroanal. Chem. 2018, 808, 114–123. [Google Scholar] [CrossRef]



| Electrode | Linear Range (μM) | Detection Limit (μM) | Sensitivity (μA mM−1 cm−2) | Working Potential (V) | Ref. |
|---|---|---|---|---|---|
| CPO-27-NiII | 40–500 | 1.46 | 585 | +0.55 | [1] |
| Ni(TPA)-SWCNT-CS | 20–4400 | 4.6 | - | +0.55 | [3] |
| Au@Ni-BTC | 5–7400 | 1.5 | 1447.1 | +0.55 | [14] |
| Ni3(HHTP)2 | 1–8000 | 0.66 | 21,744 | +0.55 | [27] |
| Ni/NiO@C | 10–2000 2000–10,000 | 0.116 | 1291 | +0.55 | [51] |
| Ni/NCNs-500 | 0.1–533.6 533.6–3030 | 0.07 | 337.32 210.56 | +0.55 | [57] |
| Ni3N@C | 1–3000 | 0.3 | 1511.59 | +0.6 | [58] |
| Ni3S2@NCNT | 0.46–3190 | 0.14 | 1447.64 | +0.55 | [59] |
| Ni/Co LDH/GNRs | 5–800 | 0.82 | 344 | +0.6 | [60] |
| MWCNT-RuBpy@Ni-MOF | 5–3500 | 1.7 | 1471.43 | +0.50 | This work |
| Sample | Original (μM) | Added (μM) | Found (μM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|---|
| Honey | 84.23 | 49.50 | 133.86 | 100.26 | 3.07 |
| 26.20 | 39.64 | 66.69 | 102.14 | 4.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Liu, C.; Yan, R.; Lei, C. Bipyridyl Ruthenium-Decorated Ni-MOFs on Carbon Nanotubes for Electrocatalytic Oxidation and Sensing of Glucose. Chemosensors 2024, 12, 39. https://doi.org/10.3390/chemosensors12030039
Zhang Y, Liu C, Yan R, Lei C. Bipyridyl Ruthenium-Decorated Ni-MOFs on Carbon Nanotubes for Electrocatalytic Oxidation and Sensing of Glucose. Chemosensors. 2024; 12(3):39. https://doi.org/10.3390/chemosensors12030039
Chicago/Turabian StyleZhang, Yu, Chang Liu, Rongqiu Yan, and Chenghong Lei. 2024. "Bipyridyl Ruthenium-Decorated Ni-MOFs on Carbon Nanotubes for Electrocatalytic Oxidation and Sensing of Glucose" Chemosensors 12, no. 3: 39. https://doi.org/10.3390/chemosensors12030039
APA StyleZhang, Y., Liu, C., Yan, R., & Lei, C. (2024). Bipyridyl Ruthenium-Decorated Ni-MOFs on Carbon Nanotubes for Electrocatalytic Oxidation and Sensing of Glucose. Chemosensors, 12(3), 39. https://doi.org/10.3390/chemosensors12030039





