Detection of Cadmium and Lead Heavy Metals in Soil Samples by Portable Laser-Induced Breakdown Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
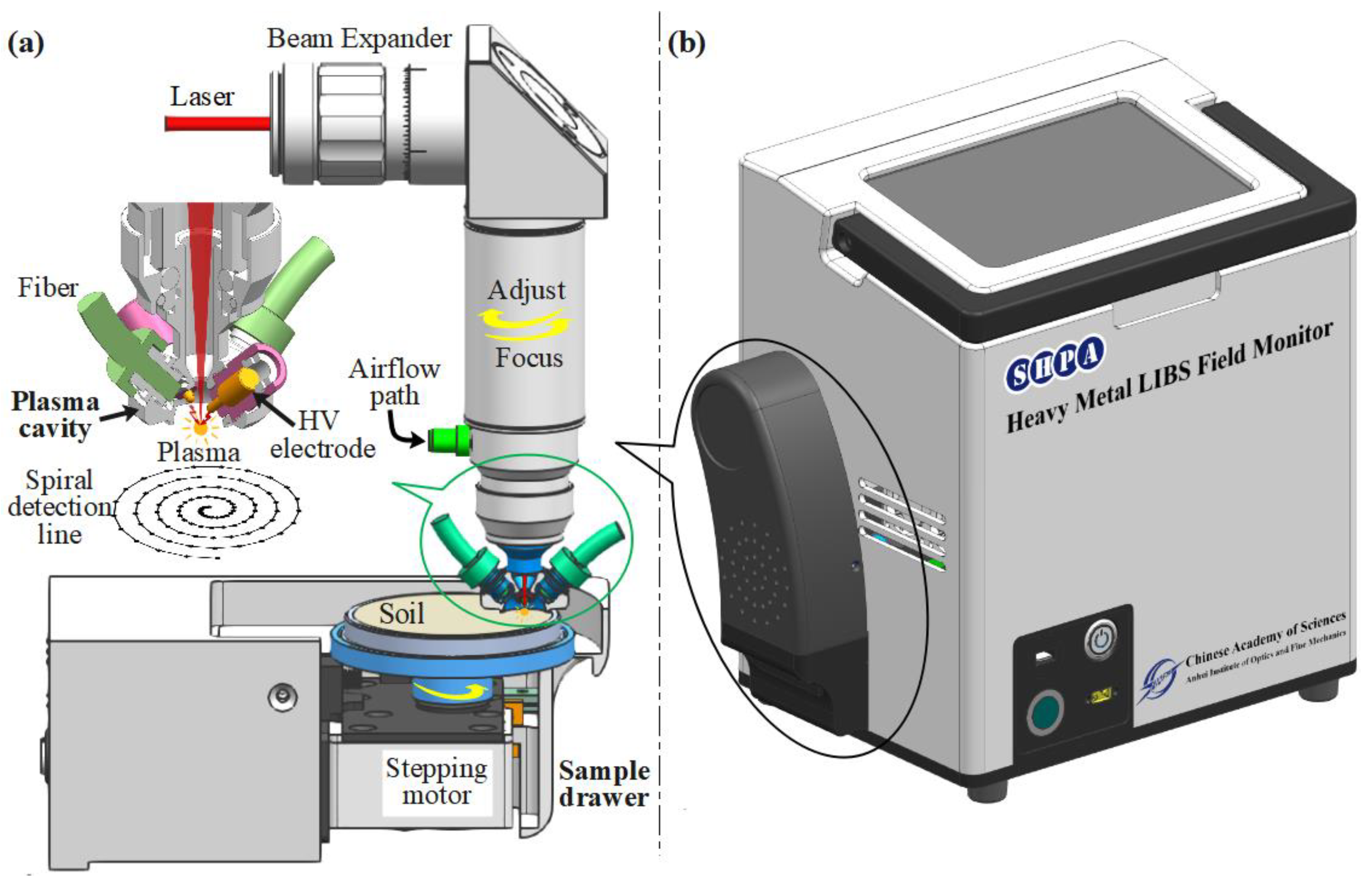
2.1. Experimental Setup
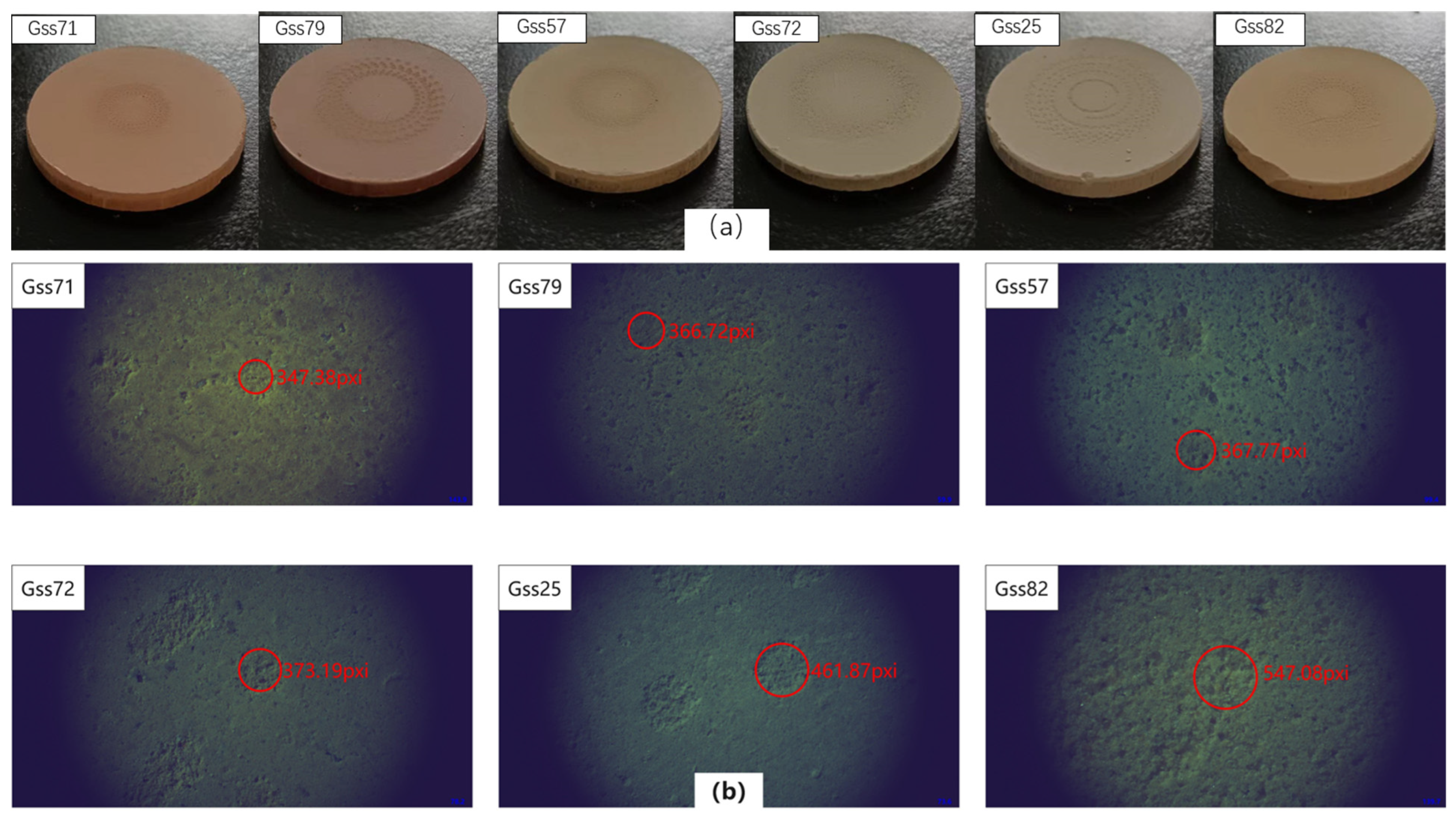
2.2. Sample Preparation
2.3. Influence of Soil Matrix Effects
2.4. Classification and Quantitative Analysis Methods
3. Results and Discussion
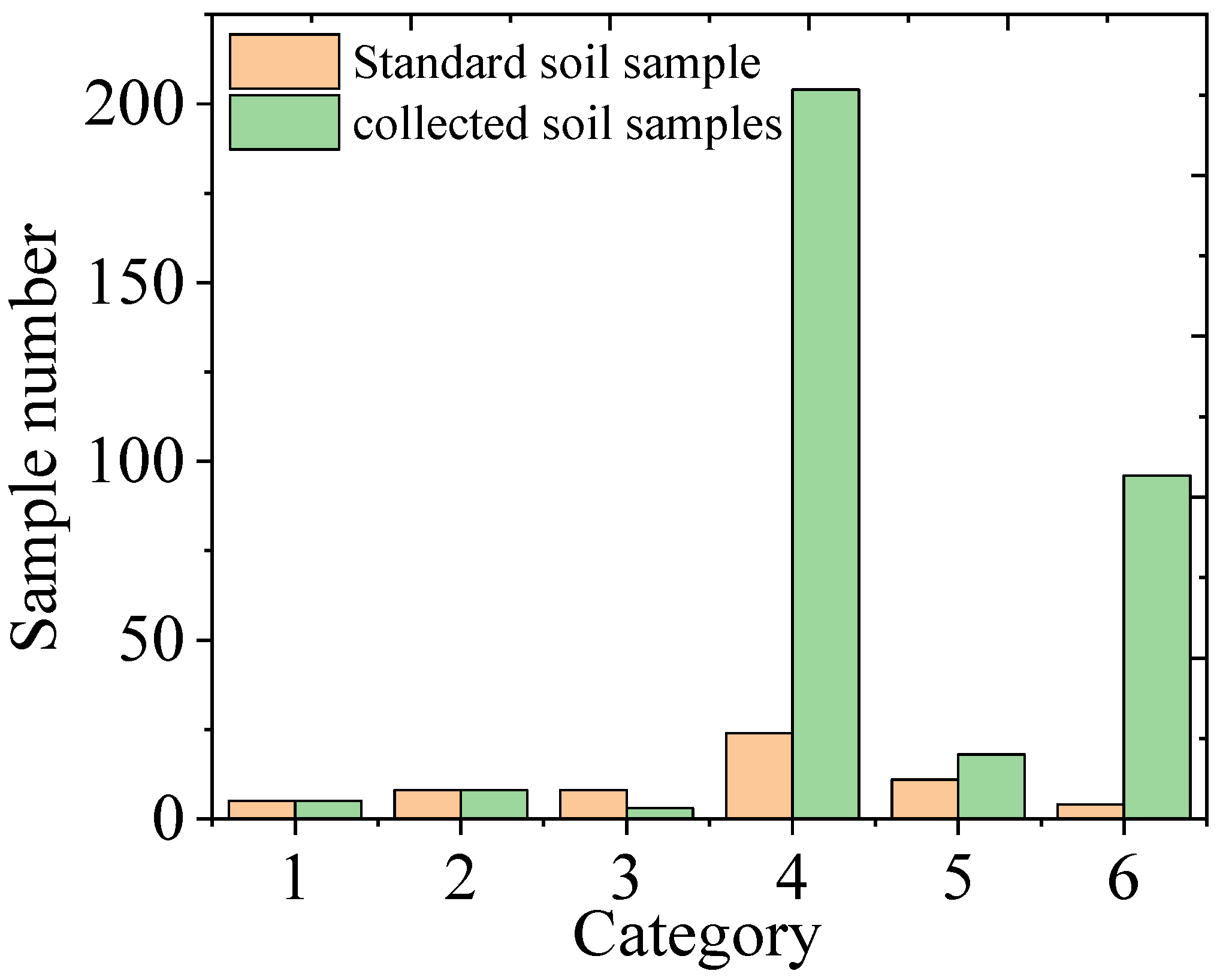
3.1. Soil Classification and Calibration Model
3.2. Classification Results of Soil Samples Collected
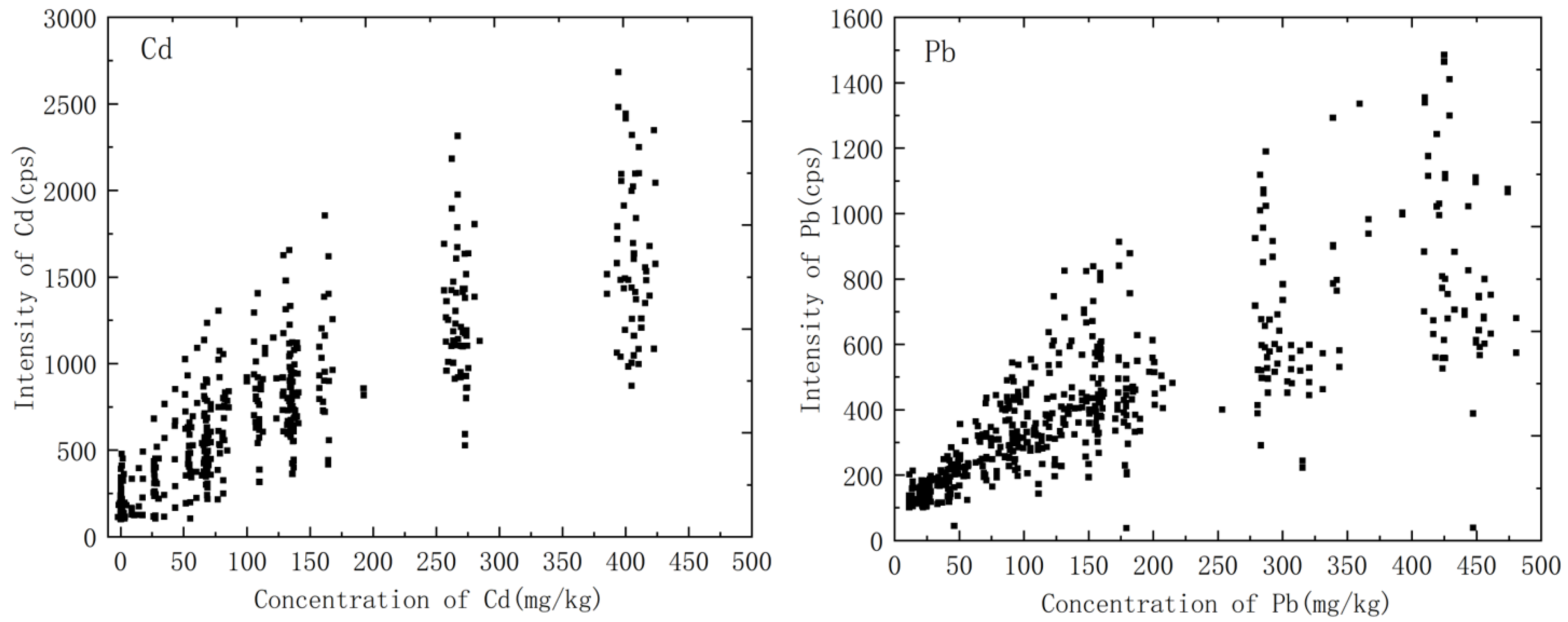
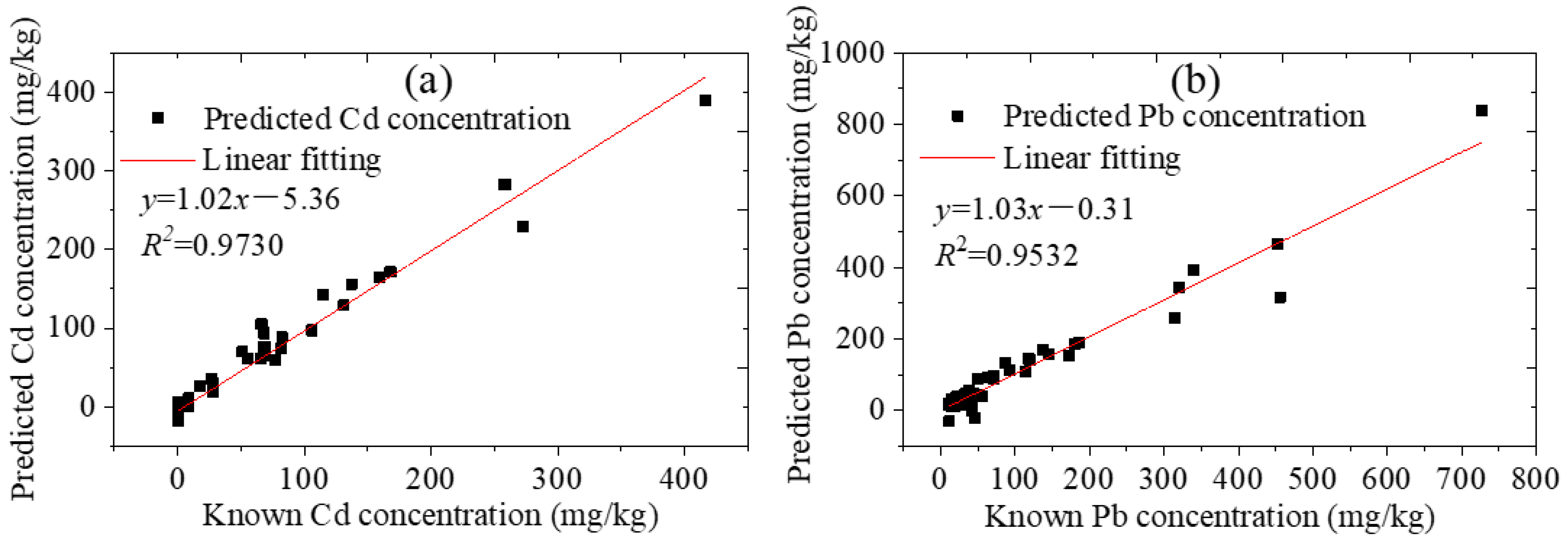
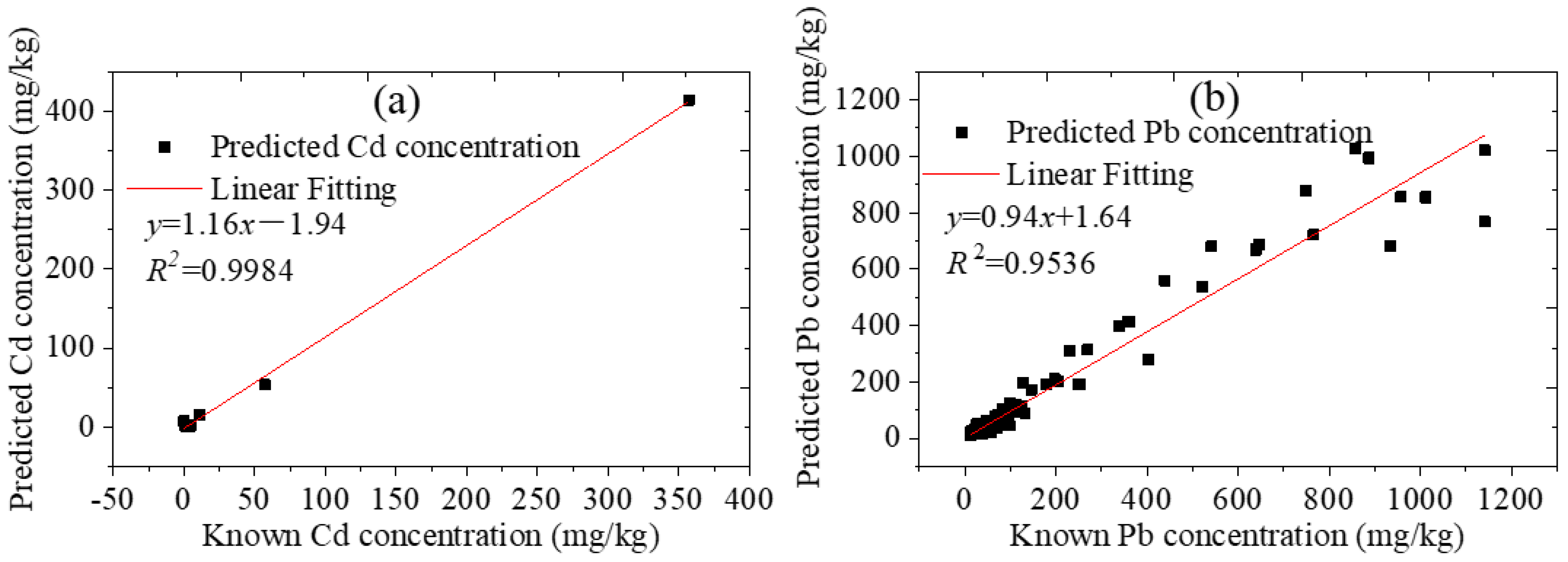
3.3. Quantitative Analysis
3.3.1. The Standard Soil Samples
3.3.2. The Collected Soil Samples
4. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, J.; He, Z.; Xie, S.; Liu, S. Health Risk Assessment of Heavy Metals (Pb, Cd, and Cr) in Farmland Soil of Hubei Province. J. Environ. Hyg. 2019, 9, 258–263. [Google Scholar]
- Gong, D.C.L.; Zheng, H. Determination of Heavy Metals in Soil, Mushroom and Plant Samples by Atomic Absorption Spectrometry. Shandong Chem. Ind. 2020, 49, 101–103. [Google Scholar]
- Li, H.; Gu, W.; Zhao, S.; Yang, M.; Wei, Z.; Zhou, N. Comparison of 6 heavy metal contents in Paris polyphylla Smith var.yunnanensis medicine and their rhizosphere soils from different geographical areas. Environ. Chem. 2021, 40, 2179–2192. [Google Scholar]
- Meng, R.; Du, J.; Liu, Y.; Luo, L.; He, K.; Liu, M.; Liu, B. Exploration of Digestion Method for Determination of Heavy Metal Elements in Soil by ICP-MS. Spectrosc. Spectr. Anal. 2021, 41, 2122–2128. [Google Scholar]
- Qasim, M.; Anwar-Ul-Haq, M.; Afgan, M.S.; Haq, S.U.; Baig, M.A. Quantitative analysis of saindha salt using laser induced breakdown spectroscopy and cross-validation with ICP-MS. Plasma Sci. Technol. 2020, 22, 074007. [Google Scholar] [CrossRef]
- Wang, Z.; Afgan, M.S.; Gu, W.; Song, Y.; Wang, Y.; Hou, Z.; Song, W.; Li, Z. Recent advances in laser-induced breakdown spectroscopy quantification: From fundamental understanding to data processing. Trends Anal. Chem. 2021, 143, 116385. [Google Scholar]
- Yan, C.; Qi, J.; Liang, J.; Zhang, T.; Li, H. Determination of coal properties using laser-induced breakdown spectroscopy combined with kernel extreme learning machine and variable selection. J. Anal. At. Spectrom. 2018, 33, 2089–2097. [Google Scholar] [CrossRef]
- Jull, H.; Künnemeyer, R.; Schaare, P. Nutrient quantification in fresh and dried mixtures of ryegrass and clover leaves using laser-induced breakdown spectroscopy. Precis. Agric. 2018, 19, 823–839. [Google Scholar] [CrossRef]
- Khalil, A.A.I.; Labib, O.A. Detection of micro-toxic elements in commercial coffee brands using optimized dual-pulsed laser-induced spectral analysis spectrometry. Appl. Opt. 2018, 57, 6729–6741. [Google Scholar]
- Zhang, D.C.; Hu, Z.Q.; Su, Y.B.; Hai, B.; Zhu, X.L.; Zhu, J.F.; Ma, X. Simple method for liquid analysis by laser-induced breakdown spectroscopy (LIBS). Opt. Express 2018, 26, 18794–18802. [Google Scholar] [CrossRef]
- Qi, J.; Zhang, T.; Tang, H.; Li, H. Rapid classification of archaeological ceramics via laser-induced breakdown spectroscopy coupled with random forest. Spectrochim. Acta B 2018, 149, 288–293. [Google Scholar] [CrossRef]
- Bhatt, C.R.; Yueh, F.Y.; Singh, J.P. Univariate and multivariate analyses of rare earth elements by laser-induced breakdown spectroscopy. Appl. Opt. 2017, 56, 2280–2287. [Google Scholar] [CrossRef]
- Gao, P.; Yang, P.; Zhou, R.; Ma, S.; Zhang, W.; Hao, Z.; Tang, S.; Li, X.; Zeng, X. Determination of antimony in soil using laser-induced breakdown spectroscopy assisted with laser-induced fluorescence. Appl. Opt. 2018, 57, 8942–8946. [Google Scholar] [CrossRef]
- Mezzacappa, A.; Melikechi, N.; Cousin, A.; Wiens, R.; Lasue, J.; Clegg, S.; Tokar, R.; Bender, S.; Lanza, N.; Maurice, S.; et al. Application of distance correction to ChemCam laser-induced breakdown spectroscopy measurements. Spectrochim. Acta B 2016, 120, 19. [Google Scholar] [CrossRef]
- Khalil, A.A.; Gondal, M.A.; Dastageer, M.A. Detection of trace elements in nondegradable organic spent clay waste using optimized dual-pulsed laser induced breakdown spectrometer. Appl. Opt. 2014, 53, 1709–1717. [Google Scholar] [CrossRef]
- Ge, F.; Gao, L.; Peng, X.; Li, Q.; Zhu, Y.; Yu, J.; Wang, Z. Atmospheric pressure glow discharge optical emission spectrometry coupled with laser ablation for direct solid quantitative determination of Zn, Pb, and Cd in soils. Talanta 2020, 218, 121119. [Google Scholar] [CrossRef]
- Umar, Z.A.; Liaqat, U.; Ahmed, R.; Baig, M.A. Detection of lead in soil implying sample heating and laser-induced breakdown spectroscopy. Appl. Opt. 2021, 60, 452–458. [Google Scholar] [CrossRef]
- Dell’Aglio, M.; Gaudiuso, R.; Senesi, G.S.; De Giacomo, A.; Zaccone, C.; Miano, T.M.; De Pascale, O. Monitoring of Cr, Cu, Pb, V and Zn in polluted soils by laser induced breakdown spectroscopy (LIBS). J. Environ. Monit. 2011, 13, 1422–1426. [Google Scholar] [CrossRef]
- He, Y.; Liu, X.; Lv, Y.; Liu, F.; Peng, J.; Shen, T.; Zhao, Y.; Tang, Y.; Luo, S. Quantitative Analysis of Nutrient Elements in Soil Using Single and Double-Pulse Laser-Induced Breakdown Spectroscopy. Sensors 2018, 18, 1526. [Google Scholar] [CrossRef]
- Rühlmann, M.; Büchele, D.; Ostermann, M.; Bald, I.; Schmid, T. Challenges in the quantification of nutrients in soils using laser-induced breakdown spectroscopy—A case study with calcium. Spectrochim. Acta B 2018, 146, 115–121. [Google Scholar] [CrossRef]
- Erler, A.; Riebe, D.; Beitz, T.; Löhmannsröben, H.-G.; Gebbers, R. Soil Nutrient Detection for Precision Agriculture Using Handheld Laser-Induced Breakdown Spectroscopy (LIBS) and Multivariate Regression Methods (PLSR, Lasso and GPR). Sensors 2020, 20, 418. [Google Scholar] [CrossRef]
- Guo, G.; Niu, G.; Shi, Q.; Lin, Q.; Tian, D.; Duan, Y. Multi-element quantitative analysis of soils by laser induced breakdown spectroscopy (LIBS) coupled with univariate and multivariate regression methods. Anal. Methods 2019, 11, 3013. [Google Scholar] [CrossRef]
- Liu, X.; Liu, F.; Huang, W.; Peng, J.; Shen, T.; He, Y. Quantitative determination of Cd in soil using laser-induced breakdown spectroscopy in Air and Ar conditions. Molecules 2018, 23, 2492. [Google Scholar] [CrossRef]
- Bousquet, B.; Sirven, J.-B.; Canioni, L. Towards quantitative laser-induced breakdown spectroscopy analysis of soil samples. Spectrochim. Acta Part B 2007, 62, 1582–1589. [Google Scholar] [CrossRef]
- Lu, C.; Wang, M.; Wang, L.; Hu, H.; Wang, R. Univariate and multivariate analyses of Sr and V in soil by laser-induced breakdown spectroscopy. Appl. Opt. 2019, 58, 7510–7516. [Google Scholar] [CrossRef]
- Honawadm, S.K.; Chinchali, S.S.; Pawar, K.; Deshpande, P. Soil Classification and Suitable Crop Prediction. In Proceedings of the National Conference on Advances in Computational Biology, Communication, and Data Analytics; 2017; pp. 25–29. [Google Scholar]
- Vrábel, J.; Képeš, E.; Duponchel, L.; Motto-Ros, V.; Fabre, C.; Connemann, S.; Schreckenberg, F.; Prasse, P.; Riebe, D.; Junjuri, R.; et al. Classification of challenging Laser-Induced Breakdown Spectroscopy soil sample data-EMSLIBS contest. Spectrochim. Acta Part B 2020, 169, 105872. [Google Scholar] [CrossRef]

| Laser (DPS-1064-BS-D) | Wavelength (nm) | 1064 |
| Pulse width (ns) | 8 | |
| Divergence (mrad) | 1.5 | |
| Repetition rate (Hz) | 5 | |
| Pulse energy (mJ) | 50 | |
| Spectrometer (AVS-MINI-USB2) | Resolution (nm) | 0.08~0.12 |
| Range (nm) | 200~450 | |
| Acquisition delay time (µs) | 1.25 | |
| Gating time (ms) | 1 |
| Properties | Cd (mg/kg) | Pb (mg/kg) |
|---|---|---|
| Minimum value | 0.03 | 11.30 |
| Maximum value | 422.45 | 1141.13 |
| Mean value | 11.77 | 55.05 |
| Standard deviation values | 50.06 | 89.96 |
| Compactness | Soils |
|---|---|
| Grade 1 | gss7, gss20, gss70, gss71, gss73 |
| Grade 2 | gss63, gss67, gss68, gss69, gss76, gss79, gss80, gss81 |
| Grade 3 | gss1a, gss54, gss56, gss57, gss62, gss64, gss65, gss66 |
| Grade 4 | gss22, gss24, gss32, gss33, gss34, gss36, gss37, gss39, gss40, gss41, gss42, gss43, gss44, gss45, gss46, gss47, gss49, gss59, gss61, gss64, gss72, gss74, gss75, nst2 |
| Grade 5 | gss2, gss3a, gss18, gss25, gss38, gss48, gss50, gss52, gss58, gss60, gss77 |
| Grade 6 | gsf2, gss17, gss78, gss82 |
| Category | Calibration Curve of Cd | R2 | Calibration Curve of Pb | R2 |
|---|---|---|---|---|
| 1 | y = 2.20x + 80.42 | 0.9823 | y = 0.76x + 273.70 | 0.9610 |
| 2 | y = 3.55x + 141.41 | 0.9872 | y = 1.35x + 181.72 | 0.9982 |
| 3 | y = 4.26x + 140.62 | 0.9965 | y = 1.49x + 208.10 | 0.9507 |
| 4 | y = 5.25x + 99.86 | 0.9954 | y = 2.01x + 216.86 | 0.9836 |
| 5 | y = 6.26x + 107.40 | 0.9927 | y = 3.85x + 131.42 | 0.9867 |
| 6 | y = 11.42x + 104.07 | 0.9937 | y = 5.32x + 40.72 | 0.9895 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, M.; Fang, L.; Zhao, N.; Ma, X. Detection of Cadmium and Lead Heavy Metals in Soil Samples by Portable Laser-Induced Breakdown Spectroscopy. Chemosensors 2024, 12, 40. https://doi.org/10.3390/chemosensors12030040
Ma M, Fang L, Zhao N, Ma X. Detection of Cadmium and Lead Heavy Metals in Soil Samples by Portable Laser-Induced Breakdown Spectroscopy. Chemosensors. 2024; 12(3):40. https://doi.org/10.3390/chemosensors12030040
Chicago/Turabian StyleMa, Mingjun, Li Fang, Nanjing Zhao, and Xiaomin Ma. 2024. "Detection of Cadmium and Lead Heavy Metals in Soil Samples by Portable Laser-Induced Breakdown Spectroscopy" Chemosensors 12, no. 3: 40. https://doi.org/10.3390/chemosensors12030040
APA StyleMa, M., Fang, L., Zhao, N., & Ma, X. (2024). Detection of Cadmium and Lead Heavy Metals in Soil Samples by Portable Laser-Induced Breakdown Spectroscopy. Chemosensors, 12(3), 40. https://doi.org/10.3390/chemosensors12030040




