Abstract
Biomedical sensing technology is developing at a tremendous pace and is expected to become an effective clinical tool for the diagnosis and monitoring of human health. The development of sensing devices has successfully transformed the specific sensor prototype designed in the laboratory into a commercially feasible clinical disease detection device. Recently, sensing devices have been accelerated and extended to various fields beyond disease detection, including the measurement of gastrointestinal physiological parameters such as pH, VOC detection, small-molecule gas sensing, and noninvasive screening of oral and lung diseases such as oral cancer, gastric cancer, and other major diseases. In this review, the applications of sensors and electronic nose devices in the diagnosis and monitoring of oral, pulmonary, and gastrointestinal diseases are reviewed, as well as the design and application of sensor materials in disease markers and in situ detection. This article also introduces the practical application of sensing devices in human disease detection, critically analyzes their detection mechanisms and clinical utility, and discusses their future development in medicine. We believe that this review will help readers, especially practitioners in the medical field, provide ideas for the development of sensing devices.
1. Introduction
Sensing technology has gained increasing attention in the biomedical field, especially in the early detection of specific diseases, noninvasive screening, post-treatment evaluation, and so on [1,2,3,4]. With the development of micro/nano fabrication technology, more chemical and biological sensor materials have been developed and used in clinical disease detection [5,6,7,8]. The miniaturization and refinement of sensor devices have attracted the attention of the healthcare industry. With the increase in clinical demand, the sensor detection of disease markers combined with pattern recognition algorithms and analysis software can enable the screening of some human diseases [9,10]. For example, the sudden appearance of an oral odor may be related to periodontal disease [11]. The presence of characteristic biomarkers in saliva may indicate the occurrence of oral cancer [12]. The detection of volatile organic compounds (VOCs) in human exhaled breath can be used to screen for gastric and intestinal diseases [13,14].
To date, medical biochemical detection and image detection equipment has been continuously upgraded, and the diversification and precision of analysis can easily lead to high costs. In addition to the robustness and adaptability of the equipment, the most common disease monitoring methods also need repeated screening steps. In order to achieve miniaturization and convenient and rapid disease detection and screening, the design of chemical and biological sensor technologies, as well as the development of material compositions, remains a significant challenge. Hence, there is a demand for further development of low-cost, simple-principle sensor designs to fulfill the requirements of high precision, ultra-trace sensitivity, rapid response, and high specificity analysis needed for large-scale clinical screening. Extensive, direct, and rapid detection of biological molecules such as proteins, miRNAs, antibodies, enzymes, volatile organic compounds, volatile sulfides, and small molecular gas markers is essential for the early detection of diseases. The research and development of new nanomaterials can also promote the transformation of surface acoustic wave (SAW) sensors, electrochemical sensors, biochemical sensors, and small electronic nose instruments in clinical applications [15,16,17,18,19,20].
A series of sensors and sensing devices have been developed over the past decade for non-invasive detection and diagnosis of oral, gastric, and intestinal-related diseases in humans, such as halitosis, periodontitis, gastritis, gastric cancer, and inflammatory bowel disease.
This review article aims to explore existing sensors and devices for non-invasive screening and diagnosis of oral diseases, lung diseases, and gastrointestinal diseases, evaluate their prospects in new disease diagnostic applications, and elucidate their potential in different diseases. The development process, technical characteristics, and advantages of sensors and devices corresponding to the field. Emphasis is placed on: (1) the analysis of disease symptoms and their detection principles and markers; (2) the provision of sensor devices and sensors related to disease diagnosis; (3) the comparison of the advantages and disadvantages of existing detection methods; and (4) the summary of the challenges in clinical application and the improvement in processing of current sensory devices.
2. Biochemical and Chemical Gas Sensors for the Detection of Oral Disease
2.1. Introduction to Oral Cancer
Oral cancer (OC) is one of the most common malignant tumors globally, with a high incidence in many countries and regions. Smoking, alcohol consumption, betel nut chewing [21], and viral infections [22] are the main contributing factors to oral cancer. Squamous cell carcinoma is the most common type of oral cancer. However, early oral lesions and carcinogenesis often present with mild symptoms, making detection challenging. Consequently, patients may receive delayed treatment, and conventional medical methods may struggle to diagnose, leading to disease progression and mortality [23].
Currently, oral cancer diagnosis primarily relies on various methods: clinical examinations [24] including direct examination and palpation of the oral cavity to detect suspicious lesions and ulcers; imaging examinations [25,26] such as X-rays, CT scans, and MRI to determine tumor size, location, invasion depth, and metastasis; histological examinations [27] involving biopsy to obtain lesion tissues for pathological examination, determining lesion nature, type, and malignancy degree; immunohistochemistry, molecular biology examinations, liquid biopsy [28], etc., are gradually becoming important diagnostic tools.
However, traditional diagnostic methods often involve invasive procedures such as blood sampling or tissue biopsy, combined with imaging modalities to confirm diagnosis. These processes are not only cumbersome but also prone to sample contamination, leading to inaccurate diagnoses. Therefore, the development of non-invasive detection methods and highly sensitive sensors has become a hot topic in early oral cancer detection. The oral cavity, as a vital medium for exchanging substances with the external environment, contains various biomarkers in saliva, including ions, proteins, enzymes, bacteria, etc. [29], which provide information on oral and internal organ diseases. Furthermore, the metabolic activity during oral lesions and carcinogenesis leads to the production of specific gas biomarkers, making oral breath analysis a promising diagnostic method. Corresponding saliva and breath biochemical sensors have been vigorously developed, offering more non-invasive, simple, and efficient detection results. Common oral cancer biomarkers include proteins, miRNAs, VOCs, volatile sulfur compounds (VSCs), etc.
In summary, early diagnosis of oral cancer is crucial. Through the development of advanced non-invasive detection methods and biochemical sensors, we hope to improve the early diagnosis rate of oral cancer, thereby providing patients with more timely and effective treatment, reducing patient suffering, and reducing societal costs.
2.2. Development of Biochemical Sensors for Oral Cancer Markers
Currently, relying solely on pathological examinations for cancer diagnosis has limitations in fully detecting early tumor progression and molecular transformation. To address this clinical need, Weigum et al. [30] developed a cell-based sensor to detect oral cancer biomarkers, such as the epidermal growth factor receptor (EGFR), which is closely associated with the occurrence and invasive cancer phenotype of early oral tumors. The sensor can capture and enrich cells with an embedded track-etched membrane (a micro-sieve) from complex biological fluids or biopsy suspensions. The cell types were then determined by immunofluorescence. These research findings support the cell sensor system as a suitable platform for rapid detection of oral cancer biomarkers and the characterization of EGFR overexpression in malignant oral tumors. Drawing from the aforementioned research, researchers also proposed a nanobiosensor chip (NBC) technology [31] for analyzing oral cancer biomarkers in exfoliated cytology specimens. This technology can monitor biochemical and morphological changes associated with early oral tumor occurrence, providing new possibilities for the early diagnosis of oral cancer.
Malhotra et al. [32] reported an ultra-sensitive electrochemical microarray technology that, after optimization, can measure a four-protein panel of biomarkers in serum and verify whether this protein panel can accurately diagnose oral cancer. The sensor consists of eight microfluidic devices loaded with different antibodies in the channels and uses 400,000 HRP paramagnetic beads to capture proteins in saliva. The bead washing and magnetic separation are used during the off-line capture to lower nonspecific binding, and the actual sample never comes into contact with the measuring sensor array. Their study of serum samples from 78 oral cancer patients and 49 control subjects demonstrated a clinical sensitivity of 89% and a specificity of 98% for oral cancer detection, indicating high diagnostic utility.
Furthermore, Shaikh et al. [33] reported the design and development of a novel pen-shaped handheld device equipped with a miniature tactile sensor at the front end and an integrated portable backend readout module for oral tissue palpation. By obtaining quantitative information about the elasticity and abnormalities of oral lesions, this device can eliminate the need for manual palpation by clinical practitioners. Due to its user-friendliness in general clinical settings, the device holds promise for early detection and improved prognosis of oral cancer.
The carriers of the above-mentioned sensor detection are cells or blood; hence, patients’ blood samples are required. The detection methods based on cells and blood have high accuracy and a strong correlation with diseases, but they are invasive and require in vitro culture. Samples are easily contaminated. The palpation method can easily cause the patient to be uncooperative. Therefore, the development of corresponding sensors tends to be non-invasive saliva sensors and exhaled breath sensors.
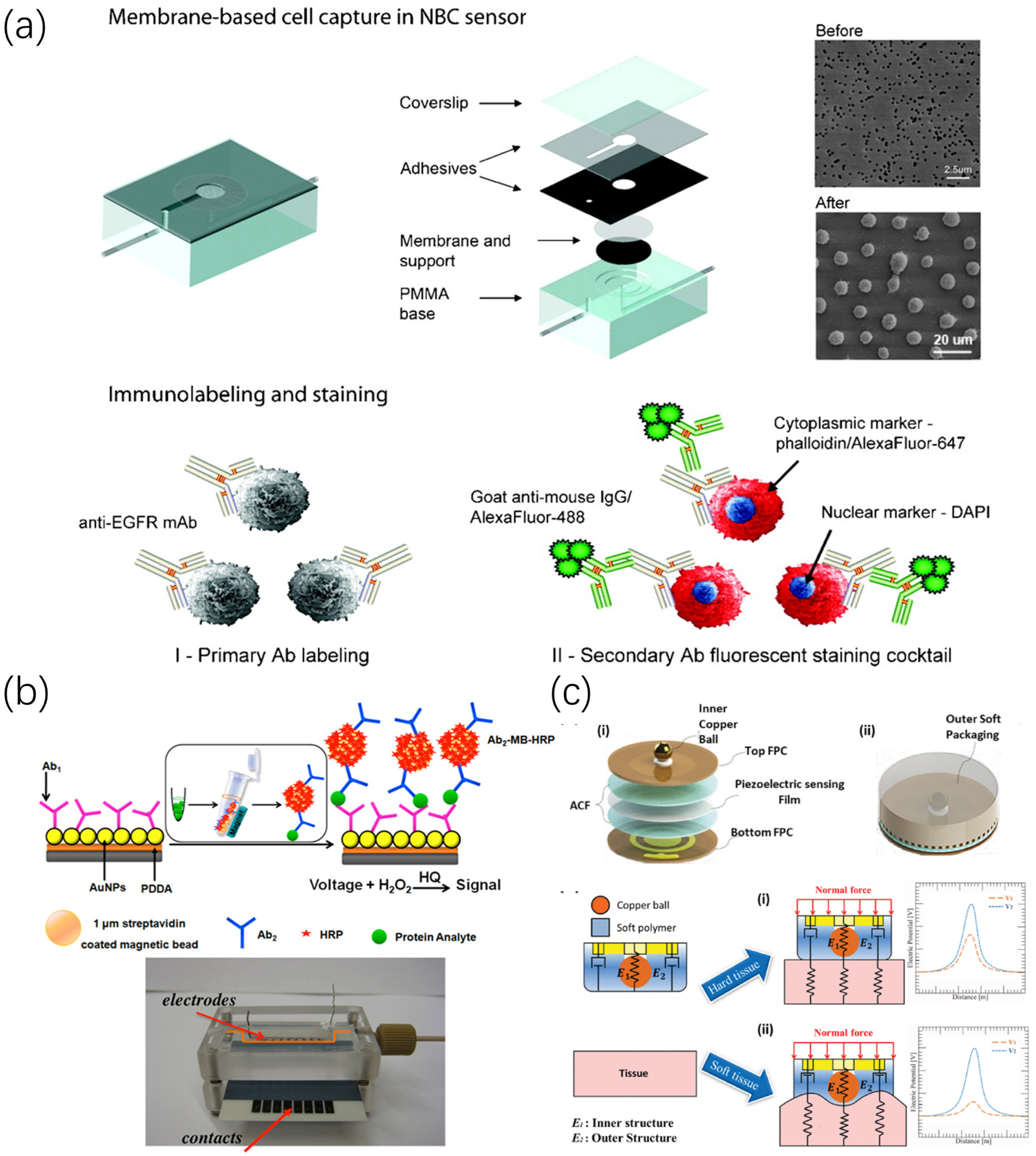
For different biomarkers, researchers have developed various sensors for the detection and early screening of oral cancer. Test samples include oral cells, blood, saliva, and exhaled breath. As shown in Figure 1, a recently developed biosensor for the detection of oral cancer is described.
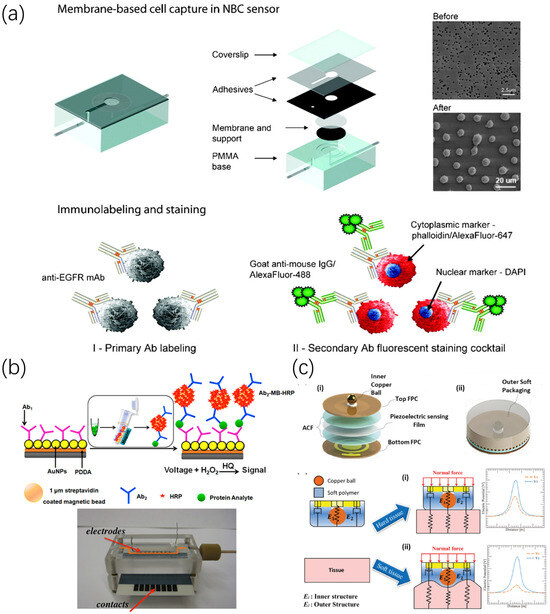
Figure 1.
Biosensor for oral cancer detection. (a) The main steps of a nano-bio-chip sensor to detect oral cells include cell capture, immunolabeling, and staining [31]. (b) Schematic diagram of microfluidic immunoarray sensor detection, which can capture specific protein antibodies in serum samples and perform ultrasensitive current detection [32]. (c) Structure and sensing mechanism of a pen-like tactile sensor for oral palpation [33].
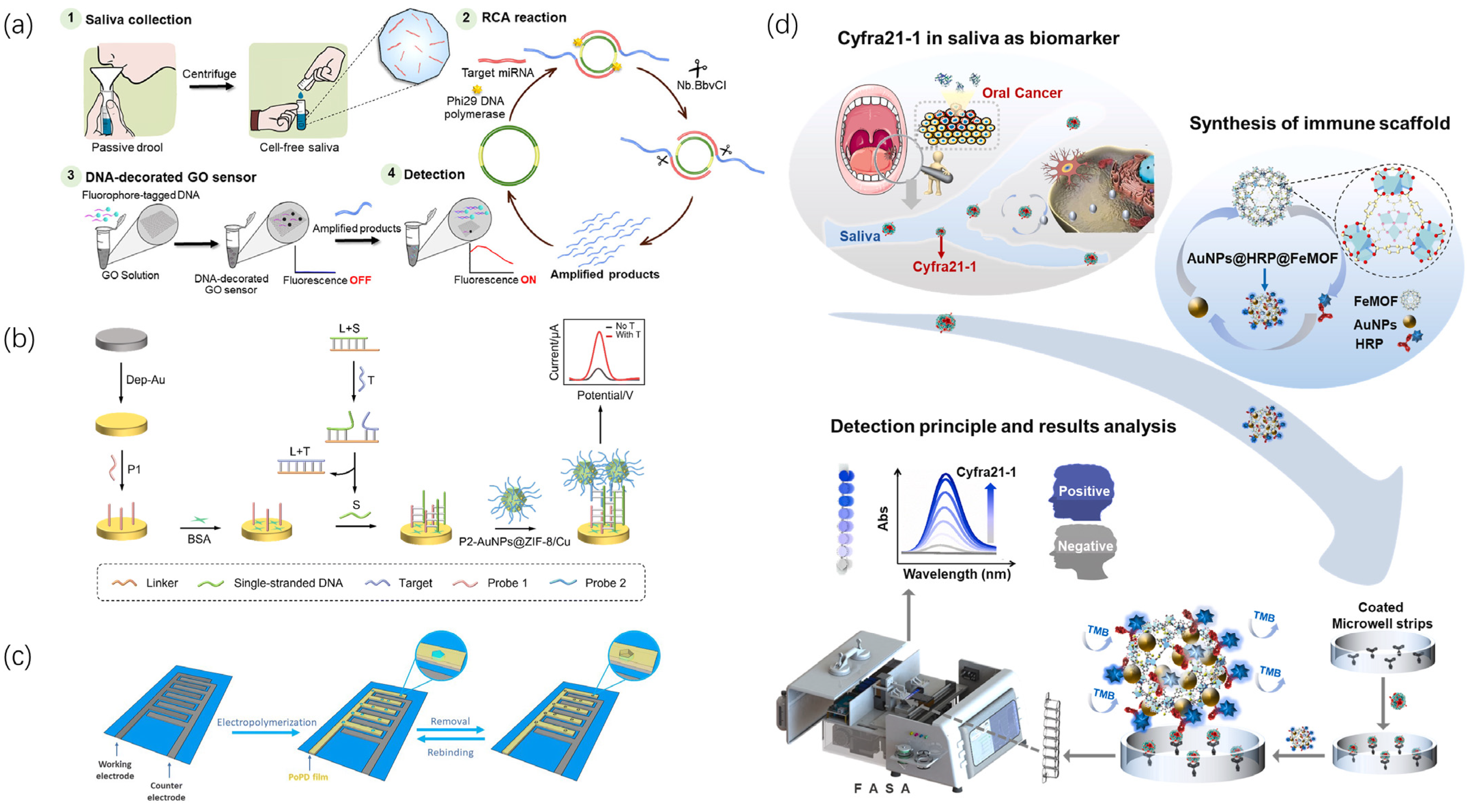
Saliva is easy to obtain and contains rich biological information about diseases; thus, research on biochemical sensors for salivary biomarkers has significantly increased, as shown in Figure 2. Tan et al. [34] studied an optical protein sensor for detecting cancer biomarkers in saliva. The proposed ultra-sensitive optical protein sensor does not require enzyme amplification and can detect IL-8 protein at a concentration of 4 fM in buffer solution. Subsequently, saliva samples were collected to validate the sensor’s performance, and the measurement results of 40 saliva samples were compared, half from oral cancer patients and half from the control group. The sensor detection results highly matched those of ELISA. Pitikultham et al. [35] proposed a novel self-assembly method based on rolling circle amplification (RCA) and graphene oxide (GO) for ultra-sensitive detection of miRNA oral cancer biomarkers—miRNA21 and miRNA16—in saliva. The detection limits were 3.85 fM and 8.81 fM, respectively. The sensor could distinguish one and three mismatched nucleotides in target miRNA, demonstrating high selectivity. Siciliano et al. [36] developed a molecularly imprinted polymer (MIP)-based electrochemical sensor for TGF-β1 detection and its application in liquid biopsy. Using TGF-β1 as the template molecule, a biomimetic surface was synthesized via the polymerization of aniline monomers on a platinum electrode, followed by detection using differential pulse voltammetry. The sensor could be used for target molecule detection in spiked saliva samples, exhibiting high recovery rates and potential for large-scale rapid screening for oral cancer diagnosis. Hu et al. [37] developed a novel electrochemical biosensor based on Cu2+-doped AuNPs@ZIF-8 composite nanomaterials for label-free detection of ORAOV1 in saliva. The sensor demonstrated a wide linear range of 0.1–104 pM and a low detection limit of 63 fM. Kumar et al. [38] developed a ZrO2-RGO-based biosensing platform for non-invasive detection of oral cancer biomarkers. They uniformly decorated nanostructured zirconia on reduced graphene oxide and coated electrodes using electrophoretic deposition. The material-modified electrochemical electrodes exhibited excellent heterogeneous electron transfer properties, and the sensing results were validated using the ELISA method. The sensor could sensitively detect the oral cancer biomarker CYFRA-21-1 in saliva for a non-invasive early diagnosis of oral cancer. Wang et al. [39] proposed a quantum dot nanofluorescent sensor based on microfluidics for on-site visualization and detection of Cd2+ biochemical markers in saliva. Based on this, they developed an AuNPs@HRP@FeMOF immunoscaffold with multiple functions, such as antibody carrier, catalytic activity, and signal amplification, for detecting the oral cancer biomarker Cyfra21-1. It could achieve highly sensitive detection of biomarkers without requiring saliva samples, thereby distinguishing oral cancer patients. For salivary miRNA biomarkers, Wang et al. designed a simple droplet microfluidic fluorescence sensor based on the CRISPR-Cas13a system. The sensor achieves highly sensitive digital quantification of individual untagged miRNA molecules without reverse transcription or sequence amplification. Designing different Cas13 for different miRNA molecules holds promise for combined miRNA detection to enhance its screening capabilities for oral cancer and other diseases.
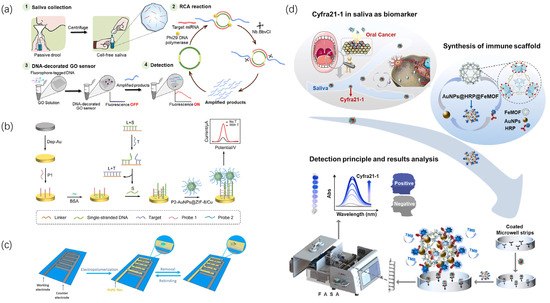
Figure 2.
Biochemical sensor for detecting oral cancer markers in saliva. (a) The fabrication process and sensing mechanism of a DNA-modified graphene oxide sensor; samples are collected and amplified through the RCA reaction [35]. (b) The nanocomposite of AuNPs@ZIF-8/Cu-based construction process of a label-free electrochemical sensor and DNA strand displacement reaction for ORAOV 1 detection [37]. (c) Fabrication of TGF-β1 MIP on primary platinum microelectrodes patterned on glass substrate receptors and detection of TGF-β1 in saliva samples through the fabricated MIP electrochemical sensor [36]. (d) Schematic diagram of Cyfra21-1 detection based on AuNPs@HRP@FeMOF immunoscaffold and FASA [40].
Research on detecting oral cancer through exhaled biomarkers has begun to develop in recent years, but the corresponding achievements are relatively few compared to salivary biomarkers. Korde et al. [41] found that nitric oxide (NO) plays a significant role in various stages from the initiation to the progression of oral cancer, serving as a validating biomarker to estimate the cancer risk in precancerous patients and smokers. Kwon et al. [42] compared the exhaled breath of oral squamous cell carcinoma (OSCC) patients and healthy controls using a gas chromatography system and found that the concentrations of hydrogen sulfide (H2S) and methyl mercaptan (CH3SH) in the exhaled breath of the OSCC group were significantly higher than those in the healthy group. Meanwhile, the total sulfur concentration in the exhaled breath samples of the OSCC group was also higher, but there was no significant difference in the ratio of CH3SH to H2S between the two groups. The researchers constructed a new variable with an area under the curve (AUC) of 0.740, a sensitivity of 68.0%, and a specificity of 72.0%, proving the feasibility of detecting VSCs in exhaled breath as a non-invasive method for oral cancer diagnosis. Bouza et al. [43] collected breath samples from 26 OSCC patients and 26 healthy controls and analyzed the samples using solid-phase microextraction and gas chromatography-mass spectrometry. Multiple VOCs were identified as breath markers for OSCC, and the linear discriminant analysis (LDA) results showed clear clustering. Xie et al. [44] demonstrated accurate diagnosis of oral cancer using surface-enhanced Raman scattering (SERS) of exhaled breath with plasmonic metal-organic framework (MOF) nanoparticles based on artificial intelligence (AI). These plasmonic MOF nanoparticles could capture the oral cancer biomarker methyl mercaptan in exhaled breath, generating distinct SERS spectra. Finally, the spectra were trained and classified using an artificial neural network (ANN) model with an accuracy of 99%.
2.3. Halitosis and Periodontal Diseases
Halitosis is a disease in which the mouth emits an unpleasant odor [45] and is relatively common. Liu et al. [46] surveyed 2000 people in China between the ages of 15 and 64 years old and found that the prevalence of halitosis was 27.5%. Yu et al. [47] surveyed 372 young people in Dunedin, New Zealand, between the ages of 18 and 30 years old and found that 31.2% of them had halitosis, which was characterized by the presence of VSCs. Teshome et al. [48] recruited 661 subjects in northwestern Ethiopia and found the prevalence of halitosis to be 44.2% upon diagnosis. Although there are some differences in the prevalence of halitosis in these findings, they all indicate that halitosis is a relatively common phenomenon. Halitosis can affect people’s normal social lives and even have a serious psychological impact on the patient. Halitosis can be categorized into the following three types: true halitosis, pseudo-halitosis, and halitophobia [49]. True halitosis refers to a noticeable oral odor that adversely affects a person’s life and may require medical treatment; pseudo-halitosis refers to the patient’s belief that he or she has halitosis but no one else can smell it; and halitophobia is a condition in which the patient insists that he or she suffers from halitosis, even after treatment for either true or pseudo-halitosis and after the patient is clinically certified as not having halitosis [49].
True halitosis can be categorized into orogenic halitosis (caused by oral diseases) and non-oral halitosis (caused by extra-oral diseases), and some studies have confirmed that 80–90% of the causes of true halitosis originate from oral diseases [50]. This article focuses on orogenic halitosis. Researchers have long been interested in the question of whether there is a relationship between periodontal disease and halitosis, and more studies have been conducted to show a strong relationship between orogenic halitosis and periodontal disease [51]. In 2023, Lee et al. [52] investigated the incidence of halitosis in 104 participants (33 healthy controls, 43 patients with gingivitis, and 28 patients with periodontitis) using gas chromatography (GC) to detect representative VSCs in exhaled breath, namely hydrogen sulfide (H2S) and methyl mercaptan (CH3SH). The prevalence of halitosis was found to be significantly higher in patients with periodontal disease than in healthy controls (p = 0.005), whereas 53.1% of participants with halitosis had gingivitis, 37.5% had periodontitis, and 90.6% had periodontal disease. The relationship between halitosis and periodontal disease is mainly established through a number of microorganisms that can produce VSCs, and the moist environment and temperature of about 37 °C in the oral cavity are very suitable for bacterial reproduction as well as efficient metabolism of sulfur-containing amino acids (e.g., cysteine, methionine, etc.), which leads to the production of VSCs [53]. The main components of oral odor in patients with halitosis are VSCs, of which H2S, CH3SH, and a relatively small amount of dimethyl sulfide account for 90% of the VSCs in halitosis [11]. H2S and CH3SH contribute to the penetration of lipopolysaccharides into the epithelium of the gingiva, which can lead to inflammation [54]. Therefore, VSCs (mainly H2S and CH3SH) can be used as biomarkers for the early diagnosis of bad breath and periodontal disease.
2.4. Chemical Gas Sensors for the Early Diagnosis of Orogenic Halitosis and Periodontal Diseases
Continuous monitoring of VSCs in human breath is important for oral disease prevention as well as the early diagnosis of related oral diseases. However, fabricating gas sensors with high selectivity and sensitivity remains a challenge. Based on the literature research, it has been found that in recent years, the main focus on detecting VSCs in human exhaled gas has been on H2S and CH3SH, especially H2S. In Table 1, we list the chemical gas sensors that have been used to detect H2S or CH3SH in recent years.

Table 1.
Sensors for detecting oral diseases.
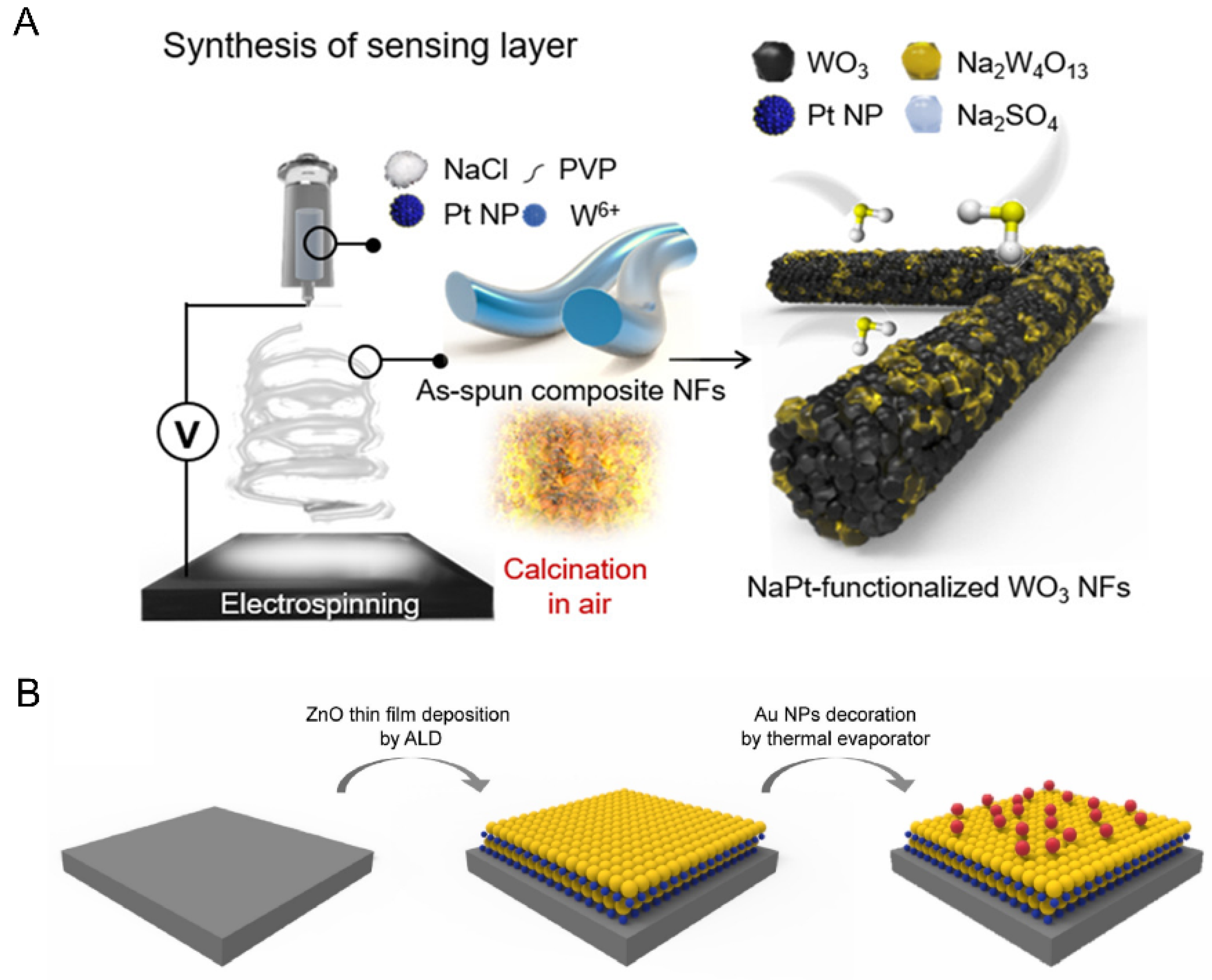
In 2021, Shin et al. [55] proposed a chemoresistive-based H2S sensor, which offers the possibility of direct, reliable, and rapid detection of H2S in real human exhaled gases. Shin et al. modulated the gas-sensing properties of WO3 nanofibers (NFs) by adding sodium chloride and platinum nanoparticles (Pt NPs) during the electrostatic spinning process (Figure 3A) to improve their sensitivity and selectivity for the detection of H2S. The improved gas-sensing performance is attributed to the electronic sensitization effect of the Na2W4O13 phase and the chemical sensitization effect (spillover effect) of the Pt NPs. They tested 80 patients using a homemade gas sensing system, compared the results with actual H2S concentrations measured by an OralChroma gas chromatography system, and found an accuracy of 86.3%, demonstrating that the sensitized material has the potential for real-time human respiratory monitoring for the prevention and early diagnosis of halitosis and oral diseases.
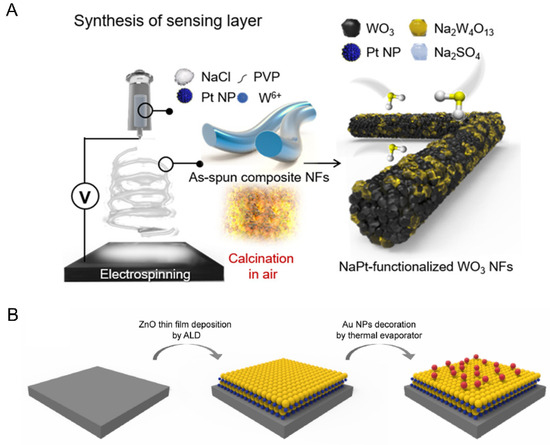
Figure 3.
(A) Schematic illustrations of Na/Pt cosensitized WO3 nanofibers [55] synthesis. (B) Schematic illustration of the Au NPs-incorporated ZnO hybrid nanofilms [61].
In 2022, in order to perform early diagnosis of periodontitis, Bae et al. [61] designed an Au NPs-ZnO hybrid gas sensor capable of selectively and accurately differentiating ppb-level CH3SH in VSCs, and the limit of detection is 50 ppb (Figure 3B). ZnO has excellent gas-sensing properties and has great potential for application in the field of chemically resistive gas sensors, and the gas-sensing performance can be further improved by the surface design of the synergistic hybridization with Au NPs, which can simultaneously improve the selectivity of CH3SH and the gas response of ppb-level VSC gases. Bae et al. experimentally demonstrated that this sensor can reliably detect (due to its high selectivity and reproducibility) low concentrations of CH3SH and can be used for exhaled gas monitoring in periodontal patients.
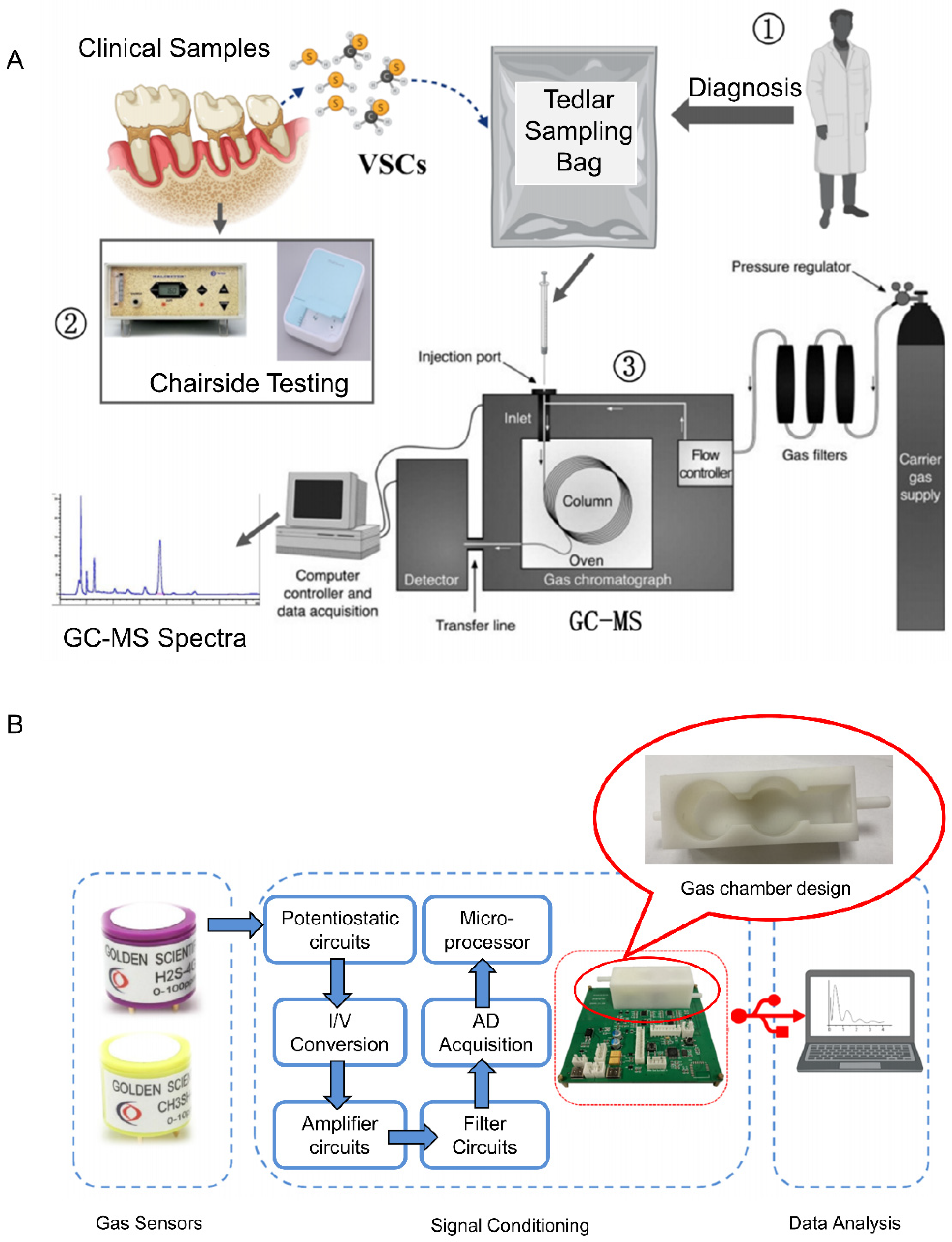
Research on electronic noses for breath-based halitosis detection and disease screening has gained high popularity internationally. H2S and CH3SH are highly reactive and have extremely low concentrations in human exhaled gases. Zhang et al. [71] collected breath samples using a chemically stable Tedlar sampling bag and analyzed VSCs in exhaled gas qualitatively and quantitatively based on gaschromatography-mass spectrometry (GC-MS) technology using single-ion detection scanning mode and an external standard method. They established a standardized methodology and procedure for breath sample collection as well as the detection of VSCs in the oral cavity by GC-MS, as shown in Figure 4A. Based on the results of GC-MS measurements, electrochemical sensors with the required range, sensitivity, resolution, and specificity were selected as the core detection elements of the e-nose, and the gas chamber and gas circuit structure of the sensor array were designed on this basis to develop an e-nose system for the detection of halitosis markers and disease screening based on the exhaled gas (Figure 4B). The basic performance of the e-nose was tested, and the results showed that the e-nose had excellent repeatability and linearity and good detection limits of 39 ppb and 48 ppb for hydrogen sulfide and methyl mercaptan, respectively. A one-dimensional convolutional neural network-based quantitative algorithm model for VSCs was also constructed, and the results showed that this e-nose discriminated between patients with orofacial diseases and healthy individuals with a sensitivity of 87.5%, a specificity of 72.7%, and an overall accuracy of 81.5%.
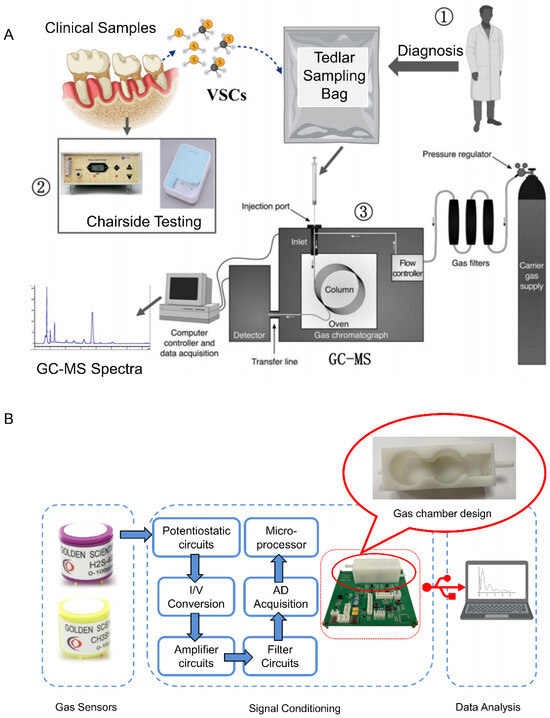
Figure 4.
(A) Schematic diagram of the breath sample collection and testing process [71]. (B) Schematic diagram of the electronic nose system [71].
3. Surface Acoustic Wave Sensor (SAW) for Detecting Lung Diseases
3.1. Introduction to Lung Diseases
Lung diseases refer to various conditions affecting the structure and function of the lungs [72]. These diseases can include lung infections [73] (such as pneumonia and tuberculosis), chronic obstructive pulmonary disease [74] (COPD), asthma [75], lung tumors [76] (such as lung cancer), pulmonary hypertension [77], pulmonary embolism [78], and others. Lung diseases may result in symptoms such as difficulty breathing, coughing, phlegm production, chest pain, and fatigue, among others, and may significantly impact the quality of life for affected individuals [79]. Early diagnosis and treatment are crucial in managing the progression of lung diseases [80]. Early screening for lung diseases can help in detecting conditions at their nascent stages when they are more amenable to treatment [81]. Screening methods may include imaging tests like chest X-rays or CT scans, pulmonary function tests, and other diagnostic procedures [82]. Additionally, research has shown that biomarker detection in biological samples such as blood, exhaled breath, condensate, or saliva can provide valuable insights into lung disease pathology and aid in early detection [83]. These biomarkers can include specific proteins, genetic markers, or metabolic byproducts associated with various lung conditions [84].
3.2. Surface Acoustic Wave Sensor
The surface acoustic wave (SAW) sensor is a new type of microacoustic sensor developed in recent years and has been widely used in the field of biosensing, especially for early screening of lung diseases. The SAW sensor is based on the principle of the piezoelectric effect and is sensitive to surface mass deposits, which affect the propagation of the acoustic wave. The measured information can be quickly converted into electrical signal output by changes in the frequency, phase, or amplitude of the surface acoustic wave (Figure 5A) [85]. Therefore, the SAW sensor has the characteristics of real-time information detection. Besides, the SAW sensor can concentrate the signal on the surface of the substrate and operate at high frequencies. It has strong compatibility with integrated circuits and has the advantages of miniaturization, integration, passive, low cost, low power consumption, and direct frequency signal output.
SAW sensors can operate on either Rayleigh or shear-level (SH) waves, and different types of surface acoustic waves have different characteristics. The Rayleigh wave is one of the most widely used surface acoustic waves and is often used for gas detection. The earliest surface acoustic wave sensors used to identify VOCs in the exhaled gas of lung cancer patients were polymer-coated SAW sensors with a frequency change response, used in conjunction with data processing for ANN algorithms [86]. Since the SAW sensor itself is not selective for VOCs in exhaled gas, gas chromatography separation technology was introduced to separate VOCs with different boiling points and polarities. Gases with different physical and chemical properties can quickly condense on the surface of the SAW sensor in sequence, causing the sensor’s resonant frequency to shift. Such a system can differentiate between hundreds of different gases.
SAW sensors, utilized for the detection of diseases, typically operate in conjunction with other components rather than being employed as standalone devices. As shown in Figure 5B, Chen et al. further proposed using the imaging recognition method to identify 11 VOCs in the exhales gas of lung cancer patients based on a kind of virtual array of SAW gas sensors [87]. Similarly, Wang and colleagues described a Rayleigh wave-type SAW gas sensor with a gas chromatography interface to detect lung cancer-specific respiratory VOCs [88,89,90]. In 2011, Wang et al. described an uncoated surface acoustic wave resonator (SAWR) sensor, which has higher sensitivity and stability, lower noise, a longer service life, and shows great potential for breath diagnosis [91]. On the basis of the previous results, Wang’s team proposed to combine MOS sensors and SAW sensors (Figure 5C) to build a hybrid lung cancer diagnosis electronic nose (HENS). The MOS gas sensor is used to detect low-molecular-weight VOCs, and the SAW sensor is used to detect higher-molecular-weight VOCs. Compared with a single sensor, such a combination can detect more VOCs on the one hand and, on the other hand, has higher sensitivity, which is more conducive to improving the accuracy of the later algorithm model. They tested the breath samples of 42 healthy people and 47 lung cancer patients and obtained a lung cancer screening sensitivity of 93.62% and a specificity of 83.37% [92]. In 2016, a novel detecting device based on an oxidized graphene SAW gas sensor was presented to detect a lung cancer-related biomarker, decane, in parts per million (ppm) concentrations [93]. In the aadvancement of high-sensitivity SAW sensors, the selection of vapor-sensitive materials is a critical and meticulously considered aspect. A considerable amount of research has been carried out to investigate sensitive materials in SAW sensors for the detection of VOCs, which are concurrently regarded as potential biomarkers.
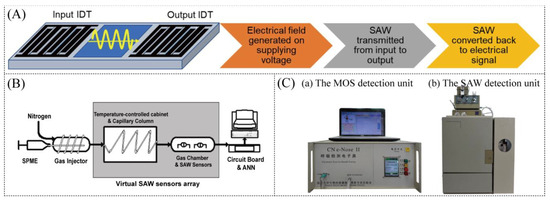
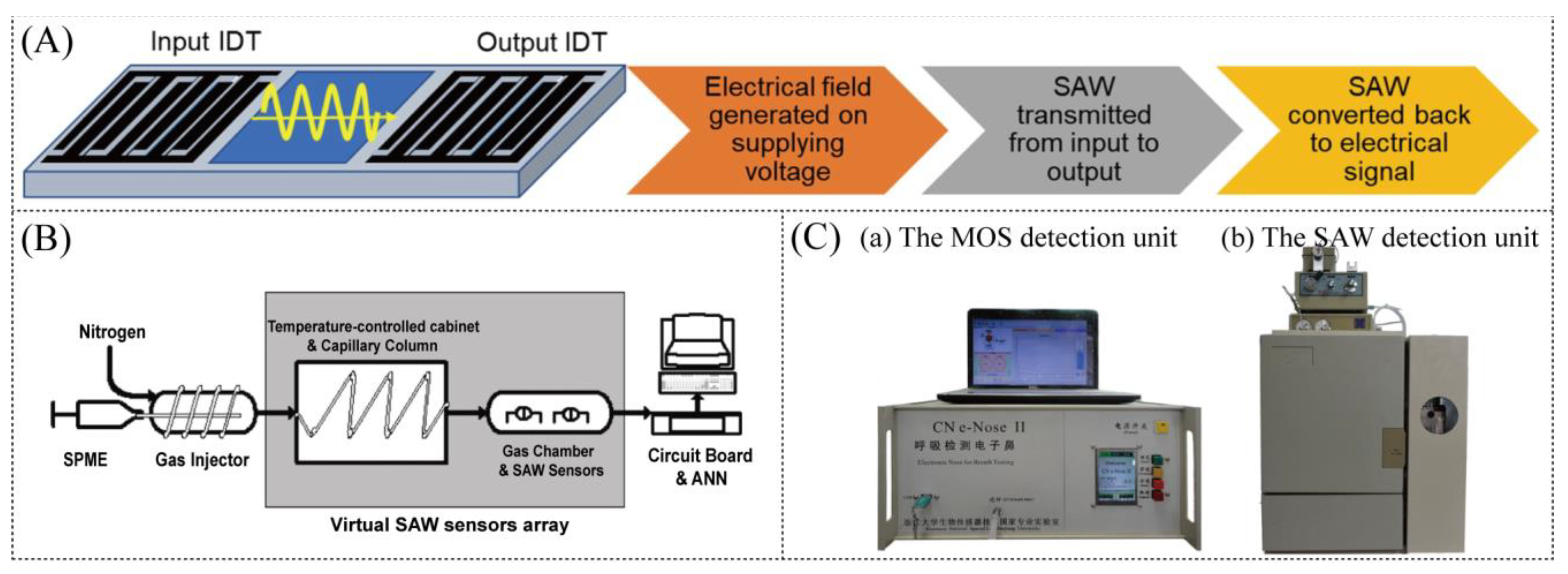
Figure 5.
(A) Sensing mechanism for detection using the SAW sensor [85]. (B) Diagram of the electronic nose system [87]. (C) Detection units of the HENS [92].
Figure 5.
(A) Sensing mechanism for detection using the SAW sensor [85]. (B) Diagram of the electronic nose system [87]. (C) Detection units of the HENS [92].
3.3. Detection of Lung Disease Markers
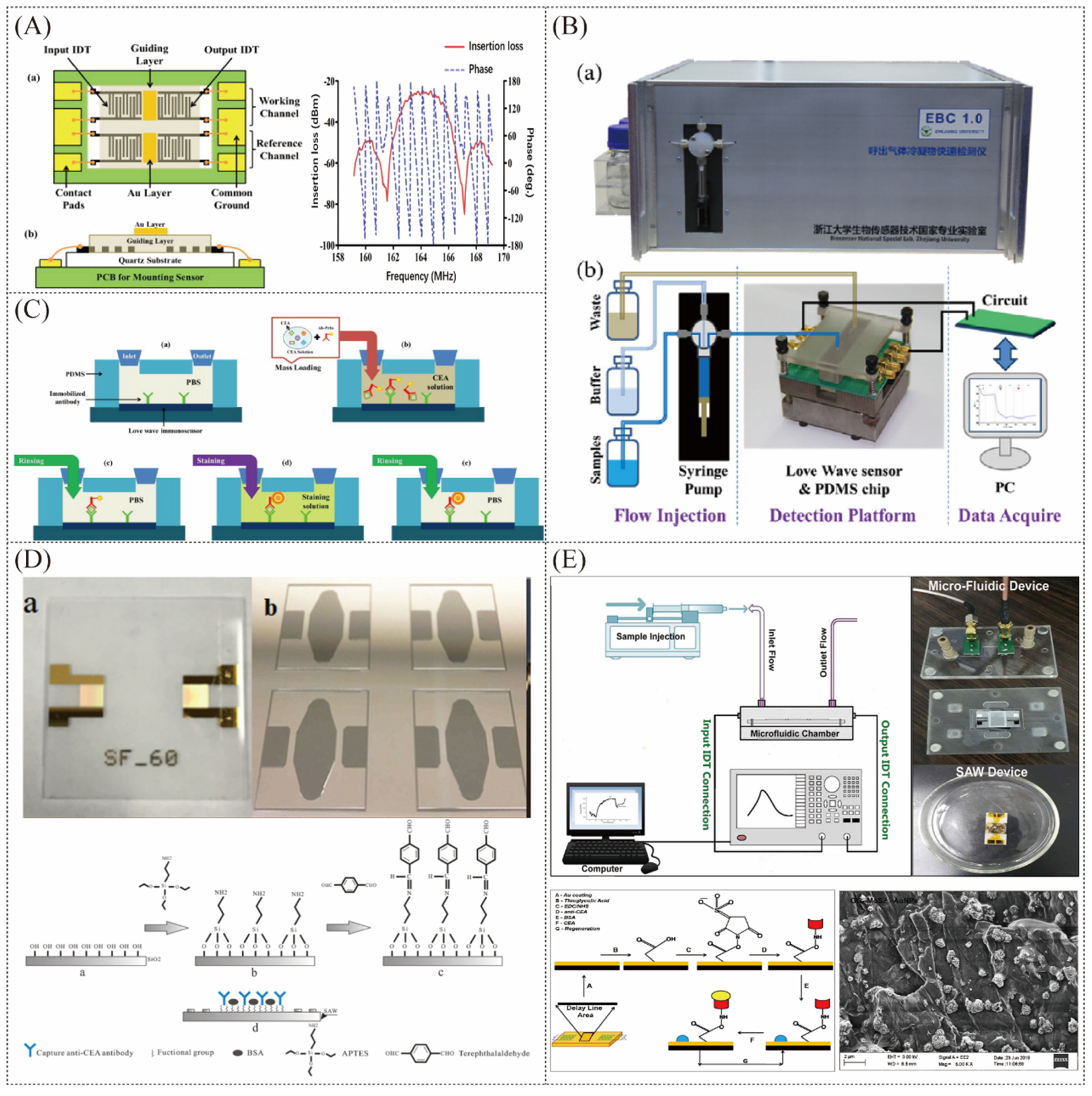
Horizontal shear wave-type SAW sensors are often used when detecting liquid samples because Rayleigh waves are easily dissipated in the liquid on the sensing surface. Among them, the Love Wave sensor further improves the sensitivity of detecting disease markers in liquid samples by adding a waveguide layer above the interdigital electrodes to gather sound wave energy (Figure 6A). In addition, the interdigital electrodes can be isolated from the liquid sample through the waveguide layer, making them less susceptible to corrosion [94]. Zhang et al. developed the Love-SAW immunosensor (Figure 6B) to detect carcinoembryonic antigen (CEA), neuron-specific enolase (NSE), and squamous cell carcinoma antigen (SCC) in exhaled breath condensate (EBC) collected from 17 patients with lung cancer and 13 healthy volunteers [95]. As shown in Figure 6C, the conventional sandwich immunoassay is employed, and the quality signal is double amplified by modifying AuNPs on the detecting antibody (Ab2) and adding gold staining solution. The sensitivity of the Love-SAW sensor to detect CEA, SCC, and NSE was 0.4798°/(ng/mL), 0.3941°/(ng/mL), and 1.1876°/(ng/mL), and the detection limits were 0.967 ng/mL, 1.598 ng/mL, and 0.663 ng/mL, respectively [96]. Later, similar studies achieved a minimum LOD of as low as 37 pg/mL for CEA detection [97]. In 2017, Li et al. modified the antibodies directly on the silicon oxide waveguide layer rather than through the gold layer (Figure 6D), reducing the influence of the mass deposition of the gold layer and thus achieving the lowest detection limit of CEA at present [98]. In 2020, Zou et al. tried to use aptamers to detect the carcinoembryonic antibody CEA in the exhaled gas condensate of lung cancer patients using a similar approach to the previous system, but obtained the limit of detection of CEA at about 1 ng/mL [99]. As shown in Figure 6E, Jandas et al. developed a SAW-based biosensor for the real-time label-free detection of CEA using a novel preparation method of anti-CEA SAM bioreceptor, obtaining the limit of detection of CEA at 0.31 ng/mL [100]. In addition, they used AuNPs-MoS2-rGO nanocluster-doped polyimide nanocomposite, which further lowered the limit of detection of CEA to 0.084 ng/mL [101], but still higher than the detection limit of the sensor without the gold layer. In 2022, Zhang et al. engineered a novel apparatus utilizing a Love Wave biosensor, with the investigation noting that this instrument facilitates multi-channel expedited detection of a spectrum of biomarkers associated with bacterial pneumonias through the analysis of human exhaled breath condensate (EBCs). The sensor integrated within the apparatus demonstrates a commendable sensitivity towards C-reactive protein (CRP) [102].
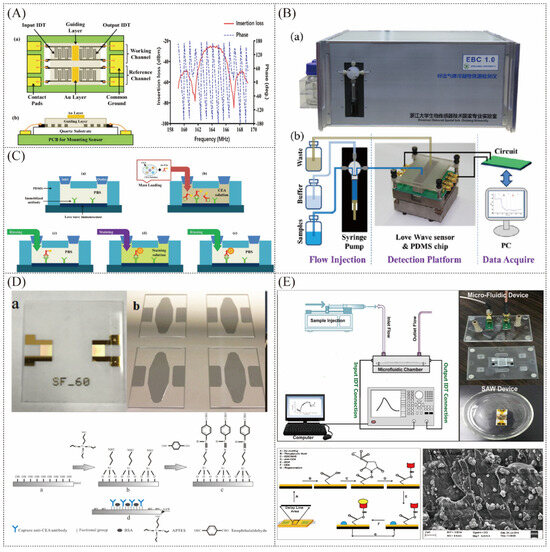
Figure 6.
(A) Love Wave sensor chip [95]. (B) Pictures of the instrumental platform for integration of the Love Wave immunosensor [95]. (C) Schematic diagram of the immunoassay process using gold staining solution to amplify the signal [95]. (D) The Love Wave chip without the top gold layer and processing steps of surface modification [98]. (E) Love-mode SAW biosensor for CEA detection using a self−assembled monolayer bioreceptor and AuNPs−MoS2−rGO [100,101].
4. Sensors and an Electronic Nose for Detecting Gastrointestinal Diseases
4.1. Gastrointestinal Diseases
Gastrointestinal (GI) diseases have a high prevalence in China. The main manifestations of gastrointestinal diseases are abdominal distension, diarrhea, abdominal pain, gastrointestinal bleeding, malabsorption, and weight loss. Nowadays, the incidence of gastrointestinal diseases is getting higher and higher with the change of lifestyle and the acceleration of the pace of life, and gastrointestinal diseases often recur. Existing methods of diagnosing many gastrointestinal diseases rely primarily on invasive, expensive, and time-consuming techniques such as colonoscopy and endoscopy [103].
4.2. Gastric Disease Sensing and Detection
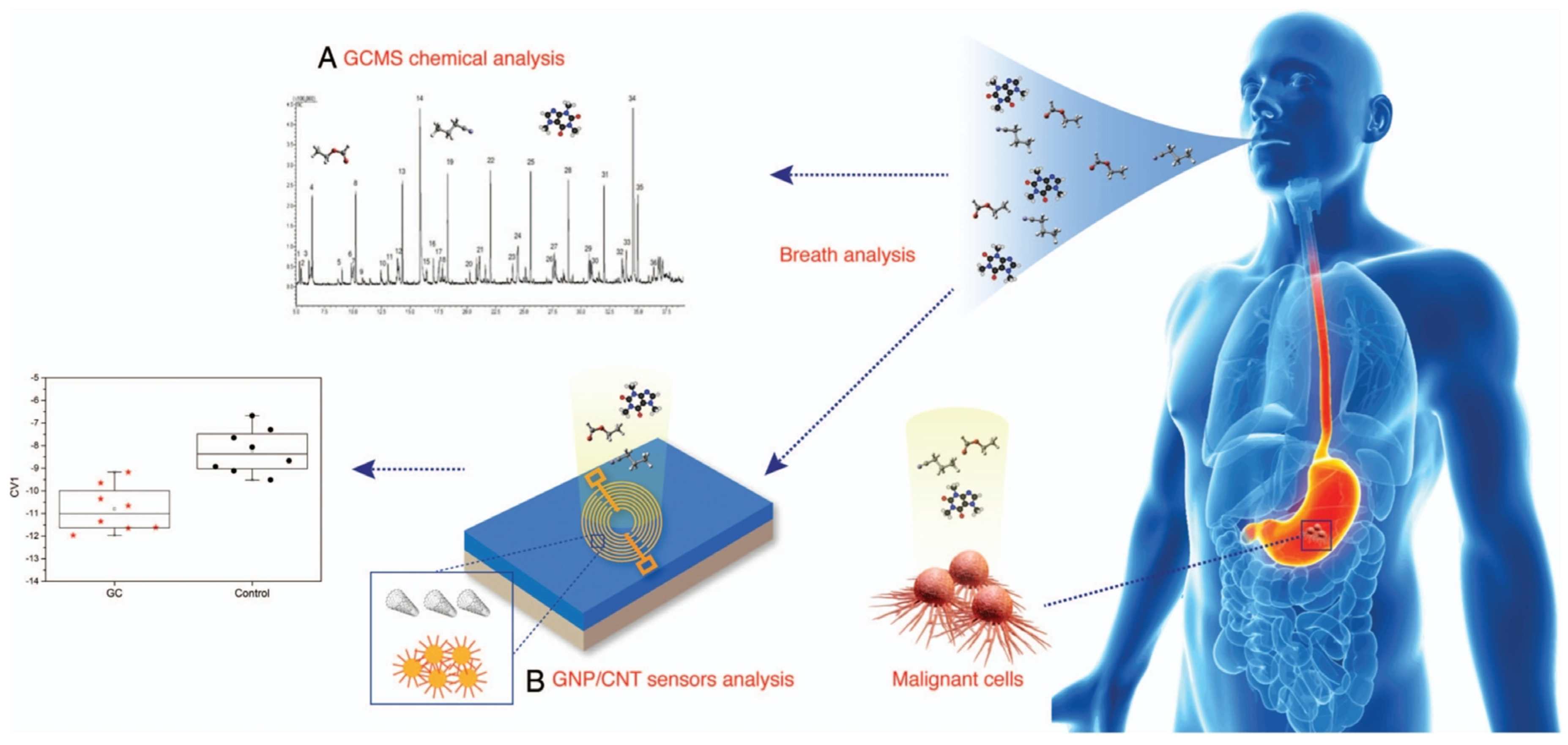
Gastric cancer is the fifth most commonly diagnosed cancer in the world, and it is more common in men than in women. Unclear clinical symptoms and a lack of clear risk factors often delay disease diagnosis, leading to poor prognosis and high recurrence rates. Xu et al. [104] have utilized 14 nanomaterial sensor arrays to detect 130 cases of gastric disease. The sensors include a gold nanoparticle layer with 11 different organic ligands and a single-walled carbon nanotube layer covered with 4 different organic covering layers, combined with a statistical pattern recognition algorithm. Patient (37 GC, 32 ulcers, and 61 less severe conditions) breath samples were characterized, and three DFA models were developed, achieving excellent discrimination between subpopulations. Amal et al. [105] collected 968 respiratory samples from 484 patients using GCMS, cross-reaction nanoarrays, and pattern recognition methods for two different analyses. Each sensor was composed of gold nanoparticles (GNPs) and single-walled carbon nanotubes (SWCNTs), with different organic thin films (ligands) covering the surface (Figure 7). In different comparisons, eight significant volatile organic compounds (p < 0.017) were detected in exhaled gas. Nanoarray analysis can distinguish GC patients from the control group with 73% sensitivity, 98% specificity, and 92% accuracy. Sharifi et al. [106] developed an electronic nose device containing eight fluorescent metal nanoclusters (NCs) sensing elements fixed on a paper substrate to detect VOCs released by the human body. After entering the headspace of the tissue, the emitted VOC reacts with the NC, causing the fluorescence intensity of the NC to change. The sensor array was able to differentiate between fresh lung cancer tissue and normal tissue in less than 4 h with 95% accuracy. Suter et al. [107] designed a sensor component tailored for the detection of gastric fluid, incorporating a hydrogel matrix interspersed with carbonate salts to elicit a distinct and uncomplicated leak detection response. Additionally, the sensor has been integrated into a bilayer patch configuration, thereby optimizing its suitability for in situ diagnostic applications.
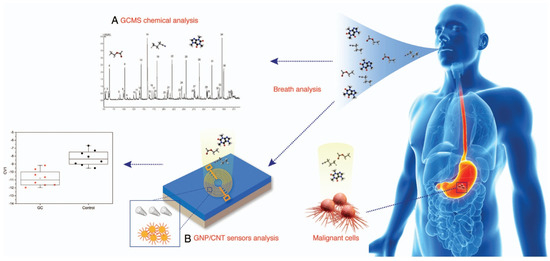
Figure 7.
There are two approaches to the analysis of volatile organic compounds from exhaled breath: (A) gas chromatography linked to mass spectrometry (GCMS) and (B) the nanoarray (sensor) method. GC, gastric cancer; GNP/CNT, gold nanoparticles/carbon nanotubes [105].
4.3. Detection of Intestinal Diseases
The prevalence of intestinal disorders has persistently remained elevated and is a subject of considerable focus among medical practitioners. Within the realm of diagnostic and therapeutic approaches to intestinal diseases, the importance of early intervention cannot be overstated [108]. Gas chromatography equipment is often used for clinical, non-invasive screening of intestinal diseases. The development of electronic nose equipment for human exhaled gas detection and sensors for gas detection are increasingly used in the early screening of intestinal diseases. Shepherd et al. [109] used headspace gas chromatography and a single metal oxide sensor coupled with artificial neural network software for stool analysis. They detected a total of 182 patient stool samples for VOCs, analyzed them with an artificial neural network, and successfully distinguished irritable bowel syndrome (IBS) patients from those with inflammatory bowel disease (IBD). For the patient sample, the sensitivity and specificity were 76% and 88%, respectively, and the overall average prediction accuracy was 76%. Chan et al. [110] conducted a systematic review of the use of fecal headspace analysis to assess gastrointestinal diseases, including celiac disease, nonalcoholic fatty liver disease, necrotizing enterocolitis, and pelvic radiation toxicity. Krishnamoorthy et al. [111] reviewed existing literature on the impact of mechanical bowel preparation on the production and measurement of volatile organic compounds. Two studies of 134 patients found no difference in the respiratory VOC spectrum measured after intestinal preparation. Another study found that after intestinal preparation in 61 patients, the level of respiratory acetone increased, but other compounds were not affected. Another study showed changes in the urine VOC spectrum, indicating limited data on the impact of intestinal preparation on VOC generation in the body. Dalis et al. [112] believed that dysbiosis or adverse changes in the composition of the organism appear before the clinical symptoms of various gastrointestinal diseases appear. Research into the diagnosis of gastrointestinal disease has led to a shift toward non-invasive methods for gastrointestinal screening, including chemical detection tests that measure changes in VOCs. Volatile organic compounds are by-products of bacterial metabolism that contribute to the distinctive odor of stool. VOCs assessment can be used as an early screening tool for discovering gastrointestinal diseases.
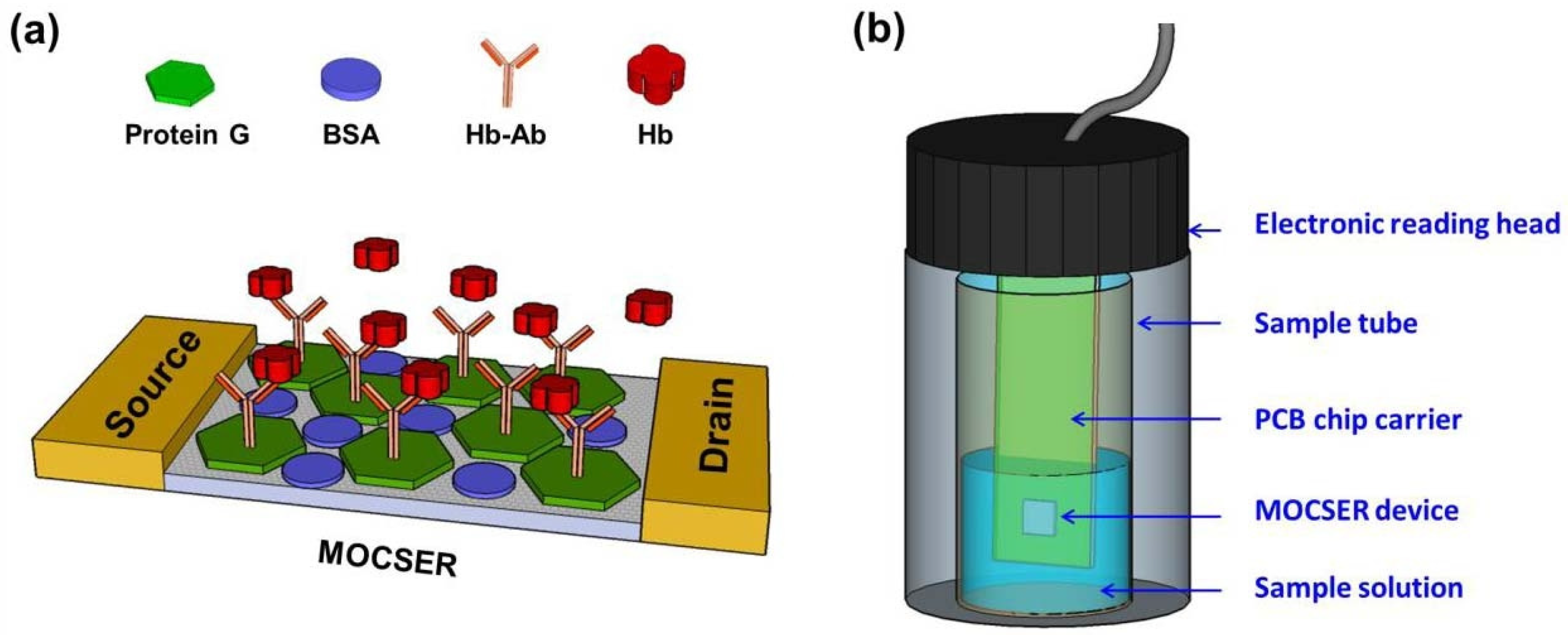
Sensor materials can help accurately diagnose the stage and status of lesions through accurate localization of intestinal lesions and sensitive analysis of disease marker levels, thereby avoiding delays in treatment. Westenbrink et al. [113] developed a new electronic nose instrument for measuring gaseous/volatile components of urine headspace based on an array of 13 commercially available electrochemical and optical sensors. A set of 92 urine samples from colorectal cancer (CRC), IBS patients, and controls were arranged in an experimental setup and run through a machine. Features were extracted from the response data and used in LDA plots, including the full three disease classifications, as well as features focused on distinguishing CRC from IBS, showing 78% sensitivity and 79% specificity for CRC. Yen et al. [114] proposed a new method and device for detecting hemoglobin in a simulated gastrointestinal environment using a GaAs-based sensor, which is termed a molecular-controlled semiconductor resistor (MOCSER). A surface protective layer of polymeric thiosilanes was deposited on top of the device to obtain a chemically passivating coating that prevents surface etching and achieves biocompatibility (Figure 8). Selective device functionalization was achieved by the subsequent adsorption of Hb antibodies on top of the protective layer. In vitro tests show that the sensor can identify fast-state simulated intestinal fluid when the Hb concentration is greater than 10 µg/mL. Furthermore, the sensor was able to detect Hb in pig intestinal fluid with the same sensitivity.
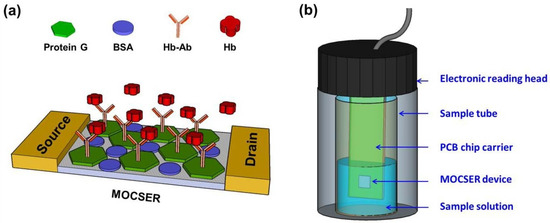
Figure 8.
(a) Schematic diagram of the molecular modifications on the GaAs-based biosensing devices. (b) The measuring setup for simulating a GI detection environment [114].
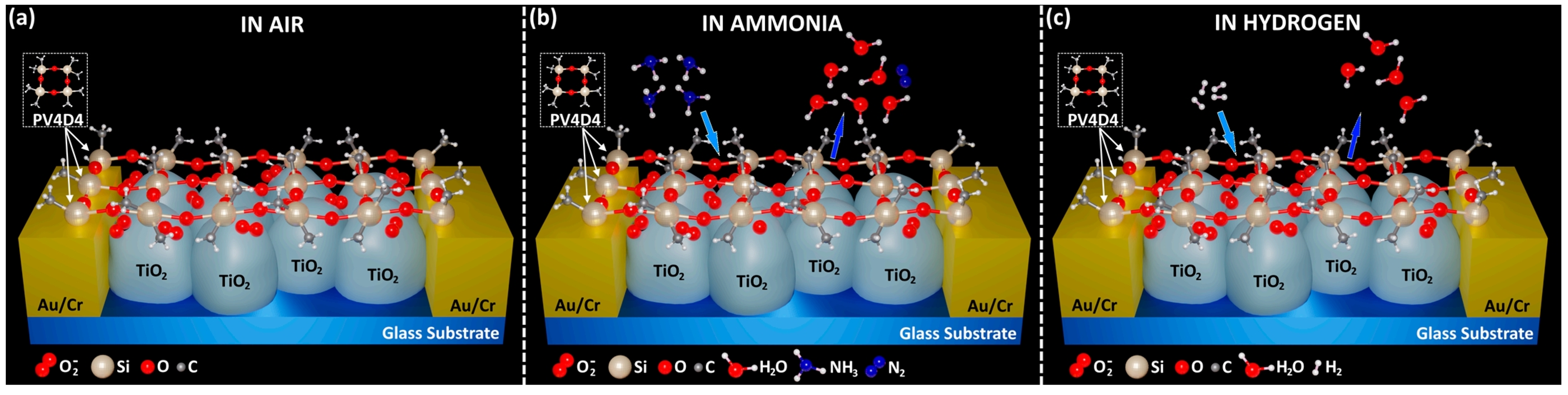
Peters et al. [115] developed an electronic nose device. In their investigation, the researchers performed analyses on a cohort of 402 patient samples, yielding a diagnostic sensitivity of 91% and a specificity of 74%. These results are adequate for differentiating patients with Barrett’s esophagus (BO). Moreover, the existence of proton pump inhibitors, hiatal hernia, and gastroesophageal reflux did not alter the differentiation efficacy, signifying that the sensor possesses considerable specificity and sensitivity. Tiele et al. [116] employed a self-constructed electronic nose in synergy with a commercial gas chromatography-ion mobility spectrometer (GC-IMS) to analyze and effectively identify VOCs in exhaled breath and biomarkers in feces, including fecal calprotectin (FCP). Additionally, this combined analytical approach was successfully applied to the detection of IBD, Crohn’s disease (CD), and ulcerative colitis (UC). Brinza et al. [117] proposed a novel dual-function sensor capable of detecting both hydrogen and ammonia gases. The sensor incorporates gas-sensitive materials at the nanoscale, which have been treated with annealing and initiated chemical vapor deposition (iCVD) techniques. This enables the sensor to respond to ammonia at room temperature, while at elevated detection temperatures, it exhibits a selective response to hydrogen gas (as depicted in Figure 9). This unique property of the sensor suggests its potential utility in the detection of gases within the human gastrointestinal system for future applications. Neetha et al. [118] prepared a CuO-Chi nanocomposite material and investigated its sensitivity to hydrogen gas by adjusting the proportional composition of the constituent materials. Utilizing a co-precipitation-probe separation technique, six distinct compositions were synthesized. It was observed that a 1:1 ratio of CuO to Chi enabled the detection of hydrogen gas at a concentration of 10 ppm at room temperature, with a detection limit as low as 0.07 ppm. These findings suggest that the material could be applied to the detection of gases associated with gastrointestinal diseases. Vasquez et al. [119] designed a chemical gas sensor based on carbon nanotubes (CNTs) coated with a thin film of polydimethylsiloxane (PDMS), which is capable of continuous gas monitoring. The sensor was demonstrated to operate continuously for 16 h under anaerobic, high-humidity, and acidic conditions, making this design suitable for continuous monitoring in the complex environment of the intestine.
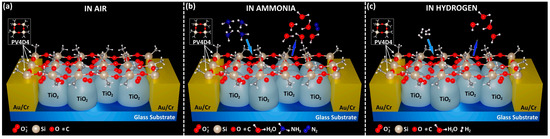
Figure 9.
The proposed sensing mechanism of PV4D4−coated TiO2 nanostructures (a) in air, (b) in ammonia, and (c) in hydrogen [117].
4.4. Ingestible Sensors
Ingestible sensing capsules are quickly becoming a key technology. Such ingestible devices are non-invasive and, therefore, very attractive to consumers. Ingestible sensors provide the opportunity to collect images and monitor luminal fluid and each segment of the intestinal tract. The ability of the contents, including electrolytes, enzymes, metabolites, hormones, and microbial communities, to gain information about an individual’s function and health through key intestinal biomarkers [120]. Smart capsules successfully transformed endoscopic capsules from laboratory prototypes into commercially viable clinical devices, and recently, this field has accelerated and expanded into various areas beyond imaging, including measurement of intestinal physiological parameters such as temperature, pH value, and pressure and gas sensing, as well as sampling devices developed to better understand gut health [121].
Kalantar-Zadeh et al. [122] reported human trials of a digestible electronic capsule that can sense oxygen, hydrogen, and carbon dioxide. The capsules use a combination of thermally conductive and semiconductor sensors, and their selectivity and sensitivity to different gases are controlled by adjusting the sensor’s heating element (Figure 10A). By changing the intake of dietary fiber to regulate the fermentation activity of intestinal microorganisms, the gas distribution of the subjects was obtained. It was found that changes in fiber intake were related to different small-intestinal and colon transit times and intestinal fermentation. Cheng et al. [123] reported a wireless ingestible capsule system for monitoring gastrointestinal pH levels (Figure 10B). They electroplated iridium oxide onto screen-printed electrodes (SPEs) and designed the capsule’s detection circuit using rigid-flex printed circuit board (RFPCB) technology. The capsule is sensitive to H+, exhibits good biocompatibility, and is suitable for in vivo monitoring. The entire capsule system, with dimensions of approximately 14.5 mm in diameter and 26 mm in height, conforms to the size requirements for in vivo gastrointestinal detection and can be used for real-time monitoring of the intestinal pH environment. Huang et al. [124] developed a pH-responsive ratiometric sensor. During the detection process, they utilized this sensor as a contrast agent, with PAI as an integrated technology, successfully measuring functional parameters of the gastrointestinal tract in tests. The sensor demonstrated high sensitivity, repeatability, and specificity, with a rapid response time to pH changes of up to 0.6 s, and it could operate continuously for 24 h. Using this sensor, they successfully observed significant disorders in motility and decreases in pH in patients with gastric and duodenal ulcers. De la Paz et al. [125] reported an ingestible sensing system for monitoring metabolites in the small intestine (Figure 10C). The sensing system is self-powered, integrating a glucose biofuel cell and biosensors into the circuitry. By utilizing a power-to-frequency conversion scheme with magnetic human-body communication, the system enables real-time in vivo power distribution from internal fuel sources and wireless detection of physiological characteristics within the gastrointestinal tract.
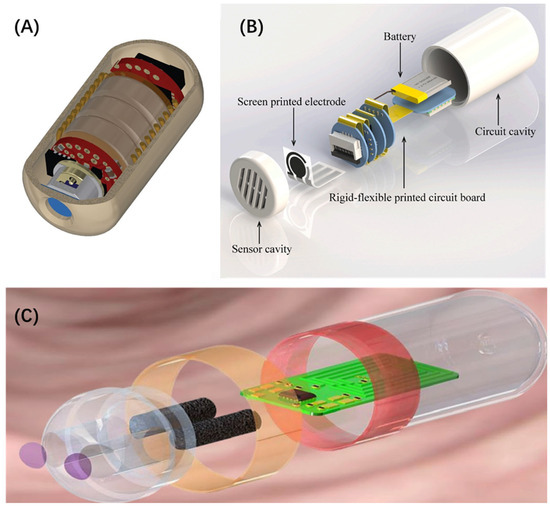
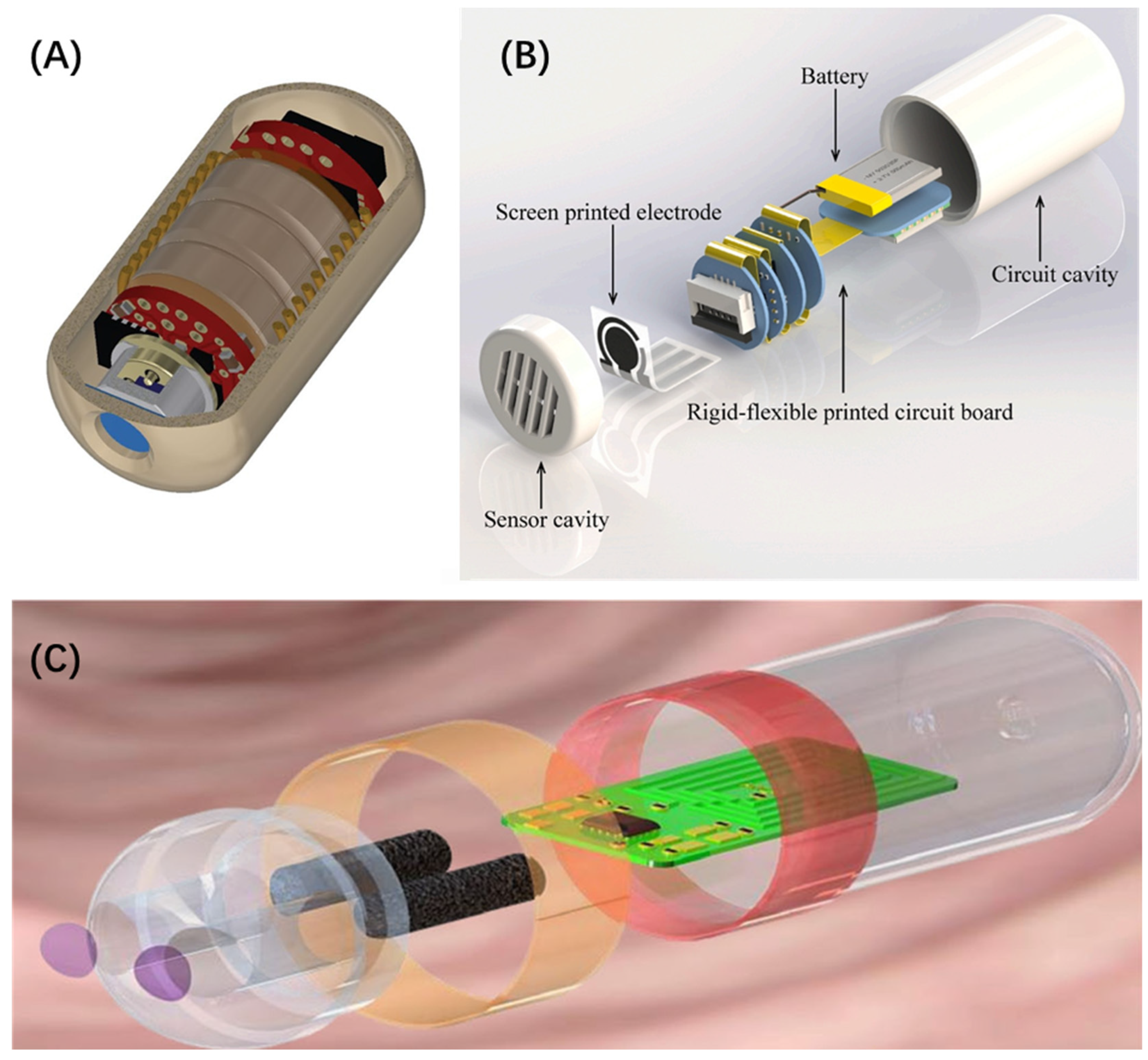
Figure 10.
(A) Three-dimensional rendering of the human gas sensing capsule sliced to demonstrate the internal components [122]. (B) The combined assembly drawing of the capsule system, including the sensor cavity, the screen-printed electrode (SPE), the RFPCB, the lithium-ion battery, and the circuit cavity [123]. (C) Schematic layout of the capsule sensor [125].
5. Conclusions and Outlook
In conclusion, through research on saliva and breath biomarkers, the early diagnosis technology for oral cancer is continuously improving and expanding. In the future, further research into oral cancer biomarkers and the development of more precise and reliable diagnostic technologies will provide solid support for the early screening and treatment of oral cancer. And with the development of these novel biosensors and detection technologies, it can offer more convenient and accurate methods for early screening and diagnosis of oral cancer, promising to play a significant role in clinical practice by providing oral cancer patients with earlier treatment and intervention opportunities, thereby improving treatment success rates and patient survival rates. Early diagnosis of halitosis and periodontal disease through the detection of exhaled gases is non-invasive, safe, convenient, and fast, and is ideal for assisting physicians in diagnosis or for patient self-examination. However, most of the chemical gas sensors used for early detection are limited to laboratory testing and have not been formally applied in clinical practice. The composition of human exhaled gas is very complex, with many interfering factors, which makes it difficult to apply the sensor to actual clinical samples, and the accuracy, stability, and sensitivity of the sensor still need to be improved. In future research, the lower detection limit of the sensor can be further reduced, and the sensitivity can be improved by improving the sensitive material and signal amplification to meet the needs of practical applications.
Surface acoustic wave sensors provide a fast, reliable, and highly sensitive detection method for the detection of lung disease markers. However, in order to achieve commercialization and rapid and accurate detection of clinical samples, it is necessary to further combine the rapidly developing MEMS manufacturing technology, nanomaterials, microfluidic chips, and electronic information technology to improve the detection performance of surface acoustic wave sensors while optimizing and standardizing the sensor preparation process and marker detection procedures. There is still huge room for development in some respects, such as surface modification, miniaturization and arraying of sensing units, portable systems with automated sample injection, flexible and wearable devices, etc.
Due to the length and particularity of the gastrointestinal tract, different types of diseases in different parts of the body often have different clinical detection methods. However, most detection methods are invasive. Patients are more willing to accept non-invasive screening. Currently, gastrointestinal disease screening, breath testing, and early screening are increasingly common. For stomach diseases, there is currently no perfect screening tool for gastric cancer and related precancerous lesions that can be widely used in different regions with different gastric cancer incidence rates around the world. Performing a Helicobacter pylori breath test can achieve early prevention. Currently, the main methods for detecting intestinal diseases include endoscopy, breath testing, stool examination, blood testing, etc. Breath testing is the most convenient. However, due to the complex composition of human exhaled gas and the large variability in samples, the correlation between diseases is not high yet. The most representative one is the detection of small intestinal bacterial overgrowth. Breath testing is currently widely used, but there is a lack of decisive markers and concentration correlations, and there is no unified international gold standard. As a non-invasive alternative to endoscopes, electronic capsules can achieve non-invasive in situ detection. However, the retention of the capsule is currently uncontrollable, and the detection range cannot be fully covered. There are also problems, such as battery life, and the clinical use coverage is not yet very optimistic. In the future, the miniaturization and precision of sensing devices, the development of specific and sensitive materials, and the emergence of high-precision integrated electronic noses will promote the further development of non-invasive and rapid screening of gastrointestinal diseases.
In the past decade, flexible sensing devices and wearable sensing devices have received increasing attention for disease detection. Many of these head wearable sensor devices are used to detect neurological/mental health-related diseases and are also used as flexible wearable sensor devices for ocular disease detection. In future research, flexible wearable devices will also be widely used in the detection of oral, lung, and gastrointestinal diseases. Miniaturized and integrated wearable sensing devices will help detect various human body data in real time, opening up new research directions for early screening and prevention of diseases, continuous home testing, and bedside testing.
Author Contributions
Conceptualization, W.Y. and S.M.; investigation, Y.X. and H.X.; writing—original draft preparation, W.Y. and S.M.; writing—review and editing, W.Y., S.M., X.Z. and J.S.; visualization, K.J.H.; supervision, H.W.; project administration, P.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Key Research and Development Program of China (Nos. 2021YFB3200801 and 2021YFC3300303), the “Pioneer” and “Leading Goose” R&D Pro-grams of Zhejiang Province (Nos. 2023C03104 and 2023C03009), and the National Science Foundation of China (Nos. 62120106004 and 32250008).
Acknowledgments
This work was supported by the National Key Research and Development Program of China (Nos. 2021YFB3200801 and 2021YFC3300303), the “Pioneer” and “Leading Goose” R&D Programs of Zhejiang Province (Nos. 2023C03104 and 2023C03009), and the National Science Foundation of China (Nos. 62120106004 and 32250008).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lee, Y.-H.; Wong, D.T. Saliva: An emerging biofluid for early detection of diseases. Am. J. Dent. 2009, 22, 241. [Google Scholar] [PubMed]
- Salek-Maghsoudi, A.; Vakhshiteh, F.; Torabi, R.; Hassani, S.; Ganjali, M.R.; Norouzi, P.; Hosseini, M.; Abdollahi, M. Recent advances in biosensor technology in assessment of early diabetes biomarkers. Biosens. Bioelectron. 2018, 99, 122–135. [Google Scholar] [CrossRef] [PubMed]
- Lino, C.; Barrias, S.; Chaves, R.; Adega, F.; Martins-Lopes, P.; Fernandes, J. Biosensors as diagnostic tools in clinical applications. Biochim. Biophys. Acta BBA-Rev. Cancer 2022, 1877, 188726. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, J.; Xiao, S.; Liu, Y.; Bai, M.; Gong, L.; Zhao, J.; Chen, D. Revolutionizing precision medicine: Exploring wearable sensors for therapeutic drug monitoring and personalized therapy. Biosensors 2023, 13, 726. [Google Scholar] [CrossRef] [PubMed]
- Waggoner, P.S.; Craighead, H.G. Micro-and nanomechanical sensors for environmental, chemical, and biological detection. Lab Chip 2007, 7, 1238–1255. [Google Scholar] [CrossRef] [PubMed]
- Rolfe, P. Micro- and Nanosensors for Medical and Biological Measurement. Sens. Mater. 2012, 24, 275–302. [Google Scholar] [CrossRef]
- Vishinkin, R.; Haick, H. Nanoscale sensor technologies for disease detection via volatolomics. Small 2015, 11, 6142–6164. [Google Scholar] [CrossRef] [PubMed]
- Tovar-Lopez, F.J. Recent Progress in Micro-and Nanotechnology-Enabled Sensors for Biomedical and Environmental Challenges. Sensors 2023, 23, 5406. [Google Scholar] [CrossRef]
- Varatharajan, R.; Manogaran, G.; Priyan, M.K.; Sundarasekar, R. Wearable sensor devices for early detection of Alzheimer disease using dynamic time warping algorithm. Clust. Comput. 2018, 21, 681–690. [Google Scholar] [CrossRef]
- Liu, T.; Gou, G.-Y.; Gao, F.; Yao, P.; Wu, H.; Guo, Y.; Yin, M.; Yang, J.; Wen, T.; Zhao, M. Multichannel Flexible Pulse Perception Array for Intelligent Disease Diagnosis System. ACS Nano 2023, 17, 5673–5685. [Google Scholar] [CrossRef]
- De Geest, S.; Laleman, I.; Teughels, W.; Dekeyser, C.; Quirynen, M. Periodontal diseases as a source of halitosis: A review of the evidence and treatment approaches for dentists and dental hygienists. Periodontol. 2000 2016, 71, 213–227. [Google Scholar] [CrossRef]
- Khurshid, Z.; Zafar, M.S.; Khan, R.S.; Najeeb, S.; Slowey, P.D.; Rehman, I.U. Role of salivary biomarkers in oral cancer detection. Adv. Clin. Chem. 2018, 86, 23–70. [Google Scholar]
- Kalantar-Zadeh, K.; Berean, K.J.; Burgell, R.E.; Muir, J.G.; Gibson, P.R. Intestinal gases: Influence on gut disorders and the role of dietary manipulations. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 733–747. [Google Scholar] [CrossRef]
- Zhang, J.; Tian, Y.; Luo, Z.; Qian, C.; Li, W.; Duan, Y. Breath volatile organic compound analysis: An emerging method for gastric cancer detection. J. Breath Res. 2021, 15, 044002. [Google Scholar] [CrossRef]
- Chen, T.; Liu, T.; Li, T.; Zhao, H.; Chen, Q. Exhaled breath analysis in disease detection. Clin. Chim. Acta 2021, 515, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Yang, A.; Yan, F. Chemical Substances. In Seamless Healthcare Monitoring: Advancements in Wearable, Attachable, and Invisible Devices; Springer: Cham, Switzerland, 2018; pp. 335–365. [Google Scholar]
- Han, Q.; Pang, J.; Li, Y.; Sun, B.; Ibarlucea, B.; Liu, X.; Gemming, T.; Cheng, Q.; Zhang, S.; Liu, H. Graphene biodevices for early disease diagnosis based on biomarker detection. ACS Sens. 2021, 6, 3841–3881. [Google Scholar] [CrossRef]
- Khan, H.; Shah, M.R.; Barek, J.; Malik, M.I. Cancer biomarkers and their biosensors: A comprehensive review. TrAC Trends Anal. Chem. 2023, 158, 116813. [Google Scholar] [CrossRef]
- Khazaei, M.; Hosseini, M.S.; Haghighi, A.M.; Misaghi, M. Nanosensors and their applications in early diagnosis of cancer. Sens. Bio-Sens. Res. 2023, 41, 100569. [Google Scholar] [CrossRef]
- Sinha, A.; Zhao, H.; Huang, Y.; Lu, X.; Chen, J.; Jain, R. MXene: An emerging material for sensing and biosensing. TrAC Trends Anal. Chem. 2018, 105, 424–435. [Google Scholar] [CrossRef]
- Neville, B.W.; Day, T.A. Oral cancer and precancerous lesions. CA-Cancer J. Clin. 2002, 52, 195–215. [Google Scholar] [CrossRef]
- Mork, J.; Lehtinen, M.; Dillner, J. Human papillomavirus infection as a risk factor for squamous-cell carcinoma of the head and neck—Reply. N. Engl. J. Med. 2001, 345, 377. [Google Scholar]
- da Silva, S.D.; Ferlito, A.; Takes, R.P.; Brakenhoff, R.H.; Valentin, M.D.; Woolgar, J.A.; Bradford, C.R.; Rodrigo, J.P.; Rinaldo, A.; Hier, M.P.; et al. Advances and applications of oral cancer basic research. Oral Oncol. 2011, 47, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.; Macey, R.; Kerr, A.R.; Lingen, M.W.; Ogden, G.R.; Warnakulasuriyas, S. Diagnostic tests for oral cancer and potentially malignant disorders in patients presenting with clinically evident lesions. Cochrane Database Syst. Rev. 2021, 7, 1465–1858. [Google Scholar] [CrossRef]
- Trojanowska, A.; Grzycka-Kowalczyk, L.; Trojanowski, P.; Klatka, J.; Drop, A. Computed tomography perfusion examination is helpful in evaluating the extent of oropharyngeal and oral cavity cancer. Pol. J. Radiol. 2011, 76, 14. [Google Scholar]
- Nae, A.; O’Leary, G.; Feeley, L.; Fives, C.; Fitzgerald, B.; Chiriac, E.; Sheahan, P. Utility of CT and MRI in assessment of mandibular involvement in oral cavity cancer. World J. Otorhinolaryngol. Head Neck Surg. 2019, 5, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Dolens, E.D.; Dourado, M.R.; Almangush, A.; Salo, T.A.; Rocha, C.A.G.; da Silva, S.D.; Brennan, P.A.; Coletta, R.D. The Impact of Histopathological Features on the Prognosis of Oral Squamous Cell Carcinoma: A Comprehensive Review and Meta-Analysis. Front. Oncol. 2021, 11, 784924. [Google Scholar] [CrossRef]
- Minami, S.; Chikazu, D.; Ochiya, T.; Yoshioka, Y. Extracellular vesicle-based liquid biopsies in cancer: Future biomarkers for oral cancer. Transl. Oncol. 2023, 38, 101786. [Google Scholar] [CrossRef]
- Goldoni, R.; Scolaro, A.; Boccalari, E.; Dolci, C.; Scarano, A.; Inchingolo, F.; Ravazzani, P.; Muti, P.; Tartaglia, G. Malignancies and Biosensors: A Focus on Oral Cancer Detection through Salivary Biomarkers. Biosensors 2021, 11, 396. [Google Scholar] [CrossRef] [PubMed]
- Weigum, S.E.; Floriano, P.N.; Christodoulides, N.; McDevitt, J.T. Cell-based sensor for analysis of EGFR biomarker expression in oral cancer. Lab Chip 2007, 7, 995–1003. [Google Scholar] [CrossRef]
- Weigum, S.E.; Floriano, P.N.; Redding, S.W.; Yeh, C.K.; Westbrook, S.D.; McGuff, H.S.; Lin, A.; Miller, F.R.; Villarreal, F.; Rowan, S.D.; et al. Nano-Bio-Chip Sensor Platform for Examination of Oral Exfoliative Cytology. Cancer Prev. Res. 2010, 3, 518–528. [Google Scholar] [CrossRef]
- Malhotra, R.; Patel, V.; Chikkaveeraiah, B.V.; Munge, B.S.; Cheong, S.C.; Zain, R.B.; Abraham, M.T.; Dey, D.K.; Gutkind, J.S.; Rusling, J.F. Ultrasensitive Detection of Cancer Biomarkers in the Clinic by Use of a Nanostructured Microfluidic Array. Anal. Chem. 2012, 84, 6249–6255. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, M.O.; Lin, C.M.; Lee, D.H.; Chiang, W.F.; Chen, I.H.; Chuang, C.H. Portable Pen-Like Device With Miniaturized Tactile Sensor for Quantitative Tissue Palpation in Oral Cancer Screening. IEEE Sens. J. 2020, 20, 9610–9617. [Google Scholar] [CrossRef]
- Tan, W.; Sabet, L.; Li, Y.; Yu, T.; Klokkevold, P.R.; Wong, D.T.; Ho, C.M. Optical protein sensor for detecting cancer markers in saliva. Biosens. Bioelectron. 2008, 24, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Pitikultham, P.; Putnin, T.; Pimalai, D.; Sathirapongsasuti, N.; Kitiyakara, C.; Jiang, Q.; Ding, B.Q.; Japrung, D. Ultrasensitive Detection of MicroRNA in Human Saliva via Rolling Circle Amplification Using a DNA-Decorated Graphene Oxide Sensor. ACS Omega 2023, 8, 15266–15275. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, G.; Chiriacò, M.S.; Ferrara, F.; Turco, A.; Velardi, L.; Signore, M.A.; Esposito, M.; Gigli, G.; Primiceri, E. Development of an MIP based electrochemical sensor for TGF-β1 detection and its application in liquid biopsy. Analyst 2023, 148, 4447–4455. [Google Scholar] [CrossRef]
- Hu, X.; Qiu, D.; Jiang, Q.; Xu, Q.; Li, J. Cu 2+-doped zeolitic imidazolate frameworks and gold nanoparticle (AuNPs@ ZIF-8/Cu) nanocomposites enable label-free and highly sensitive electrochemical detection of oral cancer-related biomarkers. Anal. Methods 2024, 16, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Sharma, J.G.; Maji, S.; Malhotra, B.D. Nanostructured zirconia decorated reduced graphene oxide based efficient biosensing platform for non-invasive oral cancer detection. Biosens. Bioelectron. 2016, 78, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Kong, L.B.; Gan, Y.; Liang, T.; Zhou, S.Q.; Sun, J.D.; Wan, H.; Wang, P. Microfluidic-based fluorescent electronic eye with CdTe/CdS core-shell quantum dots for trace detection of cadmium ions. Anal. Chim. Acta 2020, 1131, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Sun, X.Y.; Ma, C.Y.; Zhang, Y.C.; Kong, L.B.; Huang, Z.R.; Hu, Y.J.; Wan, H.; Wang, P. Multifunctional AuNPs@HRP@FeMOF immune scaffold with a fully automated saliva analyzer for oral cancer screening. Biosens. Bioelectron. 2023, 222, 114910. [Google Scholar] [CrossRef]
- Korde, S.; Sridharan, G.; Gadbail, A.; Poornima, V. Nitric oxide and oral cancer: A review. Oral Oncol. 2012, 48, 475–483. [Google Scholar] [CrossRef]
- Kwon, I.J.; Jung, T.Y.; Son, Y.; Kim, B.; Kim, S.M.; Lee, J.H. Detection of volatile sulfur compounds (VSCs) in exhaled breath as a potential diagnostic method for oral squamous cell carcinoma. BMC Oral Health 2022, 22, 268. [Google Scholar] [CrossRef] [PubMed]
- Bouza, M.; Gonzalez-Soto, J.; Pereiro, R.; de Vicente, J.C.; Sanz-Medel, A. Exhaled breath and oral cavity VOCs as potential biomarkers in oral cancer patients. J. Breath Res. 2017, 11, 016015. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Yu, W.R.; Chen, Z.X.; Wang, L.; Yang, J.J.; Liu, S.H.; Li, L.Z.; Li, Y.X.; Huang, Y.Z. Early-stage oral cancer diagnosis by artificial intelligence-based SERS using Ag NWs@ZIF core-shell nanochains. Nanoscale 2023, 15, 13466–13472. [Google Scholar] [CrossRef] [PubMed]
- Apatzidou, A.; Bakirtzoglou, E.; Vouros, I.; Karagiannis, V.; Papa, A.; Konstantinidis, A. Association between oral malodour and periodontal disease-related parameters in the general population. Acta Odontol. Scand. 2013, 71, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.N.; Shinada, K.; Chen, X.C.; Zhang, B.X.; Yaegaki, K.; Kawaguchi, Y. Oral malodor-related parameters in the Chinese general population. J. Clin. Periodontol. 2006, 33, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.W.; Goh, R.; Cheong, E.; Guan, G.; Jin, C.; Cannon, R.D.; Farella, M.; Mei, L. Prevalence of halitosis among young adults in Dunedin, New Zealand. Int. J. Dent. Hyg. 2022, 20, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Teshome, A.; Derese, K.; Andualem, G. The prevalence and determinant factors of oral halitosis in northwest ethiopia: A cross-sectional study. Clin. Cosmet. Investig. Dent. 2021, 13, 173–179. [Google Scholar] [CrossRef]
- Li, Z.; Li, J.; Fu, R.; Liu, J.; Wen, X.; Zhang, L. Halitosis: Etiology, prevention, and the role of microbiota. Clin. Oral Investig. 2023, 27, 6383–6393. [Google Scholar] [CrossRef] [PubMed]
- Setia, S.; Pannu, P.; Gambhir, R.S.; Galhotra, V.; Ahluwalia, P.; Sofat, A. Correlation of oral hygiene practices, smoking and oral health conditions with self perceived halitosis amongst undergraduate dental students. J. Nat. Sci. Biol. Med. 2014, 5, 67. [Google Scholar] [CrossRef]
- Morita, M.; Wang, H.L. Association between oral malodor and adult periodontitis: A review. J. Clin. Periodontol. 2001, 28, 813–819. [Google Scholar] [CrossRef]
- Lee, Y.H.; Shin, S.I.; Hong, J.Y. Investigation of volatile sulfur compound level and halitosis in patients with gingivitis and periodontitis. Sci. Rep. 2023, 13, 13175. [Google Scholar] [CrossRef] [PubMed]
- Aylıkcı, B.U.; Çolak, H. Halitosis: From diagnosis to management. J. Nat. Sci. Biol. Med. 2013, 4, 14. [Google Scholar] [CrossRef]
- Morita, M.; Wang, H.L. Relationship between sulcular sulfide level and oral malodor in subjects with periodontal disease. J. Periodontol. 2001, 72, 79–84. [Google Scholar] [CrossRef]
- Shin, H.; Kim, D.-H.; Jung, W.; Jang, J.-S.; Kim, Y.H.; Lee, Y.; Chang, K.; Lee, J.; Park, J.; Namkoong, K. Surface activity-tuned metal oxide chemiresistor: Toward direct and quantitative halitosis diagnosis. ACS Nano 2021, 15, 14207–14217. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, Z.; Zong, X. Flexible and highly sensitive H2S gas sensor based on in-situ polymerized SnO2/rGO/PANI ternary nanocomposite with application in halitosis diagnosis. Sens. Actuators B Chem. 2019, 289, 32–41. [Google Scholar] [CrossRef]
- Jha, R.K.; D’Costa, J.V.; Sakhuja, N.; Bhat, N. MoSe2 nanoflakes based chemiresistive sensors for ppb-level hydrogen sulfide gas detection. Sens. Actuators B Chem. 2019, 297, 126687. [Google Scholar] [CrossRef]
- Bulemo, P.M.; Cho, H.-J.; Kim, D.-H.; Kim, I.-D. Facile synthesis of Pt-functionalized meso/macroporous SnO2 hollow spheres through in situ templating with SiO2 for H2S sensors. ACS Appl. Mater. Interfaces 2018, 10, 18183–18191. [Google Scholar] [CrossRef] [PubMed]
- Asres, G.A.; Baldoví, J.J.; Dombovari, A.; Järvinen, T.; Lorite, G.S.; Mohl, M.; Shchukarev, A.; Pérez Paz, A.; Xian, L.; Mikkola, J.-P. Ultrasensitive H2S gas sensors based on p-type WS 2 hybrid materials. Nano Res. 2018, 11, 4215–4224. [Google Scholar] [CrossRef]
- Lim, K.; Jo, Y.-M.; Kim, S.; Yoon, J.-W.; Jeong, S.-Y.; Kim, J.-S.; Choi, H.J.; Cho, Y.; Park, J.; Jeong, Y.W.; et al. Selective dual detection of hydrogen sulfide and methyl mercaptan using CuO/CuFe2O4 nanopattern chemiresistors. Sens. Actuators B Chem. 2021, 348, 130665. [Google Scholar] [CrossRef]
- Bae, G.; Kim, M.; Lee, A.; Ji, S.; Jang, M.; Yim, S.; Song, W.; Lee, S.S.; Yoon, D.H.; An, K.-S. Nanometric lamination of zinc oxide nanofilms with gold nanoparticles for self-perceived periodontal disease sensors. Compos. Part B Eng. 2022, 230, 109490. [Google Scholar] [CrossRef]
- Priya, M.; Subha, P.; Aswathy, P.; Merin, K.; Jayaraj, M.; Kumar, K.R. Selective detection of hydrogen sulphide from the background of low concentration reducing gases. Mater. Chem. Phys. 2021, 260, 124038. [Google Scholar] [CrossRef]
- Cha, J.-H.; Kim, D.-H.; Choi, S.-J.; Koo, W.-T.; Kim, I.-D. Sub-parts-per-million hydrogen sulfide colorimetric sensor: Lead acetate anchored nanofibers toward halitosis diagnosis. Anal. Chem. 2018, 90, 8769–8775. [Google Scholar] [CrossRef] [PubMed]
- Rosolina, S.M.; Carpenter, T.S.; Xue, Z.-L. Bismuth-based, disposable sensor for the detection of hydrogen sulfide gas. Anal. Chem. 2016, 88, 1553–1558. [Google Scholar] [CrossRef] [PubMed]
- Gatty, H.K.; Stemme, G.; Roxhed, N. A miniaturized amperometric hydrogen sulfide sensor applicable for bad breath monitoring. Micromachines 2018, 9, 612. [Google Scholar] [CrossRef] [PubMed]
- Jafari, M.; Hasanzadeh, M. Pharmacotherapy. Non-invasive bioassay of Cytokeratin Fragment 21.1 (Cyfra 21.1) protein in human saliva samples using immunoreaction method: An efficient platform for early-stage diagnosis of oral cancer based on biomedicine. Biomed. Pharmacother. 2020, 131, 110671. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Singh, A.; Shukla, A.; Kaswan, J.; Arora, K.; Ramirez-Vick, J.; Singh, P.; Singh, S.P. Anti-IL8/AuNPs-rGO/ITO as an immunosensing platform for noninvasive electrochemical detection of oral cancer. ACS Appl. Mater. Interfaces 2017, 9, 27462–27474. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Das, S.R.; Brownlee, B.J.; Parate, K.; Davis, T.M.; Stromberg, L.R.; Chan, E.K.; Katz, J.; Iverson, B.D.; Claussen, J.C. CIP2A immunosensor comprised of vertically-aligned carbon nanotube interdigitated electrodes towards point-of-care oral cancer screening. Biosens. Bioelectron. 2018, 117, 68–74. [Google Scholar] [CrossRef]
- Mohseni, S.; Moghadam, T.T.; Dabirmanesh, B.; Jabbari, S.; Khajeh, K. Development of a label-free SPR sensor for detection of matrixmetalloproteinase-9 by antibody immobilization on carboxymethyldextran chip. Biosens. Bioelectron. 2016, 81, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, S.-H.; Du, X.-Y.; Sun, J.-J. Plasmonic Ag nanocube enhanced SERS biosensor for sensitive detection of oral cancer DNA based on nicking endonuclease signal amplification and heated electrode. Sens. Actuators B Chem. 2021, 338, 129854. [Google Scholar] [CrossRef]
- Xue, Y.; Tao, Z.; Yang, T.; Chen, Y.; Zhang, J.; Wan, H.; Ye, W.; Wang, P. Study on the Screening and Diagnosis of Oral Diseases Based on Volatile Sulfur Compounds in Human Exhaled Breath. Chin. J. Biomed. Eng. 2021, 40, 202–209. [Google Scholar] [CrossRef]
- Cho, S.J.; Stout-Delgado, H.W. Aging and lung disease. Annu. Rev. Physiol. 2020, 82, 433–459. [Google Scholar] [CrossRef]
- Cookson, W.O.; Cox, M.J.; Moffatt, M.F. New opportunities for managing acute and chronic lung infections. Nat. Rev. Microbiol. 2018, 16, 111–120. [Google Scholar] [CrossRef]
- Agustí, A.; Vogelmeier, C.; Faner, R. COPD 2020: Changes and challenges. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 319, L879–L883. [Google Scholar] [CrossRef] [PubMed]
- Lambrecht, B.N.; Hammad, H. The immunology of asthma. Nat. Immunol. 2015, 16, 45–56. [Google Scholar] [CrossRef]
- Hensley, C.T.; Faubert, B.; Yuan, Q.; Lev-Cohain, N.; Jin, E.; Kim, J.; Jiang, L.; Ko, B.; Skelton, R.; Loudat, L. Metabolic heterogeneity in human lung tumors. Cell 2016, 164, 681–694. [Google Scholar] [CrossRef]
- Hoeper, M.M.; Humbert, M.; Souza, R.; Idrees, M.; Kawut, S.M.; Sliwa-Hahnle, K.; Jing, Z.-C.; Gibbs, J.S.R. A global view of pulmonary hypertension. Lancet Respir. Med. 2016, 4, 306–322. [Google Scholar] [CrossRef] [PubMed]
- Goldhaber, S.Z.; Bounameaux, H. Pulmonary embolism and deep vein thrombosis. Lancet 2012, 379, 1835–1846. [Google Scholar] [CrossRef]
- Jones, R.; Muyinda, H.; Nyakoojo, G.; Kirenga, B.; Katagira, W.; Pooler, J. Does pulmonary rehabilitation alter patients’ experiences of living with chronic respiratory disease? A qualitative study. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 2375–2385. [Google Scholar] [CrossRef]
- Singh, D.; Agusti, A.; Anzueto, A.; Barnes, P.J.; Bourbeau, J.; Celli, B.R.; Criner, G.J.; Frith, P.; Halpin, D.M.; Han, M. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: The GOLD science committee report 2019. Eur. Respir. J. 2019, 53, 1900164. [Google Scholar] [CrossRef] [PubMed]
- Kwong, G.A.; Ghosh, S.; Gamboa, L.; Patriotis, C.; Srivastava, S.; Bhatia, S.N. Synthetic biomarkers: A twenty-first century path to early cancer detection. Nat. Rev. Cancer 2021, 21, 655–668. [Google Scholar] [CrossRef]
- Kołodziej, M.; de Veer, M.J.; Cholewa, M.; Egan, G.F.; Thompson, B.R. Lung function imaging methods in cystic fibrosis pulmonary disease. Respir. Res. 2017, 18, 96. [Google Scholar] [CrossRef] [PubMed]
- Devillier, P.; Salvator, H.; Naline, E.; Couderc, L.-J.; Grassin-Delyle, S. Metabolomics in the diagnosis and pharmacotherapy of lung diseases. Curr. Pharm. Des. 2017, 23, 2050–2059. [Google Scholar] [CrossRef]
- Kodavanti, U.P. Respiratory toxicity biomarkers. In Biomarkers in Toxicology; Elsevier: Amsterdam, The Netherlands, 2014; pp. 217–239. [Google Scholar]
- Hekiem, N.L.L.; Ralib, A.A.M.; Akmal, M.; Hattar, M.; Ahmad, F.B.; Nordin, A.N.; Rahim, R.A.; Za’bah, N.F. Advanced vapour sensing materials: Existing and latent to acoustic wave sensors for VOCs detection as the potential exhaled breath biomarkers for lung cancer. Sens. Actuators A-Phys. 2021, 329, 112792. [Google Scholar] [CrossRef]
- Yu, H.; Xu, L.; Cao, M.F.; Chen, X.; Wang, P.; Jiao, J.W.; Wang, Y.L. Detection volatile organic compounds in breath as markers of lung cancer using a novel electronic nose. In Proceedings of the IEEE Sensors 2003, Toronto, ON, Canada, 22–24 October 2003; Volumes 1 and 2, pp. 1333–1337. [Google Scholar]
- Chen, X.; Cao, M.F.; Li, Y.; Hu, W.J.; Wang, P.; Ying, K.J.; Pan, H.M. A study of an electronic nose for detection of lung cancer based on a virtual SAW gas sensors array and imaging recognition method. Meas. Sci. Technol. 2005, 16, 1535–1546. [Google Scholar] [CrossRef]
- Wang, P.; Chen, X.; Xu, F.; Lu, D.; Cai, W.; Ying, K.; Wang, Y.; Hu, Y. Development of electronic nose for diagnosis of lung cancer at early stage. In Proceedings of the 2008 International Special Topic Conference on Information Technology and Applications in Biomedicine, Shenzhen, China, 30–31 May 2008; Volumes 1 and 2, p. 402. [Google Scholar]
- Cao, M.; Chen, X.; Wang, Y.; Ying, K.; Feng-Juan, X.; Wang, P. A Novel Electronic Nose for Detection of Lung Cancer Based on Virtual SAW Gas Sensors Array. Chin. J. Biomed. Eng. 2008, 27, 102–107. [Google Scholar]
- Wang, D.; Wang, L.; Yu, J.; Wang, P.; Hu, Y.; Ying, K. A Study on Electronic Nose for Clinical Breath Diagnosis of Lung Cancer. In Proceedings of the Olfaction and Electronic Nose, Brescia, Italy, 15–17 April 2009; pp. 314–317. [Google Scholar]
- Wang, D.; Wang, L.; Yu, J.; Wang, P.; Hu, Y.J.; Ying, K.J. Characterization of a Modified Surface Acoustic Wave Sensor Used in Electronic Nose for Potential Application in Breath Diagnosis. Sens. Lett. 2011, 9, 884–889. [Google Scholar] [CrossRef]
- Wang, D.; Yu, K.; Wang, Y.S.; Hu, Y.J.; Zhao, C.; Wang, L.; Ying, K.J.; Wang, P. A Hybrid Electronic Noses’ System Based on Mos-Saw Detection Units Intended for Lung Cancer Diagnosis. J. Innov. Opt. Health Sci. 2012, 5, 1150006. [Google Scholar] [CrossRef]
- Zhang, X.F.; Zhang, Z.W.; He, Y.L.; Liu, Y.X.; Li, S.; Fang, J.Y.; Zhang, X.A.; Peng, G. Sniffing lung cancer related biomarkers using an oxidized graphene SAW sensor. Front. Phys. 2016, 11, 116801. [Google Scholar] [CrossRef]
- Saha, K.; Agasti, S.S.; Kim, C.; Li, X.N.; Rotello, V.M. Gold Nanoparticles in Chemical and Biological Sensing. Chem. Rev. 2012, 112, 2739–2779. [Google Scholar] [CrossRef]
- Zhang, X.; Zou, Y.C.; An, C.; Ying, K.J.; Chen, X.; Wang, P. A miniaturized immunosensor platform for automatic detection of carcinoembryonic antigen in EBC. Sens. Actuators B-Chem. 2014, 205, 94–101. [Google Scholar] [CrossRef]
- Zou, Y.; Zhang, X.; An, C.; Ran, C.; Ying, K.; Wang, P. A point-of-care testing system with Love-wave sensor and immunogold staining enhancement for early detection of lung cancer. Biomed. Microdevices 2014, 16, 927–935. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.; Wei, X.; Xue, Y.; Wan, H.; Wang, P. Recent advances in acoustic wave biosensors for the detection of disease-related biomarkers: A review. Anal. Chim. Acta 2021, 1164, 338321. [Google Scholar] [CrossRef] [PubMed]
- Li, S.M.; Wan, Y.; Su, Y.; Fan, C.H.; Bhethanabotla, V.R. Gold nanoparticle-based low limit of detection Love wave biosensor for carcinoembryonic antigens. Biosens. Bioelectron. 2017, 95, 48–54. [Google Scholar] [CrossRef]
- Zou, Y.; Zhou, Y.; Chen, Y.; Zhang, X.; Ran, C. Love wave based portable sensing system for on-line detection of carcinoembryonic antigen in exhaled breath condensate. Biomed. Microdevices 2020, 22, 78. [Google Scholar] [CrossRef] [PubMed]
- Jandas, P.J.; Luo, J.T.; Quan, A.J.; Qiu, C.H.; Cao, W.G.; Fu, C.; Fu, Y.Q. Highly selective and label-free Love-mode surface acoustic wave biosensor for carcinoembryonic antigen detection using a self-assembled monolayer bioreceptor. Appl. Surf. Sci. 2020, 518, 146061. [Google Scholar] [CrossRef]
- Jandas, P.J.; Luo, J.T.; Prabakaran, K.; Chen, F.; Fu, Y.Q. Highly stable, love-mode surface acoustic wave biosensor using Au nanoparticle-MoS2-rGO nano-cluster doped polyimide nanocomposite for the selective detection of carcinoembryonic antigen. Mater. Chem. Phys. 2020, 246, 122800. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.; Xiong, H.; Yu, W.; Ying, K.; Wan, H.; Wang, P. The Love Wave Biosensor for the Detection of the Bacterial Pneumonia Biomarker C-reactive Protein. In Proceedings of the 2022 IEEE International Symposium on Olfaction and Electronic Nose (ISOEN), Aveiro, Portugal, 29 May–1 June 2022; pp. 1–3. [Google Scholar]
- Wilson, A.D. Application of electronic-nose technologies and VOC-biomarkers for the noninvasive early diagnosis of gastrointestinal diseases. Sensors 2018, 18, 2613. [Google Scholar] [CrossRef]
- Xu, Z.q.; Broza, Y.Y.; Ionsecu, R.; Tisch, U.; Ding, L.; Liu, H.; Song, Q.; Pan, Y.y.; Xiong, F.x.; Gu, K.S.; et al. A nanomaterial-based breath test for distinguishing gastric cancer from benign gastric conditions. Br. J. Cancer 2013, 108, 941–950. [Google Scholar] [CrossRef]
- Amal, H.; Leja, M.; Funka, K.; Skapars, R.; Sivins, A.; Ancans, G.; Liepniece-Karele, I.; Kikuste, I.; Lasina, I.; Haick, H. Detection of precancerous gastric lesions and gastric cancer through exhaled breath. Gut 2016, 65, 400–407. [Google Scholar] [CrossRef]
- Sharifi, H.; Tashkhourian, J.; Geramizadeh, B.; Ghohestani, E.; Zangouri, V.; Asadian, F.; Hemmateenejad, B. Metallic Nanocluster-Based Sniffing Device for Identification of Malignancy in Gastric Cancer Tissues. ACS Appl. Nano Mater. 2023, 6, 5578–5590. [Google Scholar] [CrossRef]
- Suter, B.; Anthis, A.H.; Zehnder, A.K.; Mergen, V.; Rosendorf, J.; Gerken, L.R.; Schlegel, A.A.; Korcakova, E.; Liska, V.; Herrmann, I.K. Surgical Sealant with Integrated Shape-Morphing Dual Modality Ultrasound and Computed Tomography Sensors for Gastric Leak Detection. Adv. Sci. 2023, 10, 2301207. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zhang, X.; Chen, G.; Shang, L. Developing sensor materials for screening intestinal diseases. Mater. Futures 2022, 1, 022401. [Google Scholar] [CrossRef]
- Shepherd, S.; McGuire, N.; de Lacy Costello, B.; Ewen, R.; Jayasena, D.; Vaughan, K.; Ahmed, I.; Probert, C.; Ratcliffe, N. The use of a gas chromatograph coupled to a metal oxide sensor for rapid assessment of stool samples from irritable bowel syndrome and inflammatory bowel disease patients. J. Breath Res. 2014, 8, 026001. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.K.; Leggett, C.L.; Wang, K.K. Diagnosing gastrointestinal illnesses using fecal headspace volatile organic compounds. World J. Gastroenterol. 2016, 22, 1639. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, A.; Chandrapalan, S.; Bosch, S.; Bannaga, A.; De Boer, N.K.H.; De Meij, T.G.J.; Leja, M.; Hanna, G.B.; De Vietro, N.; Altomare, D.; et al. The Influence of Mechanical Bowel Preparation on Volatile Organic Compounds for the Detection of Gastrointestinal Disease—A Systematic Review. Sensors 2023, 23, 1377. [Google Scholar] [CrossRef]
- Dalis, C.; Mesfin, F.M.; Manohar, K.; Liu, J.; Shelley, W.C.; Brokaw, J.P.; Markel, T.A. Volatile Organic Compound Assessment as a Screening Tool for Early Detection of Gastrointestinal Diseases. Microorganisms 2023, 11, 1822. [Google Scholar] [CrossRef]
- Westenbrink, E.; Arasaradnam, R.P.; O’Connell, N.; Bailey, C.; Nwokolo, C.; Bardhan, K.D.; Covington, J.A. Development and application of a new electronic nose instrument for the detection of colorectal cancer. Biosens. Bioelectron. 2015, 67, 733–738. [Google Scholar] [CrossRef]
- Yen, Y.-K.; Capua, E.; Naaman, R. Application of a GaAs-based sensor for detecting hemoglobin in gastrointestinal environments. IEEE Sens. J. 2016, 17, 660–666. [Google Scholar] [CrossRef]
- Yonne, P.; Ruud, W.M.S.; Adriaan, C.T.; Sanne, K.B.; de Bart, J.; Peter, D.S. Detection of Barrett’s oesophagus through exhaled breath using an electronic nose device. Gut 2020, 69, 1169. [Google Scholar] [CrossRef]
- Tiele, A.; Wicaksono, A.; Kansara, J.; Arasaradnam, R.P.; Covington, J.A. Breath Analysis Using eNose and Ion Mobility Technology to Diagnose Inflammatory Bowel Disease—A Pilot Study. Biosensors 2019, 9, 55. [Google Scholar] [CrossRef]
- Brinza, M.; Schröder, S.; Ababii, N.; Gronenberg, M.; Strunskus, T.; Pauporte, T.; Adelung, R.; Faupel, F.; Lupan, O. Two-in-One Sensor Based on PV4D4-Coated TiO2 Films for Food Spoilage Detection and as a Breath Marker for Several Diseases. Biosensors 2023, 13, 538. [Google Scholar] [CrossRef] [PubMed]
- Neetha, A.S.; Rao, K.V. Broccoli-shaped nano florets-based gastrointestinal diseases detection by copper oxide chitosan. Mater. Today Chem. 2023, 30, 101503. [Google Scholar] [CrossRef]
- Vasquez, S.; Angeli, M.A.C.; Polo, A.; Costantini, A.; Petrelli, M.; Avancini, E.; Di Cagno, R.; Gobbetti, M.; Gaiardo, A.; Valt, M.; et al. In vitro gastrointestinal gas monitoring with carbon nanotube sensors. Sci. Rep. 2024, 14, 825. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-zadeh, K.; Ha, N.; Ou, J.Z.; Berean, K.J. Ingestible Sensors. ACS Sens. 2017, 2, 468–483. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, R.; Ibrahim, A.-B.; David, G.T.; Wayne, Y.; Leo, K.C.; Ebubekir, A. Smart capsules for sensing and sampling the gut: Status, challenges and prospects. Gut 2024, 73, 186. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Berean, K.J.; Ha, N.; Chrimes, A.F.; Xu, K.; Grando, D.; Ou, J.Z.; Pillai, N.; Campbell, J.L.; Brkljača, R.; et al. A human pilot trial of ingestible electronic capsules capable of sensing different gases in the gut. Nat. Electron. 2018, 1, 79–87. [Google Scholar] [CrossRef]
- Cheng, C.; Wu, Y.; Li, X.; An, Z.; Lu, Y.; Zhang, F.; Su, B.; Liu, Q. A wireless, ingestible pH sensing capsule system based on iridium oxide for monitoring gastrointestinal health. Sens. Actuators B Chem. 2021, 349, 130781. [Google Scholar] [CrossRef]
- Huang, W.; Chen, R.; Peng, Y.; Duan, F.; Huang, Y.; Guo, W.; Chen, X.; Nie, L. In Vivo Quantitative Photoacoustic Diagnosis of Gastric and Intestinal Dysfunctions with a Broad pH-Responsive Sensor. ACS Nano 2019, 13, 9561–9570. [Google Scholar] [CrossRef]
- De la Paz, E.; Maganti, N.H.; Trifonov, A.; Jeerapan, I.; Mahato, K.; Yin, L.; Sonsa-ard, T.; Ma, N.; Jung, W.; Burns, R.; et al. A self-powered ingestible wireless biosensing system for real-time in situ monitoring of gastrointestinal tract metabolites. Nat. Commun. 2022, 13, 7405. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).