Study of the Gas Sensing Performance of Ni-Doped Perovskite-Structured LaFeO3 Nanospheres
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Nanocomposite Synthesis
2.3. Characterizations
2.4. Test System and Test Procedure
3. Result and Discussion
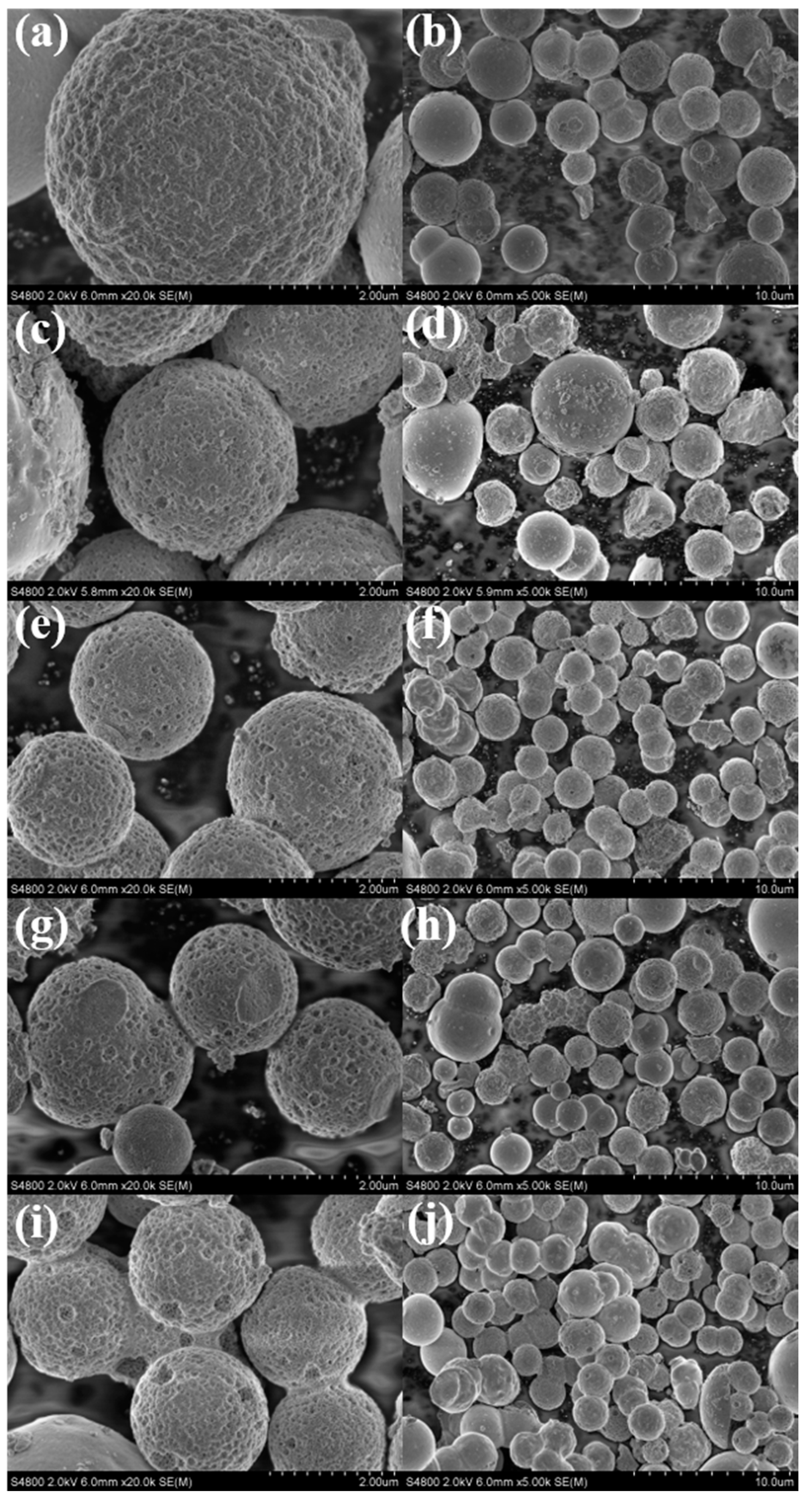
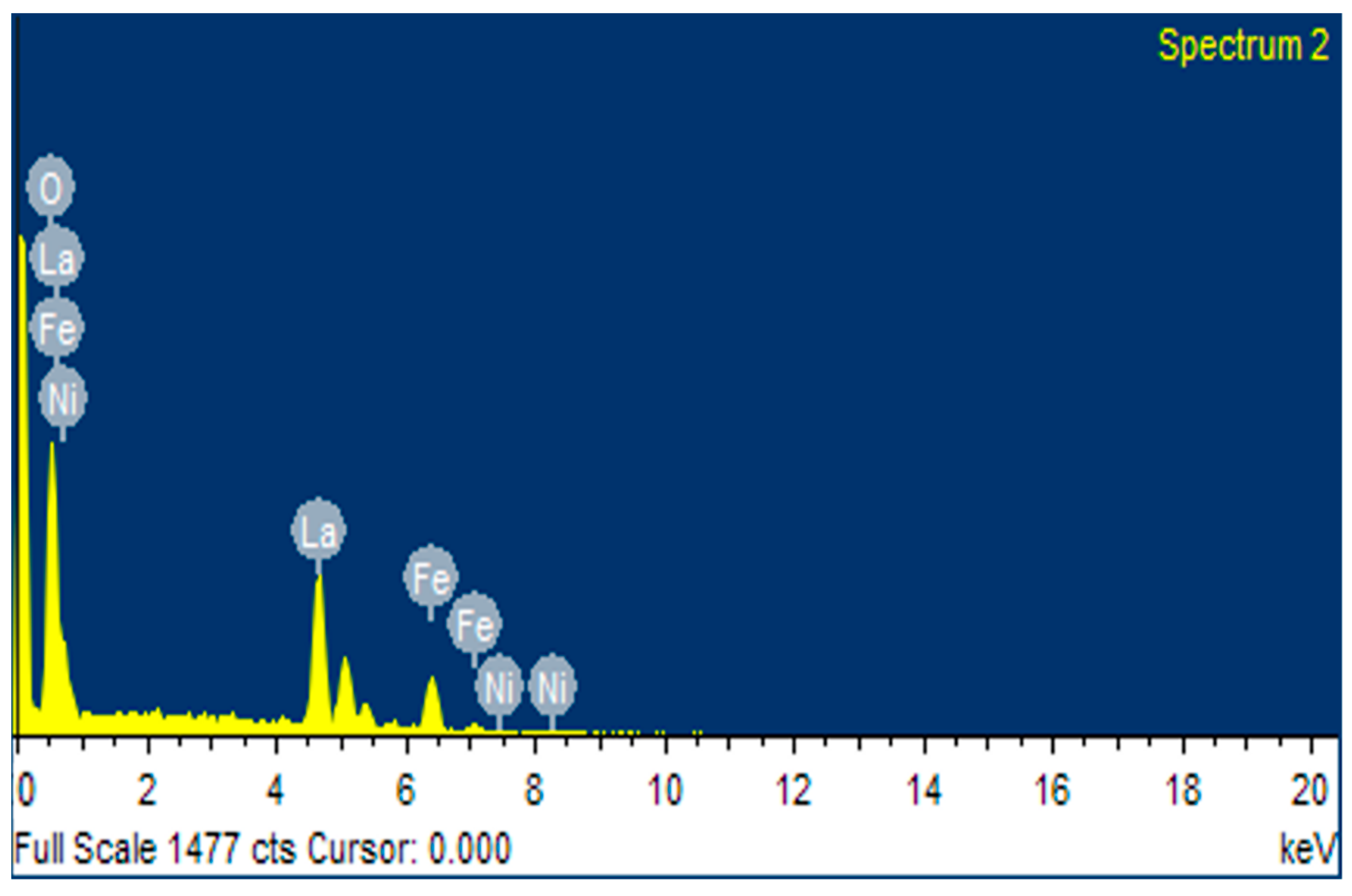
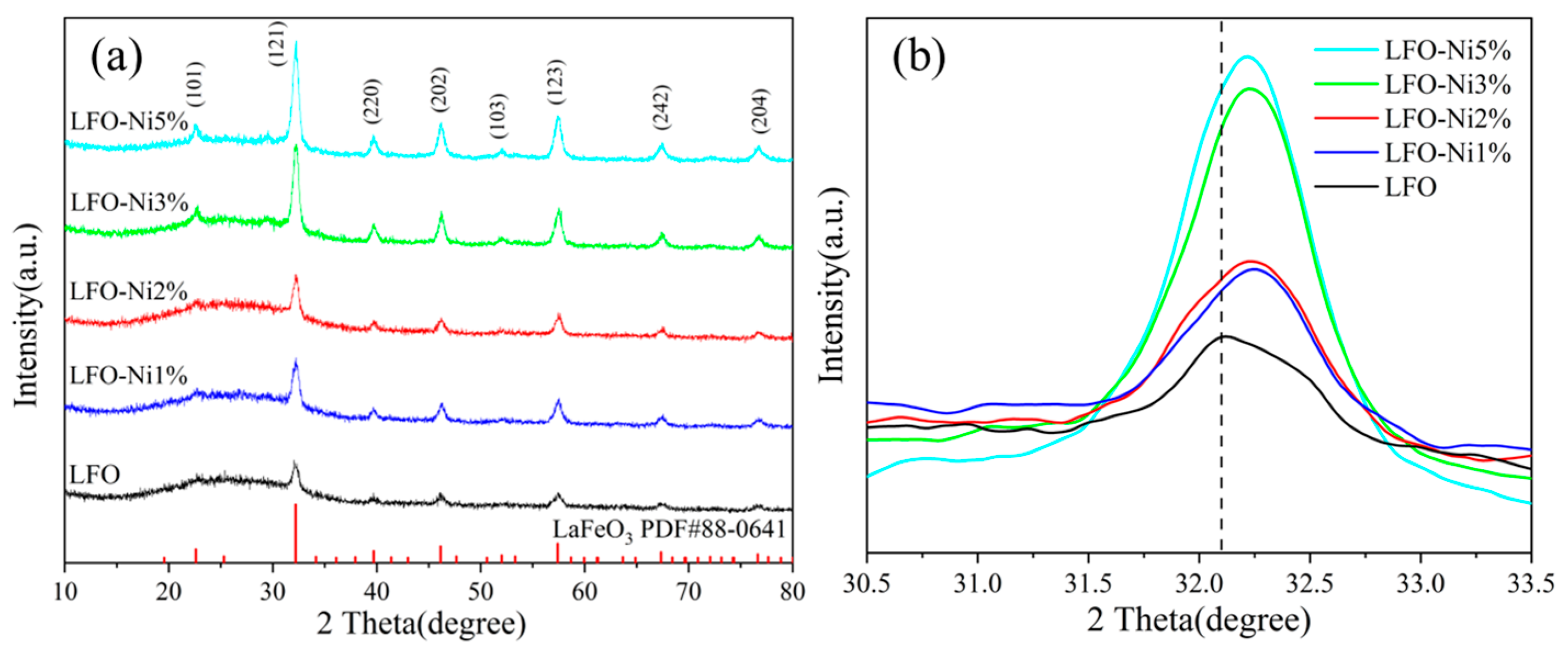
3.1. Materials Characterization
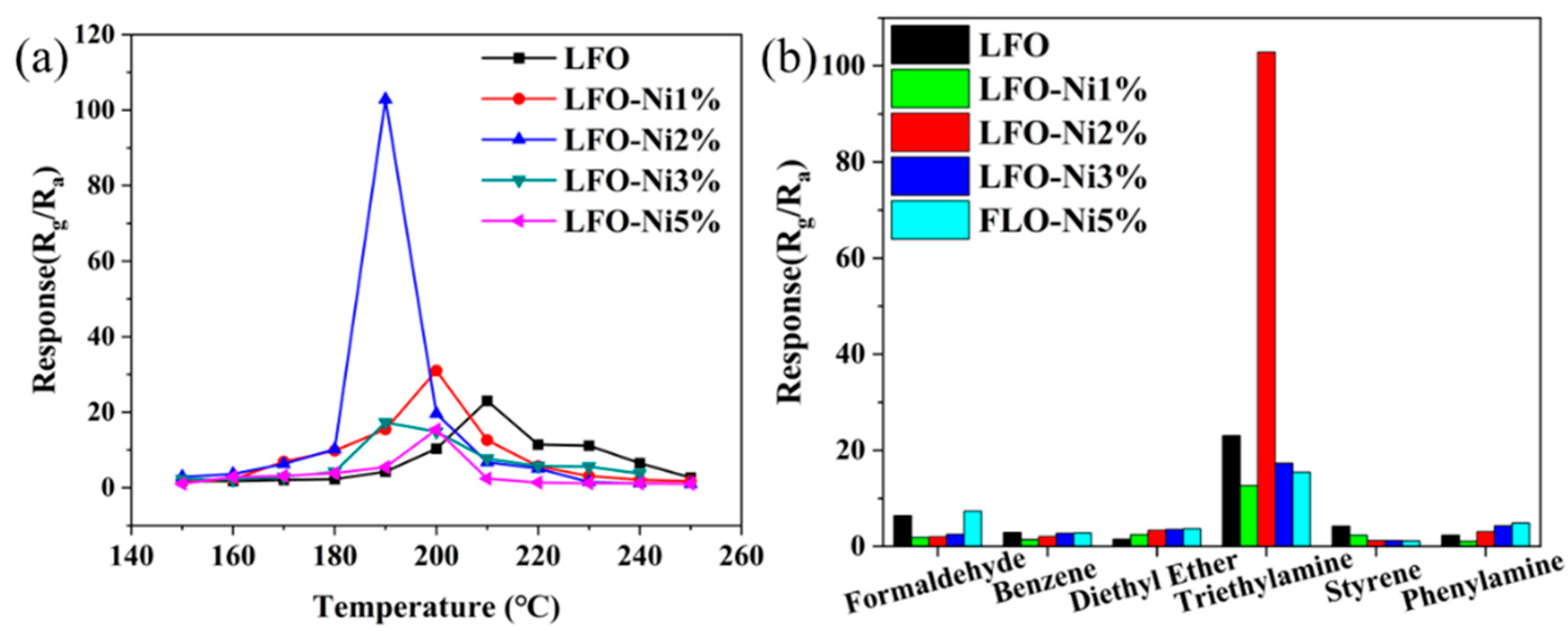
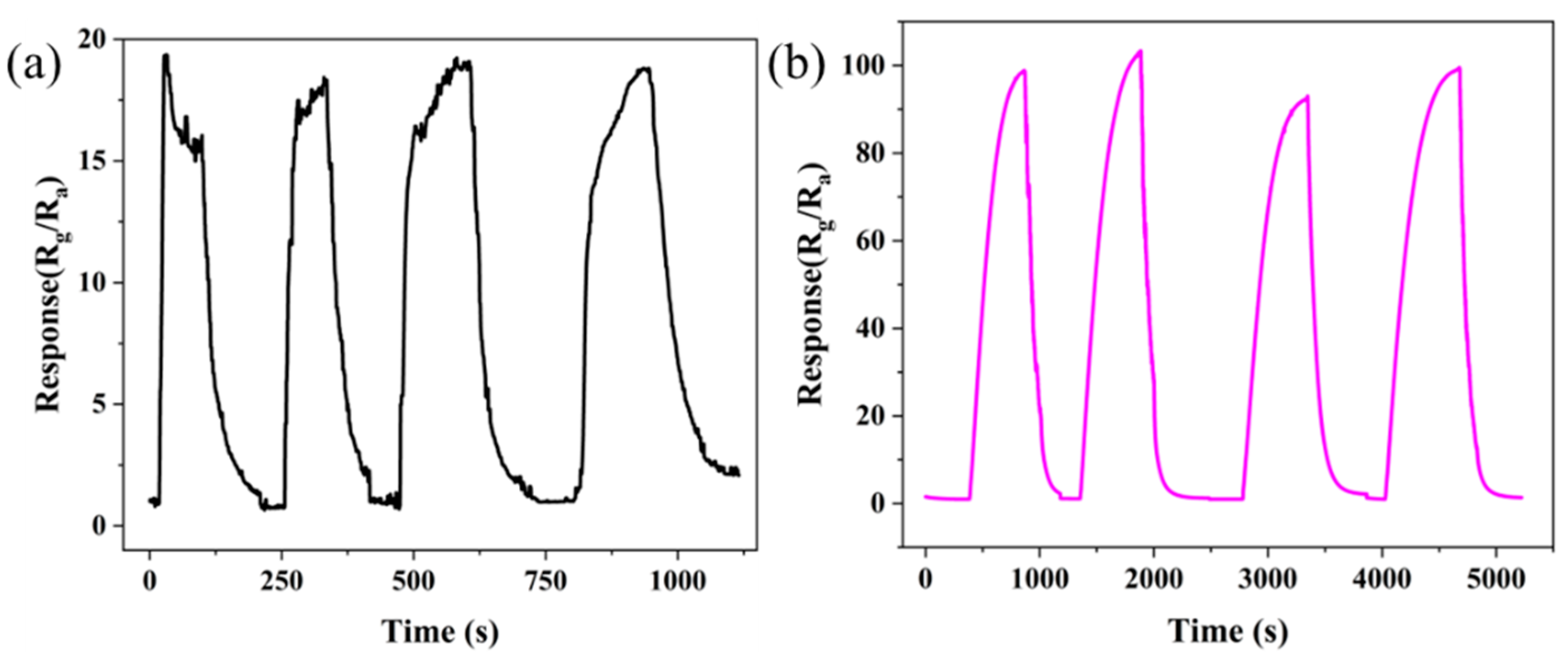
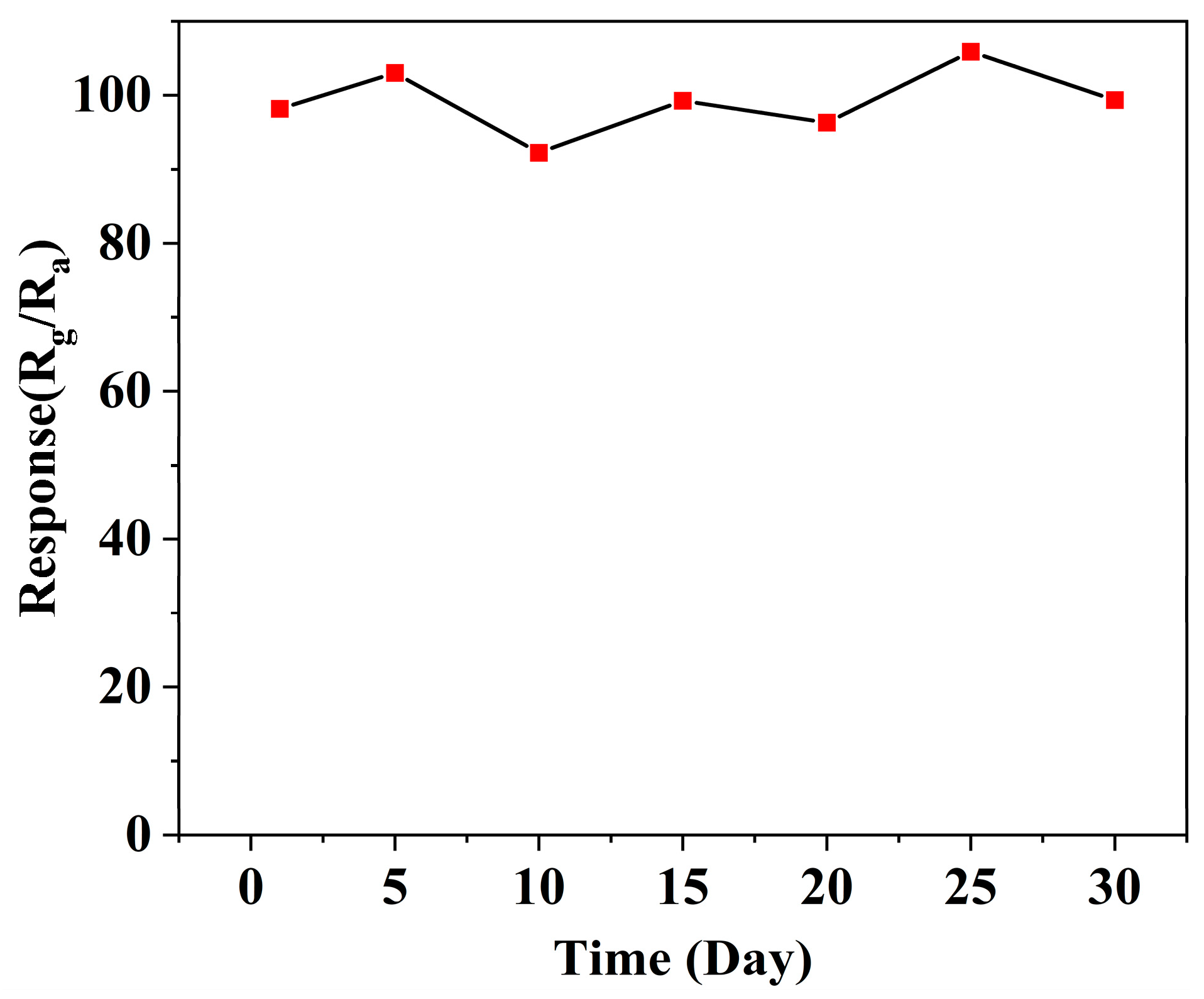
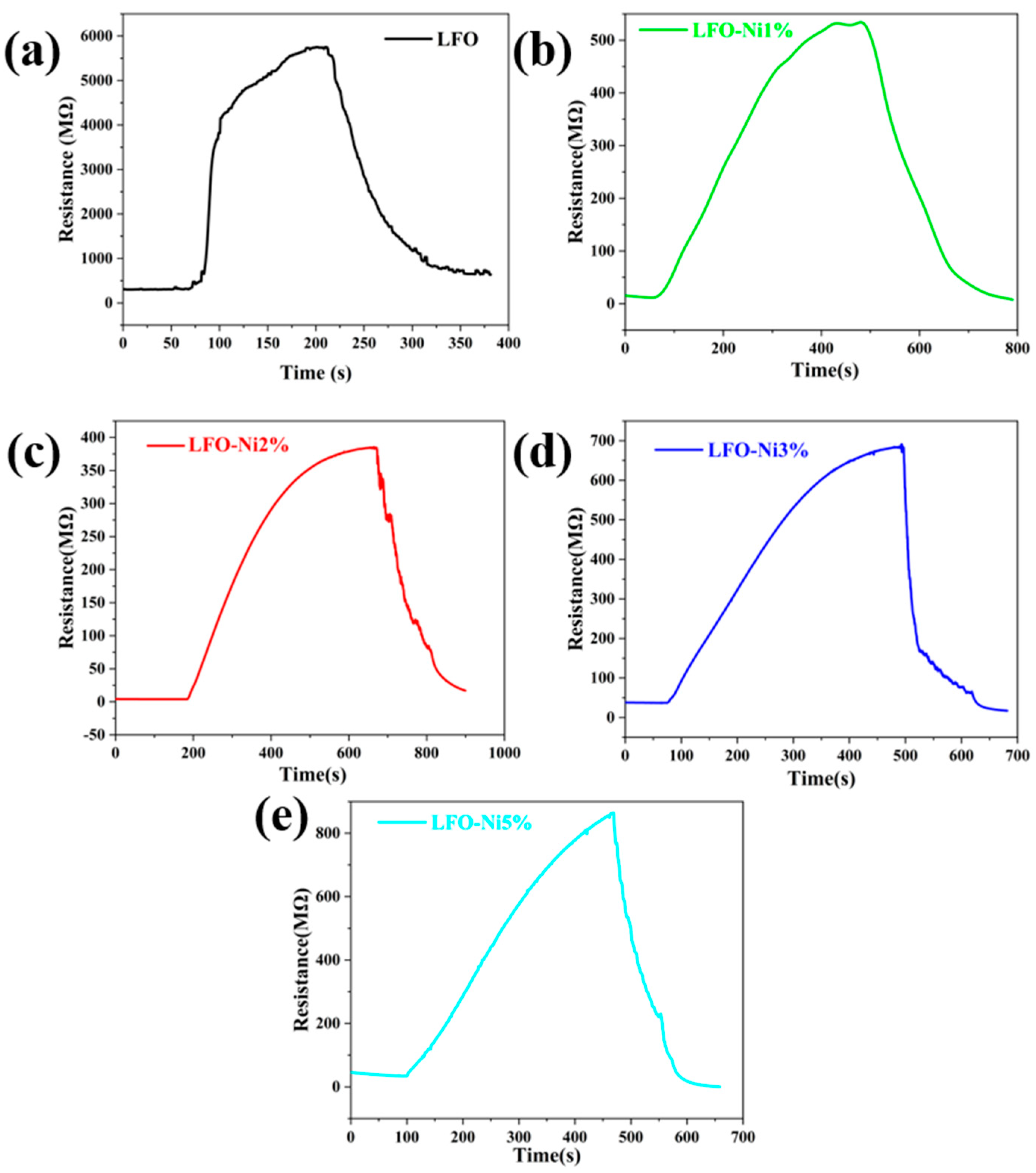
3.2. Gas Sensitive Properties of Materials
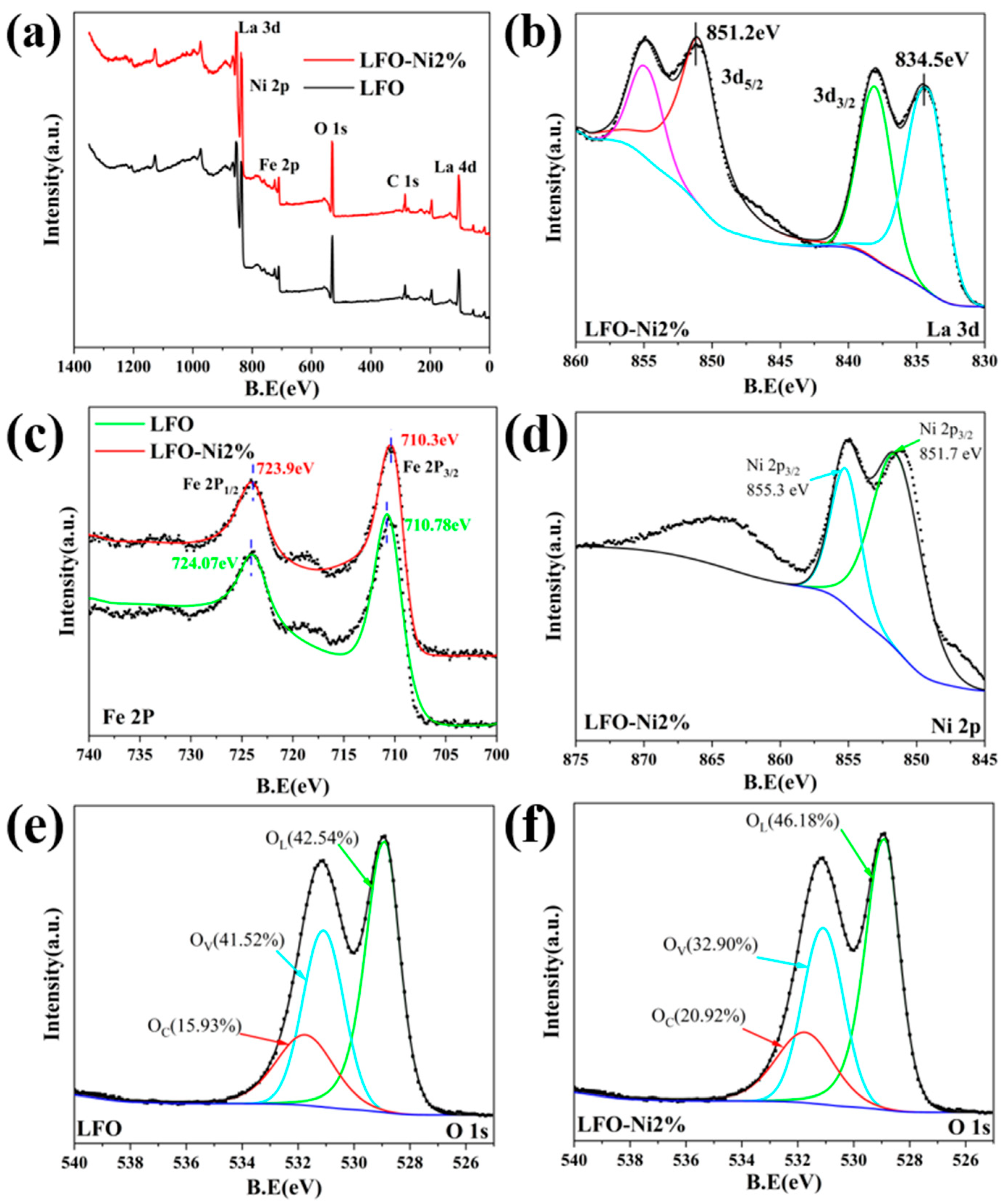
3.3. Gas-Sensitive Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, D.; Wang, Q.; Wang, W.; Chen, Y.; Ruan, S. Hollow ZnWO4/In2O3 Nanotubes for Ultrasensitive and Rapid Trace Detection of Triethylamine. ACS Appl. Nano Mater. 2023, 6, 10581–10589. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.-J.; Chen, S.-S.; Liu, C.-C.; Chen, L.; Liu, Z.-G.; Guo, Z. Chemiresistive Sensor Based on Hollow Fe2O3 Octahedrons Incorporated into Porous In2O3 Nanofibers for Enhanced Sensing Performance and Recognition toward Triethylamine. Sens. Actuators B Chem. 2023, 393, 134129. [Google Scholar] [CrossRef]
- Cai, L.-X.; Chen, L.; Sun, X.-Q.; Geng, J.; Liu, C.-C.; Wang, Y.; Guo, Z. Ultra-Sensitive Triethylamine Gas Sensors Based on Polyoxometalate-Assisted Synthesis of ZnWO4/ZnO Hetero-Structured Nanofibers. Sens. Actuators B Chem. 2022, 370, 132422. [Google Scholar] [CrossRef]
- Liu, T.; Liu, K.; Chen, X.; Liu, X.; Yin, X.-T. In-Doped ZnO/NiO Nanosheet as Highly Selective Triethylamine Sensor. J. Mater. Res. 2023, 38, 4747–4758. [Google Scholar] [CrossRef]
- Miao, G.-Y.; Chen, S.-S.; Wang, Y.-J.; Guo, Z.; Huang, X.-J. SnO2 Nanostructures Exposed with Various Crystal Facets for Temperature-Modulated Sensing of Volatile Organic Compounds. ACS Appl. Nano Mater. 2022, 5, 10636–10644. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, B.; Zhang, L.; Fan, J.; Yu, J. 0D/2D CdS/ZnO Composite with n-n Heterojunction for Efficient Detection of Triethylamine. J. Colloid Interface Sci. 2021, 600, 898–909. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Tang, X. Facile Synthesis of MOF-Derived Hierarchical Flower-Like Sr-Doped Co3O4 Structure for Effective TEA Sensing. IEEE Sens. J. 2022, 22, 23773–23779. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, L.; Fan, J.; Yu, J. Semiconductor Gas Sensor for Triethylamine Detection. Small 2022, 18, 2104984. [Google Scholar] [CrossRef]
- Ji, H.; Mi, C.; Yuan, Z.; Liu, Y.; Zhu, H.; Meng, F. Multicomponent Gas Detection Method via Dynamic Temperature Modulation Measurements Based on Semiconductor Gas Sensor. IEEE Trans. Ind. Electron. 2023, 70, 6395–6404. [Google Scholar] [CrossRef]
- Yang, D.; Gopal, R.A.; Lkhagvaa, T.; Choi, D. Metal-Oxide Gas Sensors for Exhaled-Breath Analysis: A Review. Meas. Sci. Technol. 2021, 32, 102004. [Google Scholar] [CrossRef]
- Krishna, K.G.; Parne, S.; Pothukanuri, N.; Kathirvelu, V.; Gandi, S.; Joshi, D. Nanostructured Metal Oxide Semiconductor-Based Gas Sensors: A Comprehensive Review. Sens. Actuators A Phys. 2022, 341, 113578. [Google Scholar] [CrossRef]
- Meng, F.; Qi, T.; Zhang, J.; Zhu, H.; Yuan, Z.; Liu, C.; Qin, W.; Ding, M. MoS2-Templated Porous Hollow MoO3 Microspheres for Highly Selective Ammonia Sensing via a Lewis Acid-Base Interaction. IEEE Trans. Ind. Electron. 2022, 69, 960–970. [Google Scholar] [CrossRef]
- Sun, Y.; Fan, H.; Zhu, S.; Wang, H.; Dong, W.; Al-Bahrani, M.; Wang, W.; Ma, L. Nitrogen-Doped ZnO Microspheres with a Yolk-Shell Structure for High Sensing Response of Triethylamine. Sens. Actuators B Chem. 2023, 389, 133882. [Google Scholar] [CrossRef]
- Meng, F.-J.; Xin, R.-F.; Li, S.-X. Metal Oxide Heterostructures for Improving Gas Sensing Properties: A Review. Materials 2022, 16, 263. [Google Scholar] [CrossRef] [PubMed]
- Ou, L.-X.; Liu, M.-Y.; Zhu, L.-Y.; Zhang, D.W.; Lu, H.-L. Recent Progress on Flexible Room-Temperature Gas Sensors Based on Metal Oxide Semiconductor. Nano-Micro Lett. 2022, 14, 206. [Google Scholar] [CrossRef]
- Li, F.; Lv, Q.; Gao, L.; Zhong, X.; Zhou, J.; Gao, X. Ultra-Sensitive Ag-LaFeO3 for Selective Detection of Ethanol. J. Mater. Sci. 2021, 56, 15061–15068. [Google Scholar] [CrossRef]
- Xu, D.; Zhang, Y.; Deng, Z.; Zi, B.; Zeng, J.; Song, Z.; Lu, Q.; Zhang, J.; Zhao, J.; Liu, Q. Metal-Organic Framework-Derived LaFeO3@SnO2/Ag p–n Heterojunction Nanostructures for Formaldehyde Detection. ACS Appl. Nano Mater. 2022, 5, 14367–14376. [Google Scholar] [CrossRef]
- Cao, E.; Zhang, Y.; Sun, L.; Sun, B.; Hao, W.; Zhang, Y.; Nie, Z. WO3-LaFeO3 Nanocomposites for Highly Sensitive Detection of Acetone Vapor at Low Operating Temperatures. Chemosensors 2023, 11, 439. [Google Scholar] [CrossRef]
- Jing, Z.-J.; Zhong, Z.-C.; Zhang, C.-M.; Gao, Q.-C. Co-Doped LaFeO3 Gas Sensor for Fast Low-Power Acetone Detection. J. Nanoelectron. Optoelectron. 2022, 17, 775–784. [Google Scholar] [CrossRef]
- Hao, P.; Song, P.; Yang, Z.; Wang, Q. Synthesis of Novel RuO2/LaFeO3 Porous Microspheres Its Gas Sensing Performances towards Triethylamine. J. Alloys Compd. 2019, 806, 960–967. [Google Scholar] [CrossRef]
- Li, Z.; Lou, C.; Lei, G.; Lu, G.; Pan, H.; Liu, X.; Zhang, J. Regulation of Electronic Properties of ZnO/In2O3 Heterospheres via Atomic Layer Deposition for High Performance NO2 Detection. CrystEngComm 2021, 23, 5060–5069. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Jin, X.; Sun, G.; Cao, J.; Wang, Y. The Effects of Co Doping on the Gas Sensing Performance of In2O3 Porous Nanospheres. Sens. Actuators B Chem. 2024, 403, 135155. [Google Scholar] [CrossRef]
- Tamilalagan, E.; Muthumariappan, A.; Chen, T.; Chen, S.-M.; Maheshwaran, S.; Huang, P.-J. A Highly Selective Enzyme-Free Amperometric Detection of Glucose Using Perovskite-Type Lanthanum Cobaltite (LaCoO3). J. Electrochem. Soc. 2021, 168, 086501. [Google Scholar] [CrossRef]
- Qin, W.; Zhang, R.; Yuan, Z.; Xing, C.; Meng, F. Preparation of P-LaFeO₃/n-Fe2O3 Heterojunction Composites by One-Step Hydrothermal Method and Gas Sensing Properties for Acetone. IEEE Trans. Instrum. Meas. 2022, 71, 1501809. [Google Scholar] [CrossRef]
- Qin, W.; Yuan, Z.; Gao, H.; Meng, F. Ethanol Sensors Based on Porous In2O3 Nanosheet-Assembled Micro-Flowers. Sensors 2020, 20, 3353. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yuan, Z.; Ji, H.; Zhang, H.; Meng, F.; Gao, H. Highly Sensitive Ethanol Sensor Based on Two-Dimensional Layered Mesoporous In2O3 Nanosheets. IEEE Trans. Nanotechnol. 2020, 19, 486–491. [Google Scholar] [CrossRef]
- Meng, D.; Liu, D.; Wang, G.; Shen, Y.; San, X.; Si, J.; Meng, F. In-Situ Growth of Ordered Pd-Doped ZnO Nanorod Arrays on Ceramic Tube with Enhanced Trimethylamine Sensing Performance. Appl. Surf. Sci. 2019, 463, 348–356. [Google Scholar] [CrossRef]
- Meng, D.; Qiao, T.; Wang, G.; Shen, Y.; San, X.; Pan, Y.; Meng, F. NiO-Functionalized In2O3 Flower-like Structures with Enhanced Trimethylamine Gas Sensing Performance. Appl. Surf. Sci. 2022, 577, 151877. [Google Scholar] [CrossRef]
- Qin, W.; Yuan, Z.; Shen, Y.; Meng, F. Macroporous Perovskite-Structured LaFeO3 Microspheres and Their Highly Sensitive and Selective Sensing Properties to Alcohols Gas. IEEE Trans. Ind. Electron. 2023, 70, 3188–3198. [Google Scholar] [CrossRef]
- Gu, C.; Shanshan, L.; Huang, J.; Shi, C.; Liu, J. Preferential Growth of Long ZnO Nanowires and Its Application in Gas Sensor. Sens. Actuators B Chem. 2013, 177, 453–459. [Google Scholar] [CrossRef]
- Li, H.; Yu, K.; Wan, C.; Zhu, J.; Li, X.; Tong, S.; Zhao, Y. Comparison of the Nickel Addition Patterns on the Catalytic Performances of LaCoO3 for Low-Temperature CO Oxidation. Catal. Today 2017, 281, 534–541. [Google Scholar] [CrossRef]
- Luo, Y.; Zheng, Y.; Feng, X.; Lin, D.; Qian, Q.; Wang, X.; Zhang, Y.; Chen, Q.; Zhang, X. Controllable P Doping of the LaCoO3 Catalyst for Efficient Propane Oxidation: Optimized Surface Co Distribution and Enhanced Oxygen Vacancies. ACS Appl. Mater. Interfaces 2020, 12, 23789–23799. [Google Scholar] [CrossRef]
- Cao, E.; Qin, Y.; Cui, T.; Sun, L.; Hao, W.; Zhang, Y. Influence of Na Doping on the Magnetic Properties of LaFeO3 Powders and Dielectric Properties of LaFeO3 Ceramics Prepared by Citric Sol-Gel Method. Ceram. Int. 2017, 43, 7922–7928. [Google Scholar] [CrossRef]
- Peng, Q.; Shan, B.; Wen, Y.; Chen, R. Enhanced Charge Transport of LaFeO3 via Transition Metal (Mn, Co, Cu) Doping for Visible Light Photoelectrochemical Water Oxidation. Int. J. Hydrogen Energy 2015, 40, 15423–15431. [Google Scholar] [CrossRef]
- Cao, K.; Cao, E.; Zhang, Y.; Hao, W.; Sun, L.; Peng, H. The Influence of Nonstoichiometry on Electrical Transport and Ethanol Sensing Characteristics for Nanocrystalline LaFeO3-Sensors. Sens. Actuators B Chem. 2016, 230, 592–599. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, Y.; Wang, G.; Li, S.; Wang, L.; Han, Y.; Jiang, X.; Wei, A. High Toluene Sensing Properties of NiO-SnO2 Composite Nanofiber Sensors Operating at 330 °C. Sens. Actuators B Chem. 2011, 160, 448–454. [Google Scholar] [CrossRef]
- Li, Z.; Li, S.; Song, Z.; Yang, X.; Wang, Z.; Zhang, H.; Guo, L.; Sun, C.; Liu, H.; Shao, J.; et al. Influence of Nickel Doping on Ultrahigh Toluene Sensing Performance of Core-Shell ZnO Microsphere Gas Sensor. Chemosensors 2022, 10, 327. [Google Scholar] [CrossRef]
- Zhang, J.; Li, M.; Feng, Z.; Chen, J.; Li, C. UV Raman Spectroscopic Study on TiO2. I. Phase Transformation at the Surface and in the Bulk. J. Phys. Chem. B 2006, 110, 927–935. [Google Scholar] [CrossRef]
- Xue, D.; Wang, Y.; Cao, J.; Zhang, Z. Hydrothermal Synthesis of CeO2-SnO2 Nanoflowers for Improving Triethylamine Gas Sensing Property. Nanomaterials 2018, 8, 1025. [Google Scholar] [CrossRef]
- Zheng, C.; Zhang, C.; He, L.; Zhang, K.; Zhang, J.; Jin, L.; Asiri, A.M.; Alamry, K.A.; Chu, X. ZnFe2O4/ZnO Nanosheets Assembled Microspheres for High Performance Trimethylamine Gas Sensing. J. Alloys Compd. 2020, 849, 156461. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, N.; Bu, W.; Chuai, X.; Zhou, Z.; Hu, C.; Wang, T.; Sun, P.; Liu, F.; Lu, G. Au Modified Nd-Doped In2O3 Hollow Microspheres for High Performance Triethylamine Gas Sensor. Vacuum 2023, 214, 112240. [Google Scholar] [CrossRef]
- Xu, X.; Wang, X.; Liu, W.; Wang, S.; Jiang, H.; Ma, S.; Yuan, F.; Ma, N. Triethylamine Gas Sensors Based on BiOBr Microflowers Decorated with ZnO Nanocrystals. ACS Appl. Nano Mater. 2022, 5, 15837–15846. [Google Scholar] [CrossRef]
- Wu, R.; Liu, T.; Chen, X.; Yin, X.-T. Shape-Controlled Multi-Dimensional In2O3/Mn2O3 p-n Heterojunction for Triethylamine Detection. J. Alloys Compd. 2023, 960, 170527. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, J.; Wang, Y.; Yi, G.; Sun, G.; Wang, T.; Yang, B.; Zhang, Z. Construction of ZnCo2O4 Decorated ZnO Heterostructure Materials for Sensing Triethylamine with Dramatically Enhanced Performance. New J. Chem. 2023, 47, 22226–22234. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; Han, L.; Li, X.; Xu, Y. Hierarchical Kiwifruit-like ZnO/ZnFe2O4 Heterostructure for High-Sensitive Triethylamine Gaseous Sensor. Sens. Actuators B Chem. 2021, 344, 130251. [Google Scholar] [CrossRef]
- Qin, W.; Yuan, Z.; Shen, Y.; Zhang, R.; Meng, F. Phosphorus-Doped Porous Perovskite LaFe1−xPxO3−δ Nanosheets with Rich Surface Oxygen Vacancies for ppb Level Acetone Sensing at Low Temperature. Chem. Eng. J. 2022, 431, 134280. [Google Scholar] [CrossRef]
- Yuan, Z.; Li, J.; Meng, F. High Response N-Propanol Sensor Based on Co-Modified ZnO Nanorods. J. Alloys Compd. 2022, 910, 164971. [Google Scholar] [CrossRef]
- Shuai, Y.; Peng, R.; He, Y.; Liu, X.; Wang, X.; Guo, W. NiO/BiVO4 p-n Heterojunction Microspheres for Conductometric Triethylamine Gas Sensors. Sens. Actuators B Chem. 2023, 384, 133625. [Google Scholar] [CrossRef]
- Yao, Y.; Liao, G.; Dong, W.; Cui, H.; Zhao, Z.; Han, X. Ag@ZnO Yolk-Shell Nanospheres for High-Performance Ethanol Sensor. Chem. Phys. Lett. 2022, 791, 139391. [Google Scholar] [CrossRef]
- Yin, Y.; Shen, Y.; Zhou, P.; Lu, R.; Li, A.; Zhao, S.; Liu, W.; Wei, D.; Wei, K. Fabrication, Characterization and n-Propanol Sensing Properties of Perovskite-Type ZnSnO3 Nanospheres Based Gas Sensor. Appl. Surf. Sci. 2020, 509, 145335. [Google Scholar] [CrossRef]
- Zhao, S.; Shen, Y.; Zhou, P.; Hao, F.; Xu, X.; Gao, S.; Wei, D.; Ao, Y.; Shen, Y. Enhanced NO2 Sensing Performance of ZnO Nanowires Functionalized with Ultra-Fine In2O3 Nanoparticles. Sens. Actuators B Chem. 2020, 308, 127729. [Google Scholar] [CrossRef]
- Jia, X.; Liu, T.; Feng, S.; Yu, S.; Zhang, J.; Yang, J.; Wang, S.; Li, Y.; Song, H. Construction of ZnO@ZIF-CoZn Bimetallic Core-Shell Nanospheres for Enhanced Ethanol Sensing Performance. Vacuum 2023, 215, 112271. [Google Scholar] [CrossRef]



| Materials | Temperature (°C) | Concentration (ppm) | Response | Reference |
|---|---|---|---|---|
| ZnFe2O4/ZnO | 240 | 100 | 31.5 | [40] |
| 2wt% Au/Nd-In2O3 | 250 | 100 | 61 | [41] |
| BiOBr/ZnO | 200 | 100 | 20.57 | [42] |
| In2O3/Mn2O3 | 180 | 100 | 44.3 | [43] |
| RuO2/LaFeO3 | 260 | 100 | 60.4 | [20] |
| ZC/ZnO-2 | 280 | 100 | 78.684 | [44] |
| ZnO/ZnFe2O4 | 200 | 100 | 40.15 | [45] |
| LFO-Ni2% | 190 | 100 | 102 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, F.; Yu, Z.; Zhang, R.; Gao, H.; Yuan, Z. Study of the Gas Sensing Performance of Ni-Doped Perovskite-Structured LaFeO3 Nanospheres. Chemosensors 2024, 12, 65. https://doi.org/10.3390/chemosensors12040065
Meng F, Yu Z, Zhang R, Gao H, Yuan Z. Study of the Gas Sensing Performance of Ni-Doped Perovskite-Structured LaFeO3 Nanospheres. Chemosensors. 2024; 12(4):65. https://doi.org/10.3390/chemosensors12040065
Chicago/Turabian StyleMeng, Fanli, Zhenhua Yu, Renze Zhang, Hongliang Gao, and Zhenyu Yuan. 2024. "Study of the Gas Sensing Performance of Ni-Doped Perovskite-Structured LaFeO3 Nanospheres" Chemosensors 12, no. 4: 65. https://doi.org/10.3390/chemosensors12040065
APA StyleMeng, F., Yu, Z., Zhang, R., Gao, H., & Yuan, Z. (2024). Study of the Gas Sensing Performance of Ni-Doped Perovskite-Structured LaFeO3 Nanospheres. Chemosensors, 12(4), 65. https://doi.org/10.3390/chemosensors12040065







