All-Solid-State Potentiometric Sensor Based on Graphene Oxide as Ion-to-Electron Transducer for Nitrate Detection in Water Samples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Solutions
2.2. Preparation of All-Solid-State NO3−–ISEs
2.3. Characterization of GO Solid-Contact
2.4. Evaluation of All-Solid-State NO3−–ISEs
2.5. Water Samples Analysis
3. Results and Discussion
3.1. Characterization of GO Layer
3.2. Potentiometric Response of GO-Based All-Solid-State NO3–ISEs
3.3. Electrochemical Studies
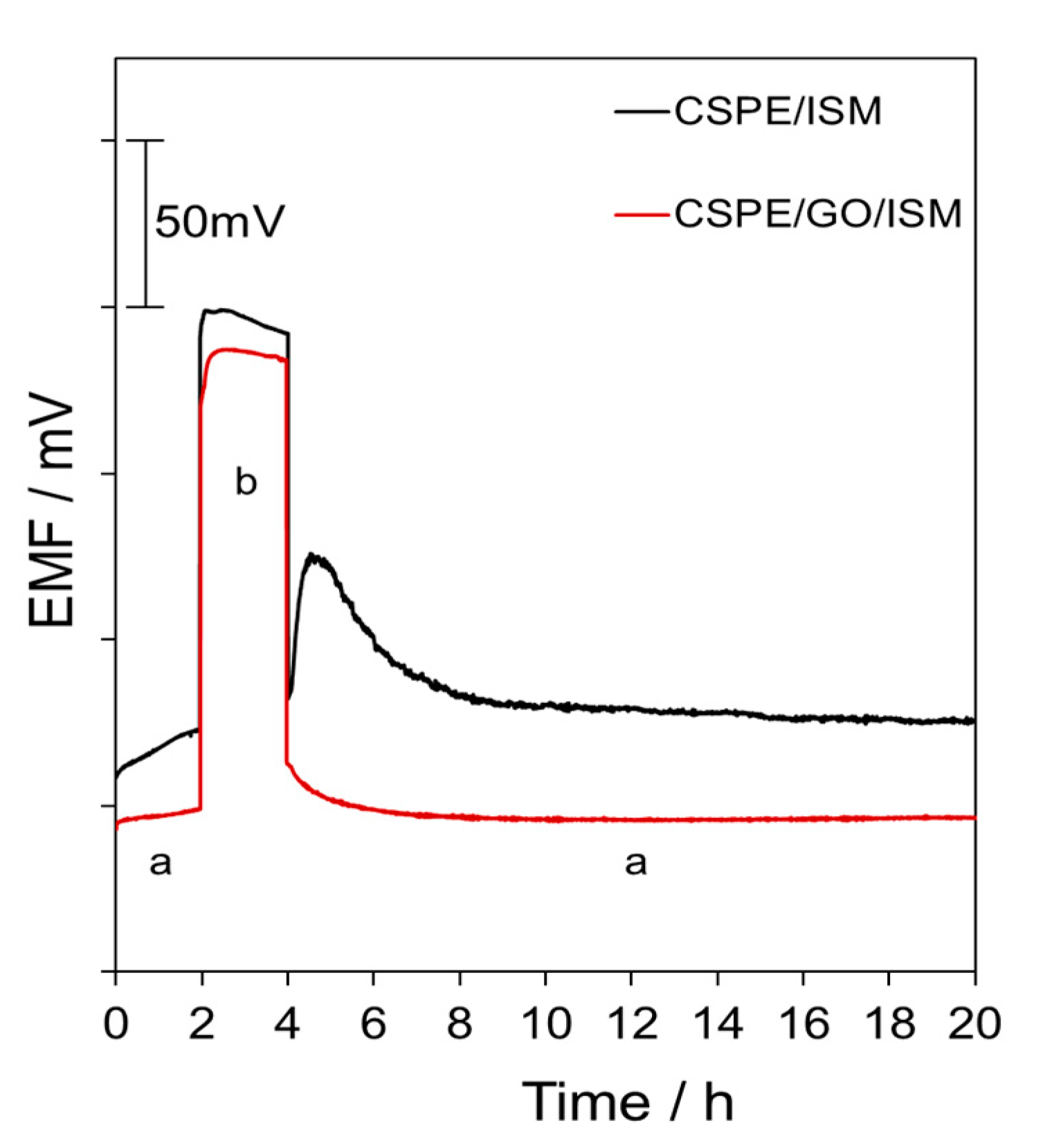
3.4. Water Layer Test and Potential Stability
3.5. Analytical Application of GO-Based All-Solid-State NO3− Electrodes
3.6. Comparison of GO-Based All-Solid-State NO3− Electrode with Previously Reported Electrodes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wetzel, R.G. (Ed.) The nitrogen cycle. In Limnology, 3rd ed.; Academic Press: San Diego, CA, USA, 2001; pp. 205–237. [Google Scholar]
- Fields, S. Global nitrogen: Cycling out of control. Environ. Health Perspect. 2004, 112, A556–A563. [Google Scholar] [CrossRef]
- Pięk, M.; Piech, R.; Paczosa-Bator, B. Improved nitrate sensing using solid contact ion selective electrodes based on TTF and its radical salt. J. Electrochem. Soc. 2015, 162, B257–B263. [Google Scholar] [CrossRef]
- Directive, C. 91/676/EEC of 12 December 1991 concerning the protection of waters against pollution caused by nitrates from agricultural sources. Off. J. 1991, 34, 375. [Google Scholar]
- Ward, M.H.; Jones, R.R.; Brender, J.D.; de Kok, T.M.; Weyer, P.J.; Nolan, B.T.; Villanueva, C.M.; van Breda, S.G. Drinking water nitrate and human health: An updated review. Int. J. Environ. Res. Public Health 2018, 15, 1557. [Google Scholar] [CrossRef] [PubMed]
- de Vries, W. Impacts of nitrogen emissions on ecosystems and human health: A mini review. Curr. Opin. Environ. Sci. Health 2021, 21, 100249. [Google Scholar] [CrossRef]
- Wang, Q.-H.; Yu, L.-J.; Liu, Y.; Lin, L.; Lu, R.-G.; Zhu, J.-P.; He, L.; Lu, Z.-L. Methods for the detection and determination of nitrite and nitrate: A review. Talanta 2017, 165, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Anil, A.G.; Kumar, V.; Kapoor, D.; Subramanian, S.; Singh, J.; Ramamurthy, P.C. Nitrates in the environment: A critical review of their distribution, sensing techniques, ecological effects and remediation. Chemosphere 2022, 287, 131996. [Google Scholar] [CrossRef]
- Gil, R.; Amorim, C.G.; Araújo, A.N.; Montenegro, M.C.B.S.M. Process analysis: Electroanalytical techniques. In Encyclopedia of Analytical Science, 3rd ed.; Worsfold, P., Poole, C., Townshend, A., Miró, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 384–388. [Google Scholar]
- Alahi, M.E.E.; Mukhopadhyay, S.C. Detection methods of nitrate in water: A review. Sens. Actuators A Phys. 2018, 280, 210–221. [Google Scholar] [CrossRef]
- Ryu, H.; Thompson, D.; Huang, Y.; Li, B.; Lei, Y. Electrochemical sensors for nitrogen species: A review. Sens. Actuators Rep. 2020, 2, 100022. [Google Scholar] [CrossRef]
- Jiang, C.; He, Y.; Liu, Y. Recent advances in sensors for electrochemical analysis of nitrate in food and environmental matrices. Analyst 2020, 145, 5400–5413. [Google Scholar] [CrossRef]
- Nielsen, H.J.; Hansen, E.H. New nitrate ion-selective electrodes based on quaternary ammonium compounds in nonporous polymer membranes. Anal. Chim. Acta 1976, 85, 1–16. [Google Scholar] [CrossRef]
- Simeonov, V.; Andreev, G.; Stoianov, A. Microdetermination of nitrate nitrogen in lake waters by potentiometry with an ion-selective electrode. Fresenius Z. Anal. Chem. 1979, 297, 418. [Google Scholar] [CrossRef]
- Ruzicka, J.; Hansen, E.H.; Zagatto, E.A. Flow injection analysis: Part VII. Use of ion-selective electrodes for rapid analysis of soil extracts and blood serum. Determination of potassium, sodium and nitrate. Anal. Chim. Acta 1977, 88, 1–16. [Google Scholar] [CrossRef]
- Cuartero, M.; Crespo, G.A. All-solid-state potentiometric sensors: A new wave for in situ aquatic research. Curr. Opin. Electrochem. 2018, 10, 98–106. [Google Scholar] [CrossRef]
- Lindner, E.; Gyurcsányi, R.E. Quality control criteria for solid-contact, solvent polymeric membrane ion-selective electrodes. J. Solid State Electrochem. 2009, 13, 51–68. [Google Scholar] [CrossRef]
- De Marco, R.; Veder, J.P.; Clarke, G.; Nelson, A.; Prince, K.; Pretsch, E.; Bakker, E. Evidence of a water layer in solid-contact polymeric ion sensors. Phys. Chem. Chem. Phys. 2008, 10, 73–76. [Google Scholar] [CrossRef]
- Fibbioli, M.; Morf, W.E.; Badertscher, M.; de Rooij, N.F.; Pretsch, E. Potential drifts of solid-contacted ion-selective electrodes due to zero-current ion fluxes through the sensor membrane. Electroanalysis 2000, 12, 1286–1292. [Google Scholar] [CrossRef]
- Hu, J.; Stein, A.; Bühlmann, P. Rational design of all-solid-state ion-selective electrodes and reference electrodes. TrAC Trends Anal. Chem. 2016, 76, 102–114. [Google Scholar] [CrossRef]
- Michalska, A. All-solid-state ion selective and all-solid-state reference electrodes. Electroanalysis 2012, 24, 1253–1265. [Google Scholar] [CrossRef]
- Lyu, Y.; Gan, S.; Bao, Y.; Zhong, L.; Xu, J.; Wang, W.; Liu, Z.; Ma, Y.; Yang, G.; Niu, L. Solid-contact ion-selective electrodes: Response mechanisms, transducer materials and wearable sensors. Membranes 2020, 10, 128. [Google Scholar] [CrossRef]
- Bobacka, J. Conducting polymer-based solid-state ion-selective electrodes. Electroanalysis 2006, 18, 7–18. [Google Scholar] [CrossRef]
- Michalska, A. Optimizing the analytical performance and construction of ion-selective electrodes with conducting polymer-based ion-to-electron transducers. Anal. Bioanal. Chem. 2006, 384, 391–406. [Google Scholar] [CrossRef] [PubMed]
- Bieg, C.; Fuchsberger, K.; Stelzle, M. Introduction to polymer-based solid-contact ion-selective electrodes-basic concepts, practical considerations, and current research topics. Anal. Bioanal. Chem. 2017, 409, 45–61. [Google Scholar] [CrossRef]
- Yuan, D.; Anthis, A.H.C.; Ghahraman Afshar, M.; Pankratova, N.; Cuartero, M.; Crespo, G.A.; Bakker, E. All-solid-state potentiometric sensors with a multiwalled carbon nanotube inner transducing layer for anion detection in environmental samples. Anal. Chem. 2015, 87, 8640–8645. [Google Scholar] [CrossRef]
- Fan, X.; Nie, W.; Tsai, H.; Wang, N.; Huang, H.; Cheng, Y.; Wen, R.; Ma, L.; Yan, F.; Xia, Y. PEDOT:PSS for flexible and stretchable electronics: Modifications, strategies, and applications. Adv. Sci. 2019, 6, 1900813. [Google Scholar] [CrossRef]
- Ozer, T.; Agir, I.; Henry, C.S. Rapid prototyping of ion-selective electrodes using a low-cost 3D printed internet-of-things (IoT) controlled robot. Talanta 2022, 247, 123544. [Google Scholar] [CrossRef]
- Yin, T.; Qin, W. Applications of nanomaterials in potentiometric sensors. Trends Anal. Chem. 2013, 51, 79–86. [Google Scholar] [CrossRef]
- Lai, C.-Z.; Fierke, M.A.; Stein, A.; Bühlmann, P. Ion-selective electrodes with three-dimensionally ordered macroporous carbon as the solid contact. Anal. Chem. 2007, 79, 4621–4626. [Google Scholar] [CrossRef] [PubMed]
- Woźnica, E.; Wójcik, M.M.; Wojciechowski, M.; Mieczkowski, J.; Bulska, E.; Maksymiuk, K.; Michalska, A. Dithizone modified gold nanoparticles films for potentiometric sensing. Anal. Chem. 2012, 84, 4437–4442. [Google Scholar] [CrossRef]
- Ping, J.; Wang, Y.; Wu, J.; Ying, Y. Development of an all-solid-state potassium ion-selective electrode using graphene as the solid-contact transducer. Electrochem. Commun. 2011, 13, 1529–1532. [Google Scholar] [CrossRef]
- Crespo, G.A. Recent advances in ion-selective membrane electrodes for in situ environmental water analysis. Electrochim. Acta 2017, 245, 1023–1034. [Google Scholar] [CrossRef]
- Hassan, S.S.M.; Eldin, A.G.; Amr, A.E.E.; Al-Omar, M.A.; Kamel, A.H.; Khalifa, N.M. Improved solid-contact nitrate ion selective electrodes based on multi-walled carbon nanotubes (MWCNTs) as an ion-to-electron transducer. Sensors 2019, 19, 3891. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Ping, J.; Fan, K.; Wang, Y.; Luo, X.; Ying, Y.; Wu, J.; Zhou, Q. All-solid-state nitrate-selective electrode and its application in drinking water. Electrochim. Acta 2012, 81, 186–190. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Meng, Z.; Qin, Y.; Jiang, D.; Xi, K.; Wang, P. Thiol-functionalized reduced graphene oxide as self-assembled ion-to-electron transducer for durable solid-contact ion-selective electrodes. Talanta 2020, 208, 120374. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-Y.; Lee, J.-W.; Park, D.J.; Lee, J.-Y.; Myung, N.V.; Kwon, S.H.; Lee, K.H. Highly stable potentiometric sensor with reduced graphene oxide aerogel as a solid contact for detection of nitrate and calcium ions. J. Electroanal. Chem. 2021, 897, 115553. [Google Scholar] [CrossRef]
- Li, D.; Wang, T.; Li, Z.; Xu, X.; Wang, C.; Duan, Y. Application of graphene-based materials for detection of nitrate and nitrite in water-a review. Sensors 2019, 20, 54. [Google Scholar] [CrossRef]
- Xu, B.; Yue, S.; Sui, Z.; Zhang, X.; Hou, S.; Cao, G.; Yang, Y. What is the choice for supercapacitors: Graphene or graphene oxide? Energy Environ. Sci. 2011, 4, 2826–2830. [Google Scholar] [CrossRef]
- Düzgün, A.; Zelada-Guillén, G.A.; Crespo, G.A.; Macho, S.; Riu, J.; Rius, F.X. Nanostructured materials in potentiometry. Anal. Bioanal. Chem. 2011, 399, 171–181. [Google Scholar] [CrossRef]
- Jaworska, E.; Lewandowski, W.; Mieczkowski, J.; Maksymiuk, K.; Michalska, A. Critical assessment of graphene as ion-to-electron transducer for all-solid-state potentiometric sensors. Talanta 2012, 97, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Meier, P.C. Two-parameter debye-hückel approximation for the evaluation of mean activity coefficients of 109 electrolytes. Anal. Chim. Acta 1982, 136, 363–368. [Google Scholar] [CrossRef]
- Buck, R.P.; Lindner, E. Recomendations for nomenclature of ion-selective electrodes (IUPAC Recommendations 1994). Pure Appl. Chem. 1994, 66, 2527–2536. [Google Scholar] [CrossRef]
- Umezawa, Y.; Buhlmann, P.; Umezawa, K.; Tohda, K.; Amemiya, S. Potentiometric selectivity coefficients of ion-selective electrodes Part I. Inorganic cations—(Technical report). Pure Appl. Chem. 2000, 72, 1851–2082. [Google Scholar] [CrossRef]
- Bobacka, J. Potential stability of all-solid-state ion-selective electrodes using conducting polymers as ion-to-electron transducers. Anal. Chem. 1999, 71, 4932–4937. [Google Scholar] [CrossRef] [PubMed]
- Hafiz, S.M.; Ritikos, R.; Whitcher, T.J.; Razib, N.M.; Bien, D.C.S.; Chanlek, N.; Nakajima, H.; Saisopa, T.; Songsiriritthigul, P.; Huang, N.M.; et al. A practical carbon dioxide gas sensor using room-temperature hydrogen plasma reduced graphene oxide. Sens. Actuators B Chem. 2014, 193, 692–700. [Google Scholar] [CrossRef]
- Ni, Z.; Wang, Y.; Yu, T.; Shen, Z. Raman spectroscopy and imaging of graphene. Nano Res. 2008, 1, 273–291. [Google Scholar] [CrossRef]
- Mehta, J.S.; Faucett, A.C.; Sharma, A.; Mativetsky, J.M. How reliable are raman spectroscopy measurements of graphene oxide? J. Phys. Chem. C 2017, 121, 16584–16591. [Google Scholar] [CrossRef]
- Elgrishi, N.; Rountree, K.J.; McCarthy, B.D.; Rountree, E.S.; Eisenhart, T.T.; Dempsey, J.L. A practical beginner’s guide to cyclic voltammetry. J. Chem. Educ. 2018, 95, 197–206. [Google Scholar] [CrossRef]
- Bakker, E.; Pretsch, E.; Buhlmann, P. Selectivity of potentiometric ion sensors. Anal. Chem. 2000, 72, 1127–1133. [Google Scholar] [CrossRef]
- Wojciechowski, K.; Kucharek, M.; Wroblewski, W.; Warszynski, P. On the origin of the Hofmeister effect in anion-selective potentiometric electrodes with tetraalkylammonium salts. J. Electroanal. Chem. 2010, 638, 204–211. [Google Scholar] [CrossRef]
- Kim, D.-W.; Jung, D.-H.; Cho, W.-J.; Sim, K.-C.; Kim, H.-J. On-site water nitrate monitoring system based on automatic sampling and direct measurement with ion-selective electrodes. J. Biosyst. Eng. 2017, 42, 350–357. [Google Scholar] [CrossRef]
- Jung, D.H.; Kim, H.J.; Kim, J.Y.; Park, S.H.; Cho, W.J. Water nitrate remote monitoring system with self-diagnostic function for ion-selective electrodes. Sensors 2021, 21, 2703. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, M.; Ren, H.; Pu, P.; Kong, P.; Zhao, H. Comparative investigation on soil nitrate-nitrogen and available potassium measurement capability by using solid-state and PVC ISE. Comput. Electron. Agric. 2015, 112, 83–91. [Google Scholar] [CrossRef]
- De Marco, R.; Jee, E.; Prince, K.; Pretsch, E.; Bakker, E. Synthesis and characterization of high-integrity solid-contact polymeric ion sensors. J. Solid State Electrochem. 2009, 13, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhang, M.; Wang, X.; Yang, Q.; Wang, M.; Liu, G.; Yao, L. An all-solid-state nitrate ion-selective electrode with nanohybrids composite films for in-situ soil nutrient monitoring. Sensors 2020, 20, 2270. [Google Scholar] [CrossRef]
- Crespo, G.A.; Macho, S.; Rius, F.X. Ion-selective electrodes using carbon nanotubes as ion-to-electron transducers. Anal. Chem. 2008, 80, 1316–1322. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Ye, J.; Zhou, M.; Gan, S.; Zhang, Q.; Han, D.; Niu, L. All-solid-state potassium-selective electrode using graphene as the solid contact. Analyst 2012, 137, 618–623. [Google Scholar] [CrossRef]
- Abascal, E.; Gómez-Coma, L.; Ortiz, I.; Ortiz, A. Global diagnosis of nitrate pollution in groundwater and review of removal technologies. Sci. Total Environ. 2022, 810, 152233. [Google Scholar] [CrossRef]
- DGADR. Zonas Vulneráveis. Available online: https://www.dgadr.gov.pt/diretiva-nitratos/zonas-vulneraveis (accessed on 27 September 2023).
- Hjort, R.G.; Soares, R.R.A.; Li, J.; Jing, D.; Hartfiel, L.; Chen, B.; Van Belle, B.; Soupir, M.; Smith, E.; McLamore, E.; et al. Hydrophobic laser-induced graphene potentiometric ion-selective electrodes for nitrate sensing. Mikrochim. Acta 2022, 189, 122. [Google Scholar] [CrossRef]
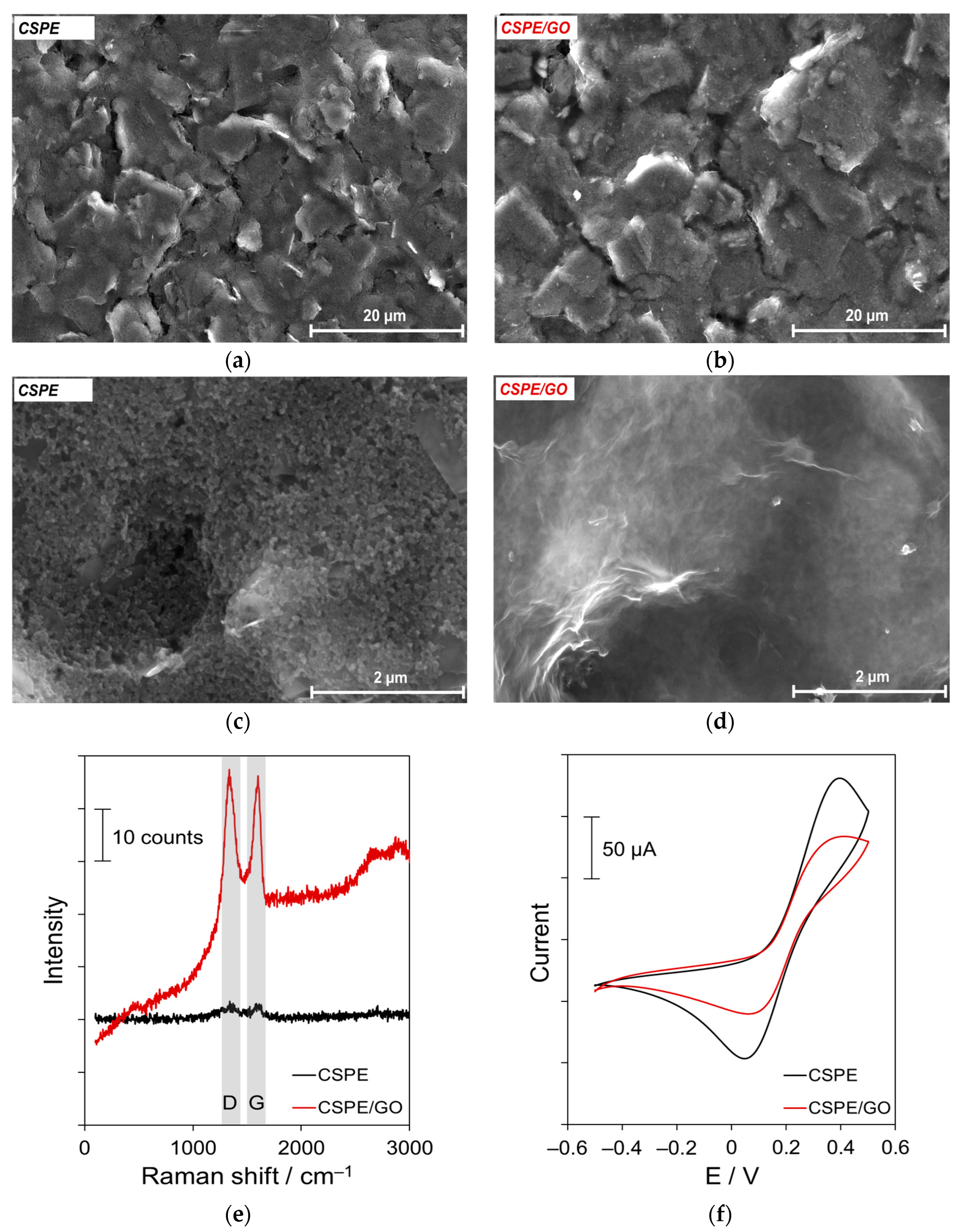
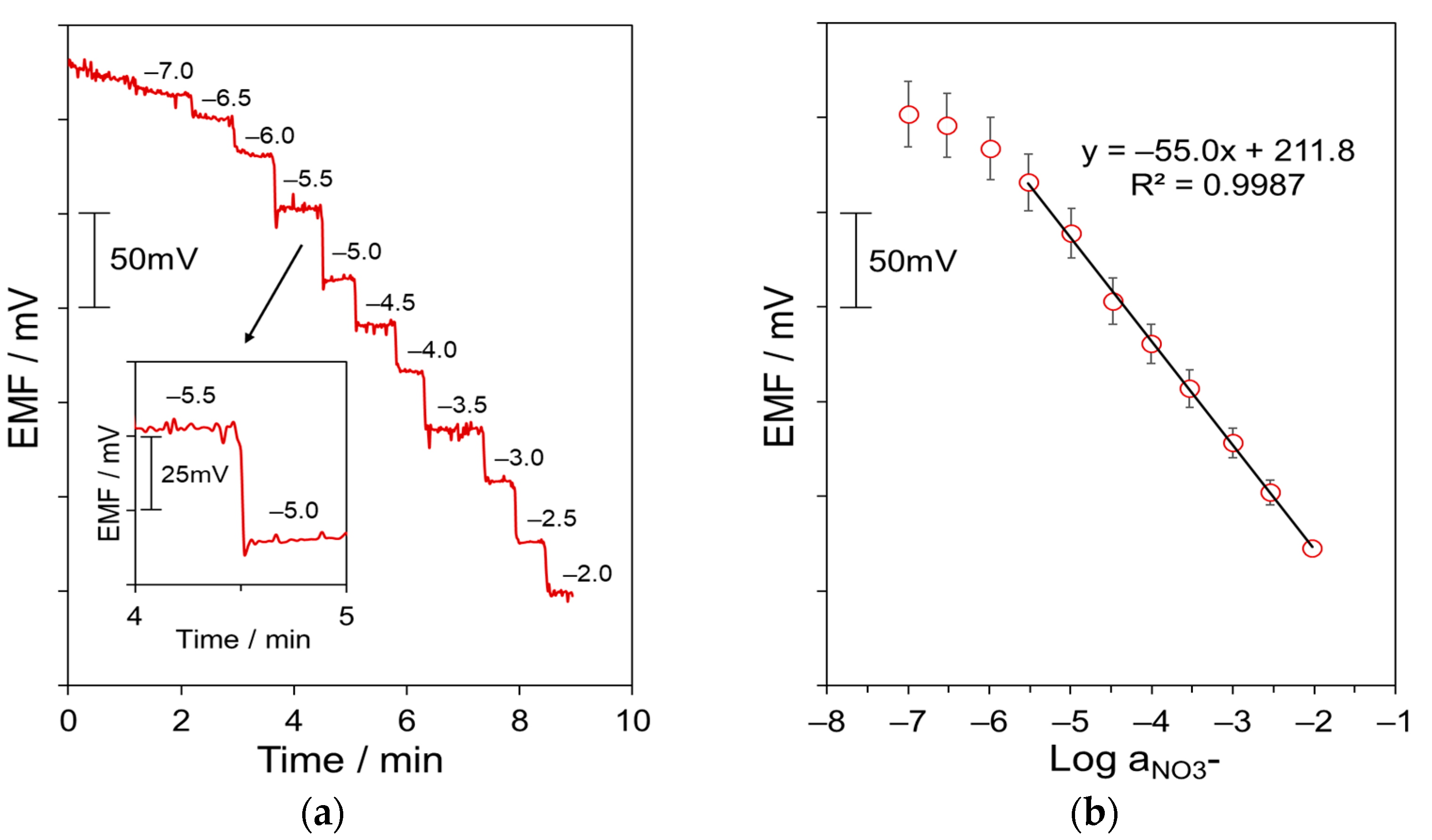
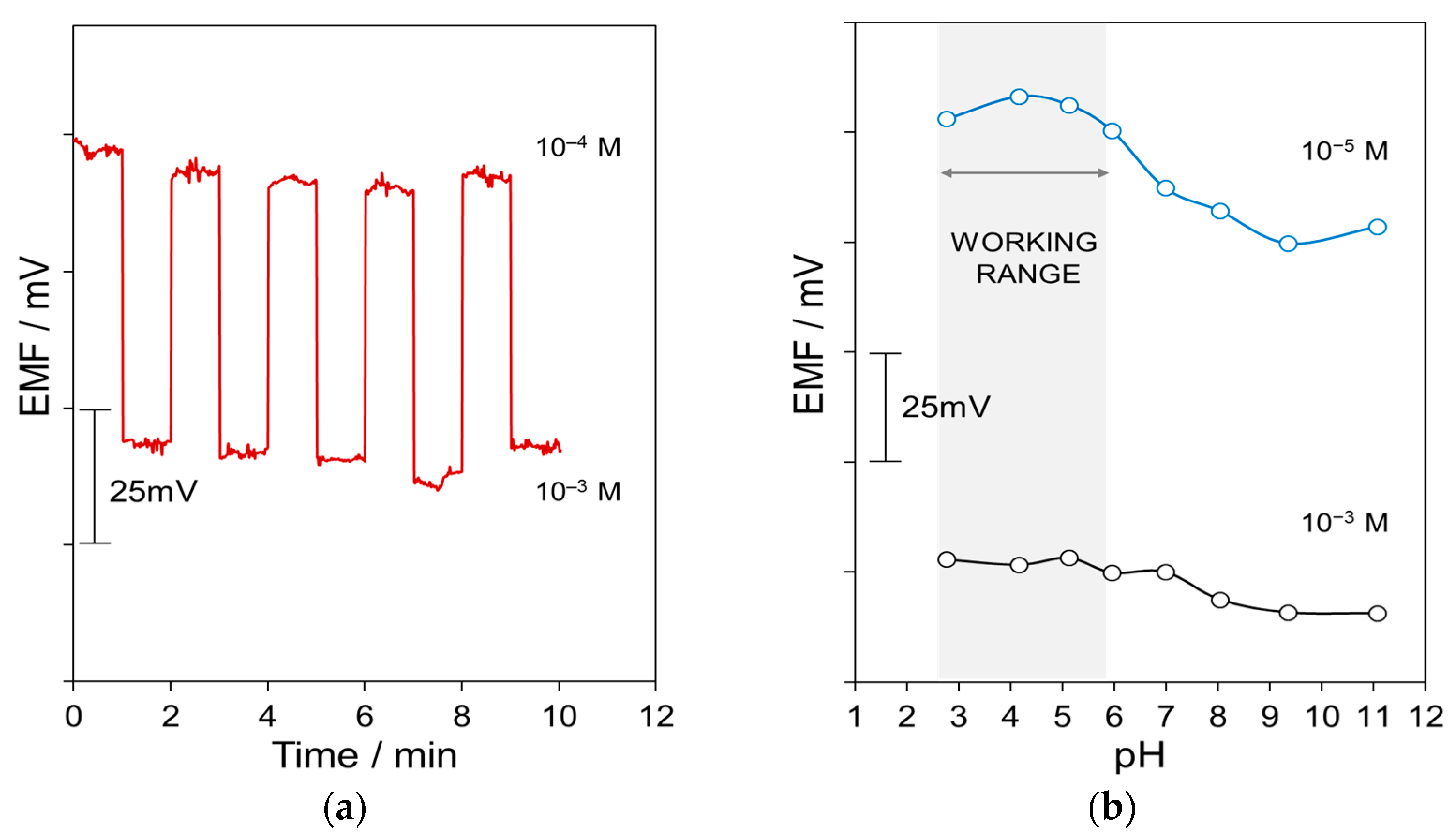
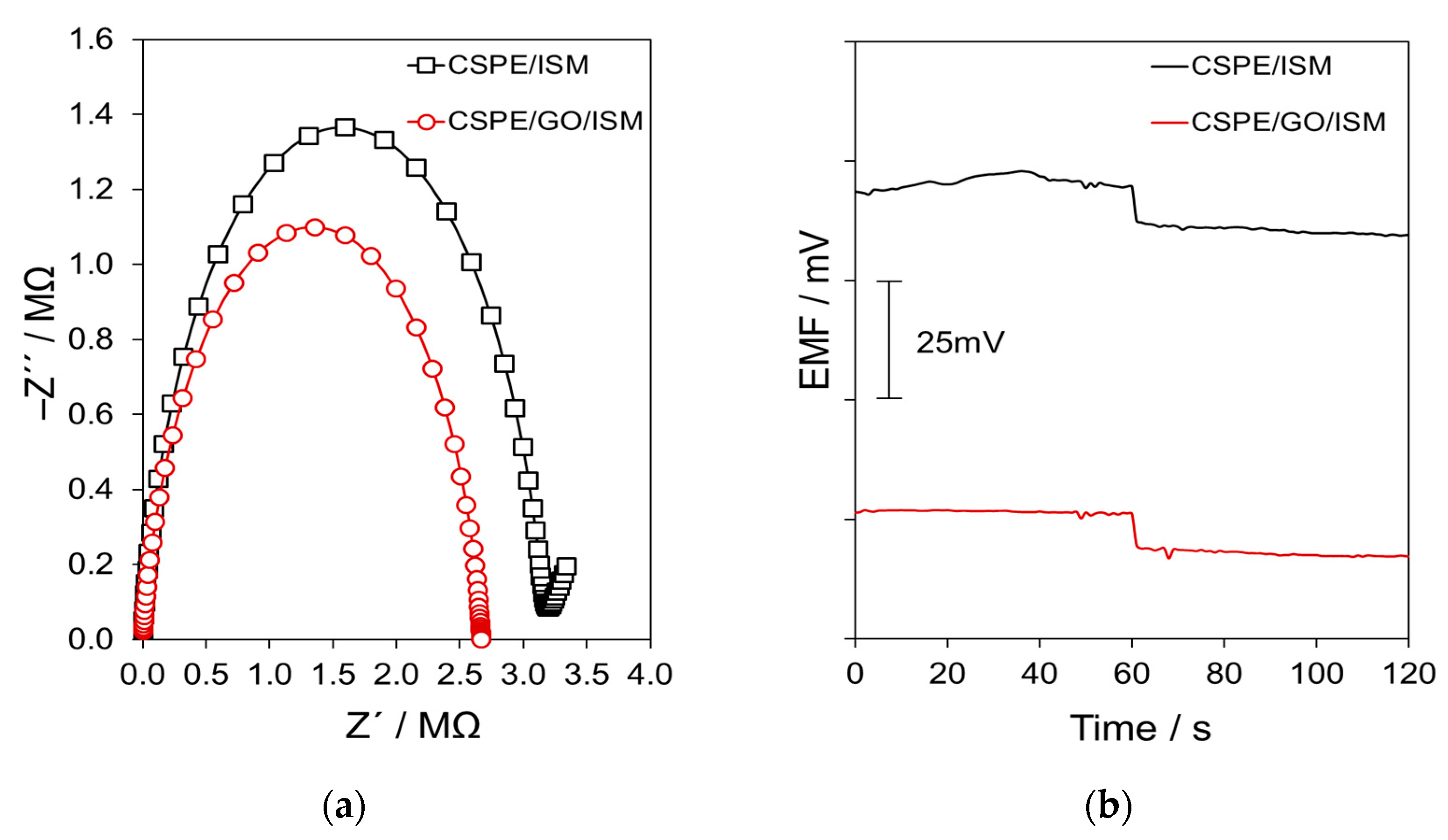
| Parameter | CSPE/GO/ISM | CSPE/ISM |
|---|---|---|
| Slope (mV dec−1) | −53.5 ± 2.4 | −55.4 ± 5.0 |
| Intercept (mV) | 215.2 ± 7.3 | 174.2 ± 33.0 |
| Coefficient of determination (R2) | 0.9976 ± 0.0015 | 0.9950 ± 0.0058 |
| Linear range of response (M) | 3.0 × 10−6–10−2 | 3.0 × 10−6–10−2 |
| Limit of detection (M) | 1.9 × 10−6 | 2.2 × 10−6 |
| Working range (pH) | 3–6 | 3–6 |
| Response time (s) | 10–20 | 10–20 |
| Reproducibility slope (SD, mV) | ||
| Intra-electrode 1 | <3.1 | <4.1 |
| Inter-electrode 2 | 2.0 | 5.0 |
| Reproducibility intercept (SD, mV) | ||
| Intra-electrode 1 | <3.8 | <16.6 |
| Inter-electrode 2 | 7.3 | 33.0 |
| ) | ||||
|---|---|---|---|---|
| CSPE/GO/ISM | CSPE/ISM | |||
| 10−4 M | 10−2 M | 10−4 M | 10−2 M | |
| CO32− | −3.2 | −4.0 | ND. | −4.5 |
| SO42− | −3.2 | −4.2 | ND. | −4.5 |
| HPO42− | −3.2 | −4.3 | ND. | −4.5 |
| Cl− | −1.3 | −2.7 | ND. | −2.7 |
| NO2− | −1.2 | −1.8 | ND. | −1.9 |
| Br− | −0.8 | −1.1 | ND. | −1.2 |
| NO3− | - | - | ND. | - |
| I− | 1.1 | 0.5 1 | ND. | 0.5 1 |
| ClO4− | 1.2 1 | 0.5 1 | ND. | 0.5 1 |
| Sample | Added (mg L−1) | CSPE/GO/ISM | Commercial ISE | ||||
|---|---|---|---|---|---|---|---|
| Found 1 (mg L−1) | CV 2 (%) | Recovery (%) | Found 1 (mg L−1) | CV 2 (%) | Recovery (%) | ||
| DW | 0 | 34.9 ± 1.0 | 2.8 | - | 37.8 ± 2.3 | 6.0 | - |
| DW.1 | 123.8 | 160.8 ± 11.4 | 7.1 | 101.7 | 160.5 ± 5.8 | 3.6 | 99.1 |
| DW.2 | 431.1 | 500.1 ± 30.0 | 6.0 | 107.9 | 488.7 ± 5.4 | 1.1 | 104.6 |
| AW | 0 | 78.5 ± 3.8 | 4.9 | - | 81.0 ± 1.9 | 2.3 | - |
| AW.1 | 123.8 | 187.2 ± 11.7 | 6.3 | 87.8 | 200.1 ± 8.9 | 4.5 | 96.2 |
| AW.2 | 431.1 | 543.5 ± 26.6 | 4.9 | 107.9 | 551.7 ± 30.6 | 5.5 | 109.2 |
| Sample | NO3− (mg L−1) 1 | % Difference | p-Value 2 | |
|---|---|---|---|---|
| CSPE/GO/ISM | Commercial ISE | |||
| Commercial | 4.7 ± 0.3 | 4.7 ± 0.2 | 0.4 | 0.370 |
| Tap | 4.3 ± 0.5 | 4.5 ± 0.1 | 6.0 | 0.443 |
| Domestic well | 34.9 ± 1.0 | 37.8 ± 2.3 | 8.5 | 0.129 |
| Agriculture well 1 | 50.5 ± 6.3 | 51.4 ± 0.2 | 1.9 | 0.152 |
| Agriculture well 2 | 78.5 ± 3.8 | 81.0 ± 1.0 | 3.3 | 0.932 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gil, R.L.; Rodriguez-Lorenzo, L.; Espiña, B.; Queirós, R.B. All-Solid-State Potentiometric Sensor Based on Graphene Oxide as Ion-to-Electron Transducer for Nitrate Detection in Water Samples. Chemosensors 2024, 12, 86. https://doi.org/10.3390/chemosensors12060086
Gil RL, Rodriguez-Lorenzo L, Espiña B, Queirós RB. All-Solid-State Potentiometric Sensor Based on Graphene Oxide as Ion-to-Electron Transducer for Nitrate Detection in Water Samples. Chemosensors. 2024; 12(6):86. https://doi.org/10.3390/chemosensors12060086
Chicago/Turabian StyleGil, Renato L., Laura Rodriguez-Lorenzo, Begoña Espiña, and Raquel B. Queirós. 2024. "All-Solid-State Potentiometric Sensor Based on Graphene Oxide as Ion-to-Electron Transducer for Nitrate Detection in Water Samples" Chemosensors 12, no. 6: 86. https://doi.org/10.3390/chemosensors12060086







