Enhancement of H2 Gas Sensing Using Pd Decoration on ZnO Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
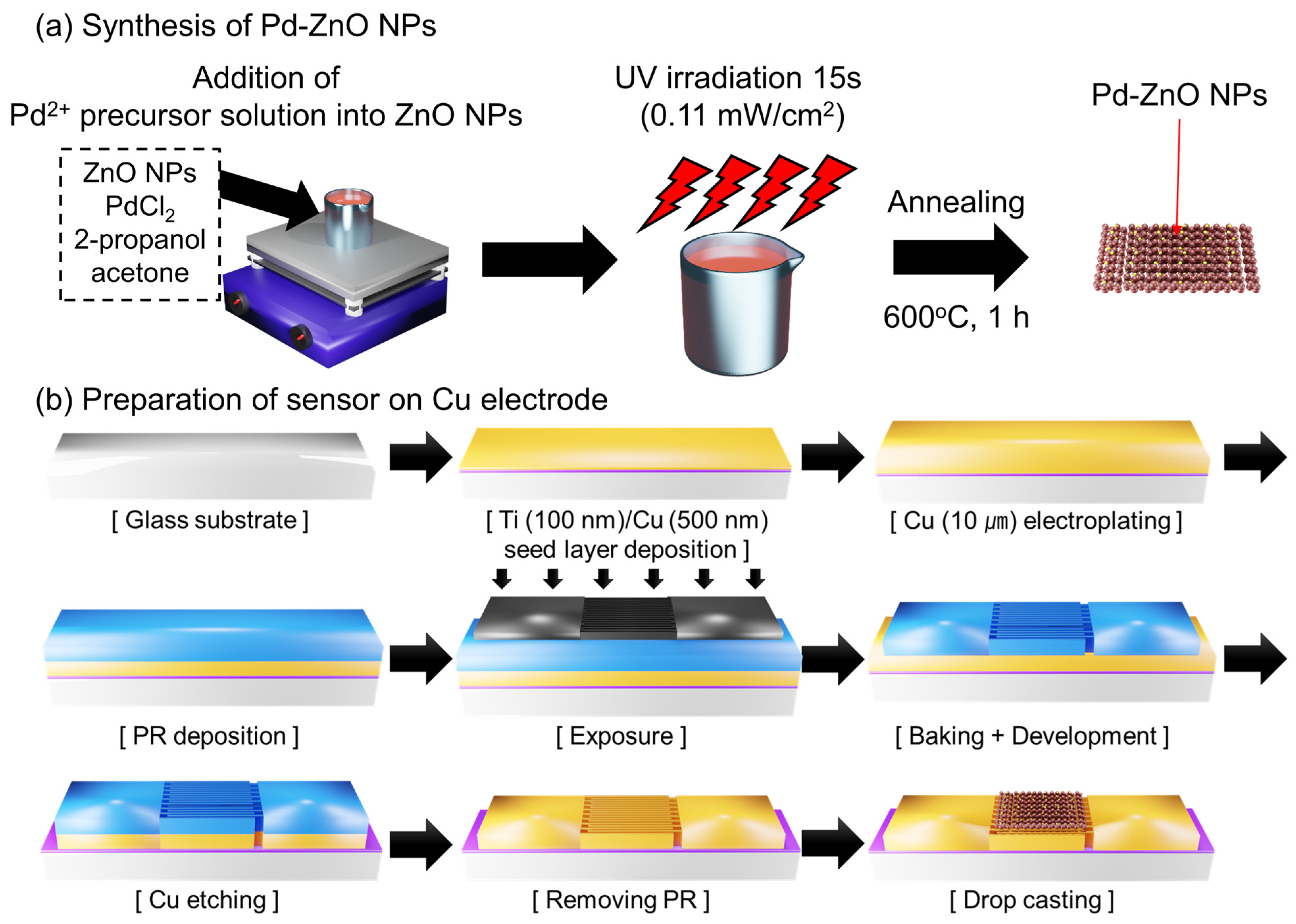
2.2. Synthesis of the Pd-Decorated ZnO NPs
2.3. Preparation of Glass Substrate
2.4. Gas-Sensing Measurements
2.5. Materials Characterization
3. Results and Discussion
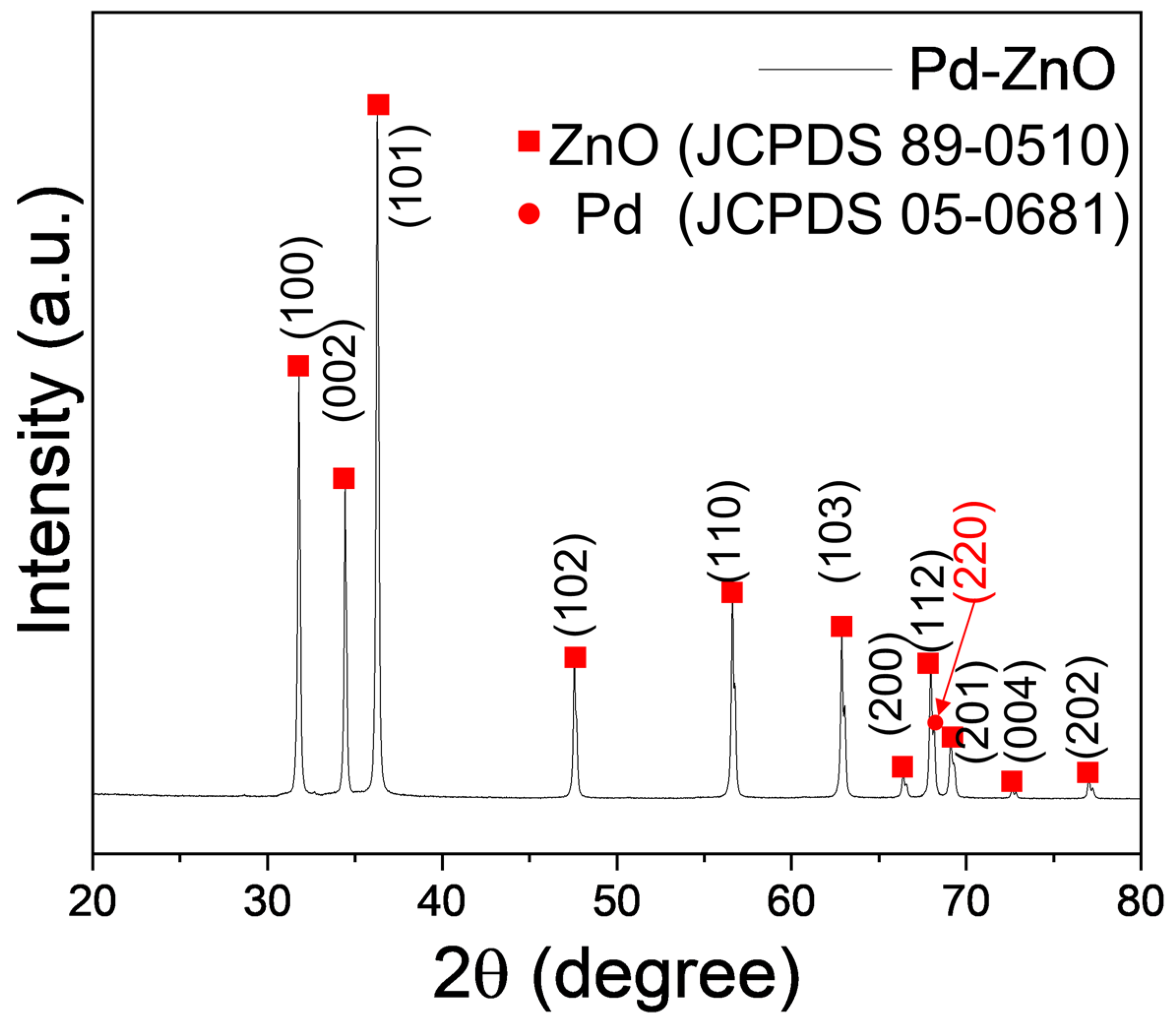
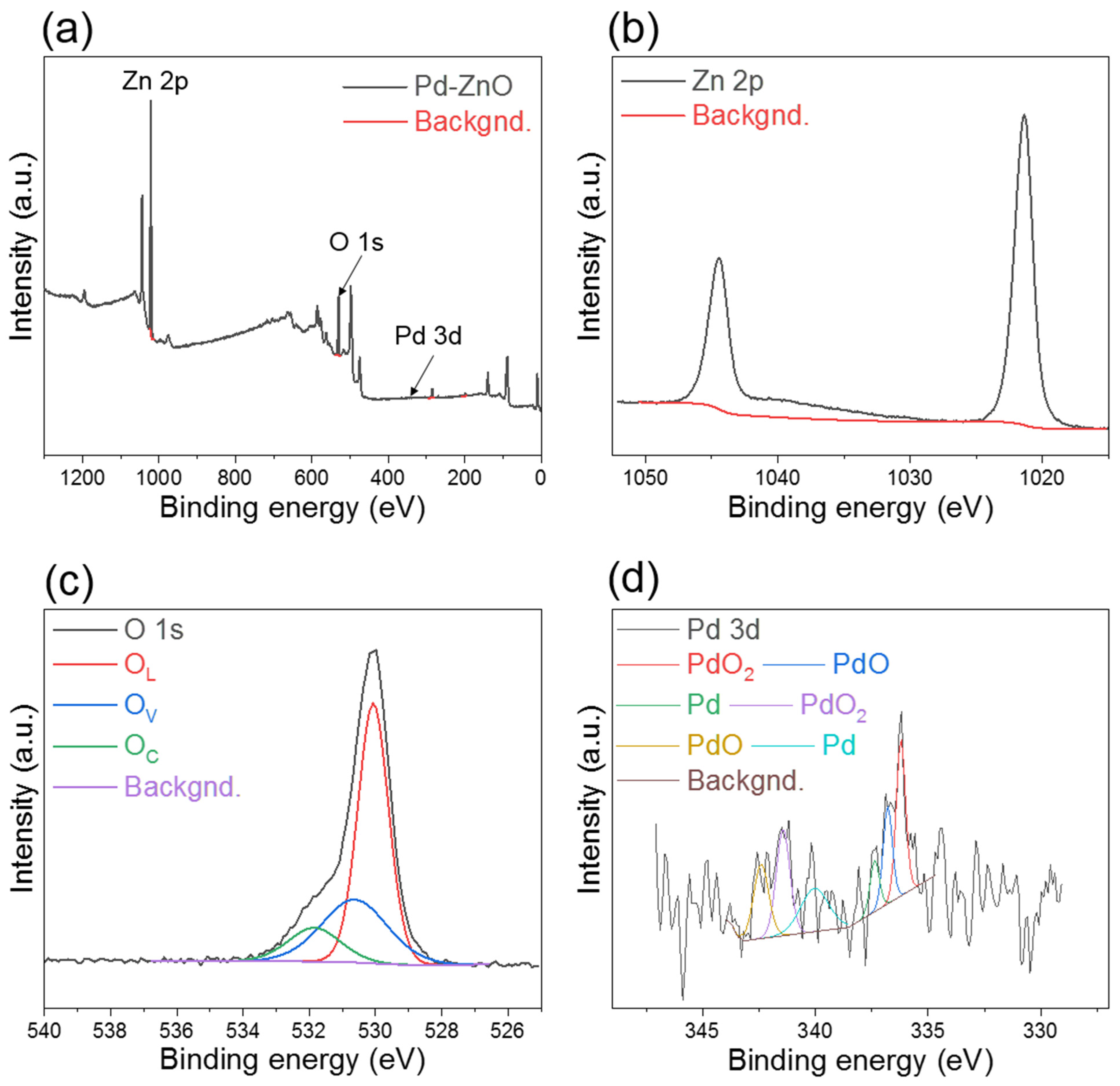
3.1. Morphological and Chemical Studies
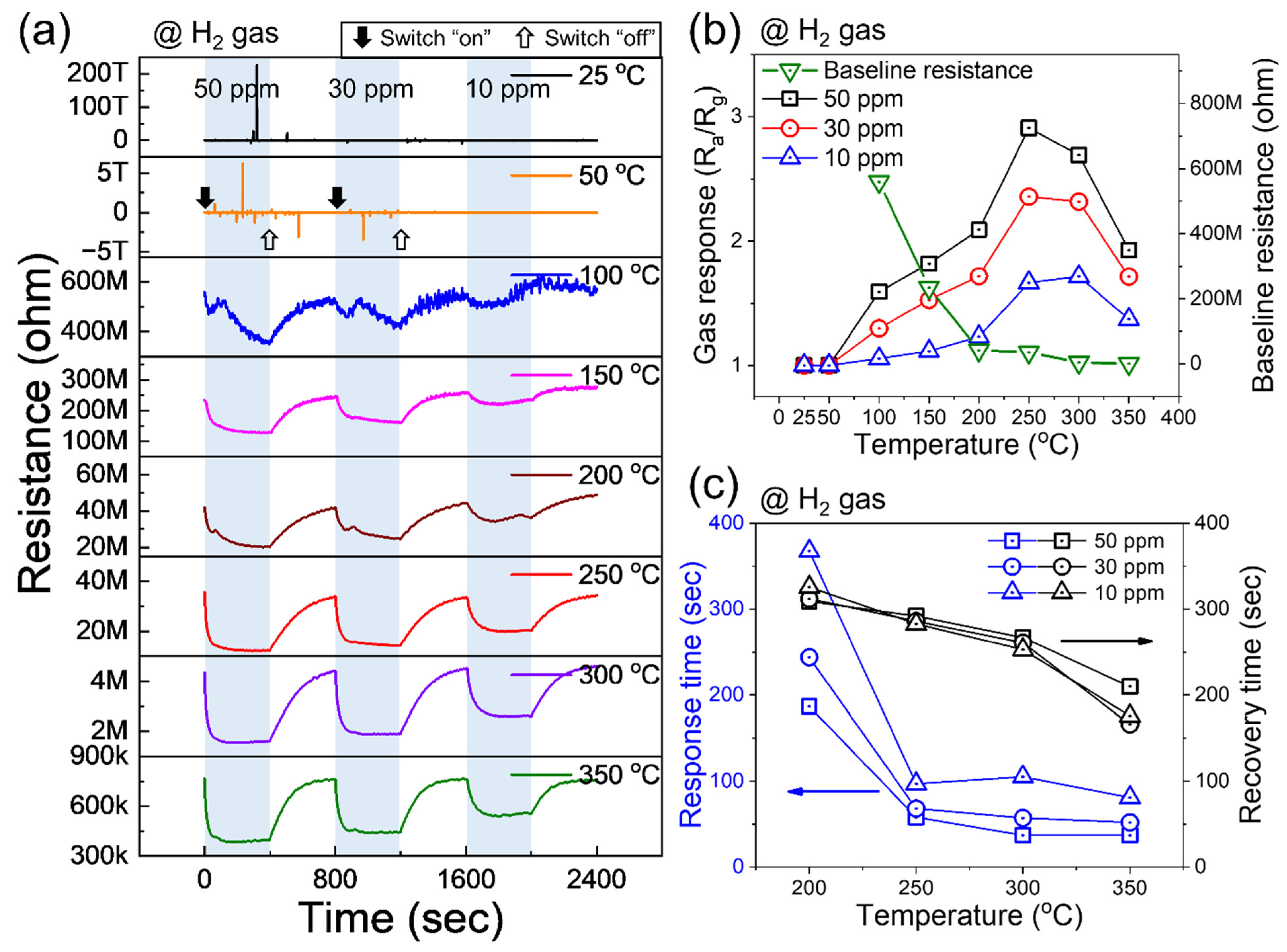
3.2. Gas-Sensing Studies
3.3. Gas Sensing Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sivaranjani, R.; Veerathai, S.; Jeoly Jenifer, K.; Sowmiya, K.; Rupesh, K.J.; Sudalai, S.; Arumnugam, A. A comprehensive review on biohydrogen production pilot scale reactor technologies: Sustainable development and future prospects. Int. J. Hydrogen Energy 2023, 48, 23785–23820. [Google Scholar] [CrossRef]
- Dzogbewu, T.C.; de Beer, D.J. Additive manufacturing of selected ecofriendly energy devices. Virtial Phys. Prototyp. 2023, 18, e2276245. [Google Scholar] [CrossRef]
- Abdin, Z.; Zafaranloo, A.; Rafiee, A.; Mérida, W.; Lipiński, W.; Khalilpour, K.R. Hydrogen as an energy vector. Renew. Sustain. Energy Rev. 2020, 120, 109620. [Google Scholar] [CrossRef]
- Tarhan, C.; Cil, M.A. A study on hydrogen, the clean energy of the future: Hydrogen storage methods. J. Energy Storage 2021, 40, 102676. [Google Scholar] [CrossRef]
- Johnston, B.; Mayo, M.C.; Khare, A. Hydrogen: The energy source for the 21st century. Technovation 2005, 25, 569–585. [Google Scholar] [CrossRef]
- Baroutaji, A.; Wilberforce, T.; Ramadan, M.; Olabi, A.G. Comprehensive investigation on hydrogen and fuel cell technology in the aviation and aerospace sectors. Renew. Sustain. Energy Rev. 2019, 106, 31–40. [Google Scholar] [CrossRef]
- Valverde, L.; Pino, F.J.; Guerra, J.; Rosa, F. Definition, analysis and experimental investigation of operation modes in hydrogen-renewable-based power plants incorporating hybrid energy storage. Energy Convers. Manag. 2016, 113, 290–311. [Google Scholar] [CrossRef]
- Belz, S. A synergetic use of hydrogen and fuel cells in human spaceflight power systems. Acta Astronaut. 2016, 121, 323–331. [Google Scholar] [CrossRef]
- Li, C.; Xu, H.; Bao, C.; Hou, S. Palladium membrane coated with zeolitic armor anchored by diffusion-piling to enhance performance. ACS Appl. Nano Mater. 2019, 2, 3377–3384. [Google Scholar] [CrossRef]
- Ma, Q.; He, Y.; You, J.; Chen, J.; Zhang, Z. Probabilistic risk assessment of fire and explosion of onboard high-pressure hydrogen system. Int. J. Hydrogen Energy 2024, 50, 1261–1273. [Google Scholar] [CrossRef]
- Abohamzeh, E.; Salehi, F.; Sheikholeslami, M.; Abbassi, R.; Khan, F. Review of hydrogen safety during storage, transmission, and applications processes. J. Loss Prev. Process Ind. 2021, 72, 104569. [Google Scholar] [CrossRef]
- Rigas, F.; Sklavounos, S. Evaluation of hazards associated with hydrogen storage facilities. Int. J. Hydrogen Energy 2005, 30, 1501–1510. [Google Scholar] [CrossRef]
- Sangchap, M.; Hashtroudi, H.; Thathsara, T.; Harrison, C.J.; Kingshott, P.; Kandjani, A.E.; Trinchi, A.; Shafiei, M. Exploring the promise of one-dimensional nanostructures: A review of hydrogen gas sensors. Int. J. Hyodrogen Energy 2024, 50, 1443–1457. [Google Scholar] [CrossRef]
- Kim, Y.K.; Hwang, S.-H.; Jeong, S.M.; Son, K.Y.; Lim, S.K. Colorimetric hydrogen gas sensor based on PdO/metal oxides hybrid nanoparticles. Talanta 2018, 188, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Nasir, M.E.; Dickson, W.; Wurtz, G.A.; Wardley, W.P.; Zayats, A.V. Hydrogen detected by the naked eye: Optical hydrogen gas sensors based on core/shell plasmonic nanorod metamaterials. Adv. Mater. 2014, 26, 3532–3537. [Google Scholar] [CrossRef] [PubMed]
- Korotcenkov, G.; Han, S.D.; Stetter, J.R. Review of electrochemical hydrogen sensors. Chem. Rev. 2009, 109, 1402–1433. [Google Scholar] [CrossRef] [PubMed]
- Phan, D.-T.; Chung, G.-S. Surface acoustic wave hydrogen sensors based on ZnO nanoparticles incorporated with a Pt catalyst. Sens. Actuators B Chem. 2012, 161, 341–348. [Google Scholar] [CrossRef]
- Zhang, Y.-N.; Peng, H.; Qian, X.; Zhang, Y.; An, G.; Zhao, Y. Recent advancements in optical fiber hydrogen sensors. Sens. Actuators B Chem. 2017, 244, 393–416. [Google Scholar] [CrossRef]
- Imai, Y.; Tadaki, D.; Ma, T.; Kimura, Y.; Hirano-Iwata, A.; Niwano, M. Response characteristics of hydrogen gas sensor with porous piezoelectric poly(vinylidene fluoride) film. Sens. Actuators B Chem. 2017, 247, 479–489. [Google Scholar] [CrossRef]
- Meng, F.; Shi, X.; Yuan, Z.; Ji, H.; Qin, W.; Shen, Y.B.; Xing, C. Detection of four alcohol homologue gases by ZnO gas sensor in dynamic interval temperature modulation mode. Sens. Actuators B Chem. 2022, 350, 130867. [Google Scholar] [CrossRef]
- Bhowmick, T.; Ghosh, A.; Nag, S.; Majumder, S.B. Sensitive and selective CO2 gas sensor based on CuO/ZnO bilayer thin-film architecture. J. Alloys Compd. 2022, 903, 163871. [Google Scholar] [CrossRef]
- Ren, X.; Xu, Z.; Liu, D.; Li, Y.; Zhang, Z.; Tang, Z. Conductometric NO2 gas sensors based on MOF-derived porous ZnO nanoparticles. Sens. Actuators B Chem. 2022, 357, 131384. [Google Scholar] [CrossRef]
- Kong, Y.; Li, Y.; Cui, X.; Su, L.; Ma, D.; Lai, T.; Yao, L.; Xiao, X.; Wang, Y. SnO2 nanostructured materials used as gas sensors for the detection of hazardous and flammable gases: A review. Nano Mater. Sci. 2022, 4, 339–350. [Google Scholar] [CrossRef]
- Gasso, S.; Sohal, M.K.; Mahajan, A. MXene modulated SnO2 gas sensor for ultra-responsive room-temperature detection of NO2. Sens. Actuators B Chem. 2022, 357, 131427. [Google Scholar] [CrossRef]
- Tian, X.; Cui, X.; Lai, T.; Ren, J.; Yang, Z.; Xiao, M.; Wang, B.; Xiao, X.; Wang, Y. Gas sensors based on TiO2 nanostructured materials for the detection of hazardous gases: A review. Nano Mater. Sci. 2021, 3, 390–403. [Google Scholar] [CrossRef]
- Zhang, D.; Yu, S.; Wang, X.; Huang, J.; Pan, W.; Zhang, J.; Meteku, B.E.; Zeng, J. UV illumination-enhanced ultrasensitive ammonia gas sensor based on (001)TiO2/MXene heterostructure for food spoilage detection. J. Hazard. Mater. 2022, 423, 127160. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wu, C.-H.; Liu, Y.-C.; Yuan, S.-H.; Chiang, Z.-X.; Zhang, S.; Wu, R.-J. Mesoporous WO3-TiO2 heterojunction for a hydrogen gas sensor. Sens. Actuators B Chem. 2021, 341, 130035. [Google Scholar] [CrossRef]
- Guo, M.; Luo, N.; Chen, Y.; Fan, Y.; Wang, X.; Xu, J. Fast-response MEMS xylene gas sensor based on CuO/WO3 hierarchical structure. J. Hazard. Mater. 2022, 429, 127471. [Google Scholar] [CrossRef] [PubMed]
- Katoch, A.; Choi, S.W.; Kim, H.W.; Kim, S.S. Highly sensitive and selective H2 sensing by ZnO nanofibers and the underlying sensing mechanism. J. Hazard. Mater. 2014, 286, 229–235. [Google Scholar] [CrossRef]
- Katoch, A.; Chou, S.-W.; Kim, J.-H.; Lee, J.H.; Lee, J.-S.; Kim, S.S. Importance of the nanograin size on the H2S-sensing properties of ZnO–CuO composite nanofibers. Sens. Actuators B Chem. 2015, 214, 111–116. [Google Scholar] [CrossRef]
- Abdullah, F.H.; Abu Bakar, N.H.H.; Abu Bakar, M. Current advancements on the fabrication, modification, and industrial application of zinc oxide as photocatalyst in the removal of organic and inorganic contaminants in aquatic systems. J. Hazard. Mater. 2022, 424, 127416. [Google Scholar] [CrossRef] [PubMed]
- Koga, K. Electronic and catalytic effects of single-atom Pd additives on the hydrogen sensing properties of Co3O4 nanoparticle films. ACS Appl. Mater. Interfaces 2020, 12, 20806–20823. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Hao, L.; Chen, L.; Guo, X.; Han, C.; Chen, J.; Jiao, W.; Wang, R.; He, X. Spray deposition of colorimetric H2 detector with Pd/MoO3 nanocomposites for rapid hydrogen leakage monitoring at room temperature. Appl. Surf. Sci. 2022, 599, 153878. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, J.-Y.; Kim, J.-H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Pd-decorated Si nano-horns as sensitive and selective hydrogen gas sensors. Mater. Res. Bull. 2020, 132, 110985. [Google Scholar] [CrossRef]
- Sun, C.; Liu, H.; Shao, J.; Pan, G.; Yang, X.; Wang, M.; Dong, J.; Zhu, M.; Qi, Y. Au-loaded Zn2SnO4/SnO2/ZnO nanosheets for fast response and highly sensitive TEA gas sensors. Sens. Actuators B Chem. 2023, 376, 132951. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Y.; Guan, K.; Liu, Y.; Li, Y.; Sun, F.; Wang, X.; Zhang, C.; Feng, S.; Zhang, T. Influence of oxygen vacancies on the performance of SnO2 gas sensing by near-ambient pressure XPS studies. Sens. Actuators B Chem. 2023, 393, 134252. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Y.; Liu, Y.; Wang, S.; Li, T.; Feng, S.; Qin, S.; Zhang, T. Heteronanostructural metal oxide-based gas microsensors. Microsyst. Nanoeng. 2022, 8, 85. [Google Scholar] [CrossRef]
- Kumar, N.; Jasani, J.; Sanvane, Y.; Korvink, J.G.; Sharma, A.; Sharma, B. Unfolding the hydrogen gas sensing mechanism across 2D Pnictogen/graphene heterostructure sensors. Sens. Actuators B Chem. 2024, 399, 134807. [Google Scholar] [CrossRef]
- Galvani, M.; Freddi, S.; Sangaletti, L. Disclosing fast detection opportunities with nanostructured chemiresistor gas sensors based on metal oxides, carbon, and transition netal dichalcogenides. Sensors 2024, 24, 584. [Google Scholar] [CrossRef]
- Lupan, O.; Postica, V.; Hoppe, M.; Wolff, N.; Polonskyi, O.; Pauporté, T.; Viana, B.; Jajérus, O.; Kienle, L.; Faupel, F.; et al. PdO/PdO2 functionalized ZnO:Pd films for lower operating temperature H2 gas sensing. Nanoscale 2018, 10, 14107–14127. [Google Scholar] [CrossRef]
- Liu, L.; Liu, C.; Li, S.; Wang, L.; Shan, H.; Zhang, X.; Guan, H.; Liu, Z. Honeycombed SnO2 with ultra sensitive properties to H2. Sens. Actuators B Chem. 2013, 177, 893–897. [Google Scholar] [CrossRef]
- Li, Z.; Yan, S.; Wu, Z.; Li, H.; Wang, J.; Shen, W.; Wang, Z.; Fu, Y. Hydrogen gas sensor based on mesoporous In2O3 with fast response/recovery and ppb level detection limit. Int. J. Hydrogen Energy 2018, 43, 22746–22755. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, W.; Li, Y. NO2 and H2 sensing properties for urchin-like hexagonal WO3 based on experimental and first-principle investigations. Ceram. Int. 2019, 45, 6043–6050. [Google Scholar] [CrossRef]
- Wang, Y.-T.; Whang, W.-T.; Chen, C.-H. Hollow V2O5 nanoassemblies for high-performance room-temperature hydrogen sensors. ACS Appl. Mater. Interfaces 2015, 7, 8480–8487. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xu, L.; Li, W.; Gou, X. α-Fe2O3 nanotubes in gas sensor and lithium-ion battery applications. Adv. Mater. 2005, 17, 582–586. [Google Scholar] [CrossRef]
- Tonezzer, M.; Iannotta, S. H2 sensing properties of two-dimensional zinc oxide nanostructures. Talanta 2014, 122, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.-J.; Li, K.-C.; Lin, Y.-C.; Pan, F.-M. A mechanistic study of hydrogen gas sensing by PdO nanoflake thin films at temperatures below 250 °C. Phys. Chem. Chem. Phys. 2015, 17, 3039–3049. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Zhang, Y.; Deng, X.; Dai, Y.; Zhang, W.; Fan, F.; Qing, B.; Zhu, C.; Fan, J.; Shi, Y. The H2 sensing properties of facets-dependent Pd nanoparticles-supported ZnO nanorods. Dalton Trans 2018, 47, 15331–15337. [Google Scholar] [CrossRef]
- Choi, S.-J.; Chattopadhyay, S.; Kim, J.J.; Kim, S.-J.; Tuller, H.L.; Rutledge, G.C.; Kim, I.-D. Coaxial electrospinning of WO3 nanotubes functionalized with bio-inspired Pd catalysts and their superior hydrogen sensing performance. Nanoscale 2016, 8, 9159–9166. [Google Scholar] [CrossRef]
- Sarica, N.; Alev, O.; Arslan, L.C.; Öztürk, Z.Z. Characterization and gas sensing performances of noble metals decorated CuO nanorods. Thin Solid Films 2019, 685, 321–328. [Google Scholar] [CrossRef]
- Peng, Y.; Zheng, L.; Zou, K.; Li, C. Enhancing performances of a resistivity-type hydrogen sensor based on Pd/SnO2/RGO nanocomposites. Nanotechnol 2017, 28, 215501. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xie, G.; Liu, F.; Ji, H. High hydrogen selectivity Pd-Ni alloy film hydrogen sensor with hybrid organosilica membranes. J. Alloys Compd. 2023, 941, 168898. [Google Scholar] [CrossRef]
- Kim, S.; Maeng, B.; Yang, Y.; Kim, K.; Jung, D. Hybrid hydrogen sensor based on Pd/WO3 showing simultaneous chemiresistive and gasochromic response. Nanomater 2023, 13, 2563. [Google Scholar] [CrossRef]
- Gong, J.; Wang, Z.; Tang, Y.; Sun, J.; Wei, X.; Zhang, Q.; Tian, G.; Wang, H. MEMS-based resistive hydrogen sensor with high performance using a palladium-gold alloy thin film. J. Alloys Compd. 2023, 930, 167398. [Google Scholar] [CrossRef]
- TGS 2626-C00-For the Detection of Hydrogen. Available online: https://www.figarosensor.com/product/docs/tgs2616-c00_product%20information%28fusa%29rev02.pdf (accessed on 30 October 2023).
- GMV-2021B MEMS H2 Sensor Manual. Available online: https://www.winsen-sensor.com/d/files/manual/gmv-2021b.pdf (accessed on 30 October 2023).

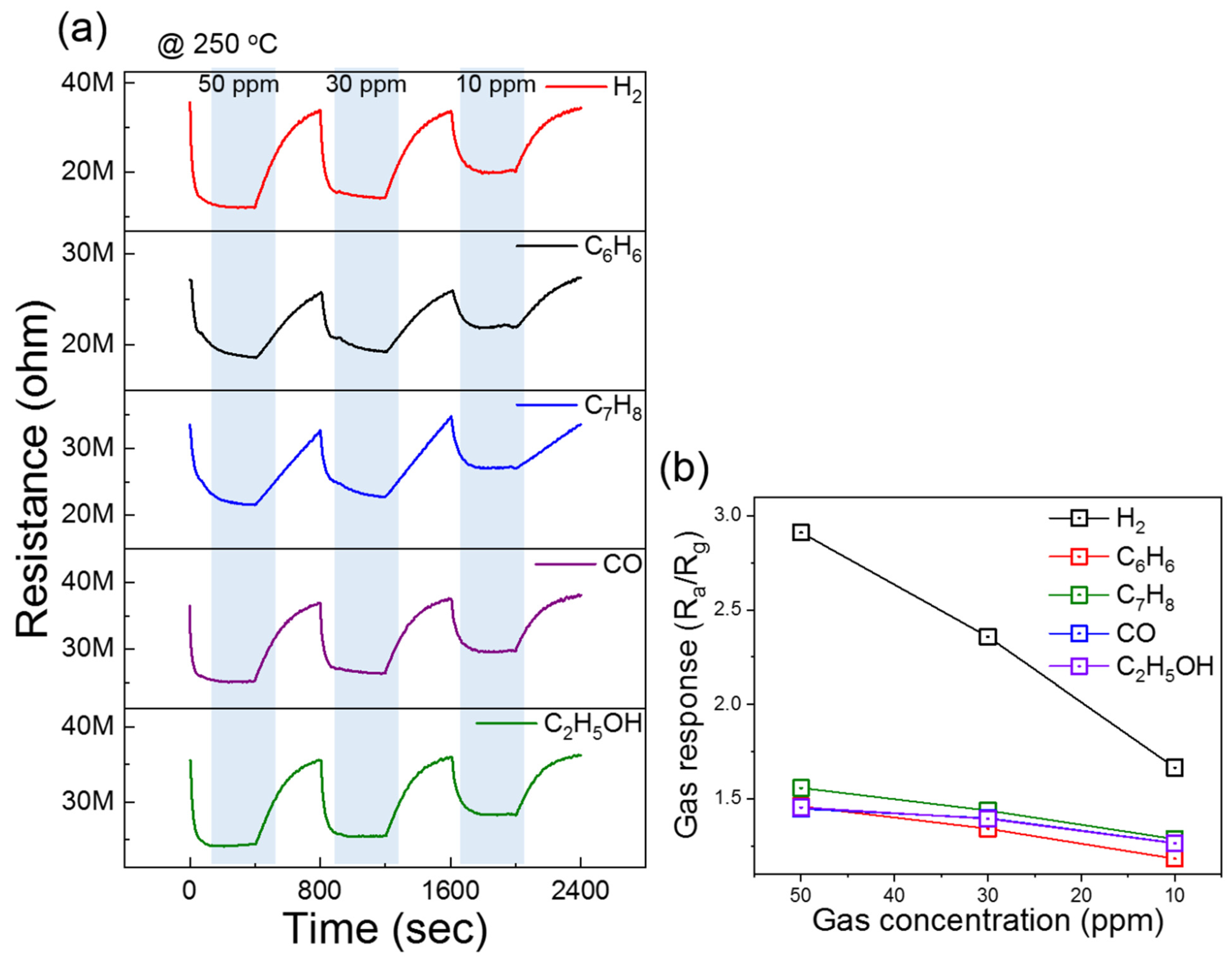


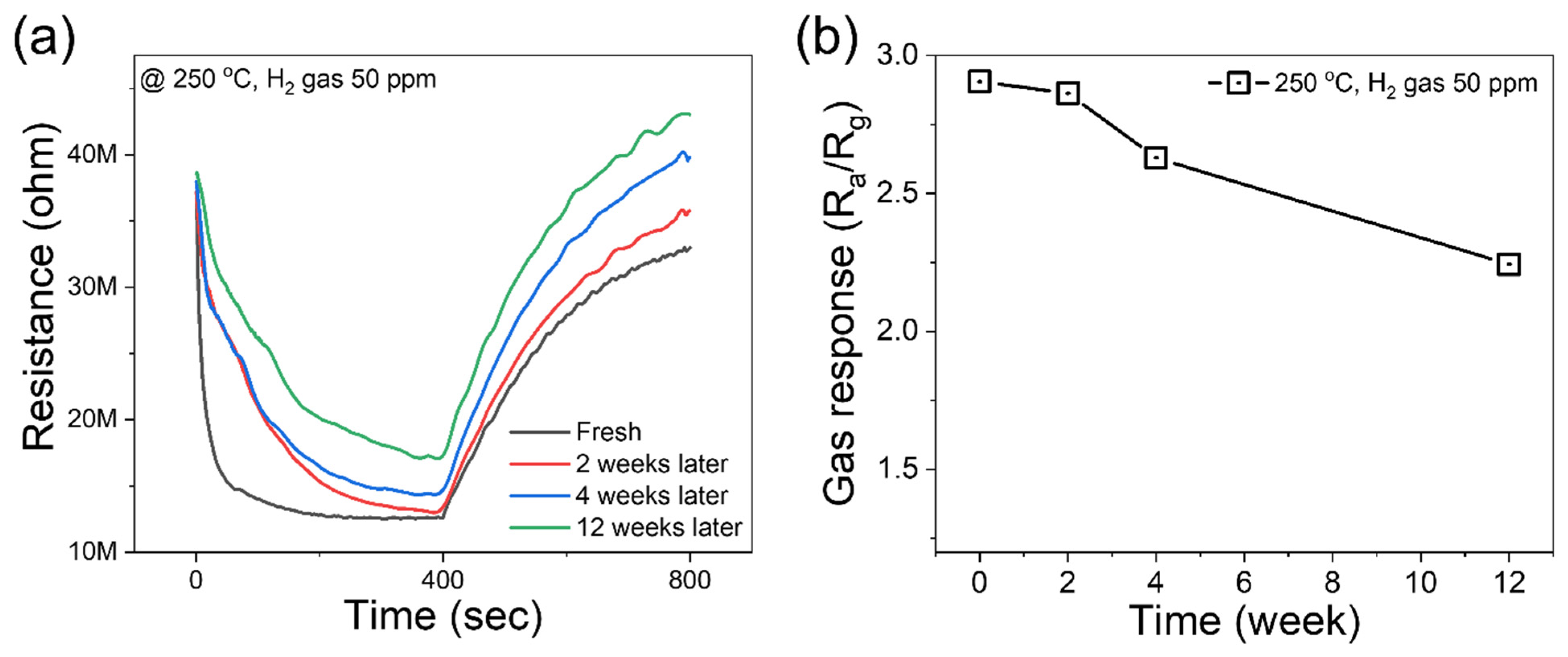
| Sensing Materials | Conc. (ppm) | T (°C) | Gas Response (Ra/Rg) or (Rg/Ra) | τres/τrec (s) | Ref. |
|---|---|---|---|---|---|
| Pd-ZnO | 50 | 250 | 2.94 | 58/292 | Present Work |
| SnO2 honeycomb | 1 | 340 | 8.4 | 4/10 | [41] |
| In2O3 nanocluster | 500 | 400 | 18 | 1.7/1.5 | [42] |
| Urchin-like WO3 nanoparticles | 50 | 250 | ~4 | -/- | [43] |
| V2O5 hollow ano sphere | 200 | 25 | 3.8 | 30/5 | [44] |
| Fe2O3 nanotube | 50 | 200 | 2.3 | -/- | [45] |
| ZnO nanohexagone | 10 | 175 | 1.089 | 11.5/14.5 | [46] |
| PdO nanoflake | 250 | 200 | 1.9 | -/- | [47] |
| Pd-ZnO nanorods | 250 | 135 | 22.5 | 1/52 | [48] |
| Pd-WO3 nanofibers | 500 | 450 | 16.3 | -/- | [49] |
| Pd-CuO nanorod | 1000 | 200 | 4.5 | 600/960 | [50] |
| Pd/SnO2/RGO | 5000 | 25 | 45.5% | >50/>1000 | [51] |
| Si-Pd-Ni | 200 | 70 | 2.21 | 107/- | [52] |
| Pd/WO3 | 1000 | 300 | 1.184 | 5/6 | [53] |
| Pd-Au | 3000 | 60 | 1.033 | 22/160 | [54] |
| TGS2616-C00 | 100 | 5 V | ~20 | -/- | [55] |
| GMV-2021B | 200 | 2.5 V | >5 | ~20/~45 | [56] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-Y.; Choi, K.; Kim, S.-W.; Park, C.-W.; Kim, S.-I.; Mirzaei, A.; Lee, J.-H.; Jeong, D.-Y. Enhancement of H2 Gas Sensing Using Pd Decoration on ZnO Nanoparticles. Chemosensors 2024, 12, 90. https://doi.org/10.3390/chemosensors12060090
Kim J-Y, Choi K, Kim S-W, Park C-W, Kim S-I, Mirzaei A, Lee J-H, Jeong D-Y. Enhancement of H2 Gas Sensing Using Pd Decoration on ZnO Nanoparticles. Chemosensors. 2024; 12(6):90. https://doi.org/10.3390/chemosensors12060090
Chicago/Turabian StyleKim, Jin-Young, Kyeonggon Choi, Seung-Wook Kim, Cheol-Woo Park, Sung-Il Kim, Ali Mirzaei, Jae-Hyoung Lee, and Dae-Yong Jeong. 2024. "Enhancement of H2 Gas Sensing Using Pd Decoration on ZnO Nanoparticles" Chemosensors 12, no. 6: 90. https://doi.org/10.3390/chemosensors12060090
APA StyleKim, J.-Y., Choi, K., Kim, S.-W., Park, C.-W., Kim, S.-I., Mirzaei, A., Lee, J.-H., & Jeong, D.-Y. (2024). Enhancement of H2 Gas Sensing Using Pd Decoration on ZnO Nanoparticles. Chemosensors, 12(6), 90. https://doi.org/10.3390/chemosensors12060090






