Research on the Detection of Hg(II) in Seawater Using GR-MWCNTs/CeO2-Modified Electrodes
Abstract
:1. Introduction
2. Experimental
2.1. Instruments and Reagents
2.2. Synthesis of Nanocomposites
2.3. GR-MWCNTs-COOH/CeO2/Nafion-Modified Electrode Preparation
2.4. Physical and Electrochemical Measurements
3. Results and Discussion
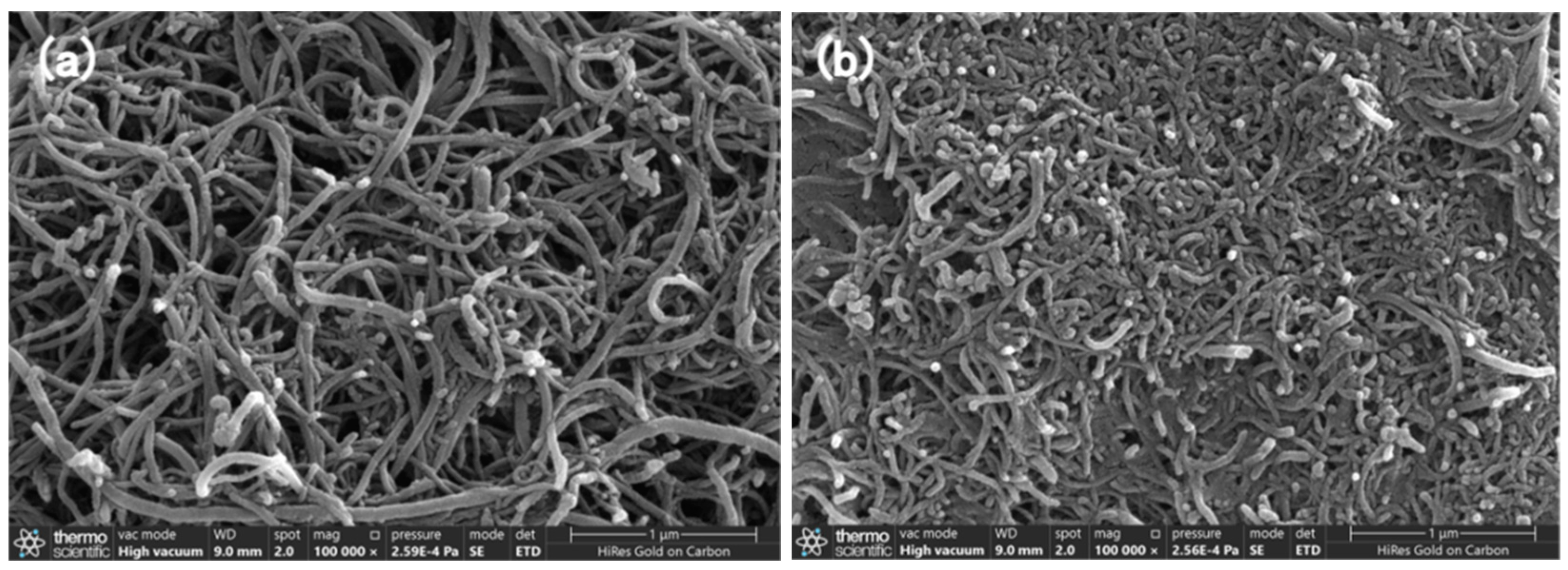
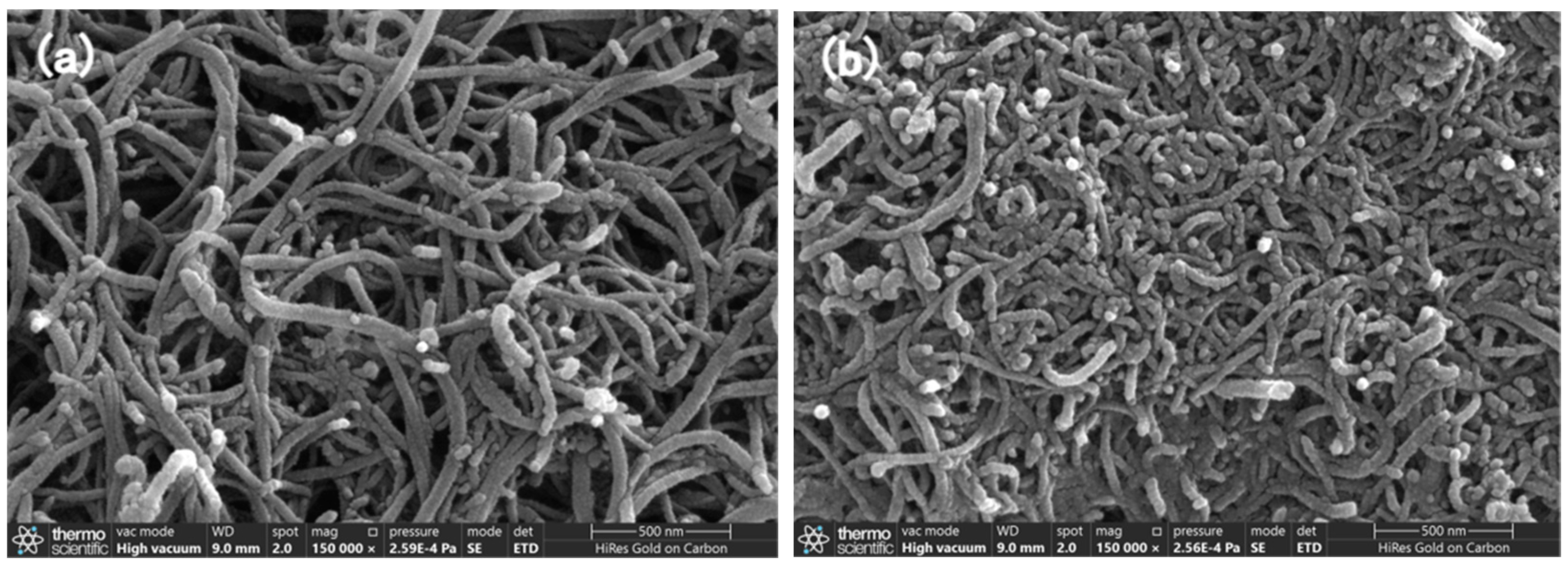
3.1. Surface Morphology of the Nanocomposites
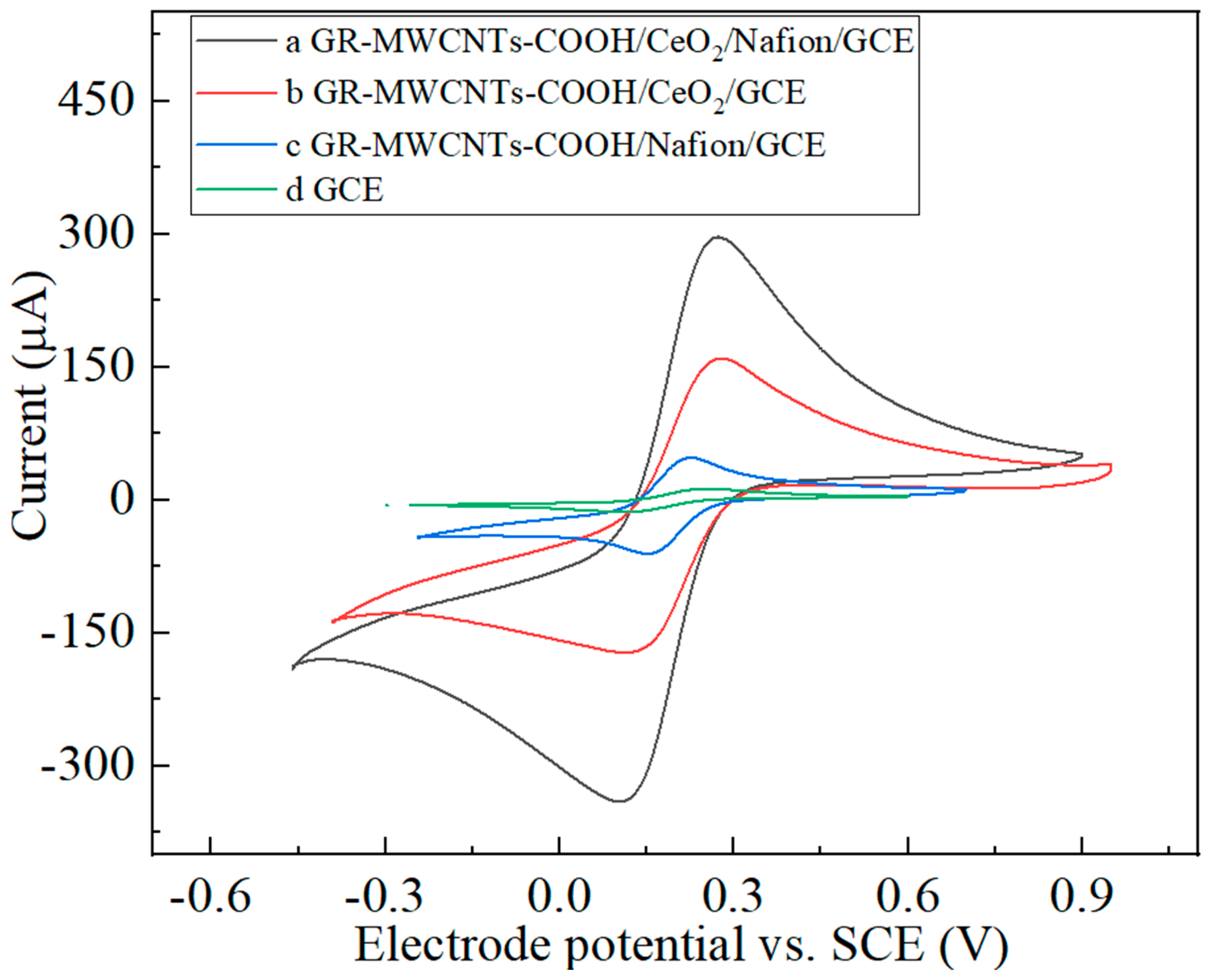
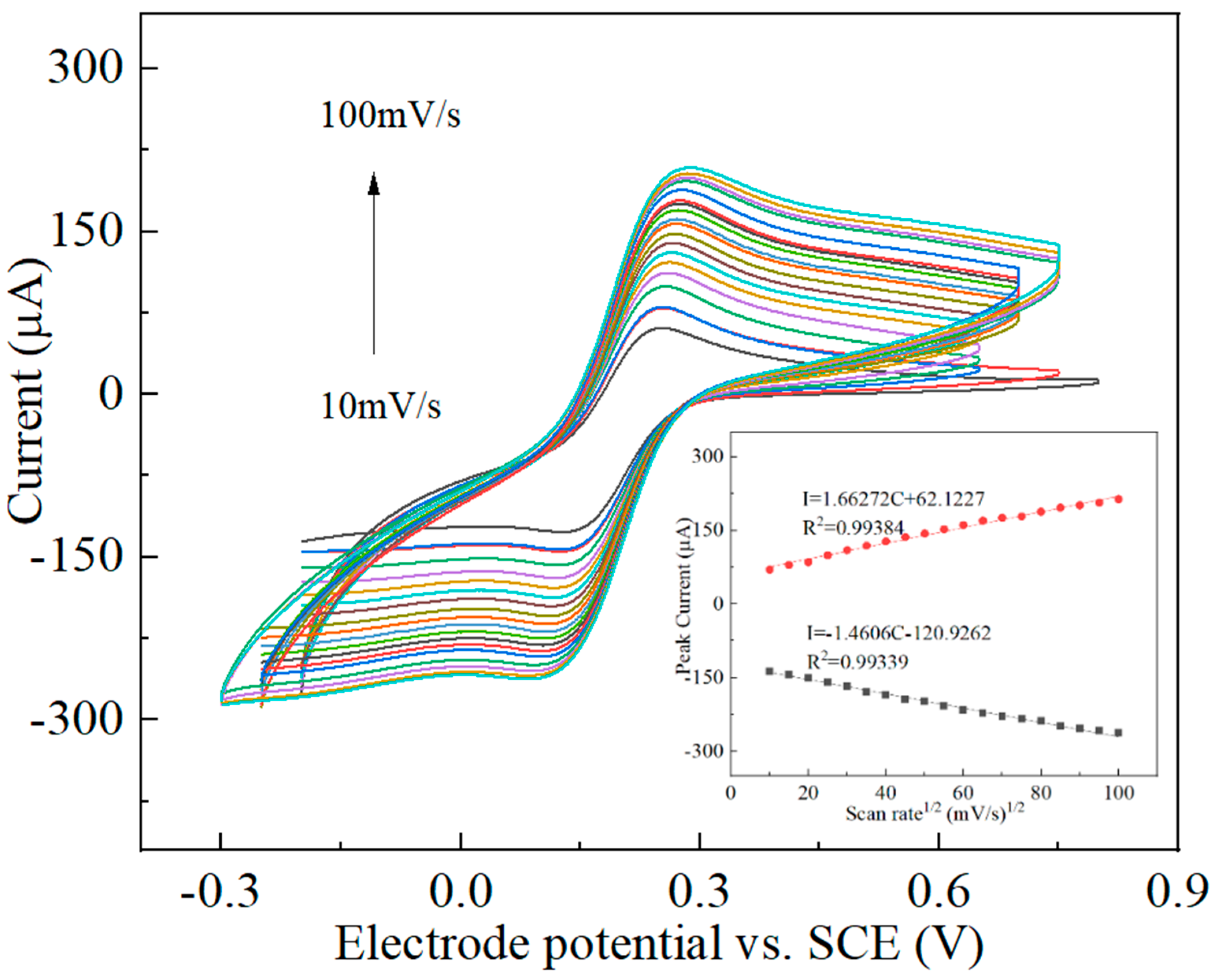
3.2. Electrochemical Characterization of the Nanocomposites
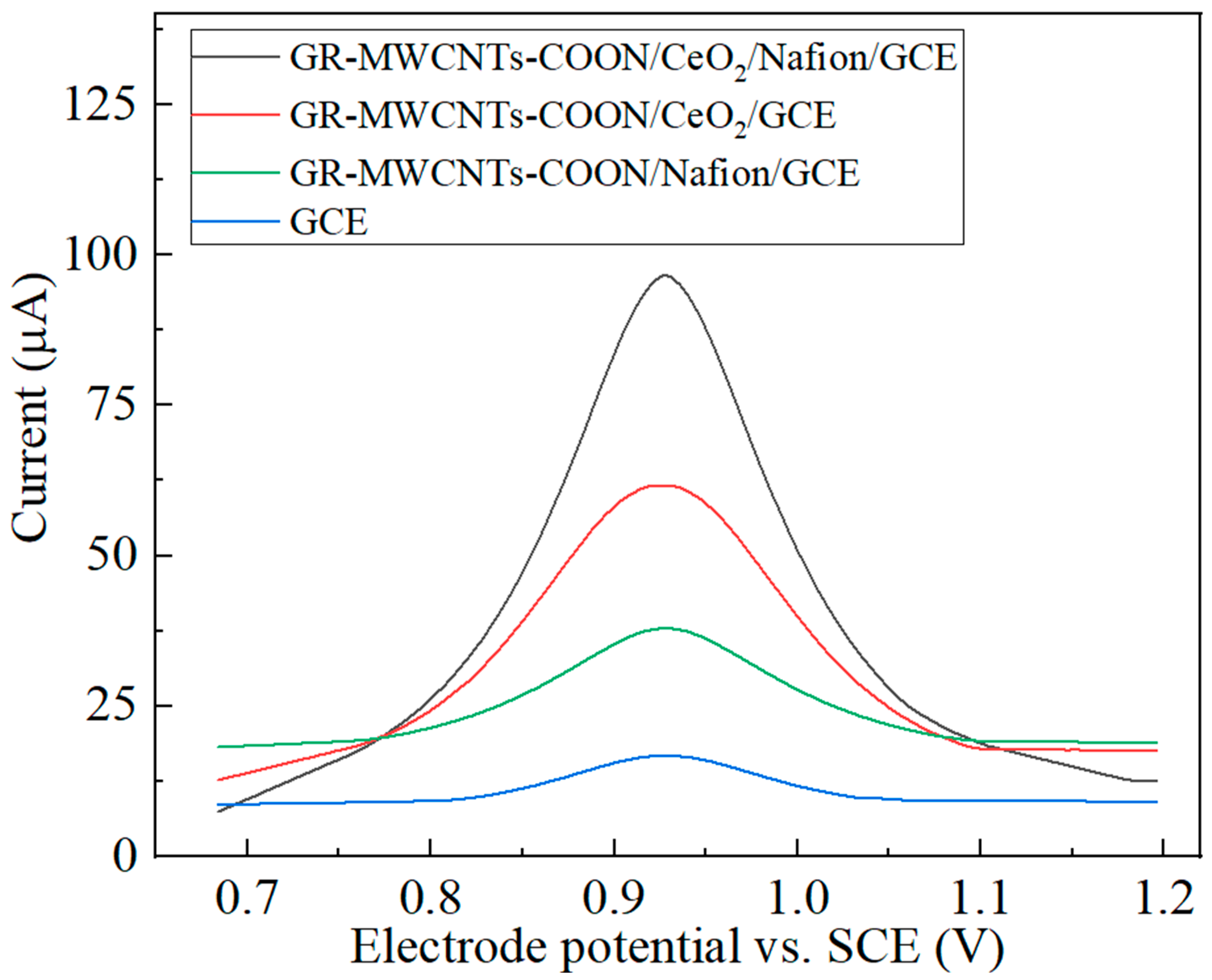
3.3. Comparison of the Performance of the Modified Electrodes
3.4. Optimization Studies
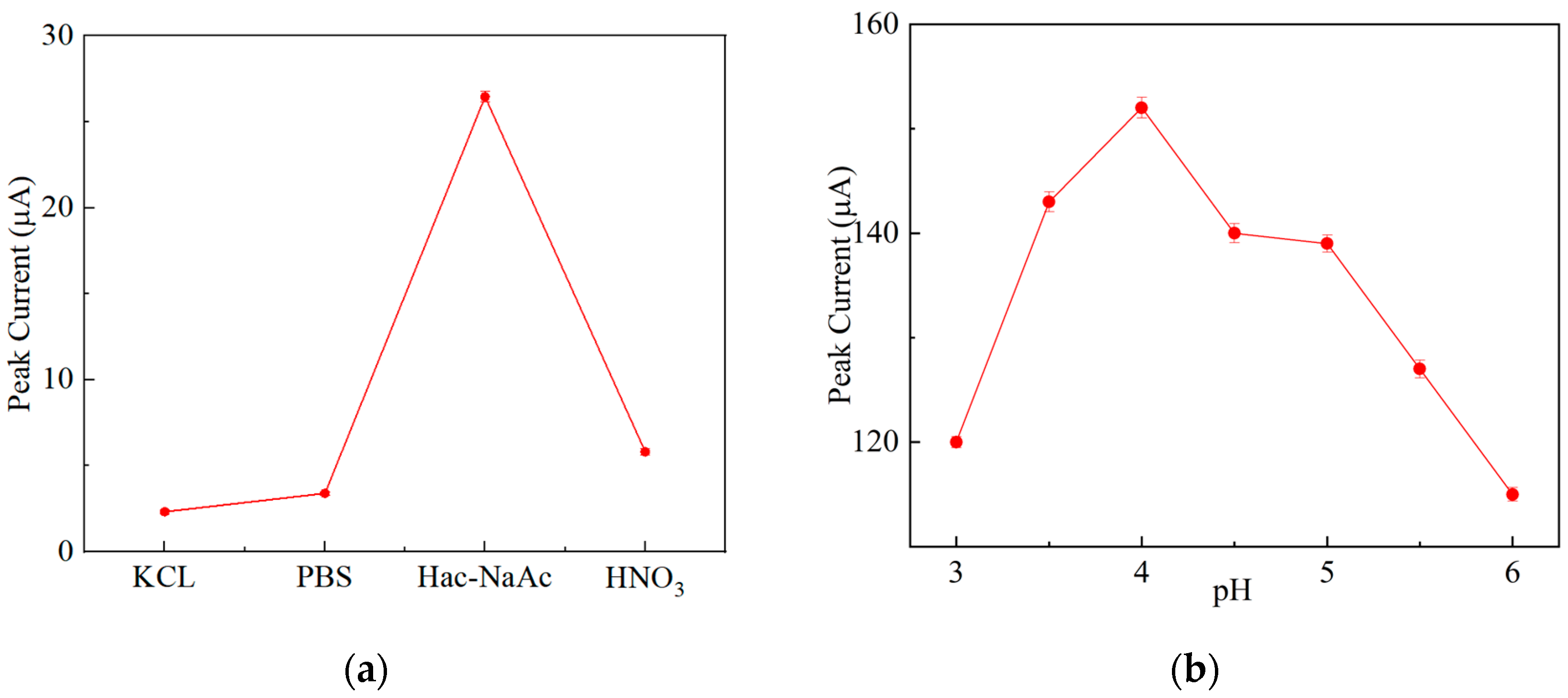
3.4.1. Effect of the Buffer Solution and pH
3.4.2. Effect of the Deposition Potential and Deposition Time
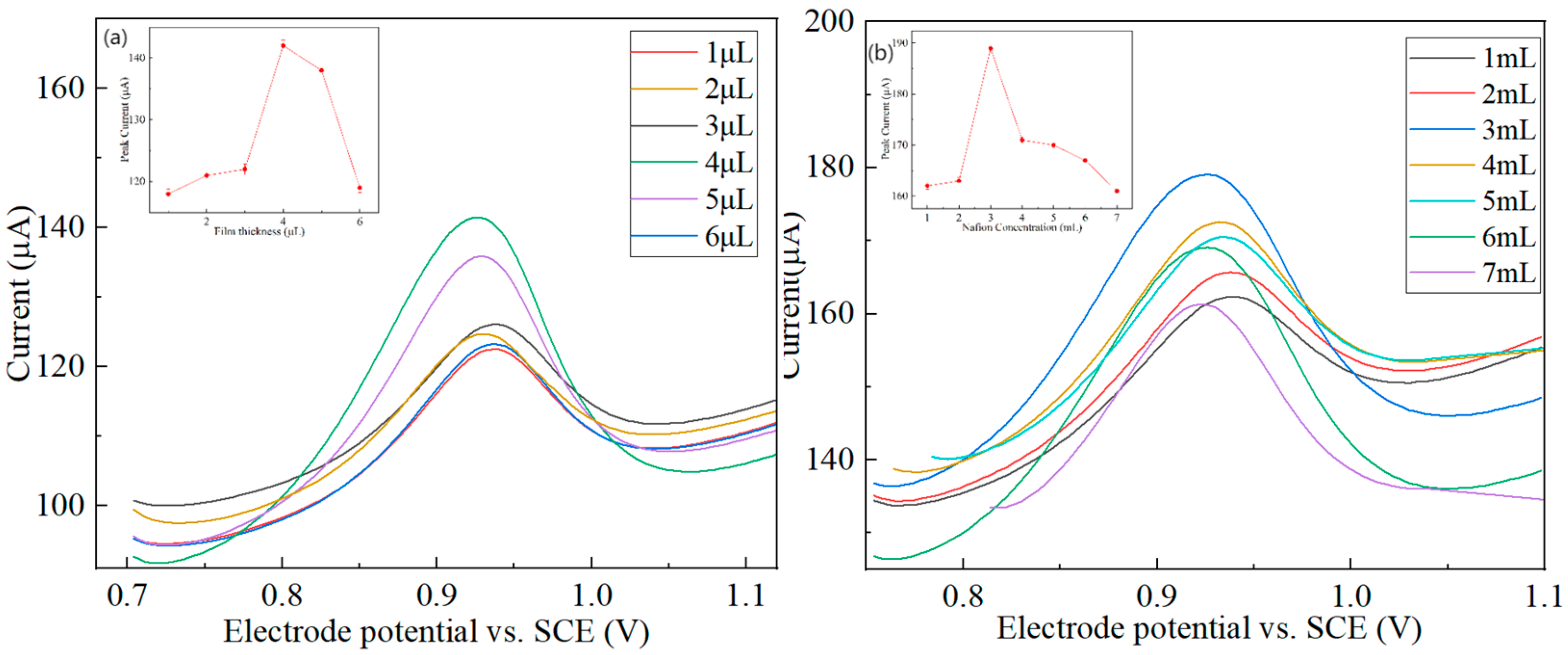
3.4.3. Effect of Membrane Thickness and Nafion Content
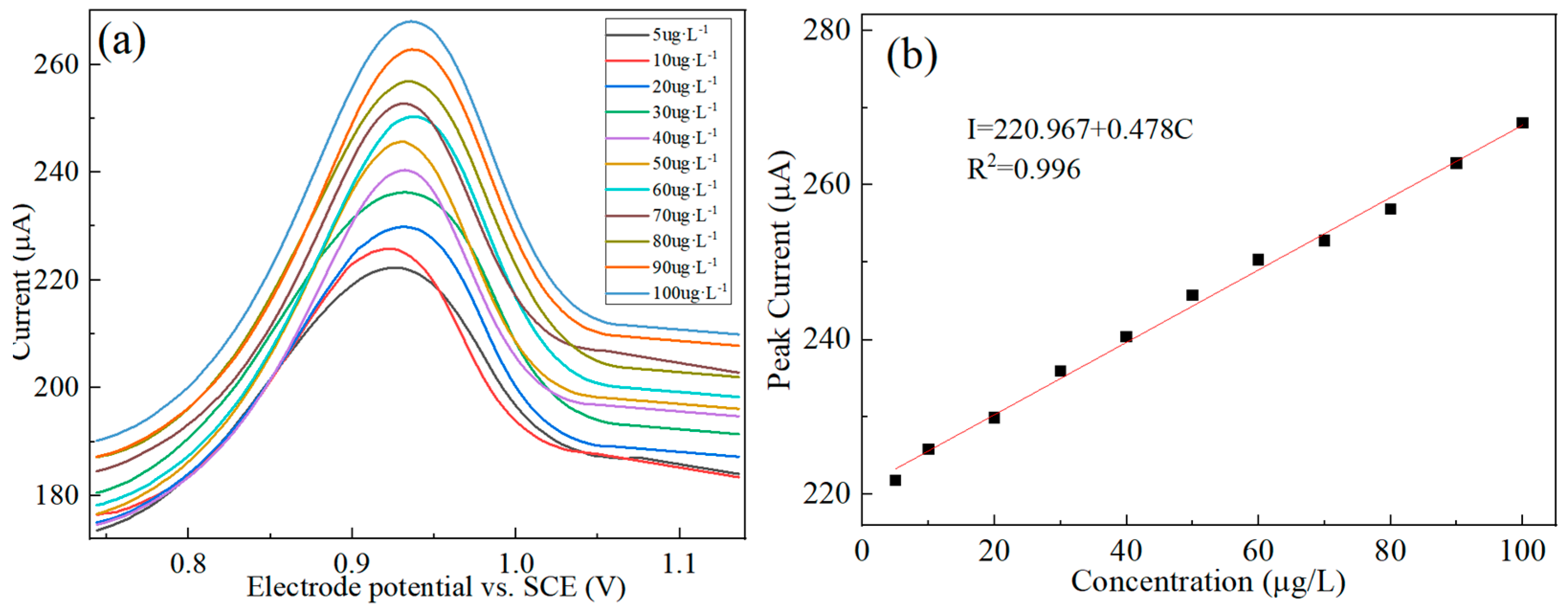
3.5. Standard Curve
3.6. Anti-Interference Capability
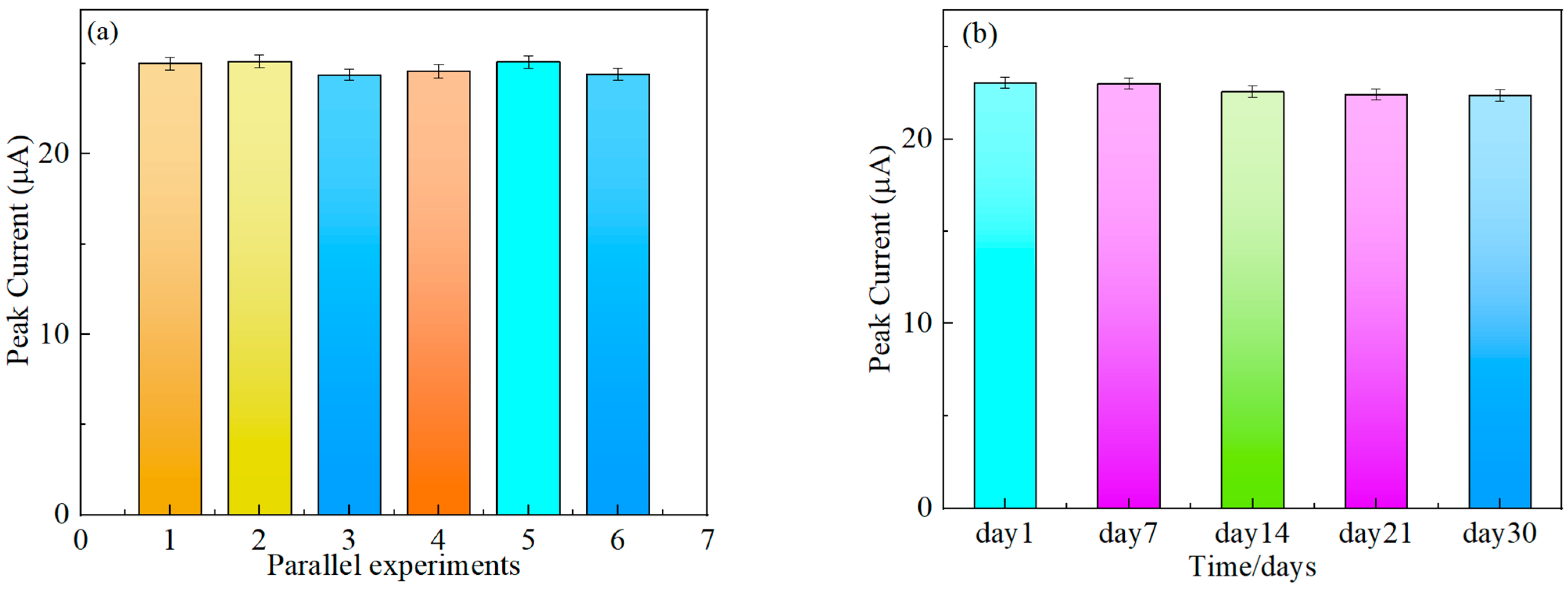
3.7. Stability
3.8. Reproducibility
3.9. Sample Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Subedi, S.; Neupane, L.N.; Yu, H.; Lee, K.-H. A New Ratiometric Fluorescent Chemodosimeter for Sensing of Hg2+ in Water Using Irreversible Reaction of Arylboronic Acid with Hg2+. Sens. Actuator B-Chem. 2021, 338, 129814. [Google Scholar] [CrossRef]
- Luo, L.; Xi, C.; Zhuo, J.; Liu, G.; Yang, S.; Nian, Y.; Sun, J.; Zhu, M.-Q.; Wang, J. A Portable Dual-Mode Colorimetric Platform for Sensitive Detection of Hg2+based on NiSe2 with Hg2+ Activated Oxidase-like Activity. Biosens. Bioelectron. 2022, 215, 114519. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wei, D.; Hu, C.; Li, S.; Ma, L.; Chen, Z.; Deng, Y. A Simple Label-Free Electrochemical Aptamer Sensor for Detection of Hg2+based on Black Phosphorus. Mater. Express 2022, 12, 802–809. [Google Scholar] [CrossRef]
- Huang, J.; Dong, R.; Habibul, M.; Zhang, Y.; Guan, M.; Li, G. An Electrochemiluminescence Aptasensor Based on Poly(Aniline-Luminol)/Graphene Oxide/Chitosan for Ultra-Sensitive Detection of Hg2+. Polym. Bull. 2023, 80, 12945–12958. [Google Scholar] [CrossRef]
- Xiao, W.; Xu, W.; Huang, W.; Zhou, Y.; Jin, Z.; Wei, X.; Li, J. Bismuth-Based BiOBrXI1-X/Ti3C2 MXene Schottky Nanocomposites for Hg2+Photoelectrochemical Sensors. ACS Appl. Nano Mater. 2022, 5, 18168–18177. [Google Scholar] [CrossRef]
- GB5749-2022; Standards for Drinking Water Quality. 2023. Available online: https://www.codeofchina.com/standard/GB5749-2022.html (accessed on 16 May 2024).
- Hao, B.; Bu, X.; Wu, J.; Ding, Y.; Zhang, L.; Zhao, B.; Tian, Y. Determination of Hg2+ in Water Based on Acriflavine Functionalized AgNPs by SERS. Microchem J. 2020, 155, 104736. [Google Scholar] [CrossRef]
- Zulhairee, M.; Zain, R.M.; Nor, A.C.M.; Jafar, N.F.; Noh, M.F.M.; Noorden, M.S.A.; Nordin, A.N.; Ab Rahim, R.; Gunawan, T.S.; Chin, L.Y.; et al. Clinical Performance of Reverse Transcription Loop-Mediated Isothermal Amplification COVID-19 Assay on Gold-Nanoparticle-Modified Screen-Printed Carbon Electrode Using Differential Pulse Voltammetry. Sens. Mater. 2023, 35, 4731–4750. [Google Scholar] [CrossRef]
- Yan, Y.; Zeng, S.; Xu, F.; Hu, Y.; Huang, H. Electrochemical Determination of Acetaminophen with a FeNi Nanoparticle Reduced Graphene Oxide (rGO) Nanocomposite and Differential Pulse Voltammetry (DPV). Anal. Lett. 2024, 57, 996–1007. [Google Scholar] [CrossRef]
- Lalmalsawmi, J.; Tiwari, D.; Kim, D.-J.; Kim, D.-J. Simultaneous Detection of Cd2+ and Pb2+ by Differential Pulse Anodic Stripping Voltammetry: Use of Highly Efficient Novel Ag0(NPs) Decorated Silane Grafted Bentonite Material. J. Electroanal. Chem. 2022, 918, 116490. [Google Scholar] [CrossRef]
- Taresh, R.R.; Roushani, M.; Karazan, Z.M. Selective Electrochemical Detection of Chromium Ions in Water Samples by Poly (Rutin)/Carbon Black-Chitosan Nanocomposite-Modified Glassy Carbon Electrode. J. Appl. Electrochem. 2024, 54, 1627–1636. [Google Scholar] [CrossRef]
- Arcos, D.; Nuno, D.; Khan, M.F.; Eom, J.; Ametller, L.; Ferrer-Anglada, N. Optoelectronic Characterization of 2D Graphene-Based Heterostructures: Gr/MoS2 and Gr/WS2. Phys. Status Solidi B-Basic Solid State Phys. 2023, 260, 2300202. [Google Scholar] [CrossRef]
- Huang, D.; Wang, L.; Zhan, Y.; Zou, L.; Ye, B. Photoelectrochemical Biosensor for CEA Detection Based on SnS2-GR with Multiple Quenching Effects of Au@CuS-GR. Biosens. Bioelectron. 2019, 140, 183–190. [Google Scholar] [CrossRef]
- Wu, Q.; Tian, L.; Shan, X.; Li, H.; Yang, S.; Li, C.; Song, Y.; Li, R.; Guo, Y.; Lu, J. A Molecule-Imprinted Electrochemiluminescence Sensor Based on CdS@MWCNTs for Ultrasensitive Detection of Fenpropathrin. Microchim. Acta 2024, 191, 269. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Y.; Li, F.; Dubovyk, V.; Guo, M.; Zhu, G.; Ran, Q.; Zhao, H. Electrochemical Sensing Platform Based on Graphitized and Carboxylated Multi-Walled Carbon Nanotubes Decorated with Cerium Oxide Nanoparticles for Sensitive Detection of Methyl Parathion. J. Mater. Res. Technol-JMRT 2022, 19, 3738–3748. [Google Scholar] [CrossRef]
- Zhu, G.; Zhang, Y.; Liu, Y.; Guo, M.; Zhang, M.; Wu, T.; Li, Y.; Zhao, H. Fabrication of Methyl Parathion Electrochemical Sensor Based on Modified Glassy Carbon Electrode with Graphitized and Carboxylated Multi-Walled Carbon Nanotubes Decorated with Zirconia Nanoparticles. Int. J. Electrochem. Sci. 2022, 17, 220678. [Google Scholar] [CrossRef]
- Wu, J.; Liu, X.; Zhang, R.; Zhang, J.; Si, H.; Wu, Z. A Novel (CaO/CeO2)@CeO2 Composite Adsorbent Based on Microinjection Titration-Calcination Strategy for CO2 Adsorption. Chem. Eng. J. 2023, 454, 140485. [Google Scholar] [CrossRef]
- Chen, Z.; Patel, R.; Berry, J.; Keyes, C.; Satterfield, C.; Simmons, C.; Neeson, A.; Cao, X.; Wu, Q. Development of Screen-Printable Nafion Dispersion for Electrochemical Sensor. Appl. Sci. 2022, 12, 6533. [Google Scholar] [CrossRef]
- Fu, X.; Xia, C.; Qu, J.; Lei, S.; Zuo, X.; Mao, Z.; Liu, Q.; Luo, P.; Zhang, R.; Hu, S. Enhanced Specific Capacitance and Stability of Polyaniline by Nafion Doping. ChemElectroChem 2022, 9, e202200312. [Google Scholar] [CrossRef]
- Yang, H.; Xu, K.; Zhang, Q.; Tao, L.; Yang, Z.; Dong, Z. Modified Nafion Membrane in Vanadium Redox Flow Battery. Prog. Chem. 2023, 35, 1595–1612. [Google Scholar] [CrossRef]
- Han, Z.-Y.; Pei, S.-P.; Yu, C.-Y.; Zhou, Y.-F. Molecular Dynamics Simulation Studies on the Micromorphology and Proton Transport of Nafion/Ti3C2Tx Composite Membrane. Chin. J. Polym. Sci. 2024, 42, 373–387. [Google Scholar] [CrossRef]
- Wen, L.; Dong, J.; Yang, H.; Zhao, J.; Hu, Z.; Han, H.; Hou, C.; Luo, X.; Huo, D. A Novel Electrochemical Sensor for Simultaneous Detection of Cd2+ and Pb2+ by MXene Aerogel-CuO/Carbon Cloth Flexible Electrode Based on Oxygen Vacancy and Bismuth Film. Sci. Total Environ. 2022, 851, 158325. [Google Scholar] [CrossRef]
- Guo, M.; Wu, T.; Zhu, G.; Liu, Y.; Zhao, M.; Shen, Y.; Zhou, Y.; Chen, L.; Guo, X.; Wang, Q.; et al. Highly Sensitive Determination of Niclosamide Based on The Graphitized and Carboxylated Multi-Walled Carbon Nanotubes Modified Glassy Carbon Electrode. Int. J. Electrochem. Sci. 2022, 17, 220772. [Google Scholar] [CrossRef]
- Kakhki, R.M. Nafion Based Biosensors: A Review of Recent Advances and Applications. Int. J. Polym. Mater. Polym. Biomat. 2023, 1–18. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, T.; Zhang, H.; Zhao, M.; Shen, Y.; Zhu, G.; Guo, M.; Liu, Y.; Li, F.; Zhao, H. Surface Optimization of Glassy Carbon Electrode with Graphitized and Carboxylated Multi-Walled Carbon Nanotubes@β-Cyclodextrin Nanocomposite for Electrochemical Determination of Methyl Parathion. Int. J. Electrochem. Sci. 2022, 17, 220653. [Google Scholar] [CrossRef]
- Somer, G.; Kalayci, S. A New and Simple Method for the Simultaneous Determination of Fe, Cu, Pb, Zn, Bi Cr, Mo, Se, and Ni in Dried Red Grapes Using Differential Pulse Polarography. Food Anal. Meth. 2015, 8, 604–611. [Google Scholar] [CrossRef]
- Gao, J.; Qiu, C.; Qu, W.; Zhuang, Y.; Wang, P.; Yan, Y.; Wu, Y.; Zeng, Z.; Huang, G.; Deng, R.; et al. Detection of Cd2+ Based on Nano-Fe3O4/MoS2/Nafion/GCE Sensor. Anal. Sci. 2023, 39, 1445–1454. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Xu, J.; Xie, F.; Xiong, S. Electrochemical Detection of As(III) by a rGO/Fe3O4-Modified Screen-Printed Carbon Electrode. Anal. Sci. 2016, 32, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.; Sahoo, P.K.; Manna, S.; Satpati, A.K. A Novel Low Cost Nonenzymatic Hydrogen Peroxide Sensor Based on CoFe2O4/CNTs Nanocomposite Modified Electrode. J. Electroanal. Chem. 2020, 876, 114504. [Google Scholar] [CrossRef]
- Manni, A.; Maallah, R.; El Haddar, A.; Chtaini, A.; Harrati, A.; Sair, S.; El Bouari, A.; El Hassani, I.-E.E.A.; Sadik, C. A Sensitive Electrochemical Sensor Using a Dolomite-Graphite Composite for the Simultaneous Detection of Pb2+ and Cd2+. Iran J. Chem. Chem. Eng.-Int. Engl. Ed. 2023, 42, 3422–3434. [Google Scholar]
- Fendrych, K.; Porada, R.; Bas, B. Electrochemical Sensing Platform Based on Zeolite/Graphite/Dimethylglyoxime Nanocomposite for Highly Selective and Ultrasensitive Determination of Nickel. J. Hazard. Mater. 2023, 448, 130953. [Google Scholar] [CrossRef]
- Mukherjee, A.G.; Renu, K.; Gopalakrishnan, A.V.; Veeraraghavan, V.P.; Vinayagam, S.; Paz-Montelongo, S.; Dey, A.; Vellingiri, B.; George, A.; Madhyastha, H.; et al. Heavy Metal and Metalloid Contamination in Food and Emerging Technologies for Its Detection. Sustainability 2023, 15, 1195. [Google Scholar] [CrossRef]
- Setiyanto, H.; Purwaningsih, D.R.; Saraswaty, V.; Mufti, N.; Zulfikar, M.A. Highly Selective Electrochemical Sensing Based on Electropolymerized Ion Imprinted Polyaniline (IIPANI) on a Bismuth Modified Carbon Paste Electrode (CPE-Bi) for Monitoring Nickel(Ii) in River Water. RSC Adv. 2022, 12, 29554–29561. [Google Scholar] [CrossRef] [PubMed]
- Gayathri, J.; Sivalingam, S.; Narayanan, S.S. Novel Synthesized SABA/MWCNTs Composite to Detect Cd2+and Pb2+ions in Real Samples of Rice Water, Tobacco Extract and Raw Milk. J. Mol. Liq. 2023, 387, 122586. [Google Scholar] [CrossRef]
- Fu, X.; Wen, J.; Xia, C.; Liu, Q.; Zhang, R.; Hu, S. Nafion Doped Polyaniline/Graphene Oxide Composites as Electrode Materials for High Performance Flexible Supercapacitors Based on Nafion Membrane. Mater. Des. 2023, 236, 112506. [Google Scholar] [CrossRef]
- Miranda, M.; Carvetta, C.; Sisodia, N.; Shirley, L.; Day, C.D.; McGuinness, K.L.; Wadhawan, J.D.; Lawrence, N.S. Nafion® Coated Electropolymerised Flavanone-Based pH Sensor. Electroanalysis 2022, 34, 1273–1279. [Google Scholar] [CrossRef]
- Khan, I.; Lee, J.H.; Park, J.; Wooh, S. Nano/Micro-Structural Engineering of Nafion Membranes for Advanced Electrochemical Applications. J. Saudi Chem. Soc. 2022, 26, 101511. [Google Scholar] [CrossRef]




| Sample | Added (μg·L−1) | Found (μg·L−1) | Recovery % | RSD % |
|---|---|---|---|---|
| 1 | 50 | 51.88 | 103.80 | 2.61 |
| 2 | 50 | 49.59 | 99.18 | 5.79 |
| 3 | 50 | 47.57 | 95.14 | 3.52 |
| 4 | 50 | 47.40 | 94.72 | 3.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, H.; Qiu, C.; Qu, W.; Zhuang, Y.; Han, X.; Tao, W.; Gu, Y.; Zhao, Z.; Liu, H.; Wang, W. Research on the Detection of Hg(II) in Seawater Using GR-MWCNTs/CeO2-Modified Electrodes. Chemosensors 2024, 12, 128. https://doi.org/10.3390/chemosensors12070128
Hao H, Qiu C, Qu W, Zhuang Y, Han X, Tao W, Gu Y, Zhao Z, Liu H, Wang W. Research on the Detection of Hg(II) in Seawater Using GR-MWCNTs/CeO2-Modified Electrodes. Chemosensors. 2024; 12(7):128. https://doi.org/10.3390/chemosensors12070128
Chicago/Turabian StyleHao, Huili, Chengjun Qiu, Wei Qu, Yuan Zhuang, Xiaochun Han, Wei Tao, Yang Gu, Zizi Zhao, Haozheng Liu, and Wenhao Wang. 2024. "Research on the Detection of Hg(II) in Seawater Using GR-MWCNTs/CeO2-Modified Electrodes" Chemosensors 12, no. 7: 128. https://doi.org/10.3390/chemosensors12070128





