Liquid-Gated Graphene Field Effect Transistor for High-Performance Label-Free Sensing of Polycyclic Aromatic Hydrocarbons
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Fabrication of Liquid-Gated Gr-FET Sensors
2.3. Characterization
3. Results and Discussion
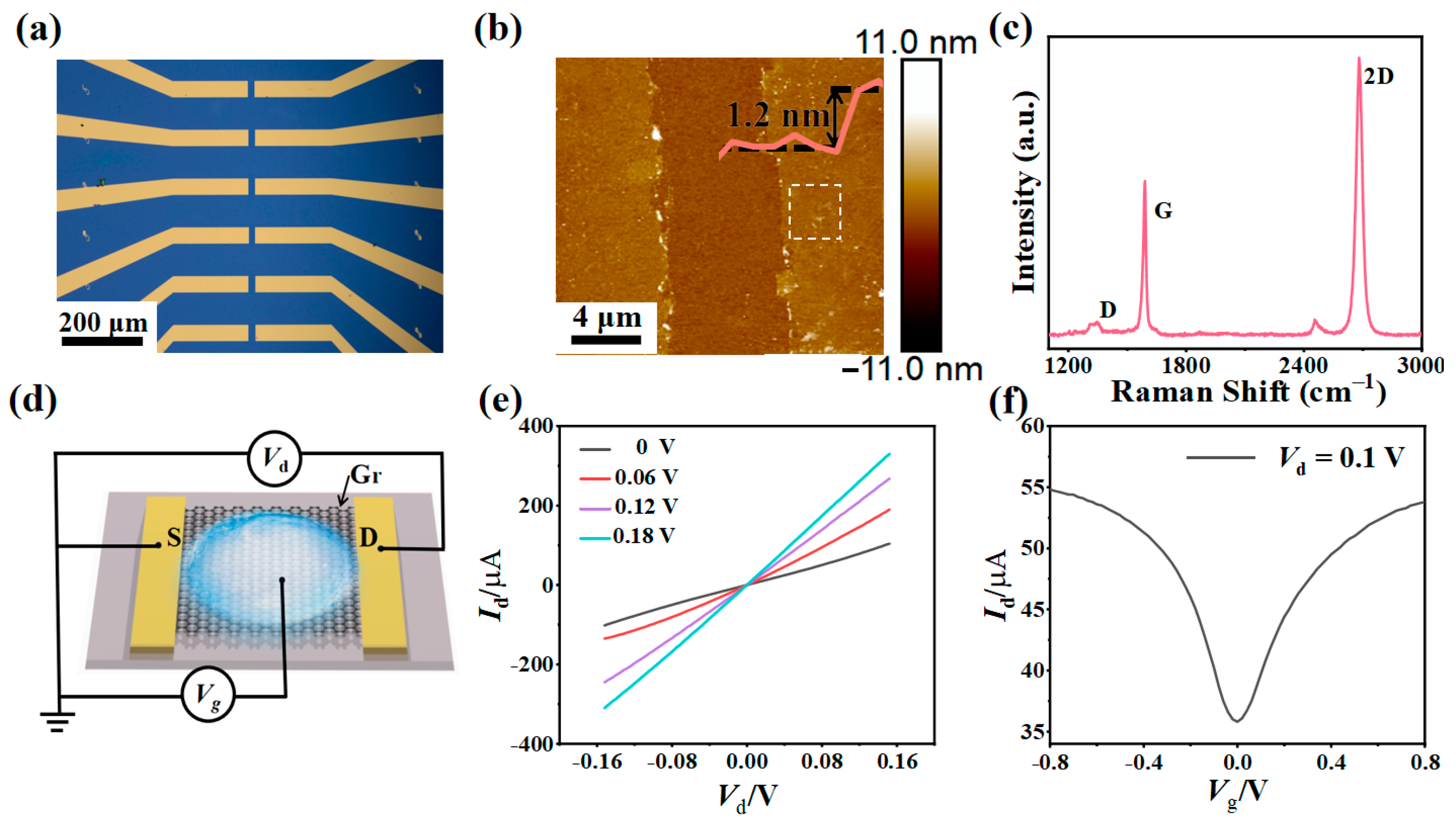
3.1. Structure and Electrical Properties of Liquid-Gated Gr-FET Sensor
3.2. Sensing Mechanism of Liquid-Gated Gr-FET Sensor
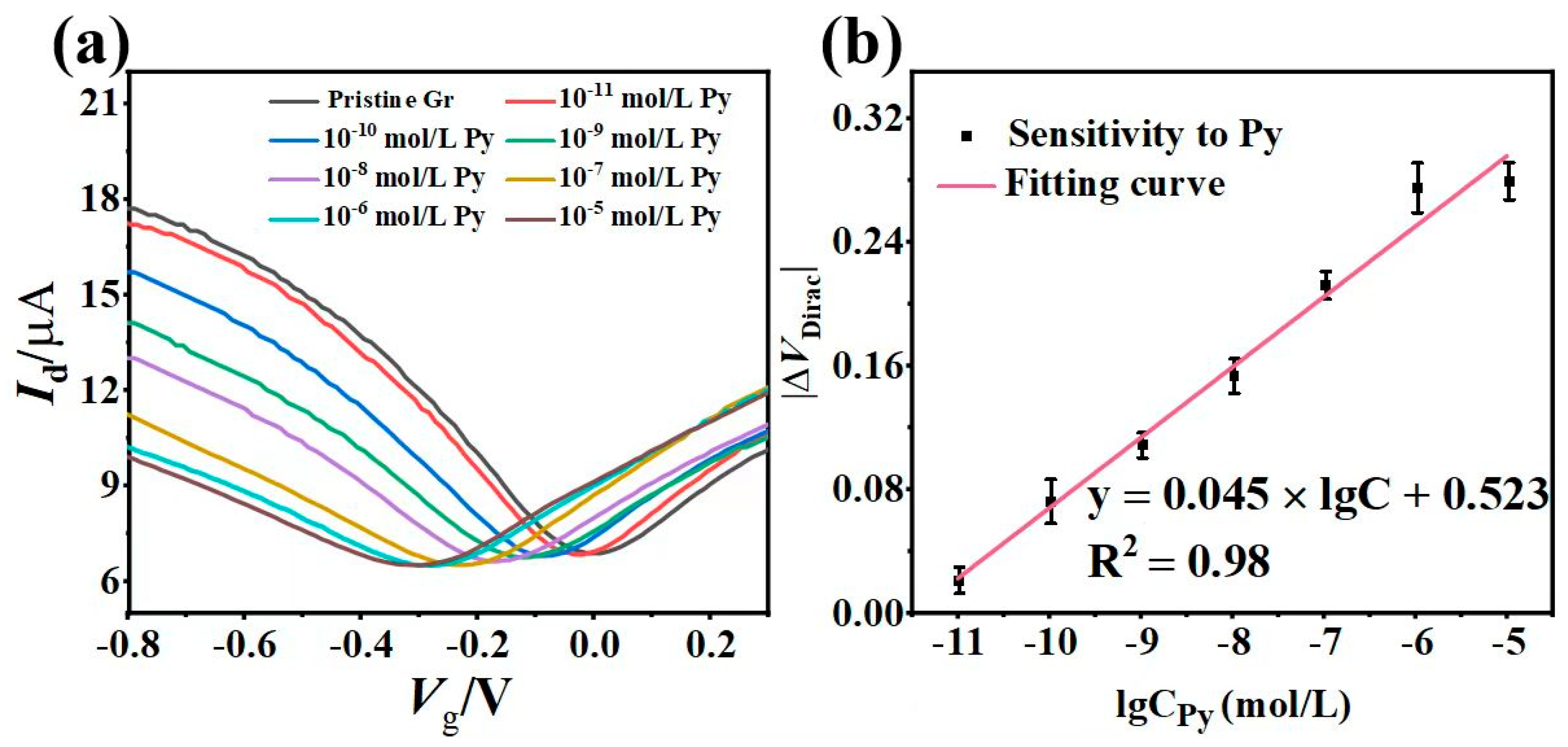
3.3. Pyrene Detection with the Liquid-Gated Gr-FET Sensor
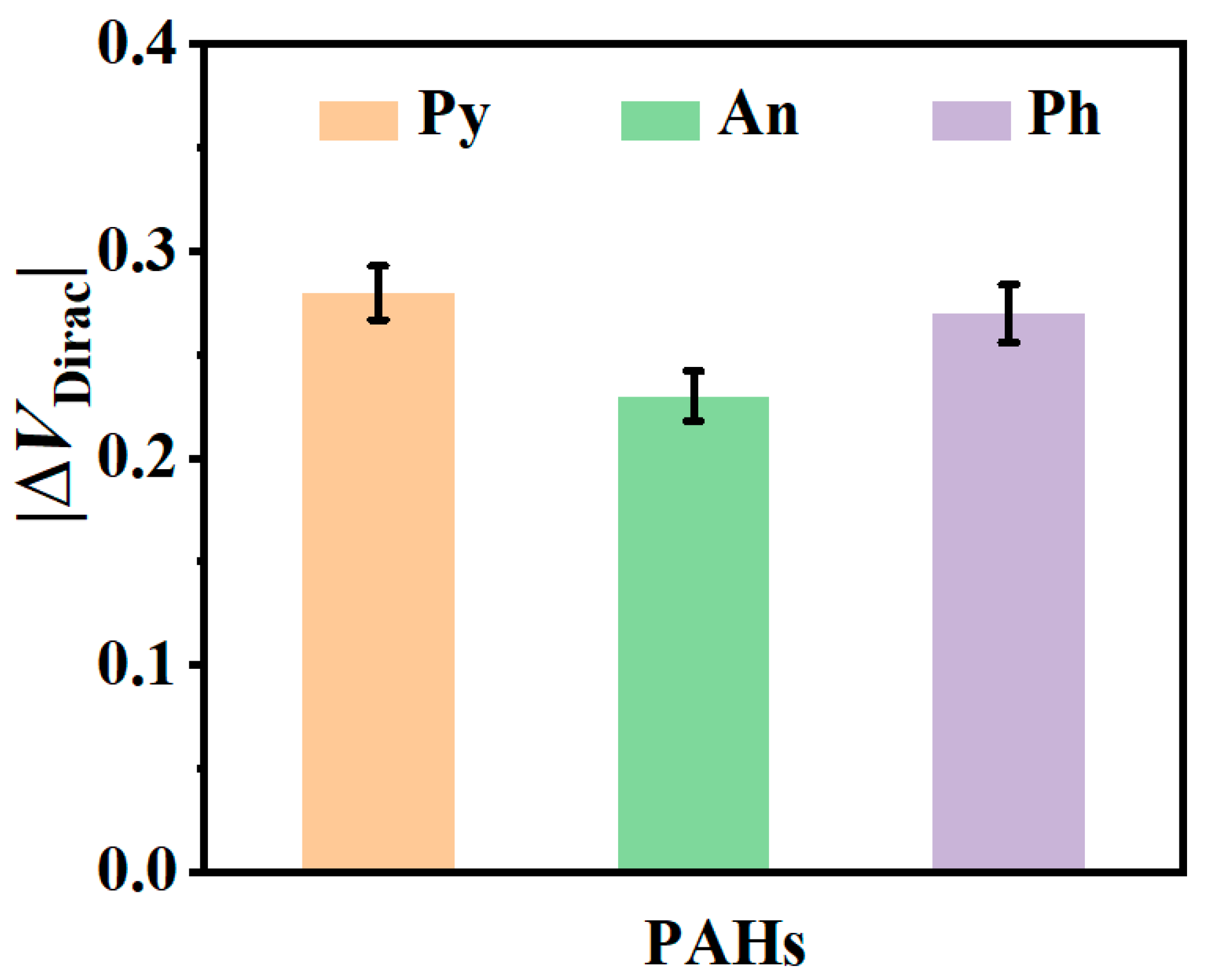
3.4. Application of Liquid Gr-FET Sensor for PHA Detection
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hao, J.N.; Yan, B. Determination of Urinary 1-Hydroxypyrene for Biomonitoring of Human Exposure to Polycyclic Aromatic Hydrocarbons Carcinogens by a Lanthanide-functionalized Metal-Organic Framework Sensor. Adv. Funct. Mater. 2016, 27, 1603856. [Google Scholar] [CrossRef]
- Li, Q.; Zheng, Q.; Shi, J.; Yan, Y.; Guo, X.; Yang, H. Magnetic optimizing surface-enhanced Raman scattering (SERS) strategy of detection and in-situ monitoring of photodegradation of Benzo[a]pyrene in water. Anal. Chim. Acta 2025, 1336, 343466. [Google Scholar] [CrossRef] [PubMed]
- Ni, N.; Li, X.; Yao, S.; Shi, R.; Kong, D.; Bian, Y.; Jiang, X.; Song, Y. Biochar applications combined with paddy-upland rotation cropping systems benefit the safe use of PAH-contaminated soils: From risk assessment to microbial ecology. J. Hazard. Mater. 2021, 404, 124123. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, X.; Chen, C.; Wang, R.; Qiao, X.; Waterhouse, G.I.N.; Xu, Z. A surface-enhanced Raman scattering sensor for the detection of benzo[a]pyrene in foods based on a gold nanostars@reduced graphene oxide substrate. Food Chem. 2023, 421, 136171. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2004; Volume 1. [Google Scholar]
- Hu, T.; Xia, Y.; Wang, Y.; Lin, L.; An, R.; Xu, L.; Qing, X. Fast Determination and Source Apportionment of Eight Polycyclic Aromatic Hydrocarbons in PM10 Using the Chemometric-Assisted HPLC-DAD Method. Chemosensors 2024, 12, 220. [Google Scholar] [CrossRef]
- Qu, B.; Li, P.; Bai, L.; Qu, Y.; Li, Z.; Zhang, Z.; Zheng, B.; Sun, J.; Jing, L. Atomically Dispersed Zn-N5 Sites Immobilized on g-C3N4 Nanosheets for Ultrasensitive Selective Detection of Phenanthrene by Dual Ratiometric Fluorescence. Adv. Mater. 2023, 35, 2211575. [Google Scholar]
- Lin, Y.-Y.; Liu, G.; Wai, C.M.; Lin, Y. Magnetic beads-based bioelectrochemical immunoassay of polycyclic aromatic hydrocarbons. Electrochem. Commun. 2007, 9, 1547–1552. [Google Scholar] [CrossRef]
- Latif, U.; Ping, L.; Dickert, F. Conductometric Sensor for PAH Detection with Molecularly Imprinted Polymer as Recognition Layer. Sensors 2018, 18, 767. [Google Scholar] [CrossRef]
- Qu, F.; Li, H. Selective molecular recognition of polycyclic aromatic hydrocarbons using CdTe quantum dots with cyclodextrin as supramolecular nano-sensitizers in water. Sens. Actuators B Chem. 2009, 135, 499–505. [Google Scholar] [CrossRef]
- Guo, Z.; Zeng, Y.; Meng, F.; Qu, H.; Zhang, S.; Hu, S.; Fan, S.; Zeng, H.; Cao, R.; Prasad, P.N.; et al. In-situ neutron-transmutation for substitutional doping in 2D layered indium selenide based phototransistor. eLight 2022, 2, 9. [Google Scholar] [CrossRef]
- Li, J.; Wu, D.; Yu, Y.; Li, T.; Li, K.; Xiao, M.-M.; Li, Y.; Zhang, Z.-Y.; Zhang, G.-J. Rapid and unamplified identification of COVID-19 with morpholino-modified graphene field-effect transistor nanosensor. Biosens. Bioelectron. 2021, 183, 113206. [Google Scholar] [CrossRef]
- Sun, M.; Zhang, C.; Lu, S.; Mahmood, S.; Wang, J.; Sun, C.; Pang, J.; Han, L.; Liu, H. Recent Advances in Graphene Field-Effect Transistor Toward Biological Detection. Adv. Funct. Mater. 2024, 34, 2405471. [Google Scholar] [CrossRef]
- Yao, L.; Gao, S.; Liu, S.; Bi, Y.; Wang, R.; Qu, H.; Wu, Y.; Mao, Y.; Zheng, L. Single-Atom Enzyme-Functionalized Solution-Gated Graphene Transistor for Real-Time Detection of Mercury Ion. ACS Appl. Mater. Interfaces 2020, 12, 6268–6275. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Hu, Y.; Han, Y.; Du, J.; Zhang, G.; Liu, Y. Electrical study of a novel pyrene boned graphene nanoribbon film and its specific aniline sensing feature. Chem. Eng. J. 2023, 471, 144443. [Google Scholar] [CrossRef]
- Lei, Y.; Zeng, R.; Li, Y.-T.; Xiao, M.-M.; Zhang, Z.-Y.; Zhang, G.-J. Real-time monitoring of cellular Ca2+ efflux with renewable graphene field effect transistor biosensor. Carbon 2023, 201, 616–623. [Google Scholar] [CrossRef]
- Lei, Y.-M.; Xiao, M.-M.; Li, Y.-T.; Xu, L.; Zhang, H.; Zhang, Z.-Y.; Zhang, G.-J. Detection of heart failure-related biomarker in whole blood with graphene field effect transistor biosensor. Biosens. Bioelectron 2017, 91, 1–7. [Google Scholar] [CrossRef]
- Ruiz, E.; Varenne, C.; De Lima, B.S.; Gueye, T.; Pauly, A.; Brunet, J.; Mastelaro, V.R.; Ndiaye, A.L. Investigating the Metallic Nanoparticles Decoration on Reduced Graphene Oxide-Based Sensors Used to Detect Sulfur Dioxide. Chemosensors 2024, 12, 24. [Google Scholar] [CrossRef]
- Huang, T.; Tang, X.; Luo, K.; Wu, Y.; Hou, X.; Tang, S. An overview of graphene-based nanoadsorbent materials for environmental contaminants detection. TrAC Trends Anal. Chem. 2021, 139, 116255. [Google Scholar] [CrossRef]
- Almeida, J.R.; Gravato, C.; Guilhermino, L. Challenges in assessing the toxic effects of polycyclic aromatic hydrocarbons to marine organisms: A case study on the acute toxicity of pyrene to the European seabass (Dicentrarchus labrax L.). Chemosphere 2012, 86, 926–937. [Google Scholar] [CrossRef]
- Wu, G.; Dai, Z.; Tang, X.; Lin, Z.; Lo, P.K.; Meyyappan, M.; Lai, K.W.C. Graphene Field-Effect Transistors for the Sensitive and Selective Detection of Escherichia coli Using Pyrene-Tagged DNA Aptamer. Adv. Healthc. Mater. 2017, 6, 1700736. [Google Scholar] [CrossRef]
- Shearer, C.J.; Slattery, A.D.; Stapleton, A.J.; Shapter, J.G.; Gibson, C.T. Accurate thickness measurement of graphene. Nanotechnology 2016, 27, 125704. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, W.; Li, Y.; Wang, H.; Qin, Z. Activated pyrene decorated graphene with enhanced performance for electrochemical energy storage. Chem. Eng. J. 2018, 334, 845–854. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman Spectrum of Graphene and Graphene Layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef] [PubMed]
- Reina, A.; Jia, X.; Ho, J.; Nezich, D.; Son, H.; Bulovic, V.; Dresselhaus, M.S.; Kong, J. Large Area, Few-Layer Graphene Films on Arbitrary Substrates by Chemical Vapor Deposition. Nano Lett. 2009, 9, 30–35. [Google Scholar] [CrossRef] [PubMed]
- McQueen, E.W.; Goldsmith, J.I. Electrochemical Analysis of Single-Walled Carbon Nanotubes Functionalized with Pyrene-Pendant Transition Metal Complexes. J. Am. Chem. Soc. 2009, 131, 17554–17556. [Google Scholar] [CrossRef] [PubMed]
- Putri, A.D.; Chotimah, N.; Ujjain, S.K.; Wang, S.; Futamura, R.; Vallejos-Burgos, F.; Khoerunnisa, F.; Morimoto, M.; Wang, Z.; Hattori, Y.; et al. Charge-transfer mediated nanopore-controlled pyrene derivatives/graphene colloids. Carbon 2018, 139, 512–521. [Google Scholar] [CrossRef]
- Wu, G.; Tang, X.; Meyyappan, M.; Lai, K.W.C. Doping effects of surface functionalization on graphene with aromatic molecule and organic solvents. Appl. Surf. Sci. 2017, 425, 713–721. [Google Scholar] [CrossRef]
- Babolghani, F.M.; Mohammadi-Manesh, E. Simulation and experimental study of FET biosensor to detect polycyclic aromatic hydrocarbons. Appl. Surf. Sci. 2019, 488, 662–670. [Google Scholar] [CrossRef]
- Munoz, J.; Campos-Lendinez, A.; Crivillers, N.; Mas-Torrent, M. Selective Discrimination of Toxic Polycyclic Aromatic Hydrocarbons in Water by Targeting π-Stacking Interactions. ACS Appl. Mater. Interfaces 2020, 12, 26688–26693. [Google Scholar] [CrossRef]
- Sehatnia, B.; Sabzi, R.E.; Kheiri, F.; Nikoo, A. Sensitive molecular determination of polycyclic aromatic hydrocarbons based on thiolated Calix[4]arene and CdSe quantum dots (QDs). J. Appl. Electrochem. 2014, 44, 727–733. [Google Scholar] [CrossRef]
- Sánchez-Alvarado, A.B.; Zhou, J.; Jin, P.; Neumann, O.; Senftle, T.P.; Nordlander, P.; Halas, N.J. Combined Surface-Enhanced Raman and Infrared Absorption Spectroscopies for Streamlined Chemical Detection of Polycyclic Aromatic Hydrocarbon-Derived Compounds. ACS Nano 2023, 17, 25697–25706. [Google Scholar] [CrossRef]


| Type of Sensing | Range (M) | Time | Matrix | Reference | |
|---|---|---|---|---|---|
| DNA/Cu2O-GS-FET | Field Effect Transistor | 2 × 10−3–3 × 10−2 | 1.5 h | Electrolyte solution | [29] |
| ITO-SAM-Nap-NH2 | Electrochemical Impedance Spectroscopy | 3.90 × 10−7–5.46 × 10−7 | 3 min | Aqueous | [30] |
| Fe3O4-Calix[4]arene @CdSe | Square Ware Voltammetry | 1.5 × 10−6–25 × 10−6 | 12 h | Water | [31] |
| SERS + SEIRA | Surface-Enhanced Raman and Infrared Absorption Spectroscopies | 3.95 × 10−8 | — | GNS | [32] |
| Liquid-gated G-FET | Field Effect Transistor | 10−10–10−6 | 2 min | PBS | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kou, C.; Xu, X.; Bao, Y.; Guo, Z.; Niu, L. Liquid-Gated Graphene Field Effect Transistor for High-Performance Label-Free Sensing of Polycyclic Aromatic Hydrocarbons. Chemosensors 2025, 13, 56. https://doi.org/10.3390/chemosensors13020056
Kou C, Xu X, Bao Y, Guo Z, Niu L. Liquid-Gated Graphene Field Effect Transistor for High-Performance Label-Free Sensing of Polycyclic Aromatic Hydrocarbons. Chemosensors. 2025; 13(2):56. https://doi.org/10.3390/chemosensors13020056
Chicago/Turabian StyleKou, Cuiyun, Xiaofeng Xu, Yu Bao, Zhinan Guo, and Li Niu. 2025. "Liquid-Gated Graphene Field Effect Transistor for High-Performance Label-Free Sensing of Polycyclic Aromatic Hydrocarbons" Chemosensors 13, no. 2: 56. https://doi.org/10.3390/chemosensors13020056
APA StyleKou, C., Xu, X., Bao, Y., Guo, Z., & Niu, L. (2025). Liquid-Gated Graphene Field Effect Transistor for High-Performance Label-Free Sensing of Polycyclic Aromatic Hydrocarbons. Chemosensors, 13(2), 56. https://doi.org/10.3390/chemosensors13020056





