Study of a Sensitive and Selective Electrochemical Biosensor for Glucose Based on Bi2Ru2O7 Pyrochlore Clusters Combined with MWCNTs
Abstract
1. Introduction
2. Materials and Methods
2.1. Apparatus
2.2. Reagents and Solutions
2.3. Synthesis and Characterization of Bi2Ru2O7 Pyrochlore Clusters
2.4. Modification of the Working Electrode and Fabrication of the Biosensor
2.5. Electrochemical Measurements
3. Results
3.1. Material Characterization
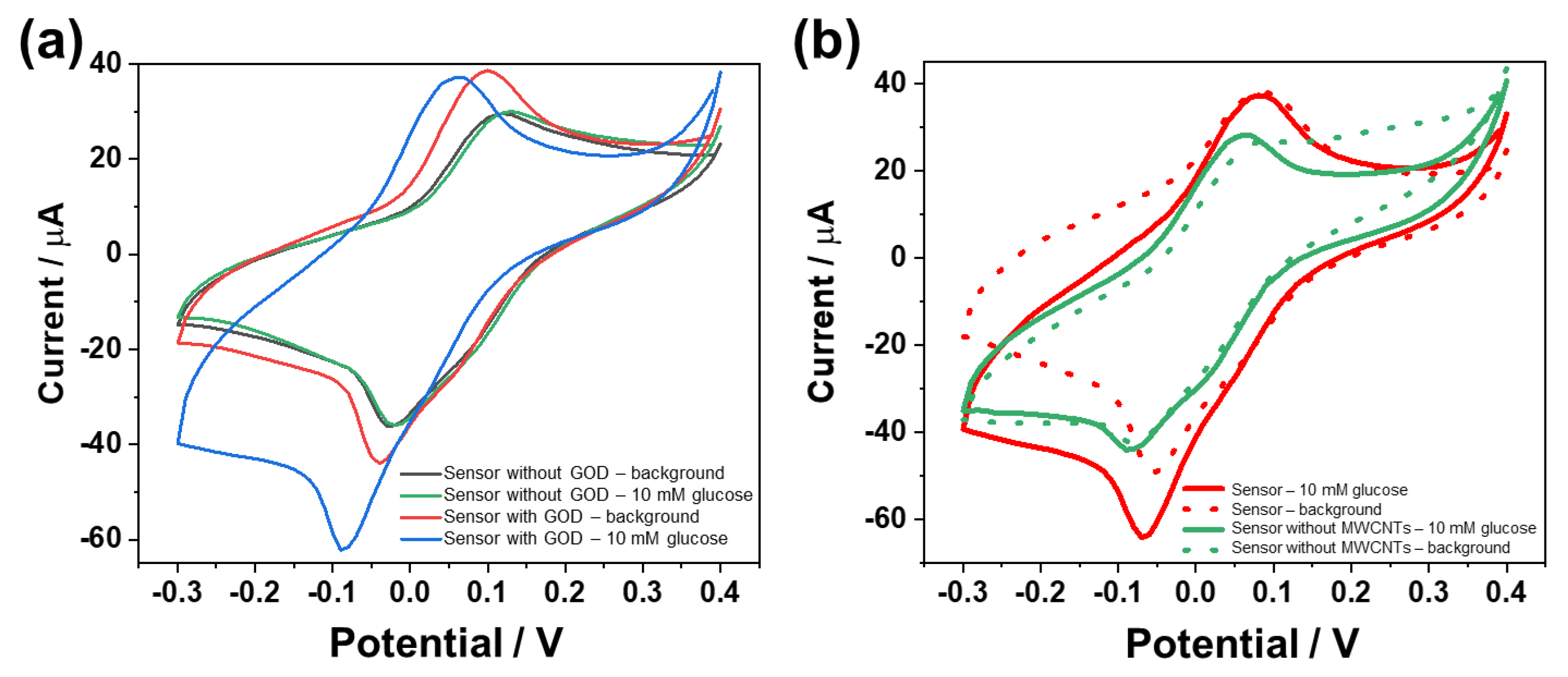
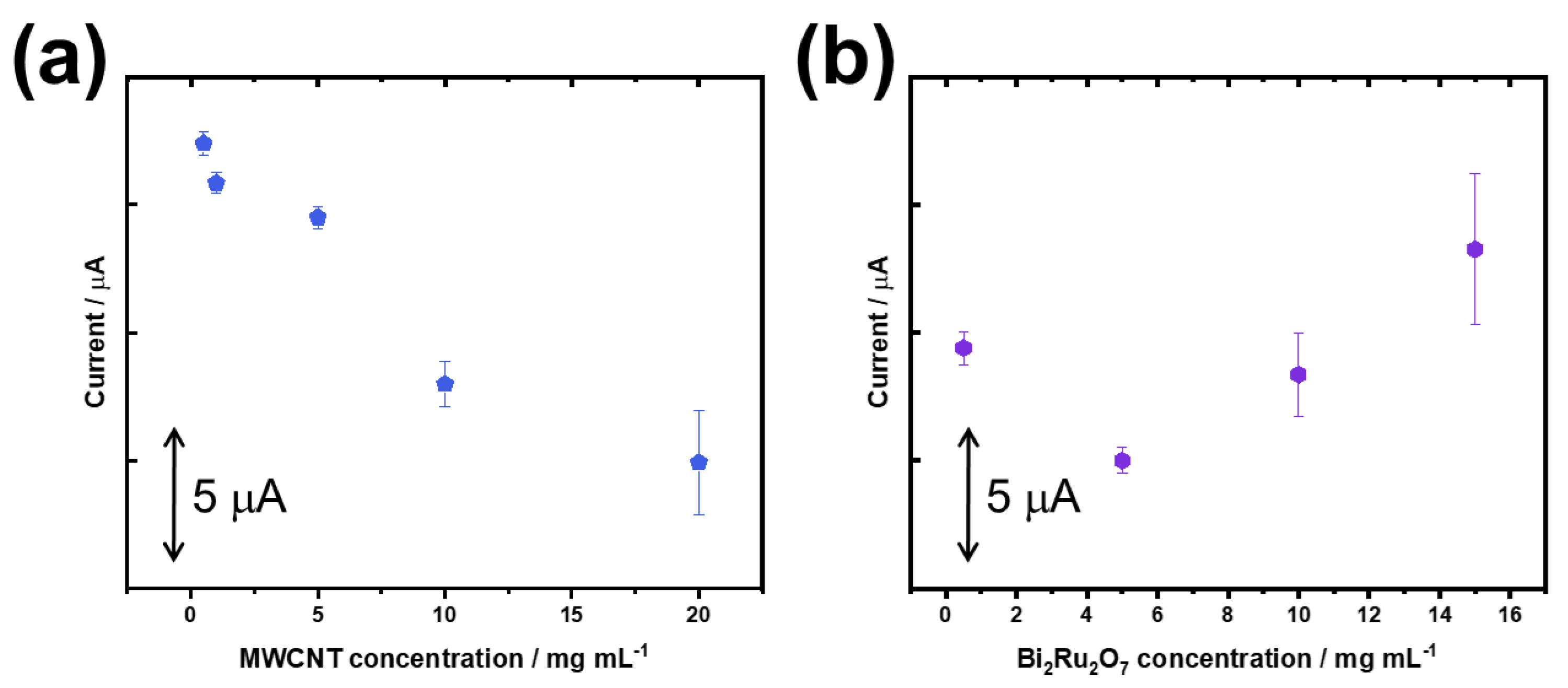
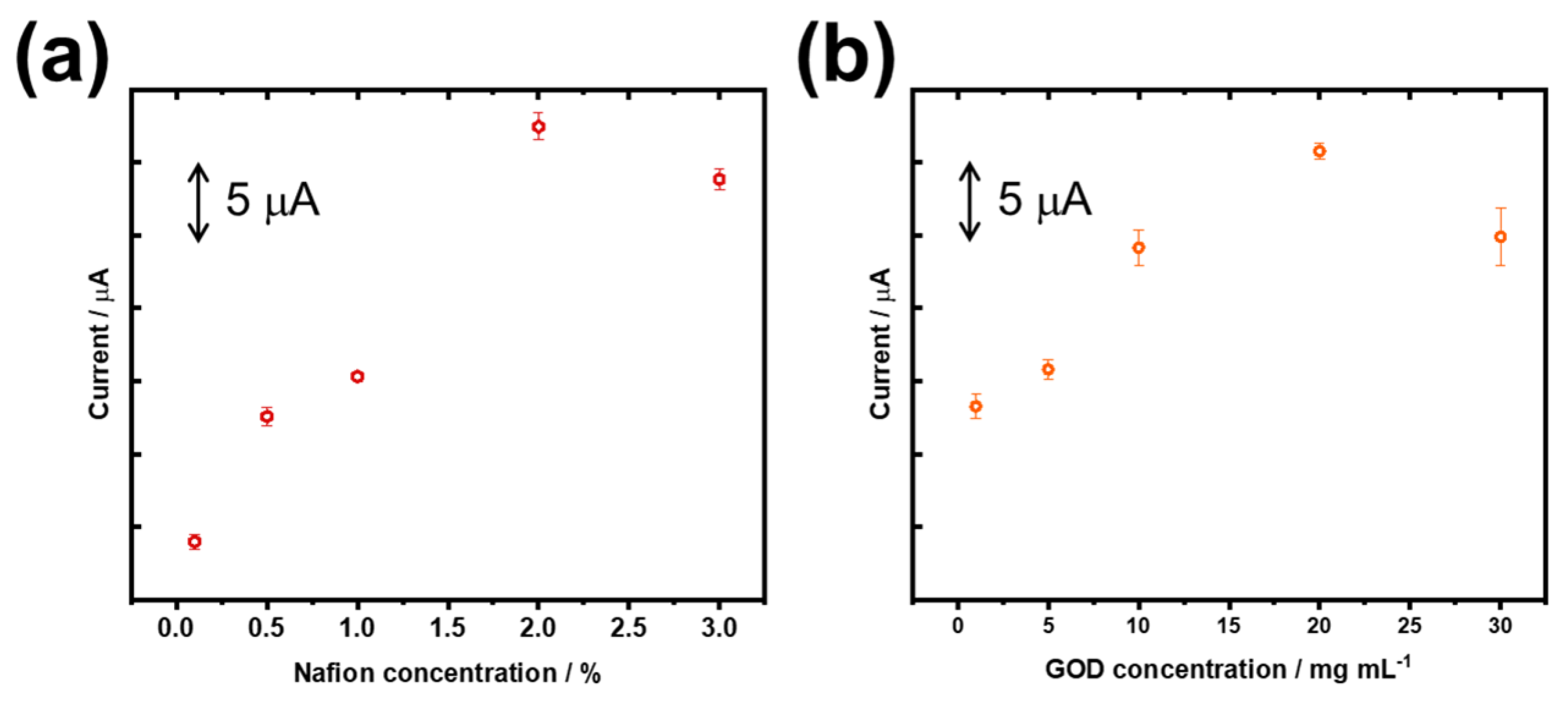
3.2. Optimization of the Glucose Biosensor
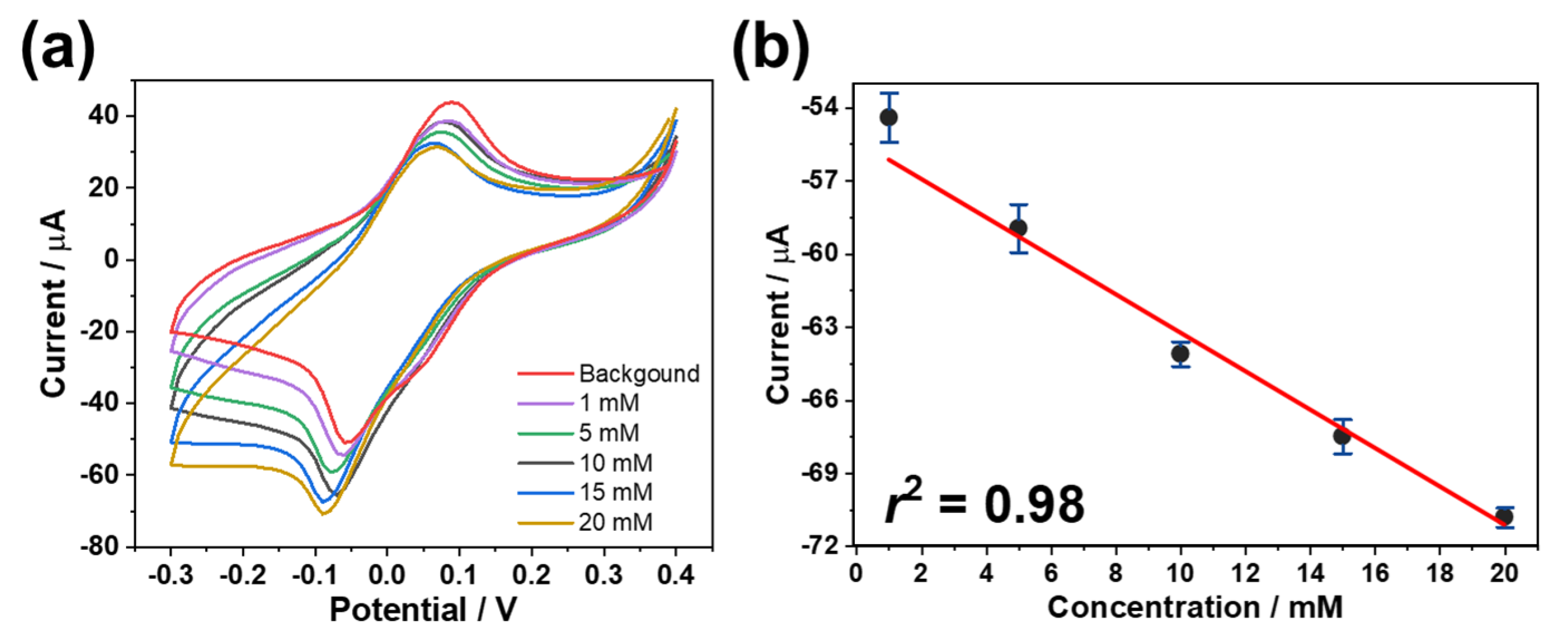
3.3. Analytical Performance of the Biosensor
3.4. Selectivity and Stability Studies
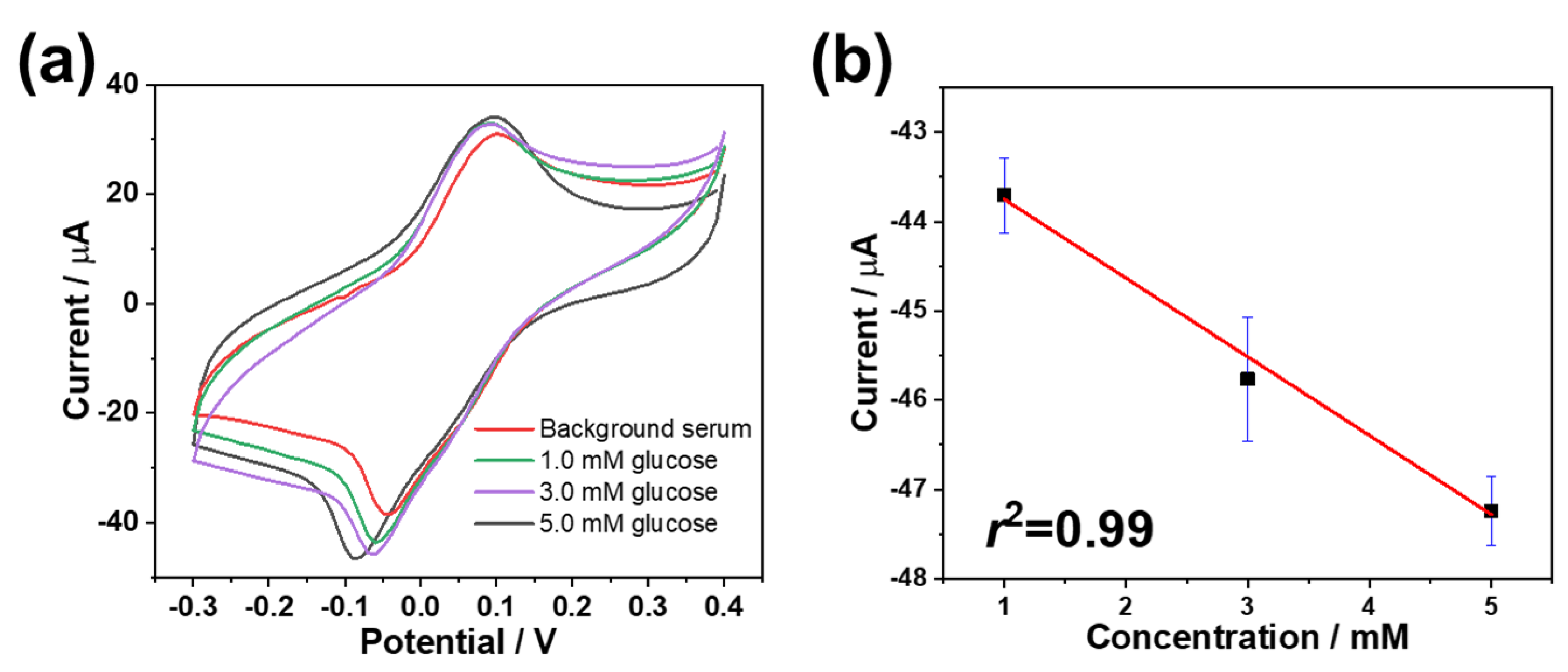
3.5. Real Sample Determination
3.6. Comparison to Other Voltammetric Glucose Biosensors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Slaughter, G.; Kulkarni, T. Detection of Human Plasma Glucose Using a Self-Powered Glucose Biosensor. Energies 2019, 12, 825. [Google Scholar] [CrossRef]
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J. Diagnostic significance of serum FGD5-AS1 and its predictive value for the development of cardiovascular diseases in patients with type 2 diabetes. Diabetol. Metab. Syndr. 2022, 14, 20. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.W.; Lee, S.; Seo, M.; Chung, T.D. Recent advances in electrochemical non-enzymatic glucose sensors—A review. Anal. Chim. Acta 2018, 29, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Bruen, D.; Delaney, C.; Florea, L.; Diamond, D. Glucose Sensing for Diabetes Monitoring: Recent Developments. Sensors 2017, 17, 1866. [Google Scholar] [CrossRef] [PubMed]
- Sridara, T.; Upan, J.; Saianand, G.; Tuantranont, A.; Karuwan, C.; Jakmunee, J. Non-Enzymatic Amperometric Glucose Sensor Based on Carbon Nanodots and Copper Oxide Nanocomposites Electrode. Sensors 2020, 20, 808. [Google Scholar] [CrossRef]
- Martín, A.; Kim, J.; Kurniawan, J.F.; Sempionatto, J.R.; Moreto, J.R.; Tang, G.; Campbell, A.S.; Shin, A.; Lee, M.Y.; Liu, X.; et al. Epidermal Microfluidic Electrochemical Detection System: Enhanced Sweat Sampling and Metabolite Detection. ACS Sens. 2017, 2, 1860–1868. [Google Scholar] [CrossRef]
- Kim, J.; Campbell, A.S.; Wang, J. Wearable non-invasive epidermal glucose sensors: A review. Talanta 2018, 177, 163–170. [Google Scholar] [CrossRef]
- Emaminejad, S.; Gao, W.; Wu, E.; Davies, Z.A.; Nyein, H.Y.Y.; Challa, S.; Ryan, S.P.; Fahad, H.M.; Chen, K.; Shahpar, Z.; et al. Autonomous sweat extraction and analysis applied to cystic fibrosis and glucose monitoring using a fully integrated wearable platform. Proc. Natl. Acad. Sci. USA 2017, 114, 4625–4630. [Google Scholar] [CrossRef]
- Mehdizadeh, B.; Maleknia, L.; Amirabadi, A.; Shabani, M. Glucose sensing by a glassy carbon electrode modified with glucose oxidase/chitosan/graphene oxide nanofibers. Diam. Relat. Mater. 2020, 109, 108073. [Google Scholar] [CrossRef]
- Mohamad, N.R.; Marzuki, N.H.C.; Buang, N.A.; Huyop, F.; Wahab, R.A. An overview of technologies for immobilization of enzymes and surface analysis techniques for immobilized enzymes. Biotechnol. Biotechnol. Equip. 2015, 29, 205–220. [Google Scholar] [CrossRef]
- Sehit, E.; Altintas, Z. Significance of nanomaterials in electrochemical glucose sensors: An updated review (2016–2020). Biosens. Bioelectron. 2020, 159, 112165. [Google Scholar] [CrossRef]
- Gorle, D.B.; Ponnada, S.; Kiai, M.S.; Nair, K.K.; Nowduri, A.; Swart, H.C.; Ang, E.H.; Nanda, K.K. Review on recent progress in metal-organic framework-based materials for fabricating electrochemical glucose sensors. J. Mater. Chem. B 2021, 9, 7927–7954. [Google Scholar] [CrossRef]
- Abellán-Llobregat, A.; Jeerapan, I.; Bandodkar, A.; Vidal, L.; Canals, A.; Wang, J.; Morallón, E. A stretchable and screen-printed electrochemical sensor for glucose determination in human perspiration. Biosens. Bioelectron. 2017, 91, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.; Wunderlich, L.; Weinzierl, F.; Lei, Y.; Duerkop, A.; Alshareef, H.N.; Baeumner, A.J. Electrochemical multi-analyte point-of-care perspiration sensors using on-chip three-dimensional graphene electrodes. Anal. Bioanal. Chem. 2021, 413, 763–777. [Google Scholar] [CrossRef]
- Jugović, B.; Grgur, B.; Antov, M.; Knežević-Jugović, Z.; Stevanović, J.; Gvozdenović, M. Polypyrrole-based Enzyme Electrode with Immobilized Glucose Oxidase for Electrochemical Determination of Glucose. Int. J. Electrochem. Sci. 2016, 11, 1152–1161. Available online: http://www.electrochemsci.org/papers/vol11/110201152.pdf (accessed on 14 January 2025). [CrossRef]
- Hrovat, M. Temperature Sensors Made by Combinations of Some Standard Thick Film Materials. J. Mater. Sci. Lett. 2000, 19, 651–655. [Google Scholar] [CrossRef]
- Dziedzic, A.; Golonka, L. Electrical Properties of Conductive Materials Used in Thick-Film Resistors. J. Mater. Sci. 1988, 23, 3151–3155. [Google Scholar] [CrossRef]
- Kim, M.; Ju, H.; Kim, J. Single Crystalline Bi2Ru2O7 Pyrochlore Oxide Nanoparticles as Efficient Bifunctional Oxygen Electrocatalyst for Hybrid Na-Air Batteries. Chem. Eng. J. 2019, 358, 11–19. [Google Scholar] [CrossRef]
- Esposito, V.; Luong, B.H.; Di Bartolomeo, E.; Wachsman, E.D.; Traversa, E. Applicability of Bi2Ru2O7 Pyrochlore Electrodes for ESB and BIMEVOX Electrolytes. J. Electrochem. Soc. 2006, 153, A2232. [Google Scholar] [CrossRef]
- Bamiduro, G.J.; Zahran, E.M. Pd@Bi2Ru2O7/BiVO4 Z-Scheme Heterojunction Nanocomposite Photocatalyst for the Degradation of Trichloroethylene. ACS Appl. Mater. Interfaces 2023, 15, 59337–59347. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, Y.; Nishi, H.; Suzuki, H.; Maeda, K. Solid-State NOx Sensor Combined with NASICON and Pb–Ru-Based Pyrochlore-Type Oxide Electrode. Sens. Actuators B Chem. 2000, 65, 141–143. [Google Scholar] [CrossRef]
- Tasić, N.; Vranešič, N.; Metarapi, D.; Mervič, K.; Žunić, M.; Dapčević, A.; Finšgar, M.; Hočevar, S.B. Protein Stacking on the APTES-Functionalized Pyrochlore Bi2Ru2O7 Clusters for Ultrasensitive and Selective Immunosensing. ACS Appl. Mater. Interfaces 2025, 17, 10792–10801. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Jang, H.; Lee, S.Y.; Jeon, J.; Kim, M.G. Redox reaction does not facilitate oxygen evolution on bismuth ruthenate pyrochlore. J. Mater. Chem. A 2022, 10, 561–569. [Google Scholar] [CrossRef]
- Jun, L.Y.; Mubarak, N.M.; Yon, L.S.; Bing, C.H.; Khalid, M.; Abdullah, E.C. Comparative Study of Acid Functionalization of Carbon Nanotube via Ultrasonic and Reflux Mechanism. J. Environ. Chem. Eng. 2018, 6, 5889–5896. [Google Scholar] [CrossRef]
- Carbonio, R.E.; Alonso, J.A.; Martínez, J.L. Oxygen Vacancy Control in the Defect Pyrochlore: A Way to Tune the Electronic Bandwidth. J. Phys. Condens. Matter 1999, 11, 361–369. [Google Scholar] [CrossRef]
- Shukla, A.K.; Kannan, A.M.; Hegde, M.S.; Gopalakrishnan, J. Effect of Counter Cations on Electrocatalytic Activity of Oxide Pyrochlores towards Oxygen Reduction/Evolution in Alkaline Medium: An Electrochemical and Spectroscopic Study. J. Power Sources 1991, 35, 163–173. [Google Scholar] [CrossRef]
- Felthouse, T. Expanded Lattice Ruthenium Pyrochlore Oxide Catalysts II. Catalyst Surface Investigations by Electron Microscopy, X-Ray Photoelectron Spectroscopy, and Temperature-Programmed Reduction and Oxidation. J. Catal. 1991, 127, 421–444. [Google Scholar] [CrossRef]
- Chipeture, A.T.; Apath, D.; Moyo, M.; Shumba, M. Multiwalled carbon nanotubes decorated with bismuth (III) oxide for electrochemical detection of an antipyretic and analgesic drug paracetamol in biological samples. J. Anal. Sci. Technol. 2019, 10, 22. [Google Scholar] [CrossRef]
- Dighole, R.P.; Munde, A.V.; Mulik, B.B.; Sathe, B.R. Bi2O3 Nanoparticles Decorated Carbon Nanotube: An Effective Nanoelectrode for Enhanced Electrocatalytic 4-Nitrophenol Reduction. Front. Chem. 2020, 8, 325. [Google Scholar] [CrossRef]
- Myndrul, V.; Iatsunskyi, I.; Babayevska, N.; Jarek, M.; Jesionowski, T. Effect of Electrode Modification with Chitosan and Nafion® on the Efficiency of Real-Time Enzyme Glucose Biosensors Based on ZnO Tetrapods. Materials 2022, 15, 4672. [Google Scholar] [CrossRef] [PubMed]
- Maity, D.; Minitha, C.R.; Kumar, R.T.R. Glucose oxidase immobilized amine terminated multiwall carbon nanotubes/reduced graphene oxide/polyaniline/gold nanoparticles modified screen-printed carbon electrode for highly sensitive amperometric glucose detection. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 105, 110075. [Google Scholar] [CrossRef] [PubMed]
- Chong, S.F.; Razak, K.A.; Nor, N.M.; Ridhuan, N.S.; Zakaria, N.D. Electrochemical Glucose Detection using Screen-Printed Carbon Electrode Modified Silica-Encapsulated Iron Oxide Nanoparticles. Mater. Today Proc. 2019, 17, 1189–1196. [Google Scholar] [CrossRef]
- Hu, T.; Wang, D.; Xu, J.; Chen, K.; Li, X.; Yi, H.; Ni, Z. Glucose sensing on screen-printed electrochemical electrodes based on porous graphene aerogel @prussian blue. Biomed. Microdevices 2022, 24, 14. [Google Scholar] [CrossRef]
- Niamsi, W.; Larpant, N.; Kalambate, P.K.; Primpray, V.; Karuwan, C.; Rodthongkum, N.; Laiwattanapaisal, W. Paper-Based Screen-Printed Ionic-Liquid/Graphene Electrode Integrated with Prussian Blue/MXene Nanocomposites Enabled Electrochemical Detection for Glucose Sensing. Biosensors 2022, 12, 852. [Google Scholar] [CrossRef]
- Phetsang, S.; Jakmunee, J.; Mungkornasawakul, P.; Laocharoensuk, R.; Ounnunkad, K. Sensitive amperometric biosensors for detection of glucose and cholesterol using a platinum/reduced graphene oxide/poly(3-aminobenzoic acid) film-modified screen-printed carbon electrode. Bioelectrochemistry 2019, 127, 125–135. [Google Scholar] [CrossRef]
- Phasuksom, K.; Sirivat, A. Chronoampermetric detection of enzymatic glucose sensor based on doped polyindole/MWCNT composites modified onto screen-printed carbon electrode as portable sensing device for diabetes. RSC Adv. 2022, 12, 28505–28518. [Google Scholar] [CrossRef]
- Ji, D.; Liu, L.; Li, S.; Chen, C.; Lu, Y.; Wu, J.; Liu, Q. Smartphone-based cyclic voltammetry system with graphene modified screen printed electrodes for glucose detection. Biosens. Bioelectron. 2017, 98, 449–456. [Google Scholar] [CrossRef]




| Spectrum | Ru, at.% | Bi, at.% | O, at.% |
|---|---|---|---|
| 1 | 38.5 | 37.7 | 23.9 |
| 2 | 42.2 | 32.0 | 25.8 |
| 3 | 15.2 | 12.7 | 72.1 |
| 4 | 14.1 | 16.6 | 69.3 |
| 5 | 16.0 | 13.5 | 70.5 |
| 6 | 20.1 | 17.1 | 62.8 |
| Sensor | Technique | LOD 1 [mM] | AR 2 [mM] | Int 3 | Ref. |
|---|---|---|---|---|---|
| GOD immobilized amine terminated MWCNTs/rGO 4/PANI 5/AuNPs 6 | CA 7 | 0.064 | 1–10 | No | [32] |
| Nafion Silica-Encapsulated Iron Oxide NPs | CA | 0.22 | <3 | / | [33] |
| Porous graphene aerogel@Prussian blue | CA | 0.15 | 0.5–6 | No | [34] |
| Ionic-Liquid/Graphene Electrode Integrated with Prussian Blue/MXene Nanocomposites | CA | 0.024 | 0.05–15 | No | [35] |
| Pt/rGO/poly(3-aminobenzoic acid) film | CA | 0.0443 | 0.25–6 | AA 8, UA 9 | [36] |
| Doped polyindole/MWCNTs composites | CA | 0.01 | 0.01–50 | No | [37] |
| Graphene-modified screen-printed electrodes | CV 10 | 0.026 | 0.1–10 | AA | [38] |
| MWCNTs/Bi2Ru2O7 +GOD-modified FCN-SPCE | CV | 0.040 | 1.0–20.0 | No | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Isailović, J.; Dapčević, A.; Žunić, M.; Finšgar, M.; Vidović, K.; Tasić, N.; Hočevar, S.B. Study of a Sensitive and Selective Electrochemical Biosensor for Glucose Based on Bi2Ru2O7 Pyrochlore Clusters Combined with MWCNTs. Chemosensors 2025, 13, 109. https://doi.org/10.3390/chemosensors13030109
Isailović J, Dapčević A, Žunić M, Finšgar M, Vidović K, Tasić N, Hočevar SB. Study of a Sensitive and Selective Electrochemical Biosensor for Glucose Based on Bi2Ru2O7 Pyrochlore Clusters Combined with MWCNTs. Chemosensors. 2025; 13(3):109. https://doi.org/10.3390/chemosensors13030109
Chicago/Turabian StyleIsailović, Jelena, Aleksandra Dapčević, Milan Žunić, Matjaž Finšgar, Kristijan Vidović, Nikola Tasić, and Samo B. Hočevar. 2025. "Study of a Sensitive and Selective Electrochemical Biosensor for Glucose Based on Bi2Ru2O7 Pyrochlore Clusters Combined with MWCNTs" Chemosensors 13, no. 3: 109. https://doi.org/10.3390/chemosensors13030109
APA StyleIsailović, J., Dapčević, A., Žunić, M., Finšgar, M., Vidović, K., Tasić, N., & Hočevar, S. B. (2025). Study of a Sensitive and Selective Electrochemical Biosensor for Glucose Based on Bi2Ru2O7 Pyrochlore Clusters Combined with MWCNTs. Chemosensors, 13(3), 109. https://doi.org/10.3390/chemosensors13030109








