Abstract
Cost-effective chemosensors have become an indispensable tool for sustainable monitoring in food safety and processing, where there is an urgent need for affordable, efficient, and real-time analytical solutions. This review discusses recent advances in low-cost chemosensor technologies, highlighting developments in materials, miniaturization, and integration into portable and accessible platforms. The focus is on applications for detecting contaminants, monitoring quality, and ensuring safety in food production and processing. This review also addresses the challenges related to sensor sensitivity, selectivity, and operational stability and provides insights into future directions and the role of low-cost chemosensors in supporting sustainable practices in these important sectors.
1. Introduction
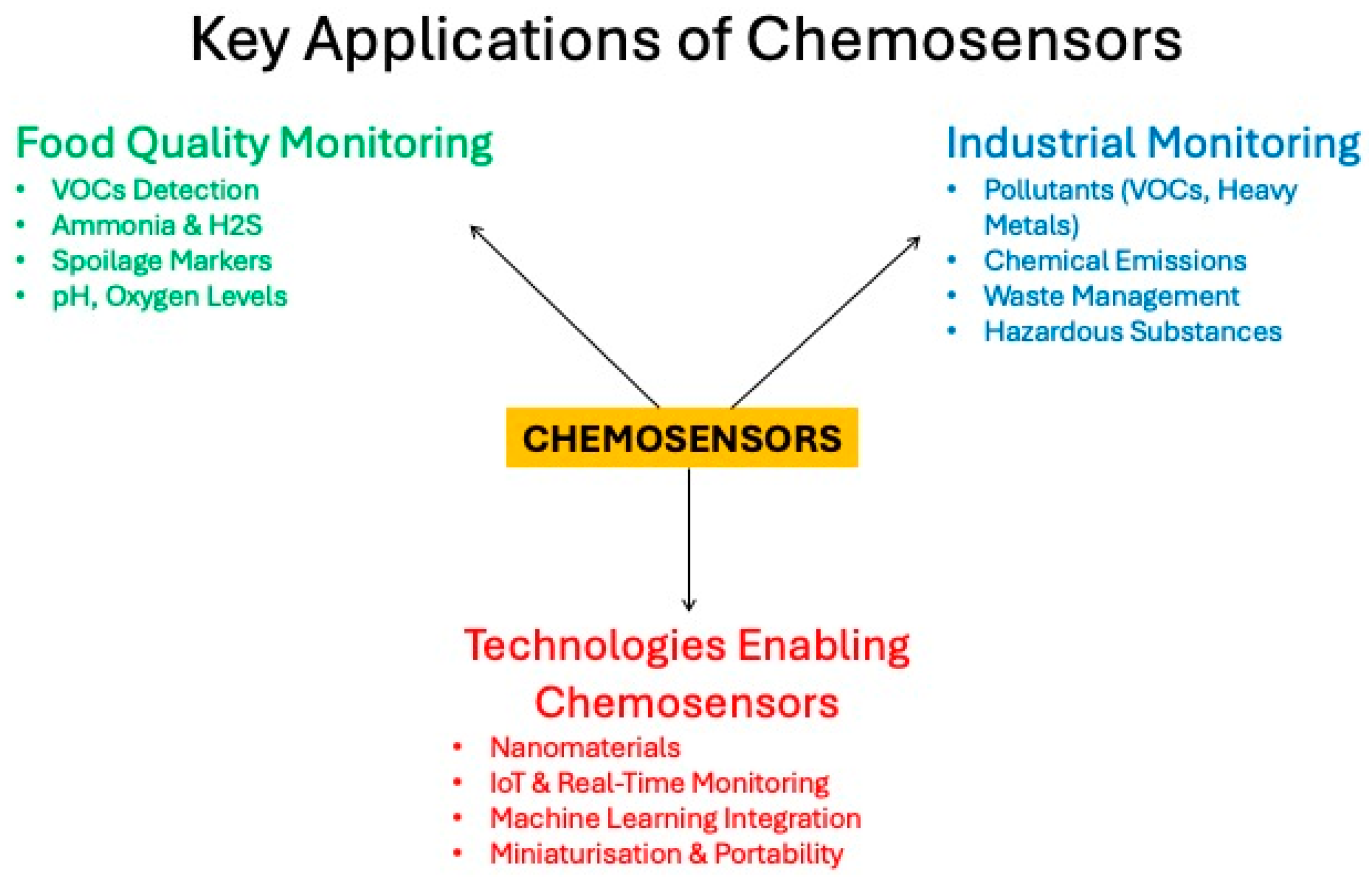
Monitoring the quality and safety of food and ensuring the efficiency of food production processes are crucial for sustainable development. These sectors face major challenges, including the increasing prevalence of contaminants, the demand for high-quality products, and the need to minimize the impact on the environment. Among various sensor technologies, chemosensors, i.e., devices capable of detecting specific chemical substances through measurable signals, have emerged as one of the most important tools to address these challenges due to their affordability, portability, and immediate analytical capabilities (Scheme 1) [1]. This review examines recent advances in low-cost chemosensor technologies, with a particular focus on their applications in food and food processing monitoring.
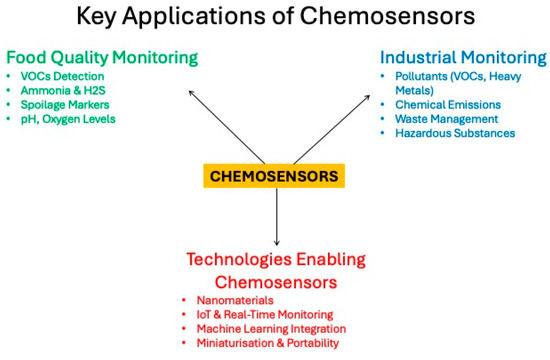
Scheme 1.
Schematic representation of key applications of chemosensors.
The global food sector is under increasing pressure to ensure product safety and quality while maintaining cost efficiency. Chemical and biological contaminants, such as pesticides, heavy metals, and pathogens, pose a significant risk to public health and undermine consumer confidence. Traditional laboratory-based methods, including chromatography and mass spectrometry, are reliable but expensive, time-consuming, and impractical for real-time applications. In contrast, chemosensors offer a fast, cost-effective alternative that enables on-site detection of hazardous substances and significantly improves monitoring efficiency [2].
To further increase their usefulness, recent advances have focused on the development of multi-analyte detection platforms, a category of chemosensors designed to simultaneously monitor various contaminants [3]. These systems can simultaneously monitor various contaminants, such as pesticide residues and microbial activity, providing a more comprehensive approach to food safety. For example, colourimetric arrays and electrochemical multiplex systems have been introduced, providing fast and accurate results without the need for complex instrumentation [4]. This progress reduces the time and cost of analysis while ensuring a thorough assessment of food quality.
Another important area is the monitoring of food quality throughout its life cycle. Chemosensors are increasingly being used to measure indicators of food spoilage such as volatile organic compounds (VOCs) [5,6], ammonia [7], or hydrogen sulfide [8,9,10] in perishable goods as well as critical parameters such as pH, oxygen content, and microbial activity. The early detection of these markers enables timely intervention, extends shelf life, prevents food waste, and ensures compliance with safety standards.
Ensuring safety in food production also involves strict adherence to national and international quality assurance protocols. Organizations such as the European Food Safety Authority (EFSA) and the US Food and Drug Administration (FDA) require continuous monitoring and transparent documentation of food safety. Chemosensors play a central role in this context as they provide reliable, repeatable measurements. Advances in wireless data transmission technologies such as Bluetooth and Internet of Things (IoT) systems further streamline these processes by enabling remote monitoring and real-time data exchange.
In food processing applications, chemosensors are equally valuable for monitoring processes such as chemical synthesis, emission control, and waste management [11]. These devices respond to chemical changes with measurable outputs such as fluorescent or electrochemical signals, enabling real-time detection of pollutants in air, water, and soil. For example, chemosensors are often used to monitor heavy metals [12], pesticides [13], and volatile organic compounds (VOCs), helping to prevent ecological damage while protecting public health. Their ability to provide rapid and accurate detection makes them indispensable tools in addressing environmental challenges. Food processing often involves the use of hazardous chemicals that require constant monitoring to comply with regulatory requirements and prevent environmental pollution [14,15].
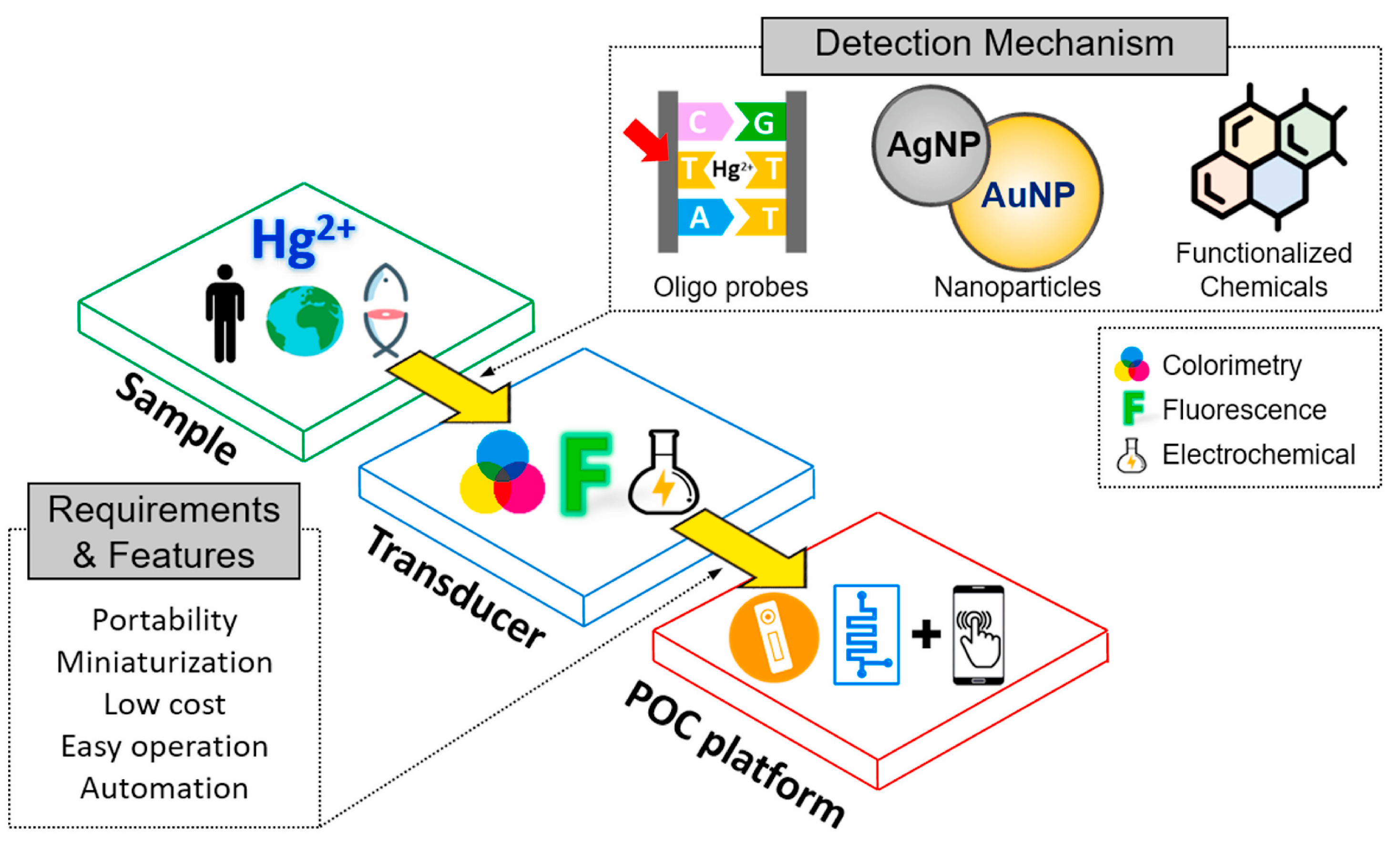
The innovations of the last decade have significantly improved the performance of chemosensors, particularly through advances in materials science and the miniaturization of devices (Figure 1) [16,17]. Novel materials such as nanostructured metals [18,19], metal–organic frameworks (MOFs) [20,21], and conducting polymers [22,23,24,25] have improved their sensitivity, selectivity, and stability. In combination with nanotechnology, these materials enable the development of compact, portable devices suitable for use in the field [26].
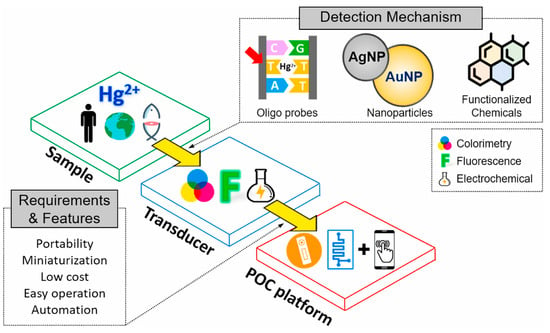
Figure 1.
Schematic flow diagram of portable sensors for mercury detection. (Reprinted with permission from [16]. Copyright © 2021, Elsevier B. V.).
Integration with digital technologies, including IoT platforms and smartphone-based systems, has further increased the applicability of chemosensors [27]. These integrated tools provide actionable insights through real-time data collection and analysis, making them particularly effective in resource-limited or remote environments where traditional laboratory infrastructure is not available.
Despite their great potential, low-cost chemosensors still face challenges in terms of sensitivity, selectivity, and operational stability [28]. Many sensors have difficulty detecting analytes at trace levels or differentiating between chemically similar substances, limiting their applications in complex environments. In addition, variations in environmental conditions, such as temperature and humidity, can affect sensor performance.
Overcoming these challenges requires interdisciplinary collaboration that incorporates advances in materials science, device technology, and data analysis. For example, machine learning algorithms are increasingly being used to process chemosensor results to improve detection accuracy and adaptive calibration under dynamic conditions. Future research should focus on the development of robust, user-friendly sensor platforms that are not only cost-effective but also reliable and durable. In addition, the integration of chemosensors with renewable energy sources and sustainable manufacturing processes holds great potential for improving their environmental performance.
2. Chemosensors: Principles and Functional Mechanisms
2.1. Definition and Working Principle
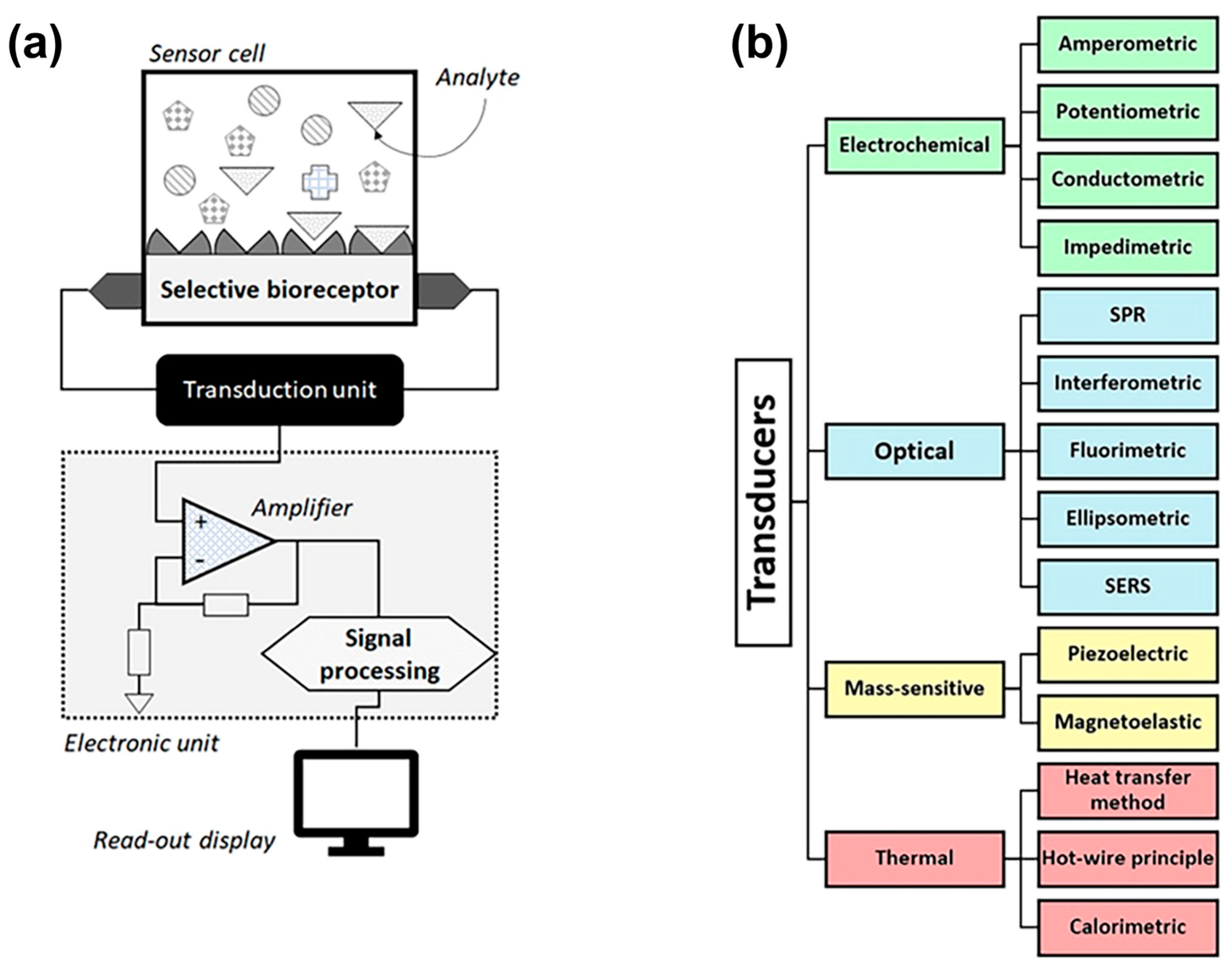
In general, a chemosensor is defined as “a sensory receptor that transduces a chemical signal to an action potential” [29]. More specifically, when the receptor is composed of chemically synthesized molecules, the resulting sensor is referred to as a chemosensor. Chemosensors represent one category of analytical devices designed to detect and quantify specific chemical substances by converting their interaction with the target analyte into a measurable signal. Other sensor types, such as biosensors and wearable sensors, also contribute to chemical detection, often complementing traditional chemosensor technology. At their core, chemosensors consist of two essential components: a recognition element, which selectively interacts with the analyte, and a transducer, which converts this interaction into an output signal (Figure 2) [30]. In the functioning of a chemosensor, the receptor plays a crucial role by converting chemical information into a measurable form of energy. The transducer, in turn, acts as the interface between this transformation and detection, converting the energy, along with the chemical information about the sample, into a meaningful analytical signal. These devices play a critical role in applications that require sensitive, selective, and real-time monitoring, particularly in fields such as food safety and industrial process control.
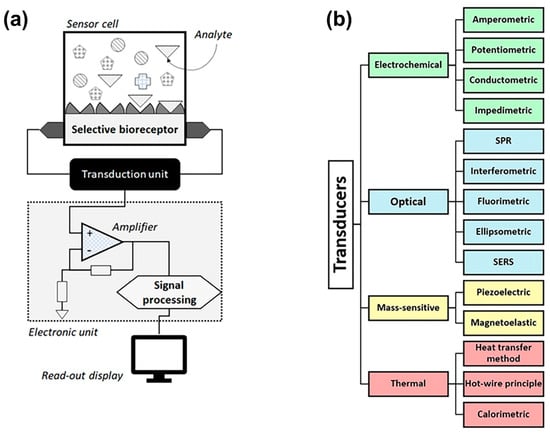
Figure 2.
(a) Schematic representation of the key elements of a generic biosensor in which the analyte (target molecules) binds selectively to receptors, serving as biorecognition elements. The transducer converts the chemical recognition to a measurable signal that is amplified and processed to render information of the analyte concentration. (b) The established transducer principles for biogenic amines (BA) detection include electrochemical, optical, mass sensitive, and thermal sensors, each with a plurality of subtypes. Chemosensors without a biorecognition element belong mostly to the electrochemical category, and a few chemosensors are equipped with an optical detection scheme. (The figure is adopted from reference [29], and it is published under the Creative Commons Attribution (CC BY 4.0) license).
The working principle of chemosensors can be broadly divided into three stages: recognition, transduction, and signal processing. In the first stage, the recognition element, or receptor, interacts with the target analyte through mechanisms such as physical adsorption, chemical bonding, or catalytic processes [31]. The receptor’s design is critical for achieving high selectivity, as it ensures that the chemosensor can distinguish the target analyte from other substances present in the sample. Non-covalent interactions, including hydrogen bonding, electrostatic forces, and π–π stacking, are often utilized to facilitate recognition [32], while more specific applications may involve covalent binding or catalytic amplification of the response. For complex targets, supramolecular systems, such as macrocycles (e.g., crown ethers and cyclodextrins) [33] or metal–organic frameworks (MOFs) [21], are employed to enhance selectivity and sensitivity.
Once the analyte interacts with the receptor, the resulting change is transmitted to the transducer, which converts the chemical or physical response into a detectable signal. Transducers play a pivotal role in the operation of chemosensors, as they act as the interface between the recognition process and signal generation. The transduction mechanism varies depending on the type of chemosensor and can be classified into optical, electrochemical, piezoelectric, or thermal systems.
- Optical transducers [34] detect changes in light properties, such as absorption, fluorescence, or luminescence, resulting from the analyte–receptor interaction. For example, a fluorophore may emit light at a specific wavelength when it binds to a contaminant like a heavy metal.
- Electrochemical transducers [35] measure changes in current, voltage, or resistance caused by the interaction. Amperometric and potentiometric sensors are widely used for detecting pesticides, heavy metals, and other analytes in food or industrial samples.
- Piezoelectric transducers [36] operate by measuring mass changes on the sensing surface through frequency shifts, making them suitable for detecting volatile organic compounds (VOCs) or small molecules.
- Thermal transducers [37] monitor temperature changes resulting from exothermic or endothermic reactions at the sensing interface.
The final stage involves signal processing and output generation. The signal produced by the transducer is amplified, processed, and interpreted by an external system, which can display the results in a user-friendly format, such as numerical values or visual outputs. Modern chemosensors often integrate microcontrollers, wireless technologies, and digital platforms, such as smartphones or Internet of Things (IoT) systems, to enable real-time data transmission, analysis, and monitoring [38]. This enhances their usability in resource-limited or remote environments where traditional laboratory equipment may be unavailable.
The operational efficiency of chemosensors depends on several critical factors, including selectivity, sensitivity, and response time. Selectivity ensures that the chemosensor targets a specific analyte while avoiding interference from other chemical species. Sensitivity determines the device’s ability to detect analytes at extremely low concentrations, which is particularly important for contaminants present in trace amounts. A rapid response time, on the other hand, enables real-time detection and intervention, making chemosensors ideal for applications that demand immediate results.
Recent innovations in materials science and nanotechnology have further enhanced the performance of chemosensors. Advanced materials, such as metal–organic frameworks (MOFs) [39], nanostructured metals [40], and conducting polymers [41], offer large surface areas and tunable properties, significantly improving the sensitivity and selectivity of these devices. For example, MOFs have been successfully employed in detecting volatile organic compounds released during food spoilage [42]. Additionally, the miniaturization of chemosensors [43] has allowed for the development of portable and field-deployable systems, further increasing their accessibility and cost-effectiveness.
2.2. Types of Chemosensors
Chemosensors come in various types, each leveraging distinct mechanisms to detect and quantify chemical substances. While this section focuses on commonly used chemosensors, other related technologies, such as electronic nose/tongue systems and magnetic sensors, also play roles in chemical sensing applications. These types include optical, electrochemical, piezoelectric, and nanomaterial-based chemosensors. Each class has unique features and advantages that make it suitable for specific applications in food safety and food processing.
Optical Chemosensors
Optical chemosensors rely on changes in the optical properties of materials to detect chemical interactions. These changes can involve fluorescence, absorbance, luminescence, or refractive index alterations. Optical sensors are widely used due to their high sensitivity and the ability to provide rapid, non-invasive measurements. For example, fluorescent chemosensors can detect contaminants like heavy metals or toxins [44] in food by emitting light at a specific wavelength upon binding to the analyte. The simplicity of optical systems and their compatibility with portable devices make them an ideal choice for on-site monitoring.
Electrochemical Chemosensors
Electrochemical chemosensors detect analytes through electrical signals, such as changes in current, voltage, or impedance. These sensors are highly versatile and can be used for the detection of a wide range of substances, including pesticides [45], heavy metals [46], and microbial byproducts [47]. Potentiometric sensors, which measure changes in ion concentration, and amperometric sensors, which detect currents generated by redox reactions, are commonly employed in food safety applications. Their low cost, ease of miniaturization, and compatibility with digital systems make electrochemical chemosensors a practical solution for real-time monitoring.
Piezoelectric Chemosensors
Piezoelectric chemosensors operate by detecting changes in the mass or mechanical properties on a sensing surface [48]. These changes cause a shift in the resonance frequency of a piezoelectric material, which can be measured to quantify the presence of the target analyte. These sensors are particularly effective in detecting volatile organic compounds (VOCs) [49], making them valuable in assessing food spoilage or monitoring industrial emissions. The high precision of piezoelectric sensors, combined with their ability to measure minute mass changes, enables their use in both qualitative and quantitative analyses.
Nanomaterial-Based Chemosensors
The integration of nanotechnology has revolutionized chemosensor design, leading to the development of nanomaterial-based sensors. Materials such as metal–organic frameworks (MOFs) [20], graphene [50], and carbon nanotubes [51] provide an exceptional surface area, tunable chemical properties, and enhanced interactions with target analytes. These properties improve the sensitivity and selectivity of chemosensors, making them suitable for detecting contaminants at trace levels. Nanomaterial-based sensors have found applications in both food and food processing settings, such as monitoring toxins, detecting VOCs, and analyzing environmental pollutants. Furthermore, their compatibility with portable devices enhances their usability in field-based scenarios.
2.3. Advantages of Cost-Effective Chemosensors
Cost-effective chemosensors offer a range of advantages that make them invaluable for sustainable monitoring in both food and food processing applications. Their key features, including affordability, portability, and real-time detection, address many of the limitations associated with traditional analytical methods, such as high costs, complexity, and time constraints.
Affordability: One of the primary advantages of cost-effective chemosensors is their economic feasibility [52], which allows widespread adoption across various sectors. Traditional laboratory-based techniques, such as gas chromatography or mass spectrometry, often require significant investment in equipment, maintenance, and skilled personnel. In contrast, chemosensors are designed to provide reliable results at a fraction of the cost. This affordability makes them particularly suitable for small-scale industries, resource-limited settings, and developing regions, where access to advanced laboratory infrastructure may be restricted. Moreover, the lower operational costs of chemosensors contribute to a reduction in the overall financial burden of continuous monitoring, enabling long-term sustainability.
Portability: Chemosensors are often compact and lightweight [53], enabling easy transport and deployment in diverse environments. Their portability is a significant advantage for on-site monitoring, especially in scenarios where samples cannot be transported to a laboratory without degradation. For example, in food supply chains, portable chemosensors can be used to check for contamination or spoilage [54] directly at the production site, during transport, or at retail locations. Similarly, in industrial settings, portable devices allow for the real-time monitoring of emissions or pollutants in remote or hazardous areas, ensuring compliance with environmental regulations without the need for complex setups.
Real-Time Detection: The ability to provide rapid, real-time detection is another critical feature of cost-effective chemosensors. Unlike traditional methods, which often involve time-consuming sample preparation and analysis, chemosensors offer near-instantaneous results. This capability is particularly beneficial in applications where immediate intervention is required, such as detecting foodborne pathogens or monitoring toxic emissions during industrial processes. Real-time data facilitate timely decision-making, minimizing risks to public health and the environment. Additionally, the integration of digital technologies, such as Bluetooth, smartphone connectivity, and IoT platforms, has further enhanced the speed and accessibility of chemosensor-based monitoring systems.
User-Friendliness and Minimal Training Requirements: Cost-effective chemosensors are typically designed for ease of use, requiring minimal training or technical expertise. This makes them accessible to a broader range of users, from field operators to non-specialists, in resource-constrained settings. The simplicity of their operation ensures that accurate monitoring can be conducted without the need for highly skilled personnel, further reducing costs and promoting widespread implementation.
Low Environmental Impact: In addition to their operational benefits, cost-effective chemosensors align with sustainability goals by reducing the environmental footprint of monitoring processes. Many modern chemosensors are designed with eco-friendly materials, minimal reagent consumption, and low energy requirements, ensuring that their use does not contribute significantly to waste or pollution. Portable devices, in particular, eliminate the need for the extensive transportation of samples, further reducing carbon emissions associated with monitoring activities.
Scalability and Versatility: The modular nature of many chemosensors makes them highly scalable and adaptable to different applications. Whether used in detecting contaminants in food, monitoring VOCs in food processing, or analyzing water quality, these sensors can be tailored to specific needs. Their scalability ensures that they can be integrated into both small-scale food production operations and large-scale food processing facilities, supporting a wide range of monitoring requirements.
The affordability, portability, and real-time detection capabilities of cost-effective chemosensors make them indispensable tools for modern monitoring systems. These features not only address practical challenges in food safety and food processing operations but also align with global sustainability goals by promoting efficient, accessible, and environmentally friendly monitoring solutions. As advancements in materials science and digital integration continue to evolve, cost-effective chemosensors are poised to play an even greater role in ensuring public health and environmental protection.
While this review primarily focuses on cost-effective chemosensors for food safety and processing, it is important to note that other sensor technologies, such as biosensors, magnetic sensors, and wearable devices, also play significant roles in chemical detection.
2.4. Biosensors
Biosensors represent a specialized subset of chemosensors that integrate biological components, such as enzymes, antibodies, nucleic acids, and living cells, with transducers to detect specific analytes [55]. The interaction between the biological component and the target analyte generates a measurable signal, which is commonly detected via electrochemical, optical, or piezoelectric methods.
- Enzyme-based biosensors are widely used in food safety [56], particularly for detecting glucose (in beverages) [57], pesticides [58], and toxins (such as aflatoxins) [59].
- Antibody-based biosensors (immunosensors) are employed for pathogen detection, such as Salmonella and Listeria in dairy products [60].
- DNA-based biosensors leverage hybridization events to detect foodborne pathogens [61] or genetically modified organisms (GMOs) [62].
While biosensors offer high specificity and sensitivity, they often require careful storage and handling due to the biological nature of their recognition elements.
2.5. Magnetic Sensors: Giant Magnetoresistance (GMR) for Foodborne Pathogens
Magnetic sensors utilize magnetoresistive materials to detect analytes based on their interaction with functionalized magnetic nanoparticles. Giant magnetoresistance (GMR) sensors, for instance, have been developed for the rapid detection of Listeria in dairy products [63], where functionalized magnetic particles bind to bacterial cells, producing a measurable change in resistance. These sensors provide a label-free, highly sensitive alternative to conventional microbiological techniques.
2.6. Electronic Nose and Tongue Systems
Electronic noses (e-noses) and electronic tongues (e-tongues) [64] are advanced multi-sensor arrays designed to mimic human olfactory and gustatory systems.
- E-noses detect volatile organic compounds (VOCs) released from food products, enabling applications such as spoilage detection [65] and flavor profiling [66].
- E-tongues analyze liquid samples to differentiate taste-related chemical compounds, providing rapid food quality assessments [67].
These systems rely on cross-reactive sensor arrays and data-driven pattern recognition techniques, such as machine learning, to interpret complex chemical signals.
2.7. Wearable Chemosensors
Wearable chemosensors are a growing class of portable, real-time sensing devices that enable the continuous monitoring of environmental and foodborne contaminants [68]. These sensors are often integrated into flexible substrates, allowing for applications in sweat, breath, or skin-based sensing to detect harmful substances. In food safety, wearable smart labels embedded with chemosensors can detect gases like ammonia (NH3) [69] and hydrogen sulfide (H2S) [70] in packaged products, providing a visual freshness indicator for consumers.
3. Advances in Chemosensors for Food Monitoring
3.1. Detection of Contaminants
Pesticides
Chemosensors have emerged as vital tools in detecting pesticide residues, offering rapid, sensitive, and on-site analysis capabilities. These sensors operate by producing a measurable signal upon interaction with specific pesticide molecules, enabling real-time monitoring of food safety.
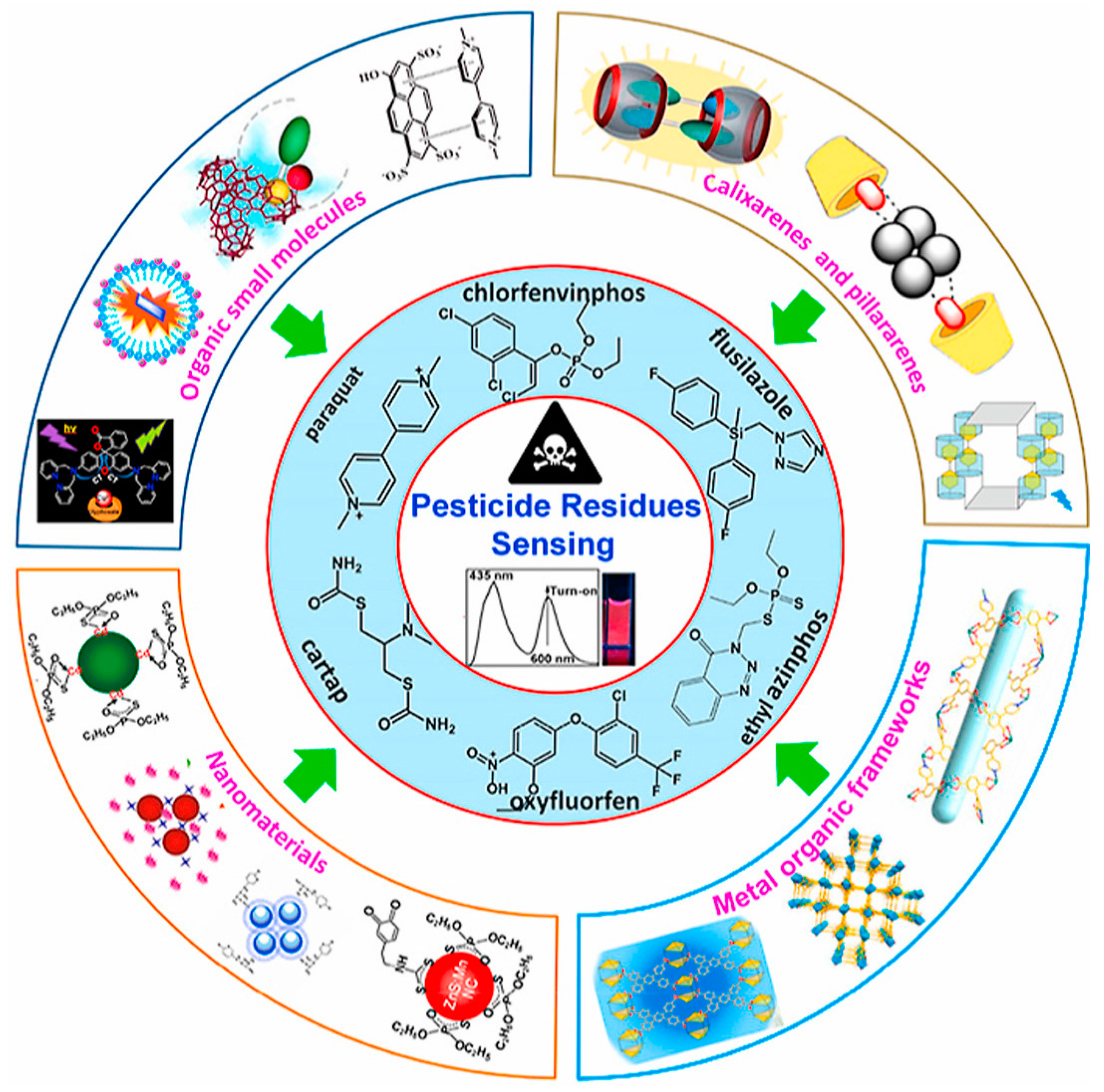
Fluorescent chemosensors utilize fluorescence changes to detect pesticides, particularly organophosphorus compounds [71]. They offer high sensitivity and selectivity, with detection limits reaching nanomolar concentrations. Recent advancements include the development of multimodal chemosensors that combine fluorescence with other detection methods to enhance real-time monitoring capabilities. The integration of nanomaterials, such as nanoparticles and nanocomposites, has significantly improved the performance of chemosensors [72]. These materials enhance sensitivity and selectivity due to their unique surface chemistry and high surface area. Nanotechnology-based chemosensors have been effectively applied to detect environmental toxic ions, including pesticide residues, contributing to more efficient environmental monitoring. Molecularly imprinted polymers (MIPs) are synthetic polymers with specific analyte-binding abilities, offering high selectivity and sensitivity in detecting pesticide residues [73]. They are chemically and thermally stable, reusable, and have been applied in various detection methods, including sensors and chromatographic techniques. Recent advances have focused on improving their extraction efficiency and detection capabilities for single and multiple pesticides. Non-biological fluorescent chemosensors, including organic functional small molecules and metal–organic frameworks, have been developed for detecting organic pesticides (Figure 3) [13]. These sensors offer advantages such as rapid detection, high sensitivity, and selectivity, with detection limits ranging from micromolar to nanomolar levels. They have been applied to detect pesticide residues in various samples, contributing to food safety and environmental monitoring. Electrochemical biosensors, particularly those utilizing nanocomposites like Ag/reduced graphene oxide/chitosan, have shown high sensitivity in detecting pesticides such as carbaryl [74]. These biosensors offer advantages like ease of use, rapid detection, portability, and cost-effectiveness, making them suitable for on-site analysis. They have demonstrated low detection limits and wide working concentration ranges, enhancing pesticide residue analysis.
Figure 3.
Fluorescent chemosensors for pesticides detection. (Reprinted with permission from [58]. Copyright © 2022, Elsevier B. V.).
In summary, the development of chemosensors for pesticide detection has advanced significantly, incorporating various materials and technologies to enhance sensitivity, selectivity, and practicality. These innovations contribute to more effective monitoring of pesticide residues, ensuring food safety and environmental protection.
Heavy metals
Heavy metal contamination, including lead (Pb), cadmium (Cd), mercury (Hg), and arsenic (As), presents significant challenges in food safety and food manufacturing. These contaminants can enter the food chain through polluted water, soil, and industrial activities, posing severe health risks, including neurotoxicity, organ damage, and carcinogenic effects [75]. Chemosensors have become pivotal tools for detecting heavy metals due to their rapid, sensitive, and cost-effective capabilities [76,77], offering an alternative to conventional analytical methods such as atomic absorption spectroscopy (AAS) and inductively coupled plasma mass spectrometry (ICP-MS). Chemosensors detect heavy metals [78] by leveraging specific interactions between metal ions and the sensor’s recognition elements, resulting in measurable optical [79] or electrochemical signals [80]. These mechanisms include the formation of complexes between heavy metal ions and ligands, which produce detectable optical or electrochemical changes, the modulation of fluorescence through interactions with fluorophores that cause quenching or enhancement, and the generation of electrical signals through the oxidation or reduction of metal ions.
Chemosensors for heavy metals can be categorized based on their detection mechanisms and formats. Optical chemosensors employ fluorescence [81], colorimetry [82], or UV–visible spectroscopy [83] to identify heavy metals, with examples including gold nanoparticles functionalized with specific ligands that exhibit colorimetric changes upon interacting with lead or mercury ions [84]. These sensors enable rapid detection and are often designed for portable, on-site use. Electrochemical chemosensors utilizing techniques like voltammetry [85] or amperometry [86] detect metal ions through variations in current, voltage, or impedance. The integration of nanostructured materials, such as graphene oxide [87] or carbon nanotubes [88], significantly enhances their sensitivity, allowing for the detection of trace-level contaminants. Paper-based sensors [89], which are frequently embedded with nanomaterials, offer a cost-effective and disposable option. These devices combine simplicity with high sensitivity, making them particularly effective for quick screening in resource-constrained environments.
Pathogens and Mycotoxins
Foodborne pathogens and mycotoxins are among the most significant threats to food safety and public health, causing widespread illnesses, economic losses, and regulatory challenges globally. Traditional methods for detecting these contaminants, such as culture-based techniques for pathogens [90] and chromatographic methods for mycotoxins [91], are often labor-intensive, time-consuming, and expensive. Recent advances in chemosensors, along with developments in biosensors and hybrid sensing platforms, have improved selectivity, sensitivity, and response times, making them effective alternatives or complementary tools to traditional laboratory-based techniques. Pathogens such as Salmonella, Escherichia coli, and Listeria monocytogenes require sensitive and specific detection methods to prevent outbreaks and ensure food safety. Chemosensors [92] have demonstrated exceptional potential in this area through mechanisms that exploit the unique biochemical signatures of these pathogens. For example, electrochemical chemosensors can detect bacterial DNA or proteins [93] by measuring electrical changes during target binding. Similarly, optical chemosensors utilizing fluorescence or colorimetric shifts offer rapid and label-free detection of pathogens in complex food matrices. The incorporation of nanomaterials like quantum dots [94], graphene [95], and metal–organic frameworks (MOFs) [96] has further enhanced the sensitivity, stability, and portability of these sensors. Notably, portable pathogen-detection devices integrated with smartphones [97] have emerged, enabling real-time monitoring in resource-constrained environments. Mycotoxins, such as aflatoxins, ochratoxins, and deoxynivalenol, are toxic secondary metabolites produced by fungi that contaminate a wide range of food products, including grains, nuts, and dairy. Chemosensors for mycotoxin detection [98] utilize a variety of mechanisms, including fluorescence quenching, electrochemical impedance spectroscopy, and surface plasmon resonance. Molecularly imprinted polymers (MIPs) have been widely adopted due to their selective recognition of mycotoxins [99], enabling high specificity even in complex food matrices. Additionally, advances in paper-based sensors have made it possible to achieve cost-effective, rapid, and on-site detection of mycotoxins, a significant step forward for large-scale food monitoring. Advances in pathogen and mycotoxin detection through chemosensors have profound implications for food monitoring and safety. These technologies are now being applied in quality control processes during food production, transportation, and storage, ensuring compliance with stringent safety regulations. Despite their progress, challenges remain in improving sensor reproducibility, overcoming matrix interferences, and scaling up production for widespread adoption. Addressing these challenges will require interdisciplinary collaboration and innovation in materials science, data analytics, and sensor engineering.
Table 1 summarizes the key performance metrics of various chemosensors, including their detection limits (LoD) and selectivity profiles, which are critical for evaluating their practical applicability. Notably, electrochemical sensors exhibit highly sensitive detection capabilities, as seen in the potentiometric detection of carbaryl, achieving an LoD as low as 4.97 × 10−15 mol L−1. Similarly, colorimetric and electrochemical approaches have been successfully applied to detect metal ions such as Co2+, with high selectivity against a wide range of potentially interfering species. Fluorescent-based chemosensors continue to demonstrate strong sensitivity, with a turn-on fluorescence probe for paraquat reaching an LoD of 5.14 × 10−1 mol L−1, while Hg2+ and Cu2+ detection has also been effectively achieved through fluorescence-based methods. Among organic contaminants, chronoamperometric detection of microcystin-LR presents an LoD of 9.65 × 10−9 mol L−1, showcasing the capabilities of electrochemical sensors in detecting hazardous pollutants at ultra-trace levels. These findings underscore the continuous advancements in chemosensor technologies, offering more reliable and selective analytical tools for environmental monitoring and food safety applications.

Table 1.
Performance metrics of chemosensors for contaminant detection.
3.2. Indicators of Food Spoilage
Volatile Organic Compounds (VOCs)
Among the most reliable indicators of food spoilage are volatile organic compounds (VOCs), which are released as metabolic byproducts during the degradation of food components. These compounds include aldehydes, ketones, alcohols, and organic acids, and their presence or concentration changes serve as measurable indicators of spoilage. Advances in chemosensors have enabled the real-time and cost-effective monitoring of VOCs, offering a sustainable solution for food safety in both food processing and consumer settings. Chemosensors detect VOCs through specific interactions between the sensor’s recognition elements and the target molecules, resulting in measurable changes in optical [100], electrical [101], or electrochemical properties [102]. The detection mechanisms often involve selective binding, where functionalized materials such as metal oxides [103], polymers [104], or nanomaterials [105] interact selectively with VOCs, leading to measurable signal transduction in the form of changes in fluorescence, conductivity, or impedance. The integration of nanostructures like graphene oxide [106], carbon nanotubes [107], or metal–organic frameworks [108] enhances the sensitivity and selectivity of these sensors. Optical sensors detect VOCs through changes in color, fluorescence, or light absorption, with examples including sensors based on colorimetric indicators or quantum dots, which exhibit detectable responses to aldehydes or alcohols released during food spoilage [54]. Electrochemical sensors, on the other hand, monitor electrical signals such as current, voltage, or resistance changes during VOC interactions, with metal oxide-based sensors particularly effective for detecting low concentrations of VOCs in complex food environments [109]. Hybrid sensors, which combine optical and electrochemical techniques [110], offer enhanced detection accuracy and versatility, making them suitable for diverse applications in food monitoring.
Ammonia and Hydrogen Sulfide
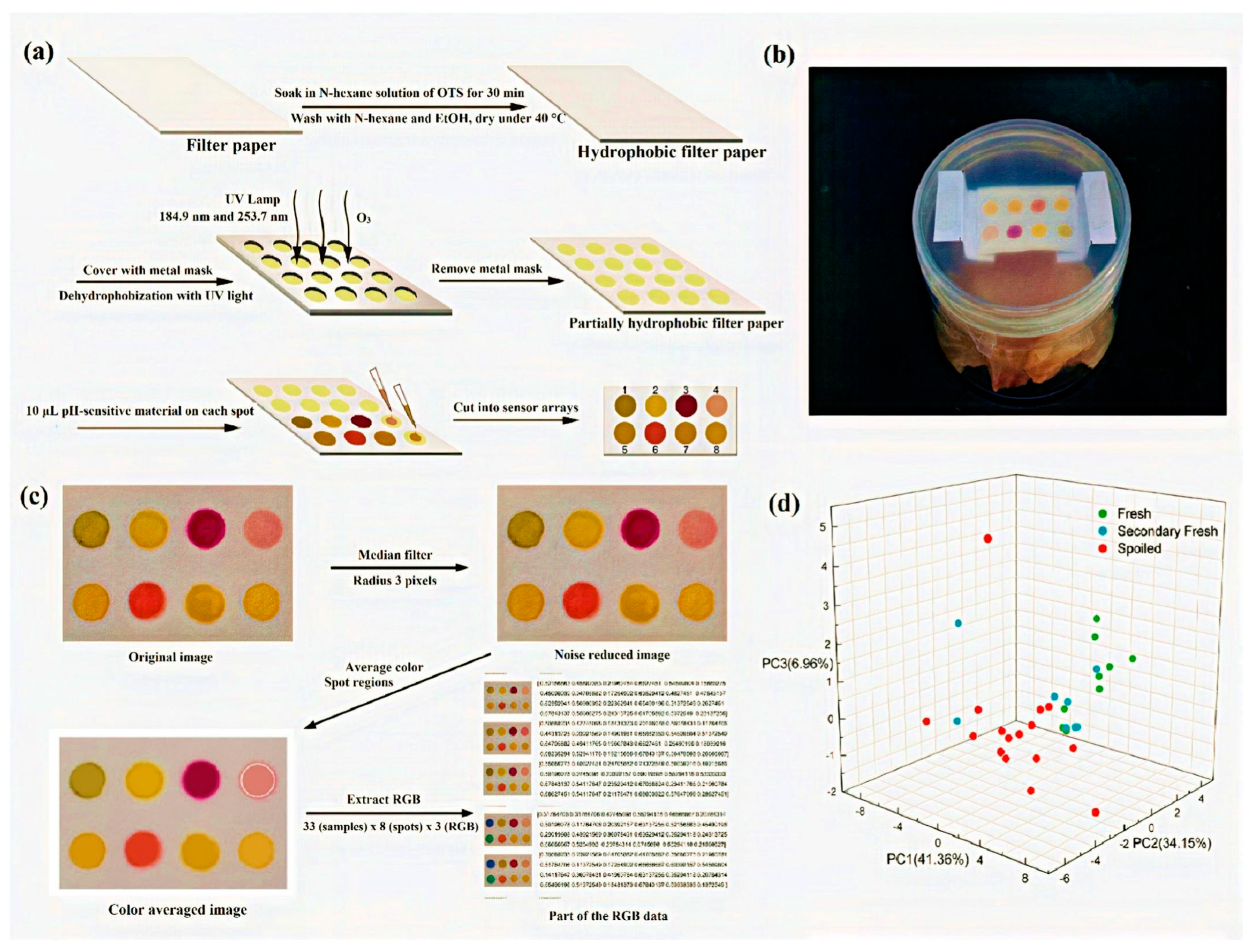
Chemosensors have emerged as valuable tools for detecting volatile compounds that indicate food spoilage, particularly ammonia (NH3) and hydrogen sulfide (H2S), which are key markers of protein degradation in perishable food products such as meat, seafood, and dairy. Figure 4 illustrates a paper-based chemosensor system designed for colorimetric detection of these volatile compounds.
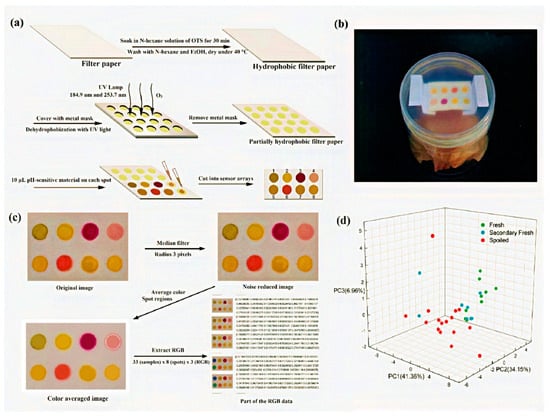
Figure 4.
(a) The fabrication process of the sensor array; (b) a packed fish sample with a colorimetric label; (c) the image processing progress of the sensor array; (d) the PCA score plot using the first three principal components. (Reprinted with permission from [93]. Copyright © 2024, Elsevier B. V.).
- Panel (a) depicts the fabrication process of the chemosensor, where hydrophilic and hydrophobic regions are created on filter paper to enable selective reagent deposition.
- Panel (b) shows a real-world setup for the sensor’s application, demonstrating its integration into a container for food monitoring.
- Panel (e) presents the image processing steps involved in analyzing the sensor’s colorimetric response, including original and processed images, median filtering, and the extraction of RGB (red, green, and blue) values, which represent the intensity of these three primary colors in digital image analysis. This approach allows for quantitative interpretation of the color changes associated with different levels of spoilage.
- Panel (d) displays a principal component analysis (PCA) plot, illustrating the classification of different spoilage levels based on the chemosensor’s colorimetric response.
This figure highlights the effectiveness of such sensors in visually detecting spoilage markers, offering a rapid and cost-effective alternative to conventional analytical techniques [111].
Ammonia is produced during the microbial decomposition of amino acids [112], while hydrogen sulfide arises from the breakdown of sulfur-containing compounds [113], including cysteine and methionine. Elevated levels of these gases indicate advanced stages of spoilage and correlate with changes in food quality and safety [114]. Traditional methods for detecting spoilage gases, such as chromatography and spectrophotometry, provide high sensitivity and accuracy but are resource-intensive and unsuitable for real-time, on-site monitoring. Chemosensors offer a more practical and cost-effective alternative by enabling rapid, in situ detection through specific interactions with these spoilage gases.
The primary mechanism by which chemosensors detect ammonia [115,116] and hydrogen sulfide [117] involves changes in optical or electrochemical signals upon exposure to these gases. Optical chemosensors often employ colorimetric or fluorescence-based systems that undergo reversible changes in color or emission intensity, while electrochemical sensors detect variations in conductivity or potential as gas molecules interact with sensor surfaces. The incorporation of nanomaterials, such as graphene oxide, metal nanoparticles, and conducting polymers, has significantly enhanced the sensitivity, selectivity, and response time of these sensors. Notably, paper-based [118] and flexible substrates functionalized with selective ligands [119] for NH3 and H2S have demonstrated potential for use in smart packaging and portable testing devices, making spoilage detection more accessible to consumers and food industry stakeholders.
Recent advancements include the integration of these sensors into “smart labels” for food packaging, which provide visual cues regarding product freshness [120]. Despite their progress, challenges such as cross-sensitivity to other gases and sensor degradation over time remain. Continued research focuses on improving sensor stability, developing eco-friendly materials, and optimizing sensor designs to minimize interference, thereby enhancing the reliability of chemosensors as indicators of food spoilage. These innovations contribute to reducing food waste and ensuring food safety in line with global sustainability goals.
pH and Oxygen Content
Chemosensors have emerged as valuable tools for monitoring food spoilage by detecting critical indicators such as pH and oxygen content, which are directly associated with the biochemical and microbial changes occurring during food degradation [121]. pH levels fluctuate significantly during spoilage due to microbial metabolism, enzymatic activity, and chemical reactions that produce or consume acidic and basic compounds [122]. For example, lactic acid bacteria in dairy and meat products lower the pH by producing organic acids [123], whereas the breakdown of proteins in seafood releases ammonia and amines, resulting in an increased pH [124]. Chemosensors designed to monitor pH [125] rely on sensitive materials that undergo colorimetric, fluorescence, or electrochemical changes in response to the hydrogen ion concentration. Recent advancements include the development of pH-responsive nanomaterials, such as metal–organic frameworks (MOFs) and graphene-based composites, which provide enhanced sensitivity, selectivity, and stability for real-time monitoring in complex food matrices.
Similarly, oxygen content plays a pivotal role in the progression of spoilage, particularly in packaged foods where oxidative reactions contribute to the degradation of lipids and other organic molecules. Oxygen-sensitive chemosensors typically use luminescent [126] or electrochemical systems that detect changes in the oxygen concentration based on the quenching of fluorescence or alterations in redox potentials. For instance, ruthenium-based complexes [127] and phosphorescent dyes [128] have been extensively employed in oxygen detection due to their high sensitivity and rapid response times. In addition, polymer-based oxygen sensors embedded in packaging films enable continuous, non-invasive monitoring of oxygen levels, ensuring the early detection of compromised storage conditions.
The integration of pH and oxygen-sensitive chemosensors in smart packaging and food quality control systems offers a cost-effective and sustainable approach to spoilage detection. These technologies provide valuable real-time insights, reducing food waste and enhancing consumer safety by enabling timely interventions before spoilage becomes irreversible.
3.3. Compliance with Food Safety Standards
The implementation of regulatory frameworks by organizations such as the European Food Safety Authority (EFSA), the U.S. Food and Drug Administration (FDA), and other international authorities is essential for ensuring food safety and consumer protection. These frameworks define maximum residue levels (MRLs) for contaminants such as pesticides, heavy metals, mycotoxins, and microbial pathogens in food products, alongside standards for monitoring food quality and shelf life. Adhering to these stringent regulations necessitates highly sensitive, specific, and rapid detection methods. In this context, chemosensors have emerged as pivotal tools that offer real-time, cost-effective monitoring solutions, bridging the gap between regulatory requirements and practical implementation.
One of the primary roles of chemosensors in regulatory compliance is their ability to detect and quantify trace contaminants at or below the MRLs established by regulatory agencies. For example, the EFSA’s stringent limits on pesticide residues in fruits and vegetables, or the FDA’s limits on mycotoxins in cereals and dairy, necessitate detection techniques with low detection thresholds and high specificity. Chemosensors, particularly those based on electrochemical, optical, and fluorescence mechanisms, have demonstrated detection limits in the nanomolar and even picomolar ranges, making them suitable for compliance with such regulations [129]. Chemosensors are proving advantageous compared to conventional laboratory techniques due to their capacity for rapid detection, which is essential in various applications, especially in food safety and environmental monitoring. Traditional methods necessitate extensive sample preparation processes, such as extraction and chromatographic separation, which can be labor-intensive and time-consuming. For instance, conventional procedures for pesticide analysis often require complex methodologies like gas chromatography–mass spectrometry (GC-MS) and solid-phase extraction that can introduce significant delays before obtaining results, as shown by Ashley et al. [130]. This lengthy sample preparation can also entail multiple steps, which impact the efficiency of contaminant detection, potentially leading to adverse economic impacts and regulatory penalties in food processing industries [131].
In contrast, chemosensors, including those based on colorimetric and fluorescence detection methods, provide quick and straightforward analytical processes that reduce the need for time-consuming protocols [132,133]. For example, fluorochromogenic chemosensors that detect contaminants without the need for extensive sample preparation have been developed, allowing for real-time monitoring of hazardous substances in various environments [134]. These approaches not only improve the speed of detection but also enhance the practicality of on-site applications, thereby making them particularly suitable for food processing environments where immediate results are critical [135].
Moreover, the integration of advanced materials in chemosensor technology has further streamlined the detection process. Markets benefit from these innovations by facilitating direct analysis with minimal sample preparation, effectively circumventing the delays associated with traditional laboratory methods [136]. As a result, chemosensors not only promise lower detection thresholds but also serve as reliable platforms for monitoring trace contaminants efficiently. This capability is particularly beneficial when regulatory compliance is paramount, ensuring that detected pollutants remain within stipulated limits [137]. In summary, the rapid detection capabilities of chemosensors provide a significant advantage over conventional analytical methods by minimizing preparation times and requiring less intricate methodologies. This efficiency addresses the pressing need for quick and reliable contaminant detection, which is crucial for compliance within stringent regulatory frameworks.
Another critical contribution of chemosensors lies in their potential for integration into hazard analysis and critical control point (HACCP) systems [138], a core component of regulatory compliance in both EFSA and FDA frameworks. Chemosensors embedded within smart packaging systems, for instance, can monitor parameters such as volatile organic compounds (VOCs) [139] released during spoilage or microbial degradation. These sensors provide real-time feedback that supports quality control during storage, transportation, and retail, ensuring that food products remain within regulatory safety limits throughout the supply chain. Moreover, wearable and portable chemosensor devices [140] facilitate on-site inspections, allowing for point-of-need testing at processing plants and distribution centers.
Chemosensors also contribute to regulatory frameworks by enhancing traceability [141], a key requirement of global food safety regulations. Their ability to provide continuous, non-destructive monitoring of food products enables the collection of detailed analytical data, which can be used to generate compliance reports and support audits. This capability aligns with the increasing adoption of digitalized quality assurance systems, where data from chemosensors can be integrated into blockchain-based traceability platforms to enhance transparency and accountability.
Despite their advantages, the widespread implementation of chemosensors for regulatory compliance faces challenges, such as variability in sensor performance due to matrix effects, the need for standardized calibration procedures, and regulatory validation of novel sensing platforms. Addressing these issues requires the harmonization of sensor validation protocols across different regulatory bodies and the development of universally accepted performance benchmarks.
In summary, chemosensors play a crucial role in supporting adherence to regulatory frameworks by providing rapid, sensitive, and real-time detection of contaminants and spoilage indicators. Their integration into food safety protocols and digital traceability systems enhances compliance with EFSA, FDA, and international regulations, contributing to safer food systems and improved public health outcomes. As sensor technologies continue to advance, they hold the potential to redefine the regulatory landscape by making food monitoring more accessible, efficient, and sustainable.
4. Chemosensors for Food Processing Monitoring
4.1. Process Optimization and Quality Control
Chemosensors have become integral to food processing monitoring, optimizing chemical synthesis and improving manufacturing efficiency. These sensors provide real-time analysis, ensuring precise control over reaction conditions and product quality while enhancing sustainability.
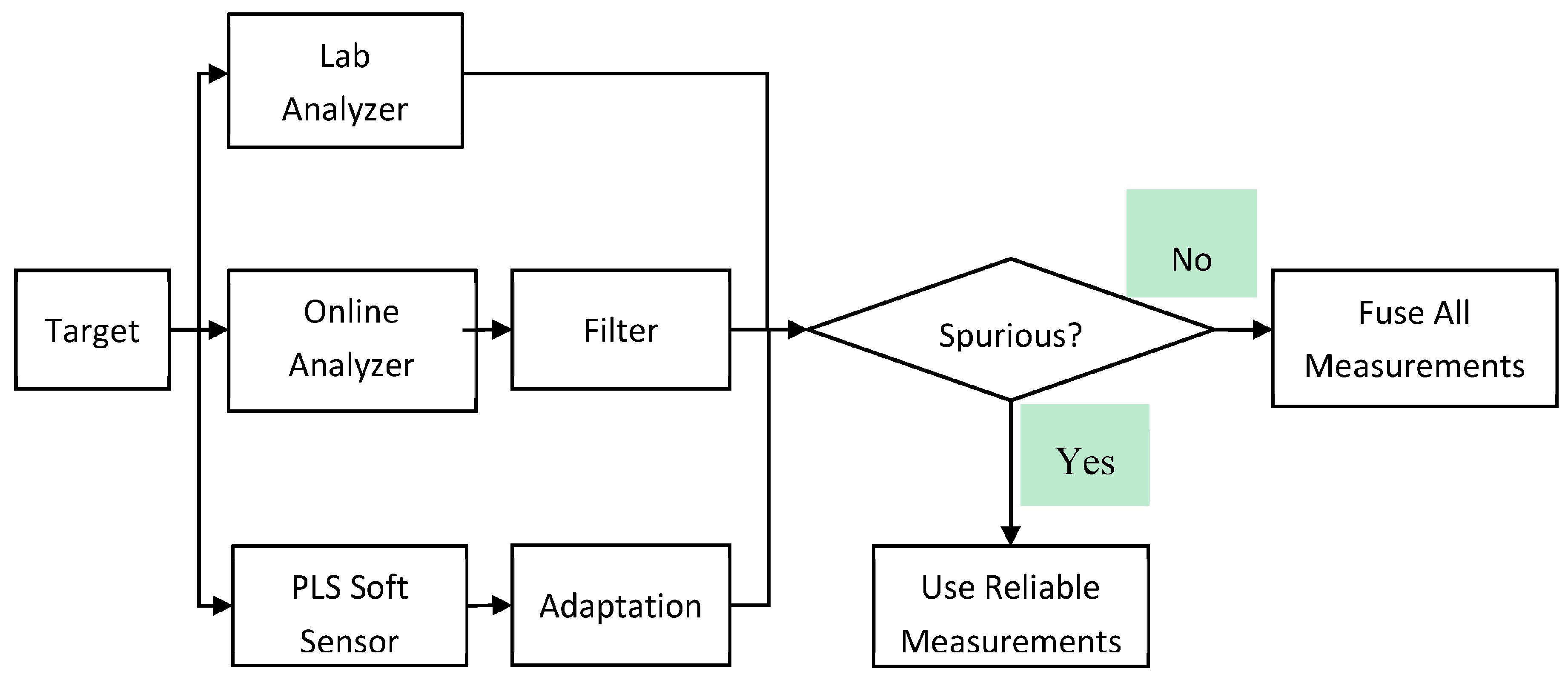
Figure 5 illustrates a sensor fusion strategy that integrates data from lab and online analyzers, filtering out spurious measurements to enhance reliability. A PLS soft sensor adapts to changing conditions, refining predictions. If no spurious data are detected, all measurements are fused for comprehensive analysis; otherwise, only reliable data are used. This approach ensures accurate, real-time process monitoring while minimizing errors and improving efficiency [142].
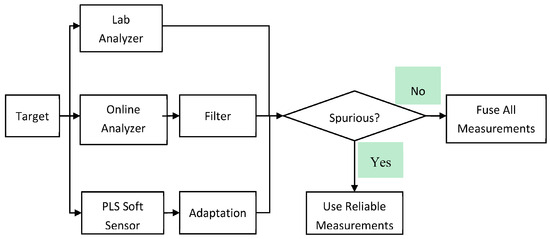
Figure 5.
Proposed multi-rate and judicious sensor fusion scheme. (The figure is adopted from reference [113], and it is published under the Creative Commons Attribution (CC BY 4.0) license).
In chemical synthesis, the application of chemosensors allows for the continuous monitoring of reactant and product concentrations, reaction intermediates, and potential impurities. This real-time data acquisition process is crucial for maintaining optimal reaction conditions, thereby enhancing yield and selectivity. For instance, integrating chemosensors with automated synthesis platforms enables dynamic adjustments during reactions, leading to more efficient and reproducible outcomes. Such integration is particularly beneficial in complex synthetic pathways where precise control over reaction parameters is essential for product quality [143].
In food production processes, chemosensors play a pivotal role in quality control by ensuring that products meet stringent specifications. They facilitate the detection of contaminants and the verification of chemical compositions, which is vital for compliance with regulatory standards and maintaining consumer safety. The deployment of chemosensors in process analytical chemistry allows for real-time monitoring, enabling immediate corrective actions and reducing the reliance on time-consuming off-line laboratory analyses [144].
The integration of chemosensors with advanced data analytics, including artificial intelligence (AI) and machine learning (ML), has further enhanced process optimization capabilities. AI-driven models can analyze complex datasets generated by chemosensors to predict optimal reaction conditions, identify potential process bottlenecks, and suggest improvements. This synergy between chemosensors and AI facilitates a more responsive and adaptive manufacturing environment, aligning with the principles of Industry 4.0 [145].
Recent advancements in chemosensor technology have focused on improving sensitivity, selectivity, and robustness to withstand the demanding conditions of food processing environments. The development of novel materials, such as metal–organic frameworks (MOFs) and nanostructured composites, has led to sensors capable of detecting trace levels of analytes with high specificity. Additionally, efforts to miniaturize sensor components have resulted in portable and cost-effective devices, broadening their applicability across various industrial sectors [117].
Despite these advancements, challenges remain in the widespread adoption of chemosensors for food processing monitoring. Issues such as sensor fouling, calibration stability, and integration with existing process control systems require ongoing research and development [15]. Addressing these challenges is essential to fully realize the potential of chemosensors in enhancing manufacturing efficiency and product quality.
In conclusion, chemosensors represent a transformative technology in food processing monitoring, offering real-time insights that drive process optimization and quality control. Their continued development and integration with digital technologies promise to further enhance efficiency, product quality, and sustainability in the food industry
4.2. Environmental Emissions and Pollution Control
The detection and control of industrial pollutants in air, water, and soil are critical components of environmental management and regulatory compliance. Industrial activities contribute to the release of hazardous pollutants, including volatile organic compounds (VOCs), heavy metals, polycyclic aromatic hydrocarbons (PAHs), and nitrogen and sulfur oxides (NOx and SOx). These pollutants pose significant risks to human health and ecosystems, necessitating accurate and real-time monitoring solutions. Chemosensors have emerged as an efficient, cost-effective, and sustainable alternative to traditional analytical techniques, such as gas chromatography and mass spectrometry, which, despite their high accuracy, are resource-intensive, require skilled operators, and are unsuitable for on-site and continuous monitoring.
Chemosensors designed for environmental monitoring operate by converting chemical interactions between target pollutants and the sensing material into detectable signals, such as changes in electrical conductivity, fluorescence intensity, or optical absorption. The sensing material plays a crucial role in determining the selectivity and sensitivity of the sensor. For air pollution monitoring [146], chemosensors utilizing nanomaterials such as graphene oxide, metal–organic frameworks (MOFs), and carbon nanotubes have shown remarkable performance in detecting VOCs and NOx at low concentrations. These sensors can be deployed in industrial facilities to monitor emissions in real time, helping to ensure compliance with environmental standards and enabling timely interventions to mitigate pollution sources.
In water monitoring, chemosensors have been developed to detect heavy metals [147], organic pollutants [148], and emerging contaminants such as pharmaceuticals [149] and microplastics [150]. Electrochemical sensors, in particular, have demonstrated high sensitivity and portability, making them ideal for field-based water quality assessments. For example, sensors based on modified electrodes with nanostructured coatings can detect trace amounts of cadmium, lead [151], and arsenic [152], with detection limits comparable to laboratory-based methods. The integration of molecularly imprinted polymers (MIPs) has further enhanced the selectivity of these sensors, allowing for the precise detection of specific pollutants even in complex aqueous matrices [153].
Soil monitoring presents additional challenges due to the heterogeneous composition of soil and the potential for matrix interferences [154]. Chemosensors used for soil analysis have evolved to address these limitations, with advances in solid-state electrochemical sensors and portable optical devices that can detect pesticides, polyaromatic hydrocarbons, and heavy metals. Such sensors are capable of rapid in-field measurements, providing a more efficient alternative to traditional soil sampling and laboratory testing.
4.3. Waste Management and Resource Efficiency
Effective waste management and resource efficiency are critical components of sustainable industrial practices. The optimization of these processes requires accurate, real-time monitoring of waste streams and by-products to minimize environmental impact and improve resource recovery. Chemosensors have emerged as valuable tools for waste analysis and process optimization due to their ability to provide rapid, sensitive, and cost-effective detection of specific analytes in complex matrices [155]. These sensors play a crucial role in monitoring parameters such as pH, heavy metal concentrations, organic pollutants, and volatile compounds, enabling more informed decision-making in industrial operations.
A significant focus of recent innovations has been the development of low-cost, disposable, and eco-friendly chemosensors. These sensors often incorporate biodegradable substrates, such as cellulose-based membranes or paper, functionalized with selective coatings to detect pollutants [156]. Such designs align with the principles of green chemistry and the circular economy by reducing the overall environmental footprint of the sensors themselves. Additionally, the combination of chemosensors with digital technologies, such as Internet of Things (IoT) platforms, has facilitated the automation of waste monitoring processes. Wireless, real-time data transmission and cloud-based analysis have enabled industries to track key waste parameters remotely, allowing for immediate corrective actions and efficient resource allocation.
Chemosensors have also proven to be instrumental in optimizing resource recovery from waste streams. In processes such as anaerobic digestion and bioreactor operations, sensors are employed to monitor the concentration of key intermediates, such as volatile fatty acids (VFAs) and ammonia [157], to prevent process imbalances and enhance biogas yields. Similarly, in chemical recycling and solvent recovery processes, chemosensors are used to assess the purity of recovered materials and to optimize separation efficiency. Innovations in multi-analyte sensing have further expanded the capabilities of chemosensors, allowing for the simultaneous detection of multiple waste components within a single sensor platform, thereby improving the overall efficiency of waste characterization.
Despite significant progress, several challenges remain in the deployment of chemosensors for food process monitoring, particularly in achieving long-term stability and resistance to harsh environmental conditions. Industrial waste streams often contain complex mixtures of contaminants, which can interfere with sensor performance. Therefore, ongoing research efforts are directed towards enhancing the robustness and durability of chemosensors through the development of protective coatings and self-cleaning surfaces. Furthermore, advancements in artificial intelligence and machine learning are expected to play a pivotal role in improving the interpretation of complex sensor data, leading to more accurate predictions and process optimizations.
In summary, innovations in chemosensor technology are contributing significantly to waste management and resource efficiency in industrial processes. By enabling real-time, precise monitoring of waste streams, these sensors support the transition to more sustainable food production practices, reduce operational costs, and enhance the recovery of valuable resources from waste, aligning with global efforts to achieve circular economy goals.
5. Challenges and Future Perspectives
5.1. Sensitivity, Selectivity, and Stability
The development of cost-effective chemosensors, which represent a large category of chemical detection tools, has made significant progress for food monitoring and food processing applications. However, challenges related to sensitivity, selectivity, and stability remain critical obstacles to their broader adoption, as is the case for many other sensor technologies. Low-cost chemosensors frequently rely on simplified detection platforms, such as paper-based assays [158] or screen-printed electrodes, which may have limitations in signal amplification compared to more complex, expensive counterparts. To enhance sensitivity, research has increasingly focused on the integration of nanomaterials, such as graphene oxide, carbon nanotubes, and metal–organic frameworks (MOFs) [159]. These materials provide a high surface area for interaction with target analytes, significantly improving signal response. However, their reproducibility and uniform performance are of concern, particularly when scaling up for commercial use.
In food matrices, the presence of complex mixtures of proteins, sugars, lipids, and other organic compounds can result in false positives or diminished signal accuracy. One approach to improve selectivity involves the use of molecularly imprinted polymers (MIPs), which are designed to have recognition sites tailored to the specific shape and functional groups of the analyte of interest. While MIPs have shown great promise in enhancing selectivity, their synthesis can be time-consuming, and their binding efficiency may degrade after repeated use, limiting their practical application. Moreover, ensuring that MIPs retain their performance under variable environmental conditions, such as changes in pH or temperature, remains a significant hurdle.
Stability is equally critical for real-world applications, as chemosensors must perform consistently over time without signal degradation or material fatigue. Many cost-effective sensors, particularly those based on organic dyes or enzymes, suffer from limited shelf life due to their susceptibility to denaturation, photobleaching, or chemical degradation. Research has aimed to address these limitations through the incorporation of robust nanocomposites and the use of synthetic recognition elements that mimic natural enzymes but are more resilient to environmental stress [160]. Additionally, encapsulation methods, such as sol–gel techniques [161], have been employed to protect sensitive components from external factors while maintaining their reactivity.
Despite these advancements, the reproducibility and scalability of high-performance, low-cost chemosensors remain key barriers to their widespread commercialization. Efforts are also being made to incorporate artificial intelligence (AI) and machine learning (ML) algorithms to improve data interpretation, particularly in distinguishing weak signals from noise. The implementation of AI in signal processing could enable predictive calibration models, enhancing sensor accuracy and enabling adaptive responses to environmental fluctuations.
Future perspectives in this field point toward the development of multifunctional, portable, and reusable sensor platforms that balance cost-effectiveness with high performance. Innovations in 3D printing and microfluidic fabrication offer potential pathways for producing customizable, high-throughput sensing devices. Moreover, further interdisciplinary collaboration between materials science, analytical chemistry, and computational modeling will be essential for addressing current limitations. Ultimately, overcoming challenges related to sensitivity, selectivity, and stability will enhance the utility of chemosensors in sustainable food monitoring and food processing quality control, ensuring both safety and compliance in a cost-efficient manner.
5.2. Adaptation to Variable Environmental Conditions
The adaptation of chemosensors to variable environmental conditions, particularly concerning humidity, temperature, and other external factors, presents significant challenges in the field of sustainable monitoring for food and food processing. The performance of chemosensors is highly sensitive to these environmental variables, which can affect their reliability and accuracy in detecting target analytes. For instance, the sensitivity and responsivity of optical chemosensors can be influenced by the functional groups present in their polymer matrices. Specifically, polar functionalities such as acids, amines, and hydroxyl groups play a crucial role in the chromism characteristics of these sensors, particularly in aqueous media, where variations in pH and temperature can lead to significant changes in sensor performance [162].
Moreover, the operational stability of chemosensors is often compromised by fluctuating environmental conditions. For example, the performance of silicon nanowire field-effect transistor (SiNW-FET) chemosensors is contingent upon effective surface modification, which enhances the binding affinity between the analytes and receptors. This modification is essential for maintaining sensitivity under varying humidity and temperature conditions, as these factors can alter the chemical interactions at the sensor surface [163]. Additionally, the detection limits of chemosensors can be adversely affected by environmental parameters, necessitating careful calibration and design to ensure consistent performance across different settings [164].
The challenges posed by environmental variability are not limited to optical and electrical sensors; they extend to the broader category of chemosensors, including those designed for detecting anions and metal ions. The selectivity and sensitivity of these sensors can be significantly impacted by external factors such as the temperature and ionic strength of the solution, which can alter the dynamics of the sensing mechanism [165,166]. For instance, the interaction between the chemosensor and the target analyte may be influenced by the charge and electron configuration of the metal ions, which can vary with temperature and other environmental conditions.
Future perspectives in the development of cost-effective chemosensors must address these environmental challenges through innovative design and material selection. The integration of advanced materials, such as polymer nanocomposites and hydrogels, may enhance the robustness of chemosensors against environmental fluctuations, thereby improving their recyclability and specificity [167,168]. Furthermore, the incorporation of responsive elements that can adapt to changes in pH or temperature could lead to more reliable monitoring systems that maintain performance across a range of conditions [169].
In conclusion, while significant progress has been made in the development of chemosensors for sustainable monitoring, addressing the challenges posed by variable environmental conditions remains a critical area for future research. By focusing on the interplay between sensor design, material properties, and environmental factors, researchers can enhance the reliability and effectiveness of chemosensors in real-world applications.
5.3. Adaptation to Variable Environmental Conditions
The field of chemosensors is experiencing significant advancements, particularly in the context of interdisciplinary research, integration with renewable energy sources, and the application of artificial intelligence (AI) and machine learning (ML) for data interpretation. These emerging trends present both challenges and opportunities that can enhance the effectiveness and sustainability of chemosensing technologies.
Interdisciplinary research is crucial for the development of innovative chemosensors that can address complex analytical challenges. By integrating knowledge from materials science, chemistry, and biology, researchers can design chemosensors with improved selectivity and sensitivity. For instance, the rational design of fluorescent and colorimetric chemosensors has been shown to enhance the detection capabilities for various analytes, including transition metal ions, by manipulating functional groups and molecular interactions [166,170]. This cross-disciplinary approach fosters the creation of multifunctional sensors capable of real-time monitoring in diverse environments, such as food safety and food processing applications.
The integration of renewable energy sources into chemosensor technology represents another promising avenue. Utilizing solar energy to power sensor systems can significantly reduce operational costs and enhance sustainability. Recent studies emphasize the potential of solar-powered chemosensors, which can operate autonomously in remote locations, thus facilitating continuous monitoring without reliance on traditional energy sources [171]. Such innovations not only contribute to environmental sustainability but also align with global efforts to reduce carbon footprints in food processing and production.
Moreover, the incorporation of AI and ML into chemosensor data interpretation is transforming how analytical data are processed and utilized. Advanced algorithms can analyze complex datasets generated by sensor arrays, enabling the rapid identification of target analytes and improving the accuracy of measurements [172]. For example, machine learning techniques have been applied to enhance the performance of chemosensor arrays in detecting toxic substances and food contaminants, demonstrating a significant leap in analytical capabilities. This trend indicates a future where intelligent systems can autonomously interpret data, leading to more efficient and reliable monitoring solutions in both food safety and industrial contexts. In conclusion, the future of chemosensors lies in embracing interdisciplinary research, renewable energy integration, and AI-driven data interpretation. These trends not only address current challenges but also pave the way for innovative solutions that can significantly enhance the sustainability and effectiveness of monitoring systems in various sectors.
5.4. Challenges and Limitations of Chemosensors in Practical Applications
Despite significant advancements in chemosensor technology, several critical challenges persist, limiting their widespread adoption in real-world applications. One of the primary concerns is selectivity, as many chemosensors struggle to differentiate between chemically similar analytes, leading to false positives or reduced accuracy in complex sample matrices. This issue is particularly relevant in food and food processing monitoring, where interference from other components can affect detection performance [173,174]. For instance, the selectivity of fluorescent chemosensors is often compromised by the presence of competing ions or molecules, which can lead to inaccurate readings [175]. Moreover, sensitivity remains a challenge, especially for contaminants present at trace levels, requiring further improvements in signal amplification strategies such as nanomaterial-enhanced sensors [176,177]. The integration of nanomaterials has shown promise in enhancing the sensitivity and selectivity of chemosensors, allowing for the detection of low-concentration analytes [178].
Another major limitation is operational stability, as many sensors experience signal drift over time due to environmental factors such as humidity, temperature variations, and pH fluctuations [179]. This is especially problematic for long-term monitoring applications, where sensors must maintain consistent performance without frequent recalibration [180]. For example, the stability of metal–organic frameworks (MOFs) in varying pH conditions is crucial for their application in aqueous environments [181]. Additionally, while miniaturization and integration with portable devices have increased the accessibility of chemosensors, the trade-off between cost-effectiveness and analytical performance remains a key barrier. Low-cost sensors often lack the robustness and reproducibility of high-end analytical instruments, posing challenges for regulatory compliance and commercialization [182]. Addressing these limitations requires interdisciplinary efforts, including advancements in materials science, machine learning-driven calibration techniques, and the development of standardized validation protocols for sensor performance in diverse real-world conditions [183].
In conclusion, while chemosensors have made significant strides in their development, challenges related to selectivity, sensitivity, operational stability, and cost-effectiveness must be addressed to facilitate their broader application in various fields, including environmental monitoring and food safety. Continued research and innovation in sensor design and materials will be essential to overcome these hurdles and enhance the practical utility of chemosensors in real-world scenarios [184,185].
5.5. Commercialization of Chemosensors: From Research to Market
The transition of chemosensors from laboratory research to commercially viable products has seen considerable progress in recent years. Several chemosensors developed for food safety and environmental monitoring have successfully reached the market, with applications in pesticide detection, heavy metal monitoring, and microbial contamination assessments. For instance, electrochemical sensors for lead (Pb2+) and cadmium (Cd2+) detection are now integrated into portable testing kits used in water quality assessments [175]. Similarly, paper-based chemosensors for pesticide residues have been developed as low-cost, disposable alternatives to conventional chromatographic methods, making rapid on-site testing more accessible.
However, the commercialization process remains complex, requiring extensive validation, regulatory approvals, and cost-effectiveness optimization. Fluorescent and colorimetric chemosensors have gained considerable attention due to their high sensitivity and potential applications in detecting metal ions and other analytes. However, their real-world performance can be significantly compromised by external conditions such as temperature [186], humidity [187], and the presence of interfering substances [188], which limits their widespread adoption. Additionally, the integration of chemosensors with digital platforms (such as smartphone-based sensing and IoT-enabled devices) has gained traction, enabling real-time data sharing and remote monitoring. Future efforts should focus on scaling up production, improving sensor durability, and ensuring compliance with food safety and environmental regulations, ultimately facilitating broader adoption in food processing and related industries
6. Conclusions
The advancements in cost-effective chemosensors for sustainable monitoring in food and food processing have significantly evolved, showcasing a variety of applications and promising future directions. Recent studies highlight the development of innovative chemosensors that leverage dual-wavelength monitoring and ratiometric fluorescent techniques, enhancing the precision of quantitative analyses in complex matrices such as water and food products [189]. These advancements not only improve detection limits but also facilitate real-time monitoring, which is crucial for ensuring food safety and quality.
Applications of chemosensors have expanded into diverse fields, including environmental monitoring and food safety. For instance, electrochemical biosensors have gained traction due to their ability to detect contaminants in food products effectively, providing high specificity and accuracy even in complex food matrices [29,190]. Moreover, colorimetric sensors utilizing gold nanoparticles have demonstrated remarkable sensitivity for detecting harmful ions like chromium and mercury, with detection limits well below regulatory thresholds [191,192]. Such capabilities underscore the potential of chemosensors to serve as vital tools in food processing, ensuring compliance with safety standards.
Looking ahead, the integration of advanced materials and technologies into chemosensor design is expected to drive further innovations. The use of metal–organic frameworks (MOFs) and organic chemosensors is particularly promising, as these materials can enhance sensitivity and selectivity while being cost-effective [193,194]. Additionally, the incorporation of smartphone technology for data collection and analysis represents a significant leap towards user-friendly, on-site monitoring solutions, making chemosensors more accessible for widespread use in both food processing and consumer settings [195]. As research continues to explore novel sensing mechanisms and materials, the future of chemosensors appears bright, with the potential to revolutionize monitoring practices across various sectors.
In conclusion, the advancements in cost-effective chemosensors are paving the way for sustainable monitoring in food safety and processing. Their diverse applications, coupled with ongoing innovations in materials and technology, promise to enhance food safety, environmental protection, and overall efficiency in food processing.
Author Contributions
Conceptualization, C.A., A.C., G.-L.R. and S.A.V.E.; investigation, C.A., A.C., G.-L.R. and S.A.V.E.; methodology, C.A., A.C., G.-L.R. and S.A.V.E.; writing—review, C.A., A.C., G.-L.R. and S.A.V.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Research, Innovation and Digitalization through the Core Program of the National Research, Development and Innovation Plan 2022–2027, project no. PN 23-02-0101-Contract No. 7N/2023.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Saccomano, S.C.; Jewell, M.P.; Cash, K.J. A Review of Chemosensors and Biosensors for Monitoring Biofilm Dynamics. Sens. Actuators Rep. 2021, 3, 100043. [Google Scholar] [CrossRef]
- Kadam, U.S.; Hong, J.C. Advances in Aptameric Biosensors Designed to Detect Toxic Contaminants from Food, Water, Human Fluids, and the Environment. Trends Environ. Anal. Chem. 2022, 36, e00184. [Google Scholar] [CrossRef]
- Ren, H.; Li, F.; Yu, S.; Wu, P. The Detection of Multiple Analytes by Using Visual Colorimetric and Fluorometric Multimodal Chemosensor Based on the Azo Dye. Heliyon 2022, 8, e10216. [Google Scholar] [CrossRef]
- Keshavarzi, P.; Abbasi-Moayed, S.; Khodabakhsh, M.; Unal, U.; Hormozi-Nezhad, M.R. Chrono-Colorimetric Sensor Array for Detection and Discrimination of Halide Ions Using an All-in-One Plasmonic Sensor Element. Talanta 2023, 259, 124528. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kulshreshtha, S.; Shrivastava, A.; Saini, A. Biosensors for Food Spoilage Detection: A Comprehensive Review of Current Advances. J. Food Chem. Nanotechnol. 2024, 10, S73–S82. [Google Scholar] [CrossRef]
- Liu, J.; Wu, D.; Wu, Y.; Shi, Y.; Liu, W.; Sun, Z.; Li, G. Recent Advances in Optical Sensors and Probes for the Detection of Freshness in Food Samples: A Comprehensive Review (2020–2023). TrAC Trends Anal. Chem. 2024, 177, 117793. [Google Scholar] [CrossRef]
- Preethichandra, D.M.G.; Gholami, M.D.; Izake, E.L.; O’Mullane, A.P.; Sonar, P. Conducting Polymer Based Ammonia and Hydrogen Sulfide Chemical Sensors and Their Suitability for Detecting Food Spoilage. Adv. Mater. Technol. 2023, 8, 2200841. [Google Scholar] [CrossRef]
- Huang, X.; Sun, W.; Li, Z.; Shi, J.; Zhang, N.; Zhang, Y.; Zhai, X.; Hu, X.; Zou, X. Hydrogen Sulfide Gas Sensing toward On-Site Monitoring of Chilled Meat Spoilage Based on Ratio-Type Fluorescent Probe. Food Chem. 2022, 396, 133654. [Google Scholar] [CrossRef]
- Chun, H.W.; Zheng, J.; Lee, E.H.; Oh, B.M.; Lee, C.B.; Min, J.S.; Kim, E.; Kim, E.; Lee, W.; Kim, J.H. Pure-Water-Soluble Colorimetric Chemosensors for Highly Sensitive and Rapid Detection of Hydrogen Sulfide: Applications to Evaluation of on-Site Water Quality and Real-Time Gas Sensors. Sens. Actuators B Chem. 2024, 402, 134989. [Google Scholar] [CrossRef]
- Dalapati, R.; Hunter, M.; Zang, L. A Dual Fluorometric and Colorimetric Sulfide Sensor Based on Coordinating Self-Assembled Nanorods: Applicable for Monitoring Meat Spoilage. Chemosensors 2022, 10, 500. [Google Scholar] [CrossRef]
- Nguyen, M.T.N.; Lee, J.S. Development of a Chemical Sensor Device for Monitoring Hazardous Gases Generated in the Semiconductor Manufacturing Process. Chemosensors 2024, 12, 233. [Google Scholar] [CrossRef]
- Pundi, A.; Chang, C.J. Recent Developments in the Preparation, Characterization, and Applications of Chemosensors for Environmental Pollutants Detection. J. Envirion Chem. Eng. 2023, 11, 110346. [Google Scholar] [CrossRef]
- Yang, J.; Chen, S.W.; Zhang, B.; Tu, Q.; Wang, J.; Yuan, M. Sen Non-Biological Fluorescent Chemosensors for Pesticides Detection. Talanta 2022, 240, 123200. [Google Scholar] [CrossRef] [PubMed]
- Keim, M.E. Industrial Chemical Disasters. In Disaster Medicine; Elsevier: Amsterdam, The Netherlands, 2006; pp. 556–562. [Google Scholar] [CrossRef]
- Nalakurthi, N.V.S.R.; Abimbola, I.; Ahmed, T.; Anton, I.; Riaz, K.; Ibrahim, Q.; Banerjee, A.; Tiwari, A.; Gharbia, S. Challenges and Opportunities in Calibrating Low-Cost Environmental Sensors. Sensors 2024, 24, 3650. [Google Scholar] [CrossRef]
- Lim, J.W.; Kim, T.Y.; Woo, M.A. Trends in Sensor Development toward Next-Generation Point-of-Care Testing for Mercury. Biosens. Bioelectron. 2021, 183, 113228. [Google Scholar] [CrossRef]
- Yang, G.; Xu, W.; Xu, B.; Yang, Y.; Li, P.; Yu, A.; Ning, S.; Fu, Q.; Zhang, R.; Liu, X. Two Decades’ Advancements and Research Trends in Needle-Type Sensor Technology: A Scientometric Analysis. Heliyon 2024, 10, e27399. [Google Scholar] [CrossRef]
- Gezahegn, T.F.; Ambaye, A.D.; Mekoyete, T.M.; Malefane, M.E.; Oyedotun, K.O.; Mokrani, T. Breakthroughs in Nanostructured-Based Chemical Sensors for the Detection of Toxic Metals. Talanta Open 2024, 10, 100354. [Google Scholar] [CrossRef]
- Arregui, F.J. Sensors Based on Nanostructured Materials. In Sensors Based on Nanostructured Materials; Springer: New York, NY, USA, 2009; pp. 1–317. [Google Scholar]
- Olorunyomi, J.F.; Geh, S.T.; Caruso, R.A.; Doherty, C.M. Metal–Organic Frameworks for Chemical Sensing Devices. Mater. Horiz. 2021, 8, 2387–2419. [Google Scholar] [CrossRef]
- Zhang, Z.; Lou, Y.; Guo, C.; Jia, Q.; Song, Y.; Tian, J.Y.; Zhang, S.; Wang, M.; He, L.; Du, M. Metal–Organic Frameworks (MOFs) Based Chemosensors/Biosensors for Analysis of Food Contaminants. Trends Food Sci. Technol. 2021, 118, 569–588. [Google Scholar] [CrossRef]
- Pilo, M.I.; Sanna, G.; Spano, N. Conducting Polymers in Amperometric Sensors: A State of the Art over the Last 15 Years with a Focus on Polypyrrole-, Polythiophene-, and Poly(3,4-Ethylenedioxythiophene)-Based Materials. Chemosensors 2024, 12, 81. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, W.; Kumar, R.; Kumar, M.; Zhang, J. Conducting Polymer-Based Nanostructures for Gas Sensors. Coord. Chem. Rev. 2022, 462, 214517. [Google Scholar] [CrossRef]
- Lange, U.; Roznyatovskaya, N.V.; Mirsky, V.M. Conducting Polymers in Chemical Sensors and Arrays. Anal. Chim. Acta. 2008, 614, 1–26. [Google Scholar] [CrossRef]
- Pavel, I.A.; Lakard, S.; Lakard, B. Flexible Sensors Based on Conductive Polymers. Chemosensors 2022, 10, 97. [Google Scholar] [CrossRef]
- Ding, X.; Srinivasan, B.; Tung, S. Development and Applications of Portable Biosensors. SLAS Technol. 2015, 20, 365–389. [Google Scholar] [CrossRef]
- Ayele, E.D.; Gavriel, S.; Gonzalez, J.F.; Teeuw, W.B.; Philimis, P.; Gillani, G. Emerging Industrial Internet of Things Open-Source Platforms and Applications in Diverse Sectors. Telecom 2024, 5, 369–399. [Google Scholar] [CrossRef]
- Virumbrales, C.; Hernández-Ruiz, R.; Trigo-López, M.; Vallejos, S.; García, J.M. Sensory Polymers: Trends, Challenges, and Prospects Ahead. Sensors 2024, 24, 3852. [Google Scholar] [CrossRef] [PubMed]
- Petrucci, R.; Pasquali, M.; Scaramuzzo, F.A.; Curulli, A. Recent Advances in Electrochemical Chitosan-Based Chemosensors and Biosensors: Applications in Food Safety. Chemosensors 2021, 9, 254. [Google Scholar] [CrossRef]
- Givanoudi, S.; Heyndrickx, M.; Depuydt, T.; Khorshid, M.; Robbens, J.; Wagner, P. A Review on Bio- and Chemosensors for the Detection of Biogenic Amines in Food Safety Applications: The Status in 2022. Sensors 2023, 23, 613. [Google Scholar] [CrossRef]
- Asefa, T.; Duncan, C.T.; Sharma, K.K. Recent Advances in Nanostructured Chemosensors and Biosensors. Analyst 2009, 134, 1980–1990. [Google Scholar] [CrossRef]
- Balamurugan, G.; Zhang, W.; Choi, Y.W.; Park, J.M.; Velmathi, S.; Park, J.S. Unmasking the Role of Anion-π Bonding—A New Paradigm for Iodide Selective Organic Probes. Dye. Pigment. 2025, 232, 112498. [Google Scholar] [CrossRef]
- Luo, H.; Chen, L.X.; Ge, Q.M.; Liu, M.; Tao, Z.; Zhou, Y.H.; Cong, H. Applications of Macrocyclic Compounds for Electrochemical Sensors to Improve Selectivity and Sensitivity. J. Incl. Phenom. Macrocycl. Chem. 2019, 95, 171–198. [Google Scholar]
- Müller, C.; Hitzmann, B.; Schubert, F.; Scheper, T. Optical Chemo- and Biosensors for Use in Clinical Applications. Sens. Actuators B Chem. 1997, 40, 71–77. [Google Scholar] [CrossRef]
- Gui, R.; Guo, H.; Jin, H. Preparation and Applications of Electrochemical Chemosensors Based on Carbon-Nanomaterial-Modified Molecularly Imprinted Polymers. Nanoscale Adv. 2019, 1, 3325–3363. [Google Scholar] [CrossRef]
- Pietrzyk-Le, A.; Suriyanarayanan, S.; Kutner, W.; Chitta, R.; D’Souza, F. Selective Histamine Piezoelectric Chemosensor Using a Recognition Film of the Molecularly Imprinted Polymer of Bis(Bithiophene) Derivatives. Anal. Chem. 2009, 81, 2633–2643. [Google Scholar] [CrossRef]
- Khorshid, M.; Losada-Pérez, P.; Cornelis, P.; Dollt, M.; Ingebrandt, S.; Glorieux, C.; Renner, F.U.; van Grinsven, B.; De Ceuninck, W.; Thoelen, R.; et al. Searching for a Common Origin of Heat-Transfer Effects in Bio- and Chemosensors: A Study on Thiols as a Model System. Sens. Actuators B Chem. 2020, 310, 127627. [Google Scholar] [CrossRef]
- Jamalipour, A.; Hossain, M.A. Smartphone Instrumentations for Public Health Safety; Springer Nature: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Li, B.; Zhao, D.; Wang, F.; Zhang, X.; Li, W.; Fan, L. Recent Advances in Molecular Logic Gate Chemosensors Based on Luminescent Metal Organic Frameworks. Dalton Trans. 2021, 50, 14967–14977. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Gou, H.; Al-Ogaidi, I.; Wu, N. Nanostructured Sensors for Detection of Heavy Metals: A Review. ACS Sustain. Chem. Eng. 2013, 1, 713–723. [Google Scholar] [CrossRef]
- Savin, M.; Mihailescu, C.M.; Moldovan, C.; Grigoroiu, A.; Ion, I.; Ion, A.C. Resistive Chemosensors for the Detection of CO Based on Conducting Polymers and Carbon Nanocomposites: A Review. Molecules 2022, 27, 821. [Google Scholar] [CrossRef]
- Li, H.Y.; Zhao, S.N.; Zang, S.Q.; Li, J. Functional Metal–Organic Frameworks as Effective Sensors of Gases and Volatile Compounds. Chem. Soc. Rev. 2020, 49, 6364–6401. [Google Scholar] [CrossRef]
- Rudzinski, C.M.; Young, A.M.; Nocera, D.G. A Supramolecular Microfluidic Optical Chemosensor. J. Am. Chem. Soc. 2002, 124, 1723–1727. [Google Scholar] [CrossRef]
- Lai, L.; Yan, F.; Chen, G.; Huang, Y.; Huang, L.; Li, D. Recent Progress on Fluorescent Probes in Heavy Metal Determinations for Food Safety: A Review. Molecules 2023, 28, 5689. [Google Scholar] [CrossRef] [PubMed]
- Ayivi, R.D.; Obare, S.O.; Wei, J. Molecularly Imprinted Polymers as Chemosensors for Organophosphate Pesticide Detection and Environmental Applications. TrAC Trends Anal. Chem. 2023, 167, 117231. [Google Scholar] [CrossRef]
- Stortini, A.M.; Baldo, M.A.; Moro, G.; Polo, F.; Moretto, L.M. Bio- and Biomimetic Receptors for Electrochemical Sensing of Heavy Metal Ions. Sensors 2020, 20, 6800. [Google Scholar] [CrossRef] [PubMed]
- Pavase, T.R.; Lin, H.; Shaikh, Q.; Hussain, S.; Li, Z.; Ahmed, I.; Lv, L.; Sun, L.; Shah, S.B.H.; Kalhoro, M.T. Recent Advances of Conjugated Polymer (CP) Nanocomposite-Based Chemical Sensors and Their Applications in Food Spoilage Detection: A Comprehensive Review. Sens. Actuators B Chem. 2018, 273, 1113–1138. [Google Scholar] [CrossRef]
- Kuchmenko, T.A.; Lvova, L.B. A Perspective on Recent Advances in Piezoelectric Chemical Sensors for Environmental Monitoring and Foodstuffs Analysis. Chemosensors 2019, 7, 39. [Google Scholar] [CrossRef]
- Kuchmenko, T.; Lvova, L. Piezelectric Chemosensors and Multisensory Systems. In Chemoresponsive Materials: Smart Materials for Chemical and Biological Stimulation; Royal Society of Chemistry: London, UK, 2022; pp. 567–603. [Google Scholar] [CrossRef]
- Pannone, A.; Raj, A.; Ravichandran, H.; Das, S.; Chen, Z.; Price, C.A.; Sultana, M.; Das, S. Robust Chemical Analysis with Graphene Chemosensors and Machine Learning. Nature 2024, 634, 572–578. [Google Scholar] [CrossRef]
- Korah, B.K.; Chacko, A.R.; Mathew, S.; Mathew, B. Carbon Nanotubes as Sensors in Food and Agricultural Applications. In Carbon Nanotubes for Energy and Environmental Applications; Apple Academic Press: Palm Bay, FL, USA, 2022; pp. 115–159. [Google Scholar]
- Subhasri, A.; Anbuselvan, C. Facile, Cost Effective Synthesis and DFT-Based Studies of Substituted Aryl Hydrazones of β-Diketones: A New Selective Fluorescent Chemosensor for Co2+. Anal. Methods 2014, 6, 5596–5609. [Google Scholar] [CrossRef]
- Bou-Maroun, E. Carbon Electrode Modified with Molecularly Imprinted Polymers for the Development of Electrochemical Sensor: Application to Pharmacy, Food Safety, Environmental Monitoring, and Biomedical Analysis. Chemosensors 2023, 11, 548. [Google Scholar] [CrossRef]
- Wanniarachchi, P.C.; Upul Kumarasinghe, K.G.; Jayathilake, C. Recent Advancements in Chemosensors for the Detection of Food Spoilage. Food Chem. 2024, 436, 137733. [Google Scholar] [CrossRef]
- Ashley, J. Biosensors. In Handbook of Dairy Foods Analysis; CRC Press: Boca Raton, FL, USA, 2021; pp. 339–359. [Google Scholar] [CrossRef]
- Kumar, H.; Neelam, R. Enzyme-Based Electrochemical Biosensors for Food Safety: A Review. Nanobiosensors Dis. Diagn. 2016, 2016, 29–39. [Google Scholar] [CrossRef]
- Cheng, N.; Shang, Y.; Xu, Y.; Wijayanti, S.D.; Tsvik, L.; Haltrich, D. Recent Advances in Electrochemical Enzyme-Based Biosensors for Food and Beverage Analysis. Foods 2023, 12, 3355. [Google Scholar] [CrossRef]
- Bucur, B.; Munteanu, F.D.; Marty, J.L.; Vasilescu, A. Advances in Enzyme-Based Biosensors for Pesticide Detection. Biosensors 2018, 8, 27. [Google Scholar] [CrossRef]
- Sharma, A.; Goud, K.Y.; Hayat, A.; Bhand, S.; Marty, J.L. Recent Advances in Electrochemical-Based Sensing Platforms for Aflatoxins Detection. Chemosensors 2017, 5, 1. [Google Scholar] [CrossRef]
- Bhunia, A.K. Biosensors and Bio-Based Methods for the Separation and Detection of Foodborne Pathogens. Adv. Food Nutr. Res. 2008, 54, 1–44. [Google Scholar] [CrossRef]
- Deng, R.; Bai, J.; Yang, H.; Ren, Y.; He, Q.; Lu, Y. Nanotechnology-Leveraged Nucleic Acid Amplification for Foodborne Pathogen Detection. Coord. Chem. Rev. 2024, 506, 215745. [Google Scholar] [CrossRef]
- Manoharan Nair Sudha Kumari, S.; Thankappan Suryabai, X. Sensing the Future—Frontiers in Biosensors: Exploring Classifications, Principles, and Recent Advances. ACS Omega 2024, 9, 48918–48987. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Sutham, P.; Wu, K.; Mallikarjunan, K.; Wang, J.-P.; Jaffrezic-Renault, N.; Liang, S.; Sutham, P.; Wu, K.; Mallikarjunan, K.; et al. Giant Magnetoresistance Biosensors for Food Safety Applications. Sensors 2022, 22, 5663. [Google Scholar] [CrossRef]
- Mahanti, N.K.; Shivashankar, S.; Chhetri, K.B.; Kumar, A.; Rao, B.B.; Aravind, J.; Swami, D.V. Enhancing Food Authentication through E-Nose and E-Tongue Technologies: Current Trends and Future Directions. Trends Food Sci. Technol. 2024, 150, 104574. [Google Scholar] [CrossRef]
- Casalinuovo, I.A.; Di Pierro, D.; Coletta, M.; Di Francesco, P. Application of Electronic Noses for Disease Diagnosis and Food Spoilage Detection. Sensors 2006, 6, 1428–1439. [Google Scholar] [CrossRef]
- Jo, Y.; Chung, N.; Park, S.W.; Noh, B.S.; Jeong, Y.J.; Kwon, J.H. Application of E-Tongue, E-Nose, and MS-E-Nose for Discriminating Aged Vinegars Based on Taste and Aroma Profiles. Food Sci. Biotechnol. 2016, 25, 1313–1318. [Google Scholar] [CrossRef]
- Magnani, G.; Giliberti, C.; Errico, D.; Stighezza, M.; Fortunati, S.; Mattarozzi, M.; Boni, A.; Bianchi, V.; Giannetto, M.; De Munari, I.; et al. Evaluation of a Voltametric E-Tongue Combined with Data Preprocessing for Fast and Effective Machine Learning-Based Classification of Tomato Purées by Cultivar. Sensors 2024, 24, 3586. [Google Scholar] [CrossRef] [PubMed]
- Koralli, P.; Mouzakis, D.E. Advances in Wearable Chemosensors. Chemosensors 2021, 9, 99. [Google Scholar] [CrossRef]
- Verma, G.; Gupta, A. Next-Generation Chemiresistive Wearable Breath Sensors for Non-Invasive Healthcare Monitoring: Advances in Composite and Hybrid Materials. Small 2025. [Google Scholar] [CrossRef]
- Hooshmand, S.; Kassanos, P.; Keshavarz, M.; Duru, P.; Kayalan, C.I.; Kale, İ.; Bayazit, M.K. Wearable Nano-Based Gas Sensors for Environmental Monitoring and Encountered Challenges in Optimization. Sensors 2023, 23, 8648. [Google Scholar] [CrossRef] [PubMed]
- Obare, S.O.; De, C.; Guo, W.; Haywood, T.L.; Samuels, T.A.; Adams, C.P.; Masika, N.O.; Murray, D.H.; Anderson, G.A.; Campbell, K.; et al. Fluorescent Chemosensors for Toxic Organophosphorus Pesticides: A Review. Sensors 2010, 10, 7018–7043. [Google Scholar] [CrossRef]
- Kim, D.Y.; Kadam, A.; Shinde, S.; Saratale, R.G.; Patra, J.; Ghodake, G. Recent Developments in Nanotechnology Transforming the Agricultural Sector: A Transition Replete with Opportunities. J. Sci. Food Agric. 2018, 98, 849–864. [Google Scholar] [CrossRef] [PubMed]
- Farooq, S.; Nie, J.; Cheng, Y.; Yan, Z.; Li, J.; Bacha, S.A.S.; Mushtaq, A.; Zhang, H. Molecularly Imprinted Polymers’ Application in Pesticide Residue Detection. Analyst 2018, 143, 3971–3989. [Google Scholar] [CrossRef]
- Mashuni, M.; Ritonga, H.; Jahiding, M.; Rubak, B.; Hamid, F.H. Highly Sensitive Detection of Carbaryl Pesticides Using Potentiometric Biosensor with Nanocomposite Ag/r-Graphene Oxide/Chitosan Immobilized Acetylcholinesterase Enzyme. Chemosensors 2022, 10, 138. [Google Scholar] [CrossRef]
- Malik, L.A.; Bashir, A.; Qureashi, A.; Pandith, A.H. Detection and Removal of Heavy Metal Ions: A Review. Envirion Chem. Lett. 2019, 17, 1495–1521. [Google Scholar] [CrossRef]
- Zhang, H.; Song, J.; Wang, S.; Song, Q.; Guo, H.; Li, Z. Recent Progress in Macrocyclic Chemosensors for Lead, Cadmium and Mercury Heavy Metal Ions. Dye. Pigment. 2023, 216, 111380. [Google Scholar] [CrossRef]
- Bumagina, N.A.; Antina, E.V. Review of Advances in Development of Fluorescent BODIPY Probes (Chemosensors and Chemodosimeters) for Cation Recognition. Coord. Chem. Rev. 2024, 505, 215688. [Google Scholar] [CrossRef]
- Dutta, M.; Das, D. Recent Developments in Fluorescent Sensors for Trace-Level Determination of Toxic-Metal Ions. TrAC Trends Anal. Chem. 2012, 32, 113–132. [Google Scholar] [CrossRef]
- Zhang, N.; Qiao, R.; Su, J.; Yan, J.; Xie, Z.; Qiao, Y.; Wang, X.; Zhong, J. Recent Advances of Electrospun Nanofibrous Membranes in the Development of Chemosensors for Heavy Metal Detection. Small 2017, 13, 1604293. [Google Scholar] [CrossRef]
- Tarapoulouzi, M.; Ortone, V.; Cinti, S. Heavy Metals Detection at Chemometrics-Powered Electrochemical (Bio)Sensors. Talanta 2022, 244, 123410. [Google Scholar] [CrossRef] [PubMed]
- Musikavanhu, B.; Liang, Y.; Xue, Z.; Feng, L.; Zhao, L. Strategies for Improving Selectivity and Sensitivity of Schiff Base Fluorescent Chemosensors for Toxic and Heavy Metals. Molecules 2023, 28, 6960. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, Z.; Qi, J.; You, J.; Ma, J.; Chen, L. Colorimetric Detection of Heavy Metal Ions with Various Chromogenic Materials: Strategies and Applications. J. Hazard. Mater. 2023, 441, 129889. [Google Scholar] [CrossRef]
- Jiang, H.; Tang, D.; Li, N.; Li, J.; Li, Z.; Han, Q.; Liu, X.; Zhu, X. A Novel Chemosensor for the Distinguishable Detections of Cu2+ and Hg2+ by off–on Fluorescence and Ratiometric UV–Visible Absorption. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 250, 119365. [Google Scholar] [CrossRef]
- Oliveira, E.; Núñez, C.; Santos, H.M.; Fernández-Lodeiro, J.; Fernández-Lodeiro, A.; Capelo, J.L.; Lodeiro, C. Revisiting the Use of Gold and Silver Functionalised Nanoparticles as Colorimetric and Fluorometric Chemosensors for Metal Ions. Sens. Actuators B Chem. 2015, 212, 297–328. [Google Scholar] [CrossRef]
- Liu, W.; Li, X.; Li, Z.; Zhang, M.; Song, M. Voltammetric Metal Cation Sensors Based on Ferrocenylthiosemicarbazone. Inorg. Chem. Commun. 2007, 10, 1485–1488. [Google Scholar] [CrossRef]
- Celestina, J.J.; Tharmaraj, P.; Jeevika, A.; Sheela, C.D. Fabrication of Triazine Based Colorimetric and Electrochemical Sensor for the Quantification of Co2+ Ion. Microchem. J. 2020, 155, 104692. [Google Scholar] [CrossRef]
- Asha, J.B.; Suresh, P. Covalently Modified Graphene Oxide as Highly Fluorescent and Sustainable Carbonaceous Chemosensor for Selective Detection of Zirconium Ion in Complete Aqueous Medium. ACS Sustain. Chem. Eng. 2020, 8, 14301–14311. [Google Scholar] [CrossRef]
- Salem, M.A.S.; Khan, A.M.; Manea, Y.K.; Saleh, H.A.M.; Ahmad, M. Carbon Nanotubes Decorated with Coordination Polymers for Fluorescence Detection of Heavy-Metal Ions and Nitroaromatic Chemicals. ACS Omega 2023, 8, 1220–1231. [Google Scholar] [CrossRef]
- Thangaraj, B.; Ponram, M.; Ranganathan, S.; Sambath, B.; Cingaram, R.; Iyer, S.K.; Natesan Sundaramurthy, K. Development of Tissue Paper-Based Chemosensor and Demonstration for the Selective Detection of Cu2+ and Hg2+ Ions. RSC Adv. 2023, 13, 26023–26030. [Google Scholar] [CrossRef]
- Souii, A.; Ben M’hadheb-Gharbi, M.; Gharbi, J. Nucleic Acid-Based Biotechnologies for Food-Borne Pathogen Detection Using Routine Time-Intensive Culture-Based Methods and Fast Molecular Diagnostics. Food Sci. Biotechnol. 2016, 25, 11–20. [Google Scholar] [PubMed]
- Shephard, G.S. Chromatographic Determination of the Fumonisin Mycotoxins. J. Chromatogr. A 1998, 815, 31–39. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, G.; Lu, C.; Deng, R.; Zhi, A.; Guo, J.; Zhao, D.; Xu, Z. Rapid Detection of Listeria Monocytogenes in Raw Milk with Loop-Mediated Isothermal Amplification and Chemosensor. J. Food Sci. 2011, 76, M611–M615. [Google Scholar] [CrossRef]
- Ding, S.; Chen, X.; Yu, B.; Liu, Z. Electrochemical Biosensors for Clinical Detection of Bacterial Pathogens: Advances, Applications, and Challenges. Chem. Commun. 2024, 60, 9513–9525. [Google Scholar] [CrossRef]
- Yuan, H.; Lin, J.H.; Dong, Z.S.; Chen, W.T.; Chan, Y.K.; Yeh, Y.C.; Chang, H.T.; Chen, C.F. Detection of Pathogens Using Graphene Quantum Dots and Gold Nanoclusters on Paper-Based Analytical Devices. Sens. Actuators B Chem. 2022, 363, 131824. [Google Scholar] [CrossRef]
- Sharma, P.S.; D’Souza, F.; Kutner, W. Graphene and Graphene Oxide Materials for Chemo- and Biosensing of Chemical and Biochemical Hazards. In Making and Exploiting Fullerenes, Graphene, and Carbon Nanotubes; Springer: Berlin/Heidelberg, Germany, 2013; pp. 237–265. [Google Scholar] [CrossRef]
- Li, Q.Q.; Wen, M.J.; Zhang, Y.S.; Guo, Z.S.; Bai, X.; Song, J.X.; Liu, P.; Wang, Y.Y.; Li, J.L. Multiple Fluorescence Response Behaviors towards Antibiotics and Bacteria Based on a Highly Stable Cd-MOF. J. Hazard. Mater. 2022, 423, 127132. [Google Scholar] [CrossRef]
- Ding, X.; Mauk, M.G.; Yin, K.; Kadimisetty, K.; Liu, C. Interfacing Pathogen Detection with Smartphones for Point-of-Care Applications. Anal. Chem. 2019, 91, 655–672. [Google Scholar] [CrossRef]
- Amadasi, A.; Dall’Asta, C.; Ingletto, G.; Pela, R.; Marchelli, R.; Cozzini, P. Explaining Cyclodextrin–Mycotoxin Interactions Using a ‘Natural’ Force Field. Bioorg. Med. Chem. 2007, 15, 4585–4594. [Google Scholar] [CrossRef]
- Hua, Y.; Ahmadi, Y.; Sonne, C.; Kim, K.H. Progress and Challenges in Sensing of Mycotoxins Using Molecularly Imprinted Polymers. Environ. Pollut. 2022, 305, 119218. [Google Scholar] [CrossRef] [PubMed]
- Azzouz, A.; Kumar, V.; Kim, K.H.; Ballesteros, E.; Rhadfi, T.; Malik, A.K. Advances in Colorimetric and Optical Sensing for Gaseous Volatile Organic Compounds. TrAC Trends Anal. Chem. 2019, 118, 502–516. [Google Scholar] [CrossRef]
- Feller, J.F.; Gatt, N.; Kumar, B.; Castro, M. Selectivity of Chemoresistive Sensors Made of Chemically Functionalized Carbon Nanotube Random Networks for Volatile Organic Compounds (VOC). Chemosensors 2014, 2, 26–40. [Google Scholar] [CrossRef]
- Li, Y.; Huang, X.; Witherspoon, E.; Wang, Z.; Dong, P.; Li, Q. Intelligent Electrochemical Sensors for Precise Identification of Volatile Organic Compounds Enabled by Neural Network Analysis. IEEE Sens. J. 2024, 24, 15011–15022. [Google Scholar] [CrossRef]
- Lawaniya, S.D.; Awasthi, A.; Menezes, P.W.; Awasthi, K. Detection of Foodborne Pathogens Through Volatile Organic Compounds Sensing via Metal Oxide Gas Sensors. Adv. Sens. Res. 2024, 4, 2400101. [Google Scholar] [CrossRef]
- Skorjanc, T.; Shetty, D.; Valant, M. Covalent Organic Polymers and Frameworks for Fluorescence-Based Sensors. ACS Sens. 2021, 6, 1461–1481. [Google Scholar] [PubMed]
- Ghawanmeh, A.A.; Tanash, S.A.; Al-Rawashdeh, N.A.F.; Albiss, B. The Recent Progress on Nanomaterial-Based Chemosensors for Diagnosis of Human Exhaled Breath: A Review. J. Mater. Sci. 2024, 59, 8573–8605. [Google Scholar] [CrossRef]
- Lu, T.; Al-Hamry, A.; Rosolen, J.M.; Hu, Z.; Hao, J.; Wang, Y.; Adiraju, A.; Yu, T.; Matsubara, E.Y.; Kanoun, O. Flexible Impedimetric Electronic Nose for High-Accurate Determination of Individual Volatile Organic Compounds by Tuning the Graphene Sensitive Properties. Chemosensors 2021, 9, 360. [Google Scholar] [CrossRef]
- Song, S.G.; Ha, S.; Cho, H.J.; Lee, M.; Jung, D.; Han, J.H.; Song, C. Single-Walled Carbon-Nanotube-Based Chemocapacitive Sensors with Molecular Receptors for Selective Detection of Chemical Warfare Agents. ACS Appl. Nano Mater. 2019, 2, 109–117. [Google Scholar] [CrossRef]
- Garg, N.; Deep, A.; Sharma, A.L. Metal-Organic Frameworks Based Nanostructure Platforms for Chemo-Resistive Sensing of Gases. Coord. Chem. Rev. 2021, 445, 214073. [Google Scholar] [CrossRef]
- Mirzaei, A.; Leonardi, S.G.; Neri, G. Detection of Hazardous Volatile Organic Compounds (VOCs) by Metal Oxide Nanostructures-Based Gas Sensors: A Review. Ceram. Int. 2016, 42, 15119–15141. [Google Scholar] [CrossRef]
- Kalkal, A.; Kadian, S.; Pradhan, R.; Manik, G.; Packirisamy, G. Recent Advances in Graphene Quantum Dot-Based Optical and Electrochemical (Bio)Analytical Sensors. Mater. Adv. 2021, 2, 5513–5541. [Google Scholar] [CrossRef]
- Jafarzadeh, S.; Yildiz, Z.; Yildiz, P.; Strachowski, P.; Forough, M.; Esmaeili, Y.; Naebe, M.; Abdollahi, M. Advanced Technologies in Biodegradable Packaging Using Intelligent Sensing to Fight Food Waste. Int. J. Biol. Macromol. 2024, 261, 129647. [Google Scholar] [CrossRef]
- Park, S.; Kim, M. Effect of Ammonia on Anaerobic Degradation of Amino Acids. KSCE J. Civ. Eng. 2016, 20, 129–136. [Google Scholar] [CrossRef]
- Dordevic, D.; Capikova, J.; Dordevic, S.; Tremlová, B.; Gajdács, M.; Kushkevych, I. Sulfur Content in Foods and Beverages and Its Role in Human and Animal Metabolism: A Scoping Review of Recent Studies. Heliyon 2023, 9, e15452. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Munekata, P.E.; Dominguez, R.; Pateiro, M.; Saraiva, J.A.; Franco, D. Main Groups of Microorganisms of Relevance for Food Safety and Stability: General Aspects and Overall Description. In Innovative Technologies for Food Preservation; Academic Press: Cambridge, MA, USA, 2017; p. 53. [Google Scholar] [CrossRef]
- Lakshmi, P.R.; Mohan, B.; Kang, P.; Nanjan, P.; Shanmugaraju, S. Recent Advances in Fluorescence Chemosensors for Ammonia Sensing in the Solution and Vapor Phases. Chem. Commun. 2023, 59, 1728–1743. [Google Scholar] [CrossRef]
- Geetha, M.; Anwar, H.; Bhattacharyya, B.; Sadasivuni, K.K. Advanced Chemosensor Techniques for Instantaneous Ammonia Monitoring. Microchem. J. 2024, 203, 110940. [Google Scholar] [CrossRef]
- Guo, Z.; Chen, G.; Zeng, G.; Li, Z.; Chen, A.; Wang, J.; Jiang, L. Fluorescence Chemosensors for Hydrogen Sulfide Detection in Biological Systems. Analyst 2015, 140, 1772–1786. [Google Scholar] [CrossRef]
- Vargas, A.P.; Gámez, F.; Roales, J.; Lopes-Costa, T.; Pedrosa, J.M. A Paper-Based Ultrasensitive Optical Sensor for the Selective Detection of H2S Vapors. Chemosensors 2021, 9, 40. [Google Scholar] [CrossRef]
- Klyamer, D.; Bonegardt, D.; Krasnov, P.; Basova, T.; Maiorova, L. Chemiresistive NH3 and H2S Sensors Based on Thin Films of Vitamin B12 Derivatives. Sens. Actuators B Chem. 2024, 418, 136268. [Google Scholar] [CrossRef]
- Nami, M.; Taheri, M.; Deen, I.A.; Packirisamy, M.; Deen, M.J. Nanomaterials in Chemiresistive and Potentiometric Gas Sensors for Intelligent Food Packaging. TrAC Trends Anal. Chem. 2024, 174, 117664. [Google Scholar] [CrossRef]
- Mafe, A.N.; Edo, G.I.; Makia, R.S.; Joshua, O.A.; Akpoghelie, P.O.; Gaaz, T.S.; Jikah, A.N.; Yousif, E.; Isoje, E.F.; Igbuku, U.A.; et al. A Review on Food Spoilage Mechanisms, Food Borne Diseases and Commercial Aspects of Food Preservation and Processing. Food Chem. Adv. 2024, 5, 100852. [Google Scholar] [CrossRef]
- Lund, P.A.; De Biase, D.; Liran, O.; Scheler, O.; Mira, N.P.; Cetecioglu, Z.; Fernández, E.N.; Bover-Cid, S.; Hall, R.; Sauer, M.; et al. Understanding How Microorganisms Respond to Acid PH Is Central to Their Control and Successful Exploitation. Front. Microbiol. 2020, 11, 556140. [Google Scholar] [CrossRef]
- Marcelli, V.; Osimani, A.; Aquilanti, L. Research Progress in the Use of Lactic Acid Bacteria as Natural Biopreservatives against Pseudomonas Spp. in Meat and Meat Products: A Review. Food Res. Int. 2024, 196, 115129. [Google Scholar] [CrossRef] [PubMed]
- Yi, Z.; Xie, J. Assessment of Spoilage Potential and Amino Acids Deamination & Decarboxylation Activities of Shewanella Putrefaciens in Bigeye Tuna (Thunnus Obesus). LWT 2022, 156, 113016. [Google Scholar] [CrossRef]
- Banik, D.; Manna, S.K.; Maiti, A.; Mahapatra, A.K. Recent Advancements in Colorimetric and Fluorescent PH Chemosensors: From Design Principles to Applications. Crit. Rev. Anal. Chem. 2023, 53, 1313–1373. [Google Scholar] [CrossRef]
- Zhang, Z.; Fan, J.; Du, J.; Peng, X. Two-Channel Responsive Luminescent Chemosensors for Dioxygen Species: Molecular Oxygen, Singlet Oxygen and Superoxide Anion. Coord. Chem. Rev. 2021, 427, 213575. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, Z.; Yong, J.; Schenk, P.M.; Tian, D.; Xu, Z.P.; Zhang, R. Determination and Imaging of Small Biomolecules and Ions Using Ruthenium(II) Complex-Based Chemosensors. Top. Curr. Chem. 2022, 380, 199–243. [Google Scholar] [CrossRef]
- Banerjee, S.; Kuznetsova, R.T.; Papkovsky, D.B. Solid-State Oxygen Sensors Based on Phosphorescent Diiodo-Borondipyrromethene Dye. Sens. Actuators B Chem. 2015, 212, 229–234. [Google Scholar] [CrossRef]
- Shah, N.S.; Thotathil, V.; Zaidi, S.A.; Sheikh, H.; Mohamed, M.; Qureshi, A.; Sadasivuni, K.K. Picomolar or beyond Limit of Detection Using Molecularly Imprinted Polymer-Based Electrochemical Sensors: A Review. Biosensors 2022, 12, 1107. [Google Scholar] [CrossRef] [PubMed]
- Ashley, J.; Wu, K.; Hansen, M.F.; Schmidt, M.S.; Boisen, A.; Sun, Y. Quantitative Detection of Trace Level Cloxacillin in Food Samples Using Magnetic Molecularly Imprinted Polymer Extraction and Surface-Enhanced Raman Spectroscopy Nanopillars. Anal. Chem. 2017, 89, 11484–11490. [Google Scholar] [CrossRef]
- Tu, X.; Chen, W. Miniaturized Salting-Out Assisted Liquid-Liquid Extraction Combined with Disposable Pipette Extraction for Fast Sample Preparation of Neonicotinoid Pesticides in Bee Pollen. Molecules 2020, 25, 5703. [Google Scholar] [CrossRef]
- Upadhyay, Y.; Bothra, S.; Kumar, R.; Sahoo, S.K. Smartphone-Assisted Colorimetric Detection of Cr3+ Using Vitamin B6 Cofactor Functionalized Gold Nanoparticles and Its Applications in Real Sample Analyses. ChemistrySelect 2018, 3, 6892–6896. [Google Scholar] [CrossRef]
- Hu, Q.; Duan, C.; Wu, J.; Su, D.; Zeng, L.; Sheng, R. Colorimetric and Ratiometric Chemosensor for Visual Detection of Gaseous Phosgene Based on Anthracene Carboxyimide Membrane. Anal. Chem. 2018, 90, 8686–8691. [Google Scholar] [CrossRef] [PubMed]
- Quang, D.T.; Kim, J.S. Fluoro- and Chromogenic Chemodosimeters for Heavy Metal Ion Detection in Solution and Biospecimens. Chem. Rev. 2010, 110, 6280–6301. [Google Scholar] [CrossRef]
- Sarkar, H.S.; Ghosh, A.; Das, S.; Maiti, P.K.; Maitra, S.; Mandal, S.; Sahoo, P. Visualisation of DCP, a Nerve Agent Mimic, in Catfish Brain by a Simple Chemosensor. Sci. Rep. 2018, 8, 3402. [Google Scholar] [CrossRef]
- Kaur, A.; Sharma, H.; Kaur, S.; Singh, N.; Kaur, N. A Counterion Displacement Assay with a Biginelli Product: A Ratiometric Sensor for Hg2+ and the Resultant Complex as a Sensor for Cl−. RSC Adv. 2013, 3, 6160–6166. [Google Scholar] [CrossRef]
- Sapozhnikova, Y.; Lehotay, S.J. Evaluation of Different Parameters in the Extraction of Incurred Pesticides and Environmental Contaminants in Fish. J. Agric. Food Chem. 2015, 63, 5163–5168. [Google Scholar] [CrossRef]
- Dragone, R.; Grasso, G.; Muccini, M.; Toffanin, S. Portable Bio/Chemosensoristic Devices: Innovative Systems for Environmental Health and Food Safety Diagnostics. Front. Public Health 2017, 5, 239110. [Google Scholar] [CrossRef]
- Rincón, E.M.; Espinosa, E.; Serrano, L.; Zuliani, A.; Cova, C.M.; Rincón, E.; Espinosa, E.; Serrano, L.; Zuliani, A. Paving the Way for a Green Transition in the Design of Sensors and Biosensors for the Detection of Volatile Organic Compounds (VOCs). Biosensors 2022, 12, 51. [Google Scholar] [CrossRef]
- Qian, R.C.; Long, Y.T. Wearable Chemosensors: A Review of Recent Progress. ChemistryOpen 2018, 7, 118–130. [Google Scholar] [CrossRef] [PubMed]
- Meliana, C.; Liu, J.; Show, P.L.; Low, S.S. Biosensor in Smart Food Traceability System for Food Safety and Security. Bioengineered 2024, 15, 2310908. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chiang, L. Monitoring Chemical Processes Using Judicious Fusion of Multi-Rate Sensor Data. Sensors 2019, 19, 2240. [Google Scholar] [CrossRef]
- Zhang, C.; Bian, T.; Jiao, K.; Su, W.; Wu, K.-J.; Su, A.A.; He, C.; Zhang, C.; Bian, T.; Jiao, K.; et al. A Review on Artificial Intelligence Enabled Design, Synthesis, and Process Optimization of Chemical Products for Industry 4.0. Processes 2023, 11, 330. [Google Scholar] [CrossRef]
- (161a) Sensor Fusion Applications in Industrial Chemical Process Monitoring|AIChE. Available online: https://www.aiche.org/academy/conferences/aiche-spring-meeting-and-global-congress-on-process-safety/2020/proceeding/paper/161a-sensor-fusion-applications-industrial-chemical-process-monitoring?utm_source=chatgpt.com (accessed on 10 January 2025).
- Flores-Cerrillo, J.; He, Q.P.; Wang, J.; Yu, H. Editorial: Smart Manufacturing: Advances and Applications of Artificial Intelligence, Machine Learning and Industrial Internet of Things in the Chemical and Biochemical Industry. Front. Chem. Eng. 2023, 5, 1309165. [Google Scholar] [CrossRef]
- Du, Y.; Yu, D.G.; Yi, T. Electrospun Nanofibers as Chemosensors for Detecting Environmental Pollutants: A Review. Chemosensors 2023, 11, 208. [Google Scholar] [CrossRef]
- Wu, D.; Huang, W.; Lin, Z.; Duan, C.; He, C.; Wu, S.; Wang, D. Highly Sensitive Multiresponsive Chemosensor for Selective Detection of Hg2+ in Natural Water and Different Monitoring Environments. Inorg. Chem. 2008, 47, 7190–7201. [Google Scholar] [CrossRef]
- Gowri, A.; Kathiravan, A. Fluorescent Chemosensor for Detection of Water Pollutants. In Sensors in Water Pollutants Monitoring: Role of Material; Springer: Singapore, 2020; pp. 147–160. [Google Scholar] [CrossRef]
- Martin, M.A.; Olives, A.I.; del Castillo, B.; Menendez, J.C. Trends in the Design and Application of Optical Chemosensors in Pharmaceutical and Biomedical Analysis. Curr. Pharm. Anal. 2008, 4, 106–117. [Google Scholar] [CrossRef]
- Lee, C.; Han, S.; Park, J.H. Electrochemical Detection of Microplastics in Water Using Ultramicroelectrodes. Chemosensors 2024, 12, 278. [Google Scholar] [CrossRef]
- Hu, C.; Wu, K.; Dai, X.; Hu, S. Simultaneous Determination of Lead(II) and Cadmium(II) at a Diacetyldioxime Modified Carbon Paste Electrode by Differential Pulse Stripping Voltammetry. Talanta 2003, 60, 17–24. [Google Scholar] [CrossRef]
- Dai, X.; Nekraseova, O.; Hyde, M.E.; Compton, R.G. Anodic Stripping Voltammetry of Arsenic(III) Using Gold Nanoparticle-Modified Electrodes. Anal. Chem. 2004, 76, 5924–5929. [Google Scholar] [CrossRef] [PubMed]
- Ayerdurai, V.; Cieplak, M.; Kutner, W. Molecularly Imprinted Polymer-Based Electrochemical Sensors for Food Contaminants Determination. TrAC Trends Anal. Chem. 2023, 158, 116830. [Google Scholar] [CrossRef]
- Nadporozhskaya, M.; Kovsh, N.; Paolesse, R.; Lvova, L. Recent Advances in Chemical Sensors for Soil Analysis: A Review. Chemosensors 2022, 10, 35. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Gupta, S.P. Sensors for Waste Management. In Sensors for Environmental Monitoring, Identification, and Assessment; IGI Global Scientific Publishing: Hershey, PA, USA, 2024; pp. 231–250. [Google Scholar] [CrossRef]
- Verdejo, B.; Inclán, M.; Clares, M.P.; Bonastre-Sabater, I.; Ruiz-Gasent, M.; García-España, E. Fluorescent Chemosensors Based on Polyamine Ligands: A Review. Chemosensors 2022, 10, 1. [Google Scholar] [CrossRef]
- Sun, H.; Xiao, K.; Zeng, Z.; Yang, B.; Duan, H.; Zhao, H.; Zhang, Y. Electroactive Biofilm-Based Sensor for Volatile Fatty Acids Monitoring: A Review. Chem. Eng. J. 2022, 449, 137833. [Google Scholar] [CrossRef]
- Mahadeva, S.K.; Walus, K.; Stoeber, B. Paper as a Platform for Sensing Applications and Other Devices: A Review. ACS Appl. Mater. Interfaces. 2015, 7, 8345–8362. [Google Scholar] [CrossRef]
- Ishihara, S.; Azzarelli, J.M.; Krikorian, M.; Swager, T.M. Ultratrace Detection of Toxic Chemicals: Triggered Disassembly of Supramolecular Nanotube Wrappers. J. Am. Chem. Soc. 2016, 138, 8221–8227. [Google Scholar] [CrossRef]
- Iskierko, Z.; Checinska, A.; Sharma, P.S.; Golebiewska, K.; Noworyta, K.; Borowicz, P.; Fronc, K.; Bandi, V.; D’Souza, F.; Kutner, W. Molecularly Imprinted Polymer Based Extended-Gate Field-Effect Transistor Chemosensors for Phenylalanine Enantioselective Sensing. J. Mater. Chem. C Mater. 2017, 5, 969–977. [Google Scholar] [CrossRef]
- Pierre, A.C. The Sol-Gel Encapsulation of Enzymes. Biocatal. Biotransformation 2004, 22, 145–170. [Google Scholar] [CrossRef]
- Babazadeh-Mamaqani, M.; Roghani-Mamaqani, H.; Abdollahi, A.; Salami-Kalajahi, M. Optical Chemosensors Based on Spiropyran-Doped Polymer Nanoparticles for Sensing PH of Aqueous Media. Langmuir 2022, 38, 9410–9420. [Google Scholar] [CrossRef]
- Lee, M.; Palanisamy, S.; Zhou, B.H.; Wang, L.Y.; Chen, C.Y.; Lee, C.Y.; Yuan, S.S.F.; Wang, Y.M. Ultrasensitive Electrical Detection of Follicle-Stimulating Hormone Using a Functionalized Silicon Nanowire Transistor Chemosensor. ACS Appl. Mater. Interfaces 2018, 10, 36120–36127. [Google Scholar] [CrossRef] [PubMed]
- Abou-Melha, K.S. Analytical Chemistry Optical Chemosensor for Spectrophotometric Determination of Nitrite in Wastewater. ChemistrySelect 2020, 5, 6216–6223. [Google Scholar] [CrossRef]
- Mansha, M.; Akram Khan, S.; Aziz, M.A.; Zeeshan Khan, A.; Ali, S.; Khan, M. Optical Chemical Sensing of Iodide Ions: A Comprehensive Review for the Synthetic Strategies of Iodide Sensing Probes, Challenges, and Future Aspects. Chem. Rec. 2022, 22, e202200059. [Google Scholar] [CrossRef] [PubMed]
- Park, J.K.; Shin, J.; Jang, S.; Seol, M.L.; Kang, J.; Choi, S.; Eom, H.; Kwon, O.; Park, S.; Noh, D.Y.; et al. Rational Design of Fluorescent/Colorimetric Chemosensors for Detecting Transition Metal Ions by Varying Functional Groups. Inorganics 2022, 10, 189. [Google Scholar] [CrossRef]
- Gao, Y.Y.; He, J.; Li, X.H.; Li, J.H.; Wu, H.; Wen, T.; Li, J.; Hao, G.F.; Yoon, J. Fluorescent Chemosensors Facilitate the Visualization of Plant Health and Their Living Environment in Sustainable Agriculture. Chem. Soc. Rev. 2024, 53, 6992–7090. [Google Scholar] [CrossRef]
- Sharma, P.; Naithani, S.; Yadav, V.; Sangeeta; Guchhait, B.; Kumar, S.; Goswami, T. Indium Nanocubes Based Recyclable Fluorescent Chemosensor for Sustainable Environmental Monitoring: PH-Induced Fluorescence Transition and Selective Detection of Pd(II) Ions. Sci. Total Environ. 2024, 920, 171043. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, N.; Bai, W.; Bao, Y. Fluorescent Chemosensors Based on Conjugated Polymers with N-Heterocyclic Moieties: Two Decades of Progress. Polym. Chem. 2020, 11, 3095–3114. [Google Scholar] [CrossRef]
- Goshisht, M.K.; Patra, G.K.; Tripathi, N. Fluorescent Schiff Base Sensors as a Versatile Tool for Metal Ion Detection: Strategies, Mechanistic Insights, and Applications. Mater. Adv. 2022, 3, 2612–2669. [Google Scholar] [CrossRef]
- Hasan, M.M.; Hossain, S.; Mofijur, M.; Kabir, Z.; Badruddin, I.A.; Yunus Khan, T.M.; Jassim, E. Harnessing Solar Power: A Review of Photovoltaic Innovations, Solar Thermal Systems, and the Dawn of Energy Storage Solutions. Energies 2023, 16, 6456. [Google Scholar] [CrossRef]
- Li, Z.; Askim, J.R.; Suslick, K.S. The Optoelectronic Nose: Colorimetric and Fluorometric Sensor Arrays. Chem. Rev. 2019, 119, 231–292. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.C.; Steinbrueck, A.; Sedgwick, A.C.; James, T.D. Fluorescent Chemosensors for Ion and Molecule Recognition: The Next Chapter. Front. Sens. 2021, 2, 731928. [Google Scholar] [CrossRef]
- Karuk Elmas, Ş.N.; Berk Gunay, I.; Karagoz, A.; Bostanci, A.; Sadi, G.; Yilmaz, I. A Novel Fluorescent Probe Based on Perylene Derivative for Hg2+ Ions and Biological Thiols and Its Application in Live Cell Imaging and Theoretical Calculations. Electroanalysis 2020, 32, 775–780. [Google Scholar] [CrossRef]
- Sharma, N.; Kaur, N. Fluorenone Appended Colorimetric Sensor for Cascade Detection of Fluoride and Calcium Gluconate with Applications in Solid State and Logic Gate Systems. ChemistrySelect 2023, 8, e202204459. [Google Scholar] [CrossRef]
- Picchetti, P.; Pearce, A.K.; Parkinson, S.J.; Grimm, L.M.; O’Reilly, R.K.; Biedermann, F. Polymersome-Encapsulated Chemosensors: New Design Strategies toward Biofluid-Applicable Cucurbit[7]Uril Indicator Displacement Assays. Macromolecules 2024, 57, 4062–4071. [Google Scholar] [CrossRef]
- Zeng, J.; Xu, X.; Xue, L.; Xu, Y.; Wang, X.; Zhang, Y.; Wang, H. A Novel Pyridine and Julolidine Based Chemosensor for Al3+ Detection. ChemistrySelect 2023, 8, e202204556. [Google Scholar] [CrossRef]
- Helal, A.; Nguyen, H.L.; Al-Ahmed, A.; Cordova, K.E.; Yamani, Z.H. An Ultrasensitive and Selective Metal-Organic Framework Chemosensor for Palladium Detection in Water. Inorg. Chem. 2019, 58, 1738–1741. [Google Scholar] [CrossRef]
- Sivakumar, R.; Lee, N.Y. Paper-Based Fluorescence Chemosensors for Metal Ion Detection in Biological and Environmental Samples. Biochip. J. 2021, 15, 216–232. [Google Scholar] [CrossRef]
- Simeonov, S.; Szekeres, A.; Covei, M.; Stroescu, H.; Nicolescu, M.; Chesler, P.; Hornoiu, C.; Gartner, M. Sol-Gel Multilayered Niobium (Vanadium)-Doped TiO2 for CO Sensing and Photocatalytic Degradation of Methylene Blue. Materials 2024, 17, 1923. [Google Scholar] [CrossRef]
- Pal, S.; Yu, S.S.; Kung, C.W. Group 4 Metal-Based Metal—Organic Frameworks for Chemical Sensors. Chemosensors 2021, 9, 306. [Google Scholar] [CrossRef]
- González-Morales, D.; Valencia, A.; Díaz-Nuñez, A.; Fuentes-Estrada, M.; López-Santos, O.; García-Beltrán, O. Development of a Low-Cost UV-Vis Spectrophotometer and Its Application for the Detection of Mercuric Ions Assisted by Chemosensors. Sensors 2020, 20, 906. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Lee, S.; Zhang, A.; Yoon, J. Self-Immolative Colorimetric, Fluorescent and Chemiluminescent Chemosensors. Chem. Soc. Rev. 2018, 47, 6900–6916. [Google Scholar] [CrossRef]
- Krämer, J.; Kang, R.; Grimm, L.M.; De Cola, L.; Picchetti, P.; Biedermann, F. Molecular Probes, Chemosensors, and Nanosensors for Optical Detection of Biorelevant Molecules and Ions in Aqueous Media and Biofluids. Chem. Rev. 2022, 122, 3459–3636. [Google Scholar] [CrossRef]
- Wang, X.; Yu, J.; Ji, W.; Arabi, M.; Fu, L.; Li, B.; Chen, L. On-Off-On Fluorescent Chemosensors Based on N/P-Codoped Carbon Dots for Detection of Microcystin-LR. ACS Appl. Nano Mater. 2021, 4, 6852–6860. [Google Scholar] [CrossRef]
- Niu, H.; Yang, Y.; Zhang, H. Efficient One-Pot Synthesis of Hydrophilic and Fluorescent Molecularly Imprinted Polymer Nanoparticles for Direct Drug Quantification in Real Biological Samples. Biosens. Bioelectron. 2015, 74, 440–446. [Google Scholar] [CrossRef] [PubMed]
- MacAgnano, A.; Perri, V.; Zampetti, E.; Bearzotti, A.; De Cesare, F. Humidity Effects on a Novel Eco-Friendly Chemosensor Based on Electrospun PANi/PHB Nanofibres. Sens. Actuators B Chem. 2016, 232, 16–27. [Google Scholar] [CrossRef]
- Kumarasinghe, K.G.U.R.; Silva, A.U.T. De Indole-Based Fluorometric and Colorimetric Chemosensors for the Selective Detection of Cu2+: A Brief Review From the Year 2011–2021. Vidyodaya J. Sci. 2023, 1, s1. [Google Scholar] [CrossRef]
- Kimoto, H.; Takahashi, M.; Masuko, M.; Sato, K.; Hirahara, Y.; Iiyama, M.; Suzuki, Y.; Hashimoto, T.; Hayashita, T. High-Throughput Analysis of Bacterial Toxic Lipopolysaccharide in Water by Dual-Wavelength Monitoring Using a Ratiometric Fluorescent Chemosensor. Anal. Chem. 2023, 95, 12349–12357. [Google Scholar] [CrossRef]
- Mishra, G.K.; Barfidokht, A.; Tehrani, F.; Mishra, R.K. Food Safety Analysis Using Electrochemical Biosensors. Foods 2018, 7, 141. [Google Scholar] [CrossRef]
- Zhuang, Y.T.; Chen, S.; Jiang, R.; Yu, Y.L.; Wang, J.H. Ultrasensitive Colorimetric Chromium Chemosensor Based on Dye Color Switching under the Cr(VI)-Stimulated Au NPs Catalytic Activity. Anal. Chem. 2019, 91, 5346–5353. [Google Scholar] [CrossRef]
- Radwan, A.; El-Sewify, I.M.; Shahat, A.; Azzazy, H.M.E.; Khalil, M.M.H.; El-Shahat, M.F. Multiuse Al-MOF Chemosensors for Visual Detection and Removal of Mercury Ions in Water and Skin-Whitening Cosmetics. ACS Sustain. Chem. Eng. 2020, 8, 15097–15107. [Google Scholar] [CrossRef]
- Abd El-Fattah, W.; Guesmi, A.; Ben Hamadi, N.; Khalil, M.A.; Shahat, A. A Highly Sensitive Chemosensor Based on a Metal-Organic Framework for Determining Zinc Ions in Cosmetics Creams and Wastewater. Appl. Organomet. Chem. 2024, 38, e7407. [Google Scholar] [CrossRef]
- Rauytanapanit, M.; Suraritdechachai, S.; Vilaivan, T.; Praneenararat, T. Introducing Students to Chemical Security Concepts through Interdisciplinary Experiments Using Organic Chemosensors in Engaging Applications of Chemistry. J. Chem. Educ. 2020, 97, 1779–1788. [Google Scholar] [CrossRef]
- Calabria, D.; Guardigli, M.; Severi, P.; Trozzi, I.; Pace, A.; Cinti, S.; Zangheri, M.; Mirasoli, M. A Smartphone-Based Chemosensor to Evaluate Antioxidants in Agri-Food Matrices by in Situ AuNP Formation. Sensors 2021, 21, 5432. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).