Abstract
Nonspecific adsorption (NSA) impacts the performance of biosensors in complex samples. Coupled electrochemical–surface plasmon resonance biosensors (EC-SPR) offer interesting opportunities to evaluate NSA. This review details the main solutions to minimize fouling in electrochemical (EC), surface plasmon resonance (SPR) and EC-SPR biosensors. The discussion was centered on blood, serum and milk as examples of complex matrices. Emphasis was placed on antifouling coatings, NSA evaluation protocols and universal functionalization strategies to obtain antifouling biosensors. In the last 5 years, various antifouling coatings were developed for EC biosensors, including new peptides, cross-linked protein films and hybrid materials. Due to the comparatively much more scarce literature, for SPR and EC-SPR biosensors the discussion was extended to the early 2010s. The analysis revealed a wide range of antifouling materials with tunable conductivity, thickness and functional groups that can be tested in the future with EC-SPR. The high-throughput screening of new materials, molecular simulations and machine learning-assisted evaluations will even further widen the range of antifouling materials available for biosensors. The minimization of NSA’s impact on the analytical signal is moreover facilitated by unique sensing mechanisms associated with the bioreceptor or the particularities of the detection method. It is hoped that this review will encourage research in the field of EC-SPR biosensors.
1. Introduction
Nonspecific adsorption (NSA) is a major barrier to the widespread adoption of biosensors, along with the limited stability of biorecognition elements and the lack of selectivity of the signal transduction step. This is emphasized also by the large research efforts made to address biosensor fouling and the number of reviews, including in the recent period [1,2,3,4,5,6]. NSA refers to the accumulation of species other than the analyte of interest on the biosensing interface, and impacts most analytical characteristics of biosensors (e.g., signal stability, selectivity, sensitivity and accuracy). These problems grow with the complexity of the analyzed sample and when the concentration of the species other than the analyte of interest increases. Various physical and chemical approaches to combatting NSA have been proposed. The resistance to fouling should be adapted to particular static or hydrodynamic operational conditions, different lengths of time, and to samples with various pH levels and ionic strengths and complex compositions, including proteolytic enzymes, fats, high protein concentrations, etc. Moreover, the antifouling coatings have to meet specific requirements of thickness and conductivity in relation to the method used for signal transduction in biosensors. The NSA and the efficacy of antifouling coatings can be studied by a variety of methods. Compared to single detection procedures, coupled EC and optical methods enable us to achieve larger detection ranges, improve spatial resolution, and acquire more detailed information on interfacial, catalytic and affinity binding events. Among the coupled detection methods, the combination of electrochemistry and surface plasmon resonance (SPR) was extensively explored. The efficient addressing of NSA in coupled EC-SPR methods requires solutions that, beside antifouling protection, ensure adequate conductivity (for the EC component), thickness (for the SPR component) and bioreceptor loading capacity (for both components). Despite the high number of scientific studies dedicated to addressing fouling in biosensors in general or specifically in EC [1,2,7,8,9,10,11,12,13] and SPR methods [14,15,16], there is not yet a systematized overview of the most successful solutions developed so far for combined EC and SPR methods. Readers interested in the potential of EC-SPR are directed to a handful of reviews that contain detailed information on the analytical capabilities enabled by the coupling of the two detection methods, including briefly investigated NSA aspects [17,18,19,20].
In the following, we first detail the impact of NSA on biosensors (Section 2.1), and introduce the mechanisms by which NSA occurs and how it can be counteracted (Section 2.2). The methods and quantitative tools used to evaluate NSA are presented (Section 2.3), as they serve in the design and efficacy assessment of new antifouling strategies. Moreover, the general experimental workflow of evaluating NSA in biosensors is detailed while cautioning about the limitations induced by superficial protocols (Section 2.4). This prologue leads to the main discussion of the strategies for preventing NSA in biosensors, with a focus on promising solutions that have been applied for the analysis of complex samples by EC (Section 3), SPR (Section 4) and combined EC-SPR biosensors (Section 5). The discussion of EC-SPR biosensors concentrates on studies wherein real samples of serum, or drug–protein interactions and relevant foulant proteins, were analyzed. It extends back to early 2000, given the limited number of available studies. For SPR and EC in particular, for which richer data are available, the most promising antifouling solutions identified from the last 10 (for SPR) or 5 years (for EC) are synthetically presented. The discussion mainly includes examples of the analysis of blood and serum (addressing the clinical field) and milk (representative for applications in food safety and quality assessment). This selection is motivated by the fact that liquid samples such as blood and serum are amenable to minimal, fast sample preparation, thus maximizing the impact of the biosensor in the overall analysis. Biosensors used for the analysis of serum and milk have also a high potential for translation into commercial devices.
2. NSA in Biosensors
2.1. Contribution of NSA to Biosensor Signal
NSA, or “fouling”, refers to the accumulation of species other than the analyte of interest on the biosensing interface. The impacts of fouling on biosensors are manifold; however, they can be simplified as follows: (1) the signal caused by non-specifically adsorbed molecules interferes with or largely outweighs the signal resulting from the specific biorecognition event, and (2) the adsorption of foulant molecules affects the ability of the bioreceptor to bind the target analyte, i.e., the specific signal. The latter occurs either when the ability of the bioreceptor to change its conformation becomes limited or when the access of the analyte of interest to the bioreceptor is difficult, causing false negatives at low analyte concentrations.
To exemplify this, the cases of an electrochemical aptamer-based (E-AB) biosensor, an immunosensor with detection by SPR and an electrochemical enzyme biosensor are depicted in Figure 1A–C.
In EC biosensors, fouling has dramatic effects on the characteristics of the sensing interface and on the rate of electron transfer at the electrode surface. It may also affect the ability of the bioreceptor to bind its specific analyte. For example, non-specifically adsorbed molecules may restrict the ability of structure-switching aptamers to undergo the large conformational change required for binding the target analyte and for producing a specific EC signal [21]. Adsorbed molecules may passivate the biosensor and, in time, cause the degradation of the biosensor coating layer, leading to more NSA (Figure 1A). NSA can show up as a signal drift that complicates the interpretation of the analytical signal, as it imposes the use of an adequate background correction procedure. Over short time spans, the contribution of NSA to the biosensor signal might be negligible due to the intrinsic detection mechanism, the implementation of drift correction measures, or a combination of these [22,23]. Nonetheless, over longer times, the progressing fouling leads to a significant degradation of the biosensor surface, which can no longer be addressed by correction algorithms (as further discussed in Section 3.2).
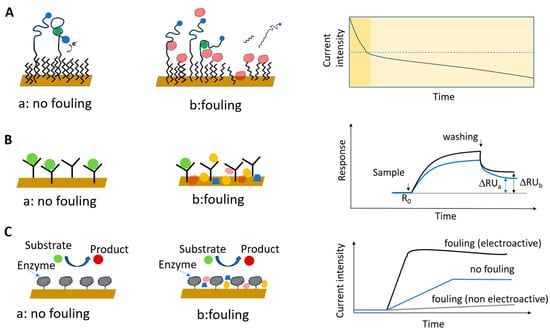
Figure 1.
Impact of fouling on the analytical signal of biosensors, illustrated for (A) an E-AB biosensor, showing the signal degradation in time, manifested as sensor drift, due to fouling and the dissolution of the coating layer, (B) an immunosensor with detection by SPR and (C) an EC enzyme biosensor. Redrawn in part from [21,24] (A). Details are given in the text.
Figure 1.
Impact of fouling on the analytical signal of biosensors, illustrated for (A) an E-AB biosensor, showing the signal degradation in time, manifested as sensor drift, due to fouling and the dissolution of the coating layer, (B) an immunosensor with detection by SPR and (C) an EC enzyme biosensor. Redrawn in part from [21,24] (A). Details are given in the text.
The adsorption of foulant molecules and the specific binding of the target analyte may lead to similar changes in the reflectivity measured with an SPR biosensor (Figure 1B). Thus, NSA contributes to the amplitude of the analytical signal, compromising its correlation with the concentration of the target analyte.
Similarly, in the case of an EC enzyme biosensor, the EC transformation of adsorbed sample components may mask the signal originating from the enzymatic reaction. Alternatively, adsorbed, passivating molecules or those interfering with the enzymatic reaction by inhibition or steric effects may lead to an underestimation of the amount of analyte of interest in the sample (Figure 1C).
An important distinction has to be made between the actual NSA and its impact on the measurable analytical signal. The perceived fouling is strictly related to the sensitivity of the method by which it is evaluated. Therefore, the dimension of NSA is better illustrated by a combination of analytical methods than by a single method [25].
2.2. Mechanisms of NSA and Ways to Counteract It
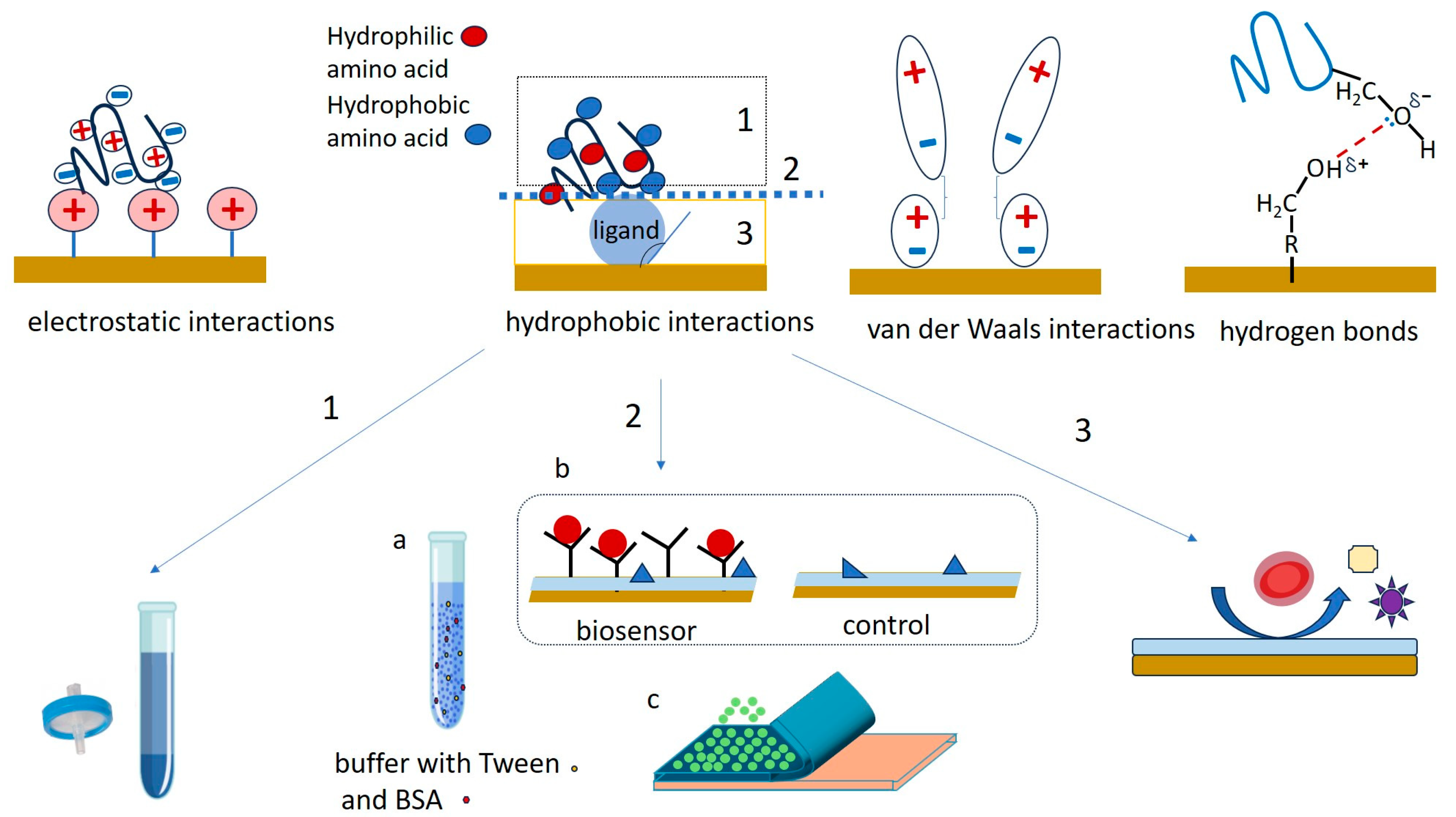
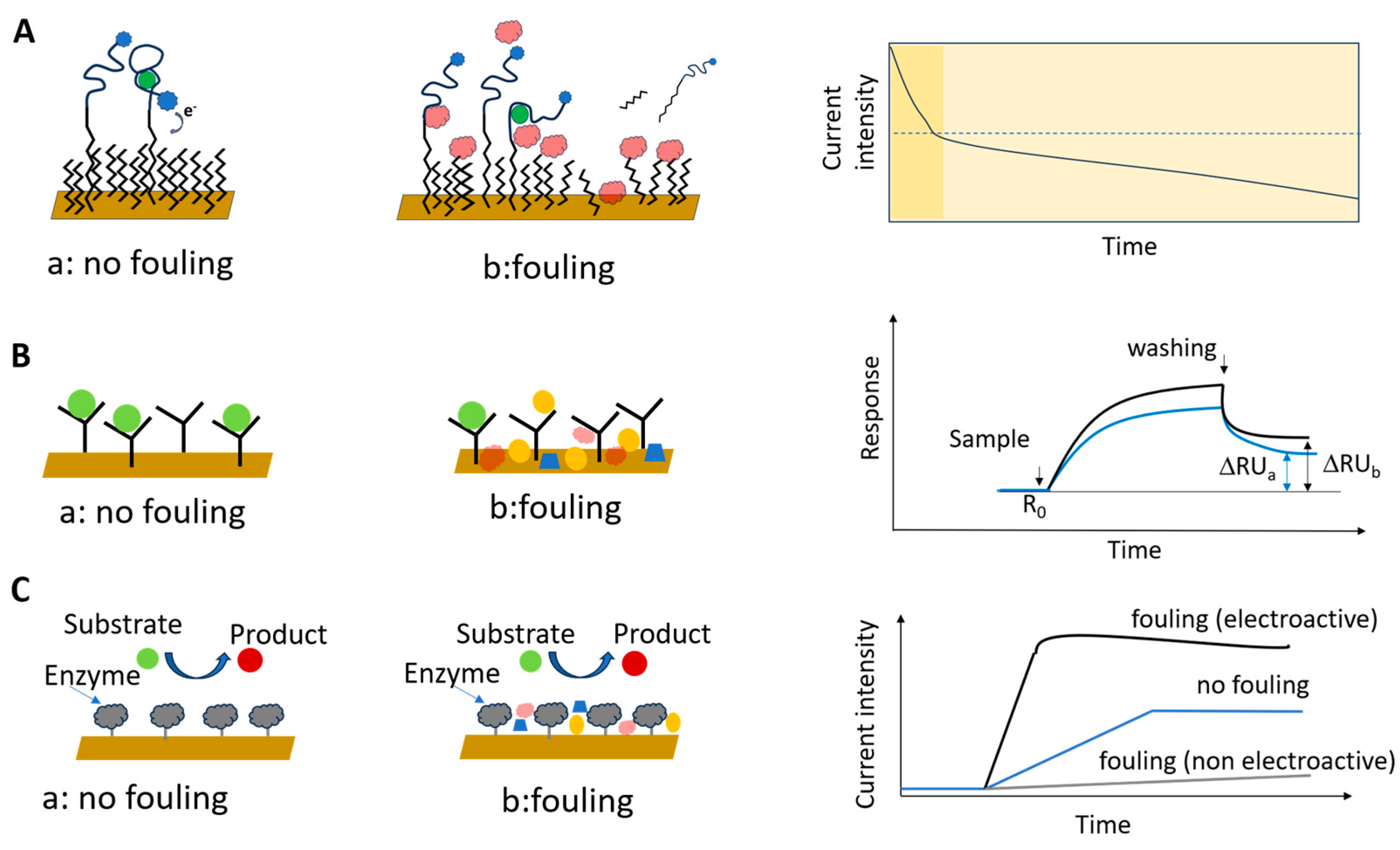
The accumulation of non-target sample components on biosensors is most often due to physical adsorption, facilitated by combinations of electrostatic interactions, hydrophobic interactions, hydrogen bonds (or other dipole–dipole interactions), and van de Walls interactions between the interface and the components of the sample matrix (Figure 2).
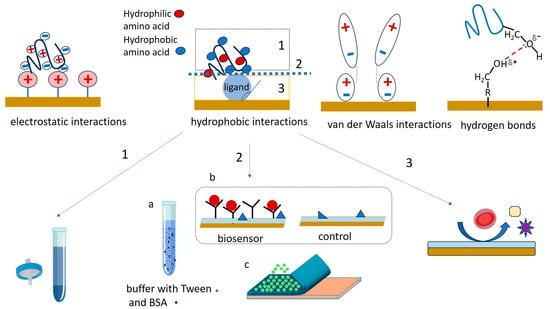
Figure 2.
Mechanisms of NSA in biosensors and the main strategies used to minimize fouling by addressing (1) sample preparation, (2) the interaction between the sample components and the biosensor interface and (3) the properties of the (coated) sensing interface. Shown are the reduction in NSA by centrifugation or filtration of the investigated sample (1, bottom left), supplementing the sample with salts, detergents and/or proteins (2a, bottom center), using reference sensors lacking biorecognition elements (2b, bottom center), using a sacrificial layer that is removed together with fouling species (2c, bottom center) and appropriate modification of the surface of the sensor with species able to repel fouling species (3, bottom right).
Understanding the contributions to NSA and minimizing the impact of NSA on the biosensor signal represent a complex, multilayered initiative that has to address (1) the foulant-containing sample, (2) the interaction between the sample matrix and the interface, and (3) the nature and coating of the biosensor surface (Figure 2). This initiative is related to the intended purpose of the biosensor and to the operational setup. For example, it has to consider whether the biosensor will be operating under static or hydrodynamic conditions, in vivo or in vitro, for single use or for repetitive measurements, if the measurement protocol involves washing steps, etc. Minimizing NSA starts most often with sample preparation steps aiming to reduce the chemical complexity of the sample. This typically involves centrifugation (e.g., for reducing the fat content of milk, to obtain serum from blood, etc.), dilution and filtration (Figure 2, “1”). To break the interaction between the sample matrix and the biosensing interface, the buffer used for sample and standards preparation can be enriched with surfactants, salts and proteins (Figure 2, “2a”). Moreover, the sensing platform may include reference (“control”) channels, spots or sensors lacking the bioreceptor, which evaluate the NSA along with the impacts of other experimental parameters, such as temperature, pH, etc. (Figure 2, “2b”). Signals of such reference channels, spots or sensors are used to correct the signals of the actual biosensor, most often by subtraction. This is only possible when the adsorption of fouling molecules is kept to a reasonably low level, compared to the binding of the specific analyte. Alternatively, the biosensor is coated with “transient” layers, onto which the adsorption of sample components is allowed (Figure 2, “2c”). The transient coatings are conveniently washed away or dissolved at a pre-determined time [26,27]. Finally, the ultimate way to protect the biosensor against NSA is to modulate its properties to adequately repel foulants (Figure 2, “3”). To this end, the surface topography may be optimized, and the diffusion of foulants towards the sensor surface can be restricted by membranes or liquid filters, taking advantage of microfluidics. The biosensing interface can be coated, in addition to the biorecognition elements, with layers of chemical species that increase the resistance of the biosensing interface to NSA. Targeting the specific processes by which NSA occurs, these “permanent”, passive, coatings achieve efficient protection against the adherence of non-target molecules by a combination of three mechanisms—the formation of a hydration layer, steric hindrances, and electrostatic repulsion [4] (Figure 3).
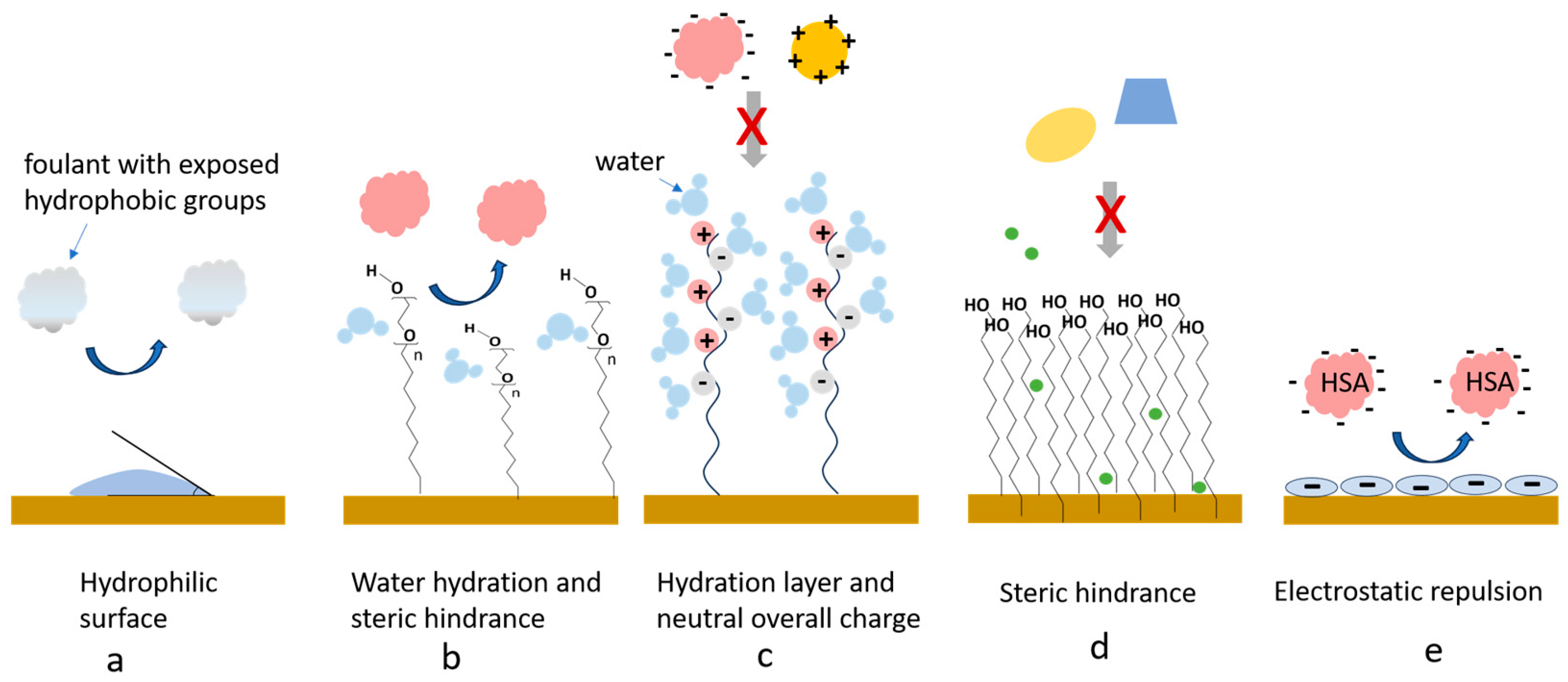
Figure 3.
The main mechanisms of counteracting NSA with antifouling coatings, illustrated for (a) a hydrophilic surface, (b) a polyethylene glycol (PEG)-coated surface, (c) a surface coated with a zwitterionic polymer, (d), a surface coated with a dense alkanethiol self-assembled monolayer (SAM) and (e) a negatively charged surface, e.g., coated with a layer of cross-linked bovine serum albumin (cBSA). Details are given in the text.
Hydrophilic biosensing surfaces discourage hydrophobic interactions and the adsorption of unwanted sample components such as partially or totally denatured proteins (Figure 3a). Most of the fouling-resistant interfaces have water contact angles lower than 25°, displaying good hydrophilicity.
A structured hydration layer is formed as result of ion solvation at the interface between the sensing surface and the sample solution. It provides an efficient barrier, repelling the foulants as these are unable to displace the strongly bound water molecules. Tight hydration layers are formed, e.g., by PEG and oligoethyleneglycol (OEG) polymers and by zwitterionic materials (Figure 3b,c).
Steric hindrances refer to the physical limitation of the diffusion of molecules towards the sensor’s surface as they have to pass through dense layers of tightly packed molecules or long chains. They arise, for example, from the compression of PEG and OEG-derivative chains, as proteins reaching the vicinity of the surface are attracted via van de Waals interaction towards the PEG/OEG coating (e.g., [28], Figure 3b). Additionally, alkanethiols with longer chain (eight or more C atoms) form stable SAMs, stabilized by the interactions between the neighboring molecules. Dense and thick coatings induce stronger steric effects (Figure 3d). However, as along with the unwanted, large-sized foulants, the access of small signaling probes or target molecules to the sensor surface is also reduced to some extent. Therefore, the composition, thickness and density of the coatings should be optimized for each application.
Proteins and other fouling molecules in the sample carry various electrical charges, and may in consequence adhere onto sensing interfaces by electrostatic interactions. An overall neutral interface minimizes electrostatic interactions [29]. Alternatively, e.g., for biosensors operating at neutral pH in serum, containing 40–60 mg/mL of negatively charged human serum albumin (HSA), coatings based on BSA were also shown to be very efficient in minimizing NSA. The electrostatic repulsion between BSA and HSA (both negatively charged at neutral pH) contributes to this effect (Figure 3e).
Often, the combination of several approaches, including those using antifouling coatings, adequate sample preparation and reference (“control”) interfaces, is required to ensure accurate measurements with the biosensors. The present review will focus on interface modifications to reduce NSA in cases of EC, SPR and EC-SPR biosensors. As we will show, few of the interface modifications employed to reduce NSA work well enough to facilitate quantitative analyses of unprocessed or minimally processed samples.
2.3. Methods Used to Evaluate NSA
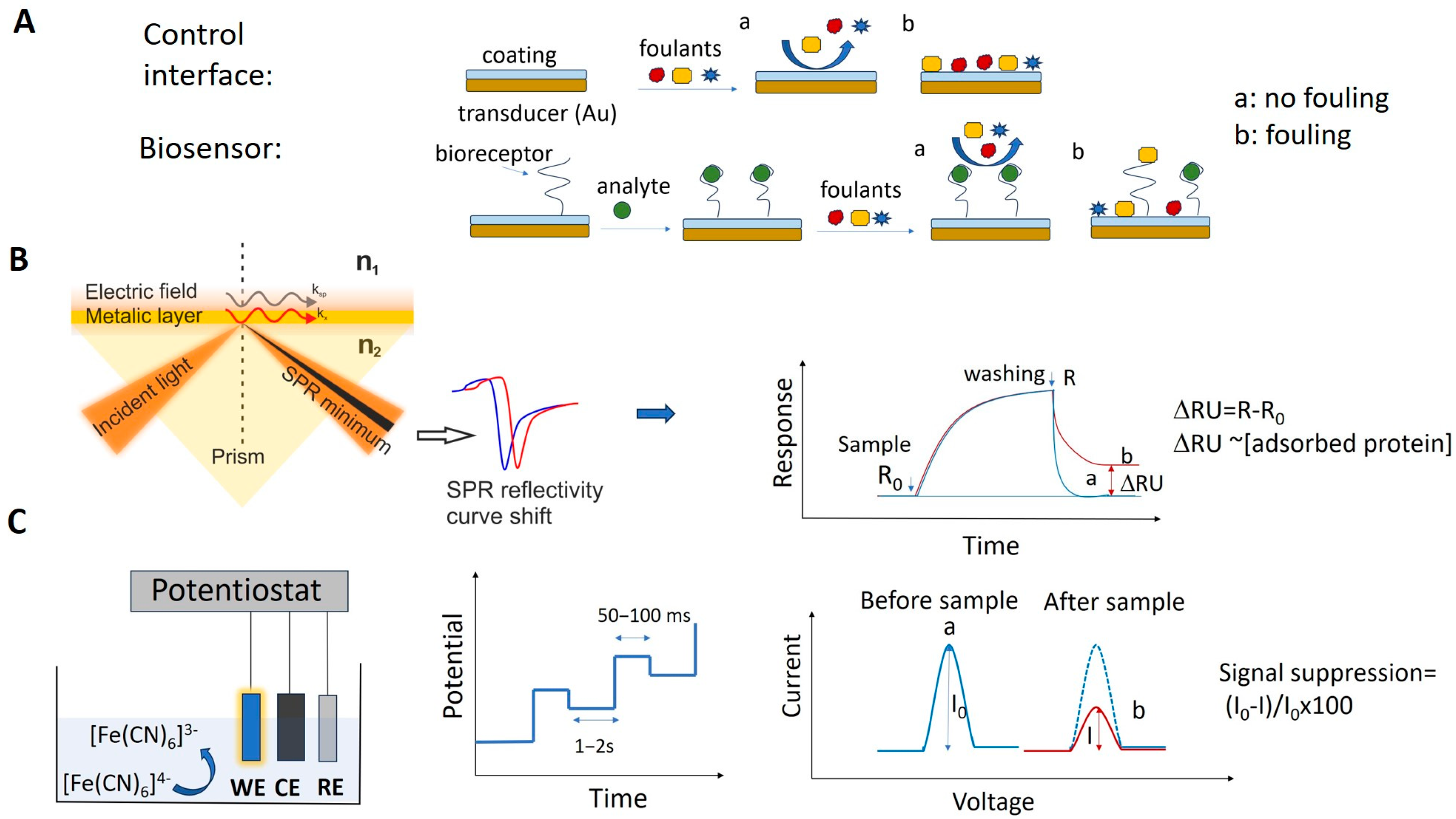
Among the biosensors used to analyze real samples, there are a few “no wash”, reagentless devices that can be used for the real-time monitoring of blood or other complex matrices. These E-AB biosensors, discussed further in Section 3.2, represent the exception. Most biosensors used for the analysis of complex biological fluids or food samples are designed for discrete measurements. Their operation includes incubation with the sample solution for a specified time, followed by washing with buffer and measuring the analytical signal. The changes in the optical, EC or mass-related properties of the biosensors after incubation with the sample are quantitatively correlated with the concentration of target analyte in the sample. The contribution of NSA to the analytical signal is determined in the same way and by the same analytical method as is used to evaluate the biosensor signal, but measures are taken to discount any specific signal due to the target analyte. This can be achieved, e.g., by using control interfaces, i.e., those lacking the bioreceptor, or by first pre-saturating the biosensor with the target molecule, before exposing it to foulants (Figure 4A).
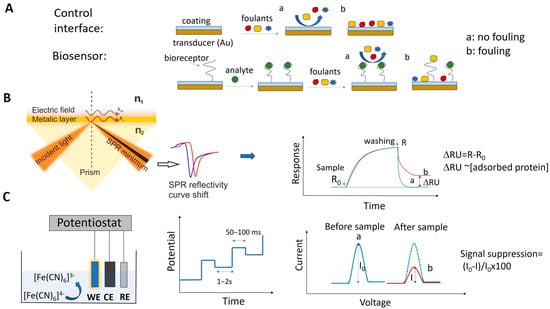
Figure 4.
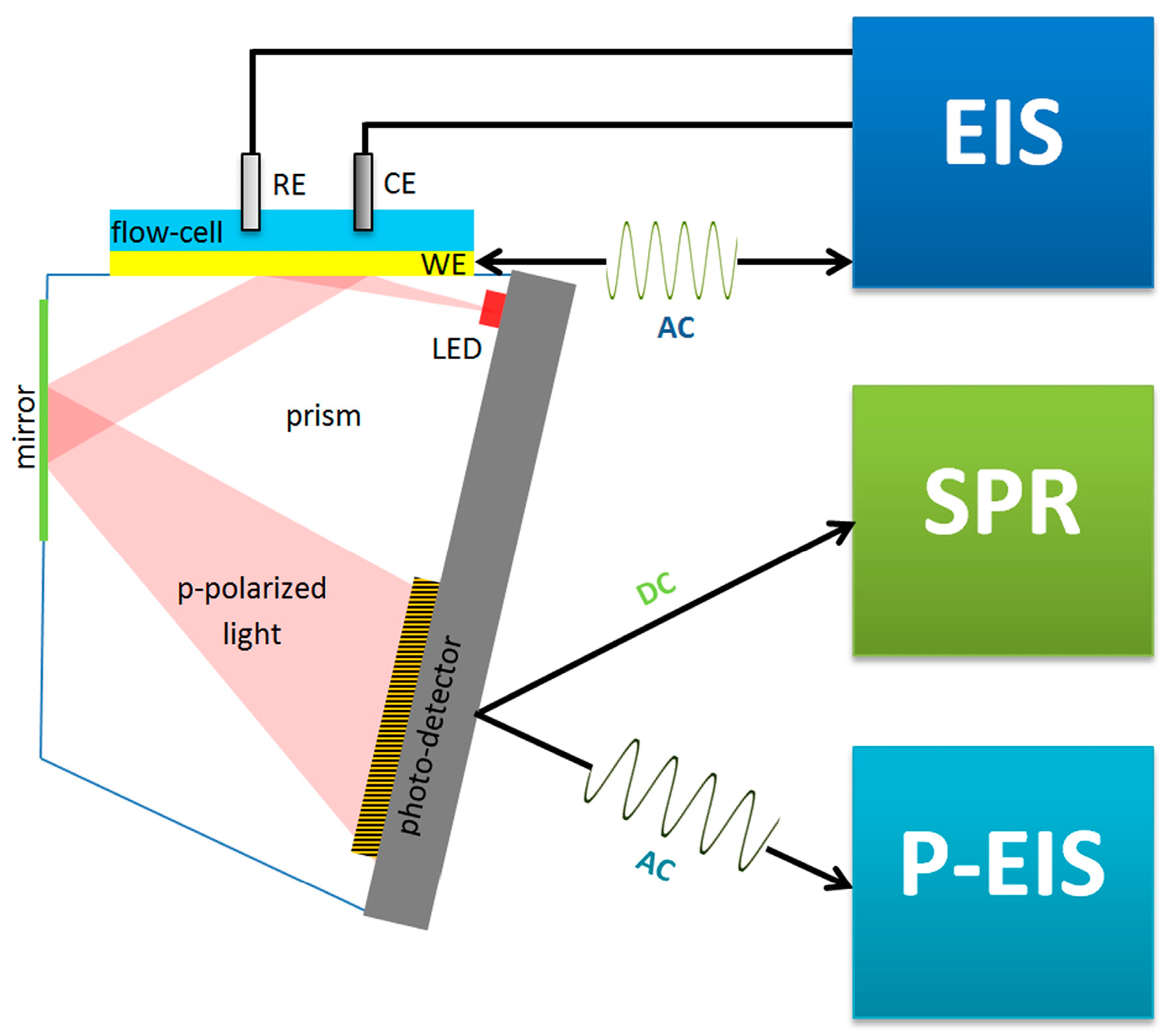
(A) Schematic illustration of the fouling testing process with biosensor and control coated interface. (B) Typical SPR experimental setup based on the Kretschmann configuration and sensorgrams showing the signal change in SPR due to NSA from sample solution. (C) Typical setup used with EC biosensors and a frequently used way to measure the NSA by differential pulse voltammetry (DPV) using ferrocyanide/ferricyanide. Representation of the potential pulses applied in DPV and of the measured change in the EC signal (“signal suppression”) resulting from the electrode fouling.
It is important to note that some NSA always occurs, even with so called “non-fouling” interfaces. The adsorbed molecules are detected with different degrees of sensitivity by the various analytical methods.
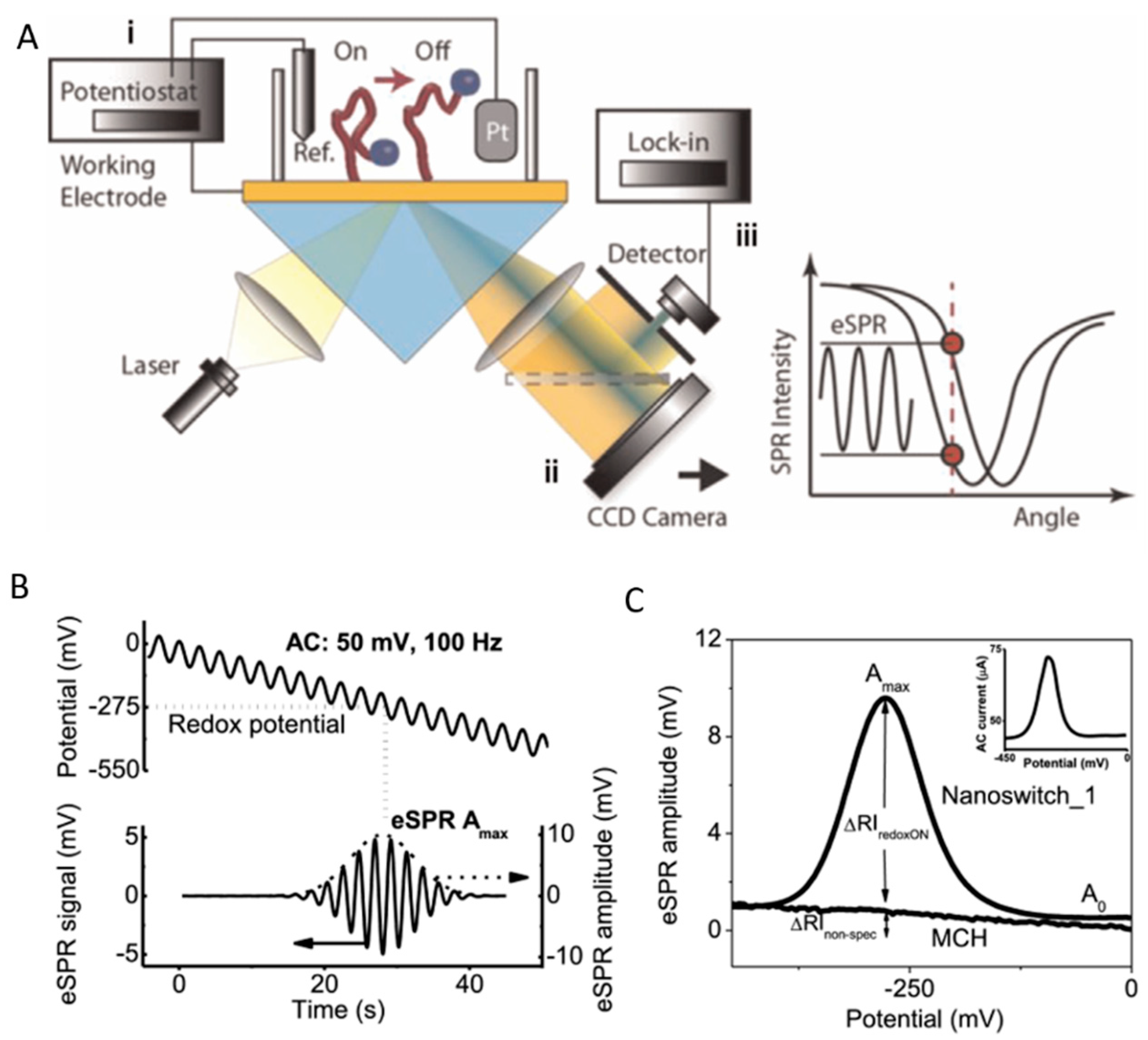
SPR biosensing is representative of a powerful class of surface-sensitive techniques that enable the trace and ultra-trace detection of various analytes through affinity pairing [30]. This affinity pairing must take place in the close vicinity of light-excited plasmons that are an integral part of the biosensor. These collective electron oscillations occur in metals at nanoscale as either propagating surface plasmons (SPs) that travel along special metal–dielectric interfaces, or as localized surface plasmons (LSPs) that are confined to the surface of metallic nanostructures. The electromagnetic field corresponding to both SPs and LSPs is localized at the surface and decays exponentially into the ambient medium with half-lives of below 300 nm and 10 nm, respectively, making SPR-based techniques more attuned to changes occurring close to plasmonic interfaces.
SPR detects, with high sensitivity, minute changes in the refractive index at the interface between a thin metallic layer (typically ~50 nm of Au) and a sample. The SPR signal is not only related to specific binding events, but also to adsorption/desorption phenomena, conformational changes, etc., occurring in close proximity to the surface.
Many SPR biosensors rely on the Kretschmann configuration (Figure 4B), whereby a metal film with plasmonic properties is evaporated onto a high-index prism, and monochromatic light is directed to the metal surface at several incidence angles.
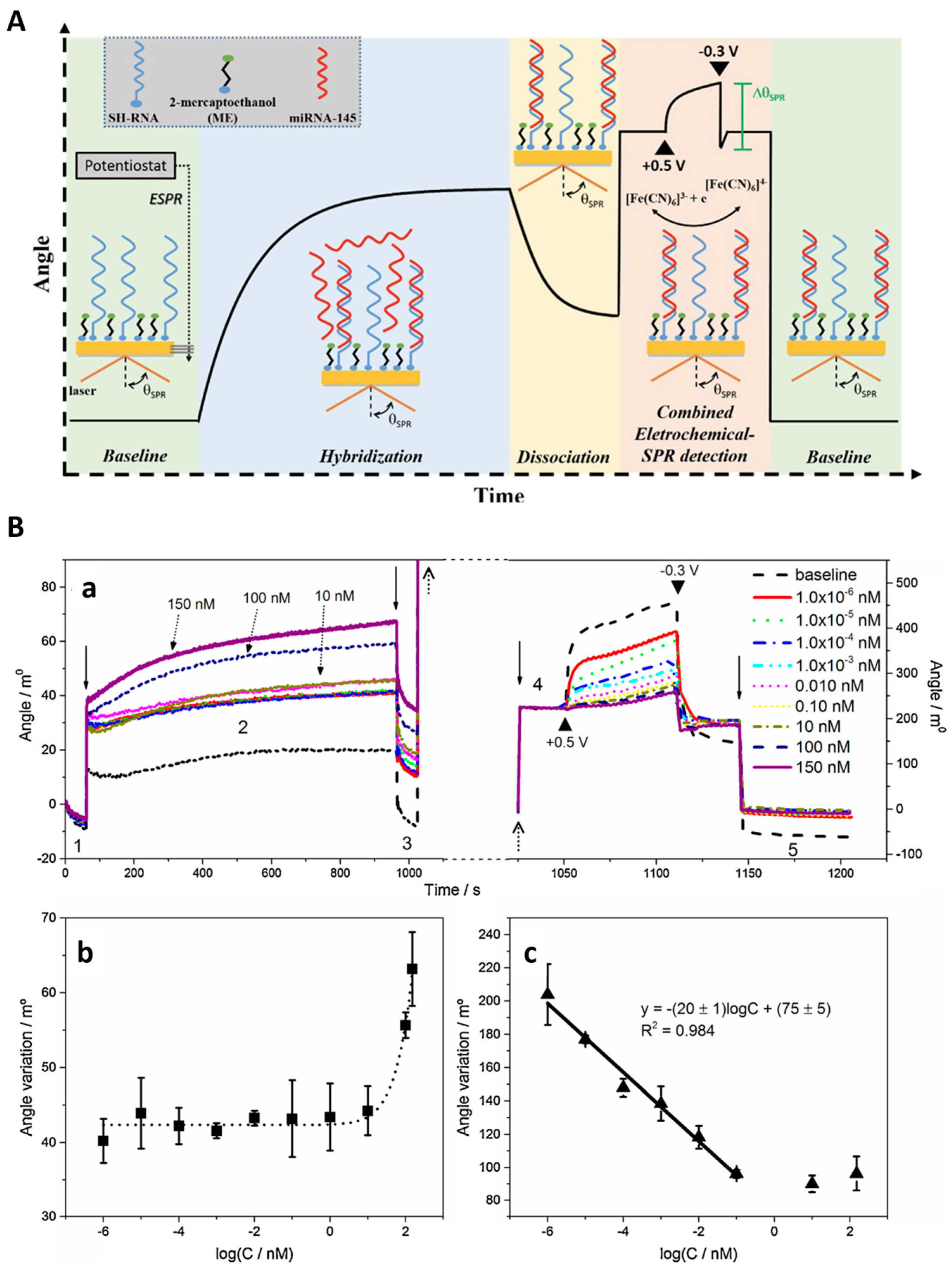
The ligand molecules are immobilized on the metal surface, while the molecules of the target analyte are typically dispersed in a mobile phase. In this classical format, the intensity of the incident light that is reflected from the interface drops, producing the SPR “dip” due to resonant coupling with surface plasmons modulated by local effective refractive index characteristics. Several factors, such as the prism adopted, the wavelength of the incident light, the metal type and the ligands, determine the precise angular location of the reflectance dip. Upon affinity pairing, chemical reaction, adsorption/desorption, temperature [31], pH [32] or NSA processes, the associated SPR angle shifts as a function of local effective refractive index changes. This may be tracked in real-time, as typically evidenced by sensorgrams.
In biosensors, SPR allows us to monitor in real time and in a label-free way the steps of sample testing (incubation and washing), and to evaluate the binding kinetics and the residual amounts of surface-bound material (Figure 4B). The resulting variation in the SPR parameters (typically, the shift in minimum SPR angle or the shift in wavelength, depending on the system) can be quantitatively expressed as an amount of protein adsorbed per surface unit. The sensitivity of this correlation depends on the experimental setup. For improved clarity, we will restrict the focus to the particular case of optical sensors based on propagating SPR, and to angle-resolved ways to achieve label-free detection, as powerful information complementary to other, more general recent reviews on plasmonic nanosensors [33,34]. For example, in one experimental setup, a 1 nm shift corresponded to a surface coverage of 17 ng/mm2, and the detection limit was 0.3 ng/mm2 of adsorbed protein [35].
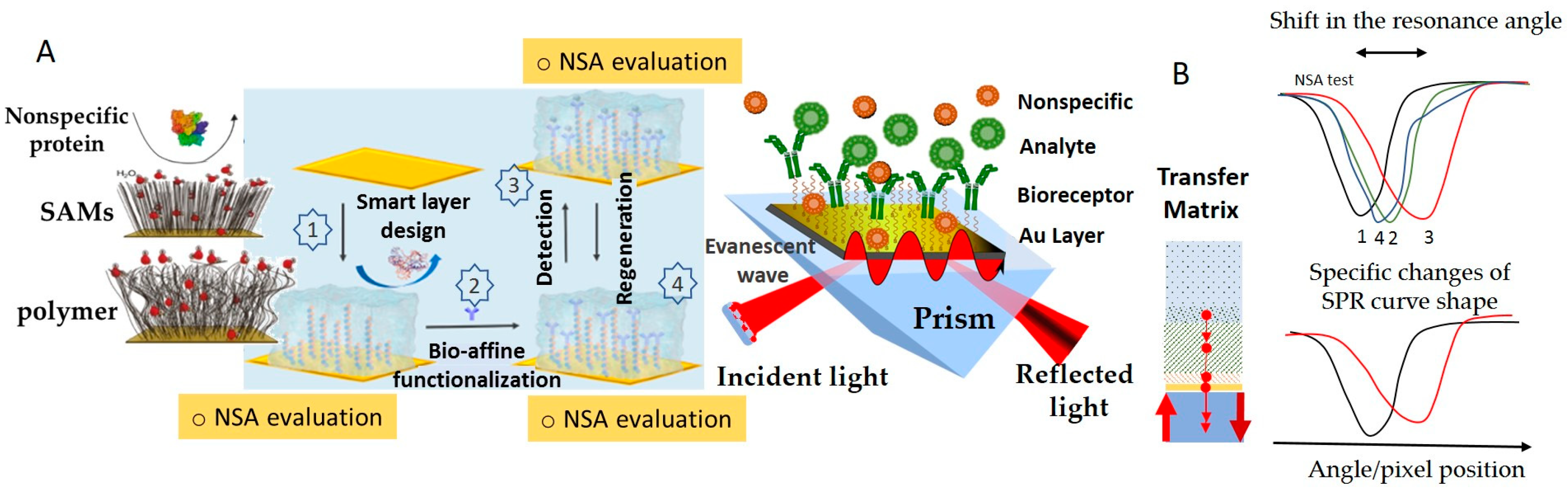
SPR is one of the methods that is most widely used to evaluate NSA, allowing the user to compare the antifouling capacities of various coatings based on quantitative protein adsorption data. Moreover, it heavily assists in the efforts directed at the development of appropriate strategies for tailored surface design [16,29,30,36,37]. SPR detection is characterized by the exponential decay of the evanescent plasmonic field. The evanescent field makes the layers closer to the plasmonic surface better-performing in terms of detection sensitivity than the more distant layers. This in turn imposes strict limits on the thickness of all intermediary layers above the SPR metallic sensor surface, down to minima customized to adapt to the overall thickness of the SPR sensing layer estimated at the level of a few hundreds of nanometers (~37% of SPR wavelength). Accordingly, the layers with a thickness ranging from 30 to 70 nm, usually polymeric, which are typically deposited on the active surfaces of biosensors to introduce antifouling properties, are seldom appropriate for SPR detection. The custom design of SPR biosensors, supported by Transfer Matrix computations, involves the selection of target thicknesses and optical properties of all functionalization layers, and even of the plasmonic layer structure. SPR was also used to show how the functionalization with biorecognition elements affects the biorecognition capacity and the resistance to the NSA of low-fouling coatings [38], to compare coatings obtained by different grafting procedures [39], etc. Adsorbed amounts of less than a few ng/cm2 indicate good resistance to NSA, and for some coatings exposed to undiluted complex real samples, the adsorbed amounts were even lower than 1 ng/cm2 [40].
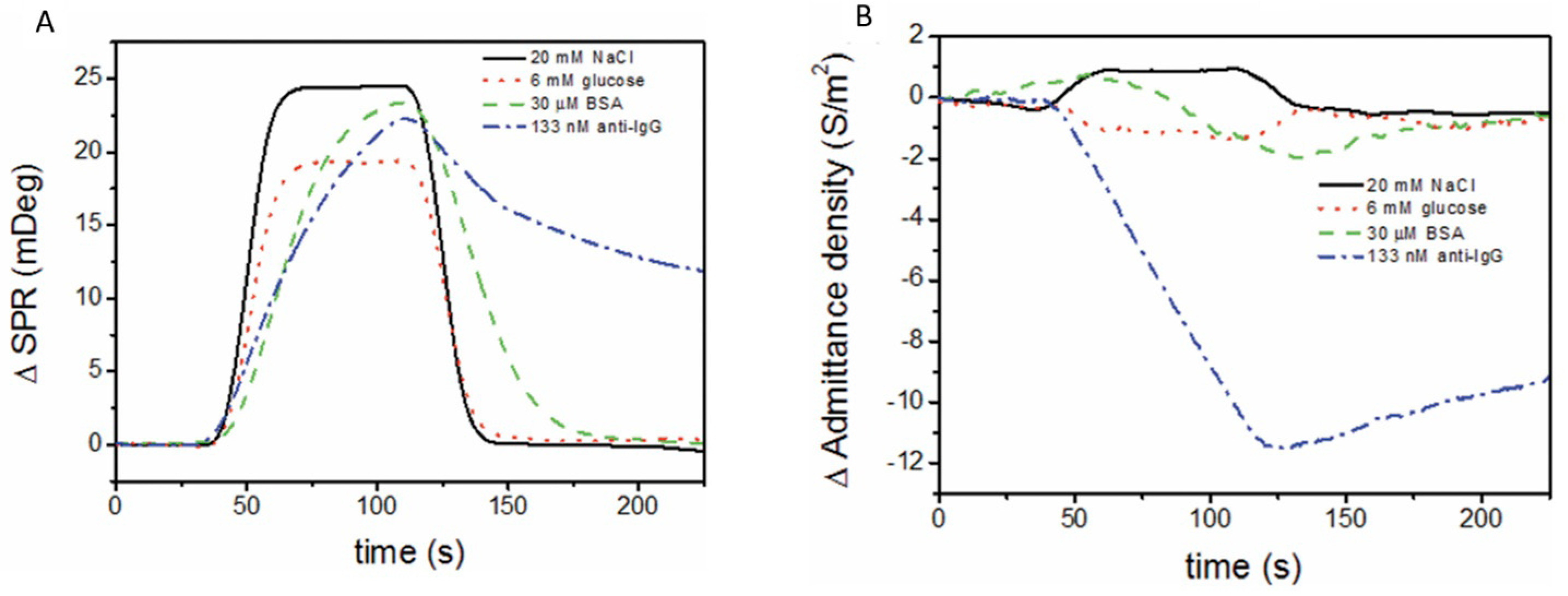
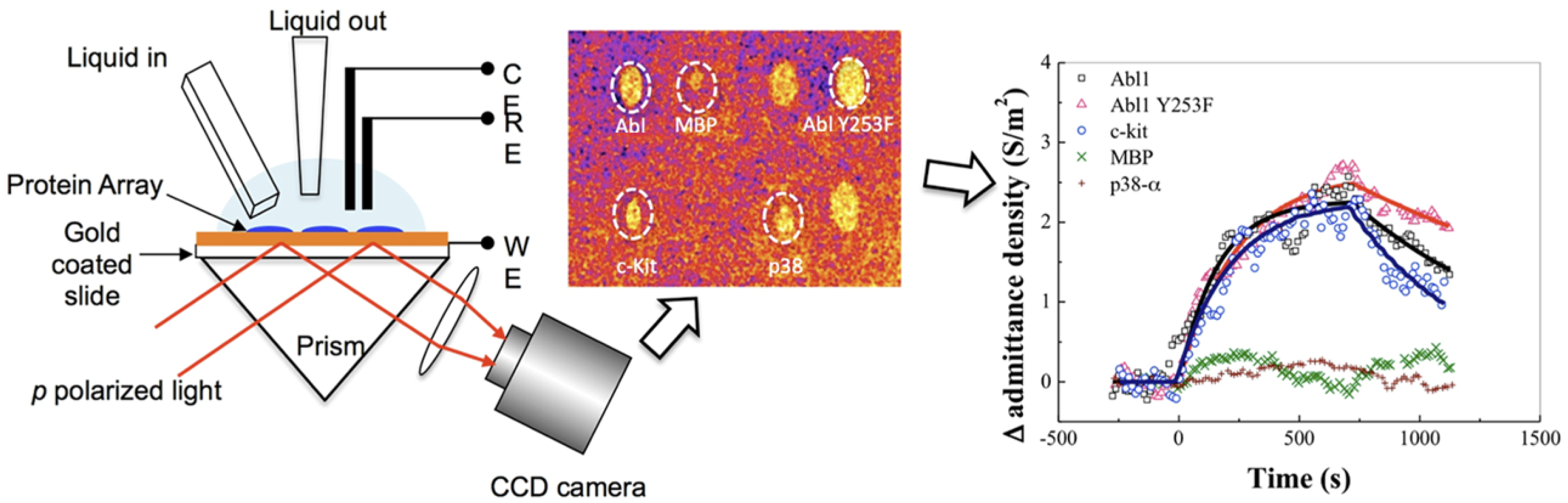
EC biosensors use a variety of EC detection methods, such as electrochemical impedance spectroscopy (EIS), constant potential amperometry, voltammetry (cyclic, linear or pulse methods), potentiometry and conductometry [8]. A classic EC setup includes three electrodes (working, counter and reference) in an EC cell, connected to a potentiostat. The biosensor is the working electrode (Figure 4C). The biorecognition event causes changes in the current intensity, impedance, charge or potential that are correlated with the amount of material bound to the biosensor. Both non-faradaic and faradaic processes were exploited in EC biosensors. In non-faradaic processes, ions in the electrolyte solution are the contributors to the EC signal, whereas in faradaic processes, the signal originates from an electroactive probe that is added into the sample or built/immobilized into the sensing layer of the biosensor. One of the preferred measurement strategies in EC biosensors implies DPV and the use of a ferrocyanide/ferricyanide redox couple as the exogeneous probe. In DPV, a series of potential pulses are superposed to a linear potential scan (Figure 4C). The current is measured at the end of each pulse, minimizing the influence of the electrical double layer charging current and, thus, increasing the sensitivity of detection. The intensity of currents produced by ferrocyanide oxidation when scanning the potential in a suitable range via DPV is compared before and after incubation with the sample and biosensor washing steps. To evaluate NSA, signal suppression was used as the quantitative indicator in EC biosensors. The signal suppression percentage is calculated as (I0 − I)/I0 × 100 (Figure 4C), where I0 and I represent the EC signal (magnitude of the peak current) measured before and after the incubation with the sample, respectively. The signal suppression occurs due to the steric barriers and electrostatic repulsions induced by the fouling layer for the negatively charged ferroyanide/ferricyanide redox probe, diffusing to the electrode. Typically, values lower than 5–10% were considered by the authors as indicating a good antifouling capacity in the case of biosensors operating in undiluted complex samples, or prepared with low dilution factors and subjected to longer biosensor storage [41].
Besides the direct evaluation by SPR or electrochemistry, various other methods have been used to determine the extent of NSA and the characteristics contributing to the antifouling behavior of the biosensor coatings. Hydrophilic, neutrally charged coatings are associated with a good antifouling capacity. Therefore, most coatings were characterized by contact angle goniometry (where low angle values indicate hydrophilicity) and zeta potential measurements (indicating the electrical charge of the material). For biosensors intended for measurements in blood and serum, fluorescence spectroscopy-based imaging methods have often been used along with fluorescent dye tags to visualize the adsorption of proteins (e.g., BSA) and blood cells on the coatings [42,43,44,45]. Total internal reflection fluorescence microscopy studies on the absorption of BSA and fibrinogen from solutions with low concentrations onto PEG and polysaccharide-modified interfaces have emphasized protein adsorption on polysaccharide coatings, while SPR measurements did not detect adsorption even at higher concentrations. At the same time, the PEG coatings have protected from adsorption at low protein concentrations, based on total internal reflection fluorescence data, but were no longer efficient at higher concentrations [46], as revealed by SPR. This is one example of the studies cautioning against using the surface coverage, calculated based on SPR data, as an exclusive indicator on whether a coating is low-fouling. The authors of this study argued that a better fouling indicator would be the rate of protein adsorption [46].
Additional analytical methods have been used for more detailed investigations of the antifouling coatings. Similar to SPR, QCM-D (quartz crystal microbalance with energy dissipation) allows one to study the resistance to NSA in real time, in static or hydrodynamic conditions. QCM-D is an acoustic method, which exploits the piezoelectric effect and uses gold-coated, AT-cut quartz crystals, which oscillate in thickness shear mode. This technology allows one to evaluate phenomena at the interface between the crystal and the solution, such as fouling, which are associated with both the mass of foulants adsorbed at the interface and with the contribution of the solvent (water) molecules. This contribution leads to changes in the viscoelastic properties at the interface between the sensor and the measurement medium. Examples of such changes include modifications in conformation or swelling [47]. QCM-D was used to, among other things, study the effect of surface density on the resistance to NSA of zwitterionic polymers [48], evaluate the antifouling capacity of the antifouling coating [49], and measure on-site and in a reagentless way the levels of E. coli O157:H7 in food products [50]. The thickness shear mode with dissipation monitoring was also recently used in an aptasensor for the detection of Staphylococcus aureus in undiluted milk [51]. The technique served both for quantitative purposes and for evaluating the resistance to NSA of layers obtained from an antifouling anchoring molecule (3-dithiothreitol propanoic acid) and a small backfilling molecule (2-(2-mercaptoethoxy)ethan-1-ol) [51].
Spectral ellipsometry is a nondestructive, real-time, optical method that measures changes in the polarized light upon reflection by surfaces. It enables one to sensitively measure the thickness of thin films, along with the refractive index, surface roughness and other parameters. It has been widely used for characterizing antifouling coatings in studies where the coating thickness and refractive index were correlated, e.g., with information provided by SPR [49,52] and infrared spectroscopy [52]. For example, the disruption of the tight, ordered structure of poly(2-hydroxyethyl methacrylate) (pHEMA) polymer brushes during functionalization, when hydroxyl groups were transformed into succinimide carbonate intermediates with higher mass and volume, was emphasized by ellipsometry through the simultaneous enhancement of coating thickness and refractive index [52]. The ordered structure and the antifouling capacity were restored to a certain degree upon functionalization with antibodies and the deactivation of residual activated groups, as indicated by corroborated ellipsometry, SPR and Fourier-Transformed Infrared Reflection Absorption Spectroscopy.
X-ray Photoelectron Spectroscopy is a surface-sensitive method that provides information on the elements present on a surface and the chemical bonds in which they are involved, allowing to confirm, e.g., the successful attachment of coatings to surfaces, and the coatings’ resistance to the adherence of foulants [46].
Low field nuclear magnetic resonance enables one to study the abundance of water molecules bound to various materials and the structure of the hydration layer. Among other things, the method has been used to show that water molecules are bound tighter by poly(sulfobetaine methacrylate) (polySBMA) polymers than by PEG [53].
Vibrational spectroscopy methods (infrared spectroscopy, Raman spectroscopy and sum frequency generation) are optical, non-destructive methods that offer complementary information on surface adsorbates based on characteristic spectra that reflect the chemical bonds in the adsorbed species. Infrared reflection–absorption spectroscopy was used to characterize various antifouling coatings, and to highlight changes upon storage or exposure to fouling agents, by comparing the spectra recorded in various conditions. For example, Vrabcova et al. [54] used Infrared Reflection Absorption Spectroscopy to emphasize the relative abundances of ionized and nonionized carboxyl groups in poly(carboxybetaine) layers grafted on gold, and to monitor the detachment of polymer chains from the supporting surface. Surface-enhanced Raman scattering was used both as a detection method for melamine in undiluted milk and to emphasize the antifouling properties of a coating based on the glycoprotein lubricin [55]. In yet another study, the interfacial hydration of several polymers was studied in situ by sum frequency generation [56]. The method provided evidence of hydrogen-bonded water at the polymer brush–water interface, and indicated the “lack” of NSA, based on the similarity between the spectra of the polymer when exposed to protein solutions and water, respectively.
There are also other methods, e.g., those based on mass spectrometry [57], which are very useful for studying the fouling mechanism and for evaluating new NSA-resistant materials. In a pragmatic approach, in biosensors, the evaluation of NSA should be carried out by a combination of methods, while keeping in mind the limitations of each procedure. The evaluations have to be performed in conditions (duration, temperature, sample dilution, etc.) equal to or exceeding the severity of those used for quantitative purposes.
2.4. NSA-Resistant Coatings
2.4.1. Overview of Fouling-Resistant Materials
An ideal anti-fouling strategy minimizes NSA to levels lower than the detection limits of the analytical methods, e.g., lower than 0.3 ng/cm2 proteins [4,58], while enabling the high specific binding of the target analyte, in conditions of no or minimal sample pre-treatment. The ideal antifouling coating is hydrophilic, has zero overall electric charge, and enables functionalization and high loading capacity with biomolecules. Moreover, it preserves its NSA-resistant capacity after bioreceptor attachment and during the entire biosensor operation, as well as upon storage.
The most efficient antifouling coatings used in biosensors are based on PEG and OEG derivatives, zwitterionic polymers, SAMs of alkanethiols and polyaromatic thiols, antifouling peptides, polysaccharides, BSA-based layers and DNA-based coatings (Figure 5). Complex materials mimicking the architecture of the cell membrane, hydrogels, or hybrid materials including several components from these classes have also been used.
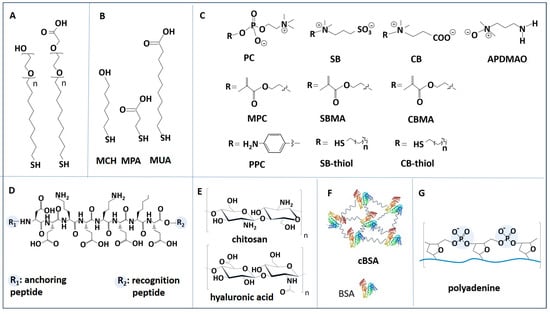
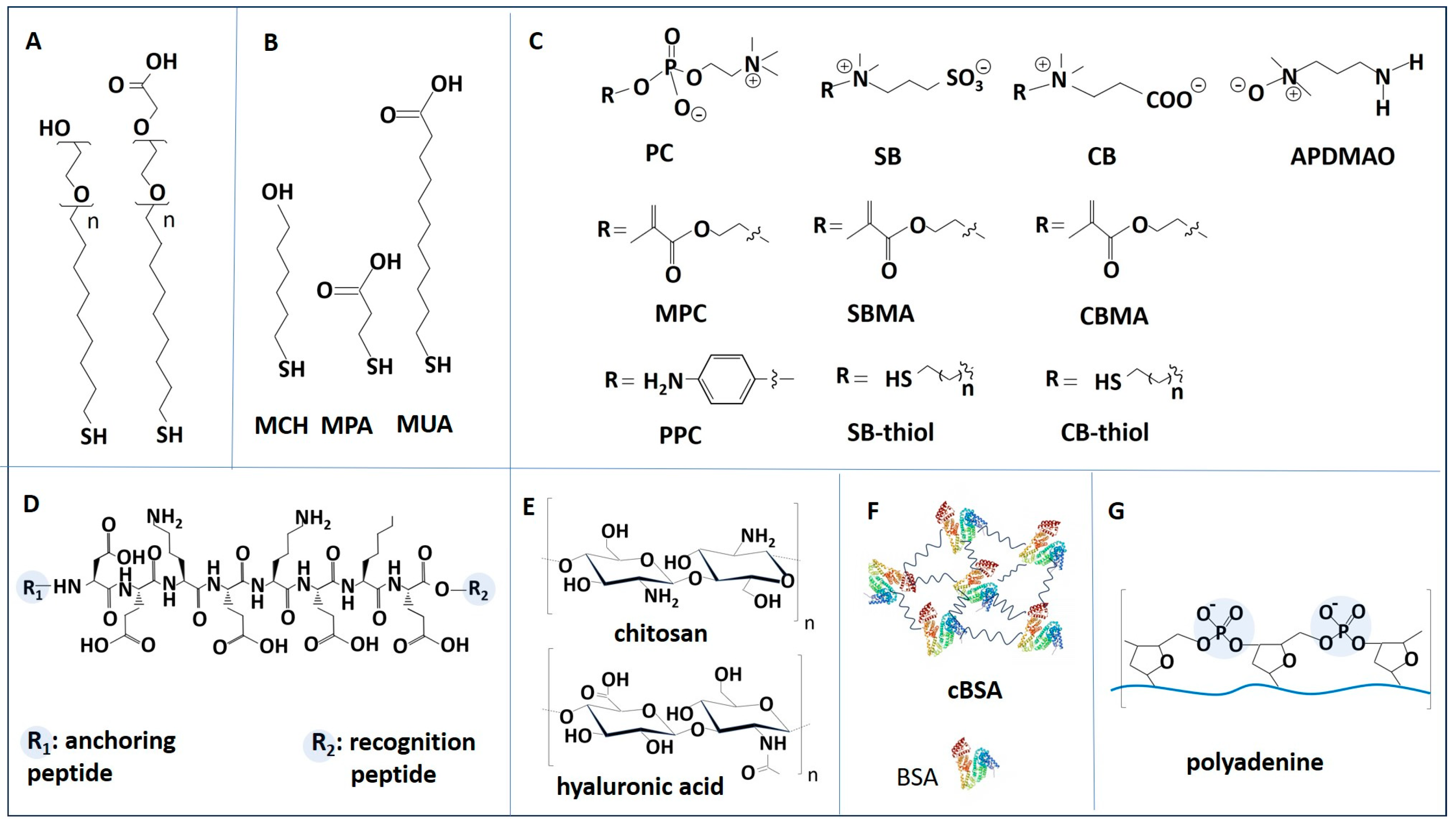
Figure 5.
Examples of molecules often used in passive antifouling coatings for biosensors. (A) PEG-based thiols. (B) Alkanethiols: 6-mercapto-1-hexanol (MCH), 3-mercapto-1-propionic acid (MPA) and 11-mercapto-1-undecanoic acid (MUA). (C) Zwitterionic polymers and thiols. PC: phosphoryl choline. SB: sulfobetaine. CB: carboxybetaine. MPC: 2-methacryloyl phosphorylcholine. SBMA: sulfobetaine methacrylate. CBMA: carboxybetaine methacrylate. PPC: phenyl phosphoryl choline. APDMAO: 3-aminopropyldimethylamine oxide. (D) Antifouling peptide EKEKEKE. (E) Polysaccharides: chitosan and hyaluronic acid (HA). (F) Cross-linked BSA (cBSA). (G) Polyadenine.
Each class of materials has advantages and drawbacks, as detailed below. Coating the sensing interface with antifouling layers can be achieved by several methods, such as self-assembly, grafting or electrochemical deposition [4]. The coating procedure and experimental parameters chosen lead to different compositions, thicknesses and densities, affecting the ability of the resulting layer to resist NSA. Passive antifouling coatings have been extensively researched, and interested readers can find details in various reviews [1,2,3,4,5,6,9,10,11,12,13,29,40,59,60,61]. As we are not aiming for a comprehensive presentation here, in the following, we introduce some of the preferred solutions to reduce NSA in EC-, SPR- and EC-SPR-based biosensing, for which specific applications are highlighted further in Section 3, Section 4 and Section 5.
2.4.2. PEG and OEG Derivatives
PEG and OEG derivatives, e.g., the thiols shown in Figure 5A, which are hydrophilic and biocompatible, were considered the gold standard in antifouling materials [62]. As ethylene glycol groups form hydrogen bonds with water molecules, each PEG molecule binds several water molecules. The strongly bound water molecules form a tight hydration layer, blocking the diffusion of fouling molecules towards the sensor surface. Moreover, PEG and OEG have long and flexible chains, and therefore have high conformational mobility. When foulant proteins approach the hydration layer formed on PEG/OEG, the polymer chains first compress and then return to the original conformation, repelling the foulants. In essence, the antifouling capacity of PEG/OEG is explained by a combination of hydration layer and steric hindrance. Due to these characteristics, SAMs with ethylene glycol terminal groups have higher resistance to NSA compared to alkanethiol SAMs with a similar number of C atoms and methyl or hydroxyl end groups, as indicated by molecular dynamics simulation [63]. Many PEG-containing thiolated molecules with various functional end groups, commercially available from different vendors, can be used by biosensor developers for further functionalization with bioreceptors and the prevention of NSA. However, PEG-based SAMs are not stable for extended periods due to their oxidation in air.
2.4.3. Alkanethiols
Alkanethiols (Figure 5B) adsorb strongly onto the Au surface by chemisorption, and form SAMs that provide the quantitative coverage of sensing interfaces and impede the adherence of fouling molecules. A SAM with mixed carboxyl/hydroxyl functional groups provides an easy method to attach bioreceptors by covalent binding to the carboxyl groups, and prevents fouling thanks to the thiols with hydroxyl end groups. A common approach in biosensor development is to first functionalize the Au interface with bioreceptors (either directly or via “anchor” molecules such as carboxyl-ended thiols), then incubate the sensing surface with an alkanethiol solution (µM–mM) for 30 min up to several hours. This “backfilling” procedure ensures that the surface that remains unoccupied by the bioreceptor or anchor molecules gets quantitatively covered by (ideally) defect-free alkanethiol SAM. In addition, backfilling helps to remove the loosely adsorbed bioreceptor and orient the attached bioreceptor molecules away from the surface, facilitating conditions favorable for the specific recognition event.
The stability and capacity to resist fouling of alkanethiol SAMs is hampered by defects in the monolayer leading to incomplete coverage. In addition to optimizing the coating procedure, the packing density, uniformity and stability of the antifouling SAM can be improved by changing the head group of the alkylthiol (e.g., hydrophobic methyl instead of hydrophilic hydroxyl [64]) or the length of the chain (e.g., the longer 8-mercapto-1-octanol instead of MCH, increasing the van der Waals interactions between neighboring thiols [21]). Longer chain alkanethiols produce ordered, uniform, high-quality monolayers wherein the van der Waals interactions between neighboring alkanethiol molecules are increased, compared to shorter alkanethiols. As a consequence, the SAMs are very efficient in restricting the access of foulant molecules to the sensing interface by steric effects, and the stability of coatings exposed to foulants is enhanced as well [21]. For example, backfilling with 8-mercapto-1-octanol instead of MCH prolonged the operational stability of an E-AB biosensor (in the detection of vancomycin in serum at 37 °C) to 1 week [21].
As noted by Shaver et al. [23], the operational and thermal stability of the thiol SAMs can be also enhanced by cross-linking [65] or by increasing the number of attachment points of the monolayer by using multi-dentate thiols [66].
Thiols can desorb or oxidize in certain experimental conditions. This needs to be considered when optimizing the biosensor’s operational conditions [56]. Additional measures are sometimes taken, e.g., including membrane or hydrogel overcoats, to ensure the accuracy of biosensors passivated with alkanethiol SAMs operating in complex protein-rich samples, such as whole blood.
2.4.4. Zwitterionic Materials
Zwitterionic materials (Figure 5C) contain repeating pairs of negatively and positively charged functional groups, distributed in a way that ensures overall electrical neutrality [8,60]. Their structure promotes the solvation of the charged groups in water, resulting in the formation of a dense hydration layer that physically blocks the NSA of molecules. Electrostatic interactions with sample components are moreover prevented thanks to the neutral charge of the material at its isoelectric point. Steric hindrances induced by the compact layers, particularly in long-chain or high-grafting-density polymers, also contribute to the resistance to fouling [60].
Polymers, peptides and other zwitterionic molecules have been integrated into biosensor coatings to enhance their resistance to NSA. The most widely used zwitterionic polymers are poly(2-methacryloyloxyethyl phosphoryl choline), poly(sulfobetaine methacrylate) (PSBMA), and poly(carboxybetaine methacrylate) (PCBMA).
The capacity of zwitterionic materials to resist NSA depends on pH, salt concentrations, the cations and anions in the material, the distance between the charged groups in the material unit, the molecular weight, the grafting density and the thickness of the coating layer [60]. In general, materials, which include cations such as guanidine and ammonium groups and anions such as sulfonic and phosphonic acids with high pKa, are the most efficient in resisting NSA [60]. Additionally, zwitterionic materials with a short distance between the charged groups present tighter interactions between neighboring chains, leading to the formation of more compact layers, which prevent the diffusion of potential interfering molecules through steric effects. Related to this, more recently proposed 3-aminopropyldimethylamine oxide (APDMAO)-based polymers, in which positively charged N+ are directly linked to negatively charged O−, have a higher capacity for forming hydrogen bonds than some of the classic polymers, as indicated by molecular dynamics simulations [67]. This leads to tight hydration layers and high resistance to NSA, i.e., less than 3 ng/mm2 adsorbed proteins from blood [68]. Moreover, the coatings are characterized by very high hydrophilicity, e.g., the water contact angle of a coating of polydopamine-APDMAO deposited on a glassy carbon electrode was shown to be 10.9° [67].
Zwitterionic materials integrating several functionalities can form stable antifouling coatings with high capacity for loading with bioreceptors, enabling a high number of repetitive tests in complex matrices. For example, a polymer brush containing N-(2-hydroxypropyl) methacrylamide (HPMAA), carboxybetaine methacrylamide (CBMAA) and sulfobetaine methacrylamide (SBMAA), obtained by surface-initiated atom-transfer radical polymerization, served in the development of a QCM immunosensor for E. coli. The biosensor was used to perform more than 60 tests on hamburger samples and displayed good sensitivity in milk, where it detected E. coli down to 700 cfu/mL [50].
The influences of pH and salt are particularly important when analyzing food samples, considering, e.g., the typical pH values of milk, fruit juices and wine of 6.5–6.9, 3.2–4.3 and 3.0–3.6, respectively. When the pH differs from the isoelectric point of the zwitterionic material, the material becomes charged, which encourages NSA. In solutions containing high concentrations of salt, the counter ions diffuse into the polymer brush and minimize the electrostatic interactions between the adjacent chains, causing them to adopt a less compact conformation, which favors NSA. In consequence, the biosensor development process should consider the preservation of the biosensor’s resistance to fouling under the optimum measurement conditions (pH and ionic strength), where the specific signal produced by the biorecognition event is the maximum.
2.4.5. Antifouling Peptides
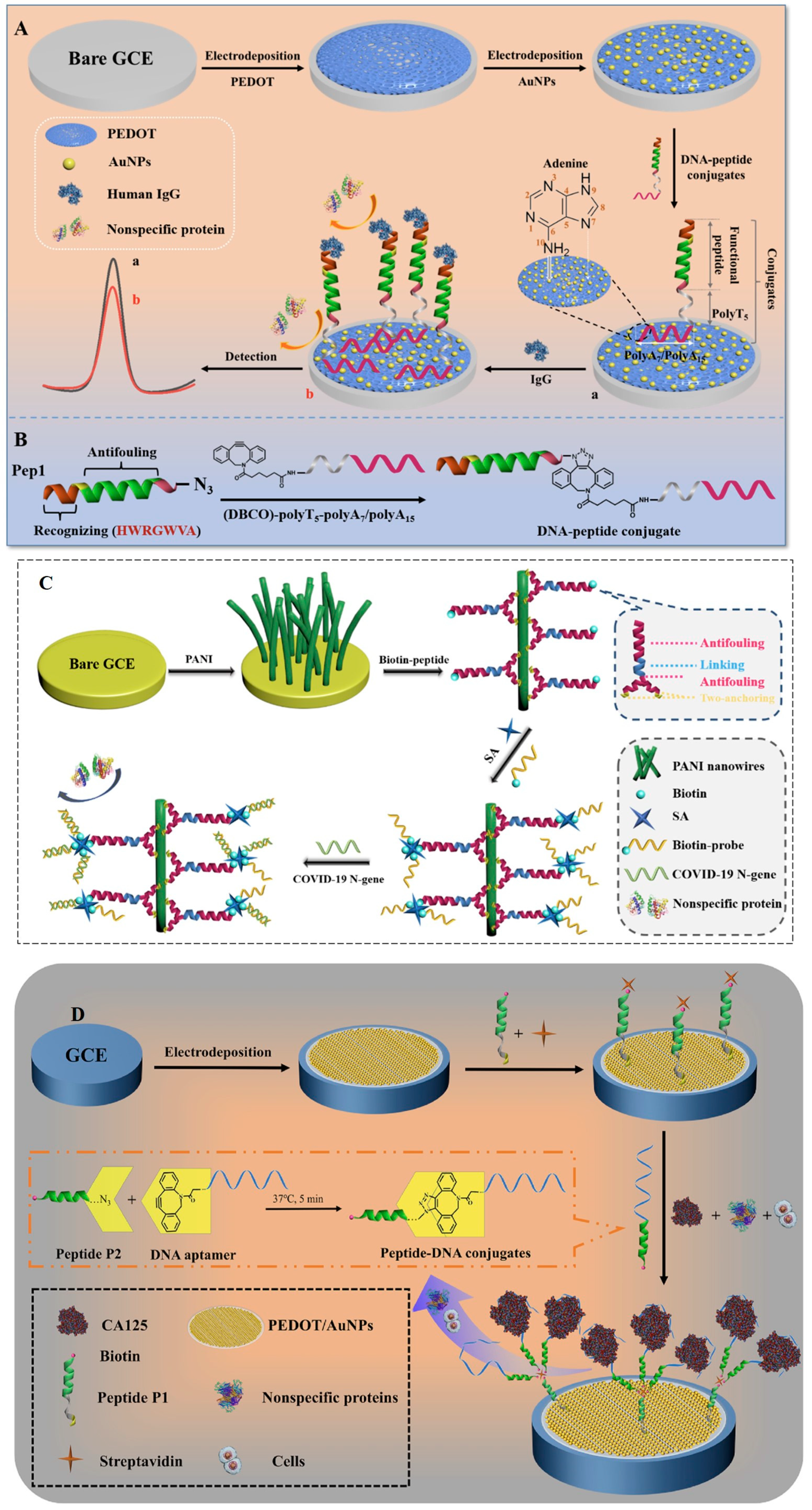
Antifouling peptides (e.g., the EKEKEKE peptide shown in Figure 5D) have been intensively researched in recent years in biosensors due to their versatility for use as biosensor components [1,10]. Zwitterionic peptides with motifs of alternating positively charged amino acids (e.g., lysine, one letter amino acid code “K”) and negatively charged amino acids (e.g., glutamic acid, one letter amino acid code “E”) have shown a good antifouling ability in complex media [35,69]. Starting from the zwitterionic peptide EKEKEKE reported in 2009 [35], many other antifouling sequences have been discovered—not only zwitterionic, but also amphiphilic peptides [1,10]. Various complex, all-in-one peptide constructs have been developed in which peptides with antifouling properties are completed with an anchoring part, a specific recognition part, and even an antibacterial component [70]. The functionalization of the sensing interfaces with antifouling peptides was most often carried out by chemisorption, facilitated by the terminal cysteine in peptides (e.g., [41,69]). Alternatively, the peptides were attached on the electrode surface by avidin–biotin interaction [71] or via oligo 3,4-dihydroxyphenylalanine [72].
The amino acid compositions of the peptides (including unnatural amino acids and D-amino acids), as well as their shapes (linear, cyclic, branched, loop, Y-shaped, U-shaped) [58,70,73,74,75] and their sizes (from small sequences to nanoparticles [11]), have been studied with respect to antifouling capacity and resistance to enzymatic hydrolysis. The ability of the peptide to resist enzymatic hydrolysis is a key issue to consider when designing biosensors with an adequate operational lifetime in biological fluids or food matrices (in which proteolytic enzymes are usually present). Chemical modifications by cyclization [75], the introduction of pseudo-peptide bonds or unnatural amino acids (e.g., α-aminoisobutyric acid [76]), or replacing L- with D-amino acids [77], promote the stability of peptides in proteolytic media [78]. Moreover, nanoparticles with enhanced resistance to NSA and high stability in biological media were obtained by the self-assembly of peptides that include specific antifouling, linker and hydrophobic domains [79].
While versatile, and amenable to inclusion in complex constructs with multiple roles, peptides require chemical synthesis, which adds to the costs associated with biosensor development. The low conductivity of peptide-based antifouling layers is a drawback for EC biosensors. The integration of conductive polymers (e.g., poly(3,4-ethylenedioxythiophene), PEDOT, polyaniline, etc.) or metallic particles (Au, Pt, etc.) solves this problem, yet it adds to biosensor complexity and costs.
2.4.6. Polysaccharides
Polysaccharides such as dextran, HA (Figure 5E), chitosan, chondroitin sulfate, etc., are large, complex carbohydrates made from monosaccharides (e.g., glucose), linked through glycosidic bonds. They contain numerous amide, hydroxyl and carboxyl functional groups, which are able to form hydrogen bonds and impart hydrophilicity to polysaccharide coatings. For example, a matrix of carboxylated HA enabled the quantitative attachment of anti-BSA antibodies (780 ng/cm2) and demonstrated low levels of NSA, i.e., less than 17 ng/cm2 after exposure to cow milk or 10% blood serum [80]. Most often, the antifouling capacity of HA-based layers alone is not enough for measurements in real samples at low dilution factors. Therefore, HA was integrated with zwitterionic or other antifouling materials to achieve the necessary protection against NSA, as reviewed, among others, in [4].
2.4.7. BSA
BSA is a 66.5 kDa protein with an isoelectric point of 4.7, which is widely used to prevent fouling in biosensors [6]. A protective layer of adsorbed BSA is typically obtained by incubating the biosensor in a concentrated solution of the protein (1–10 mg/mL) for 30–60 min. The popularity of this NSA-preventing solution, implemented mostly in biosensors for discrete measurements, is related to its effectiveness and easiness, combined with the large availability and relatively low cost of the protein. The efficiency of BSA blocking layers in serum samples is attributed to the electrostatic repulsion between the coating, with a net negative charge at physiological pH, and the HSA, the most abundant protein in serum, present at concentrations of 45–60 mg/mL. The quality of the adsorbed BSA layer depends on the nature and the surface roughness of the supporting material. The protective effect is moreover linked to the experimental conditions and the nature of the sample. Repetitive biosensor regeneration procedures using acidic or basic solutions, as frequently employed in SPR measurements, may denature the protein coating, affecting the biosensor’s resistance to NSA. In addition, complex samples such as blood or serum contain other large molecules that may replace the non-covalently adsorbed BSA.
Compared to a monolayer of adsorbed protein, cross-linked films (cBSA, Figure 5F) form a stable 3D coating on the surfaces of Au films, which is thin enough for SPR measurements and provides for both the prevention of NSA and the presence of a high number of functional groups for attaching bioreceptors [25,81]. The integration of conductive nanomaterials into cBSA films leads to porous 3D coatings that are adequate for EC biosensors, and display a high antifouling ability in serum or plasma. Various nanocomposites of cBSA with Au nanowires (AuNW) [82,83], aminated reduced graphene oxide [84], amino ferrocene-modified graphene nanosheets [85], carbon black [86] or MXene [87] have been reported.
2.4.8. DNA-Based Coatings
DNA-based antifouling electrode coatings (e.g., polyadenine in Figure 5G) exploit the affinity of polyadenine for Au surfaces, to which it binds with a strength similar to the Au–S bond [88]. Polyadenine binds to Au through the DNA bases while the phosphate groups are exposed to the solvent, imparting to the coated surface hydrophilicity and resistance to fouling. Due to adenine’s much higher affinity for gold compared to thymine [88], polyadenine–polythymine sequences adsorb on gold surfaces in an L-shaped conformation, with the adenine part laid flat and the thymine part perpendicular to the surface, forming DNA brushes [89,90]. The grafting density with polythymine is achieved by controlling the length of the polyadenine part. Steric hindrances can thus be easily avoided to enable high hybridization yields with complementary oligonucleotides; moreover, additional polyadenine sequences of adequate length can be added as diluents to achieve the best signal-to-noise ratio.
2.4.9. Biomimetic and Nature-Inspired Materials
Inspired by the natural mechanisms of cell membranes, various cell membrane-mimicking antifouling coatings have been proposed for biosensors. These range from SAMs with phosphatidylcholine end groups [91], inspired by the zwitterionic phospholipids contained in the outer cell membrane, to more complex layer architectures [92]. The benefits of resistance to fouling have to be balanced against the difficulties of carrying out some of the more complicated designs, and the limited stability of the coatings.
2.5. Experimental Protocols for Studying NSA in Biosensors
For evaluating the NSA, the experimental workflow should consider the type of sample and any pre-treatment or dilution that might be necessary to bring the sample into the dynamic range of the biosensor. Often, in the particular case of liquid samples analyzed using highly sensitive biosensors, the NSA problem is simplified by the high dilution factors associated with sample preparation. Uniform procedures and guidelines for evaluating the NSA are needed in the biosensor development field.
There are two extreme strategies used by researchers when it comes to the investigation of antifouling coatings in biosensors.
According to the first, used particularly when proposing new antifouling materials, the NSA prevention strategy is optimized before attaching the bioreceptor to the sensing interface. The effects of functionalizing the antifouling coatings with the bioreceptor are evaluated next, and adjustments are made, if necessary, to achieve the best specific/non-specific signal ratio [38]. “Control” sensing interfaces that are similar in all aspects to the biosensors, except that they do not include the specific bioreceptor, are very useful in evaluating NSA and optimizing the measurement protocol and antifouling strategy [82]. The evaluation of NSA is carried out using both solutions of single proteins of different sizes and charges, in 0.1–50 mg/mL concentrations, and relevant sample matrices (serum, blood, milk, fruit juices, river water, etc.) at different dilution factors, depending on the application. Lysozyme (14.3 kDa, pI = 11.0), BSA (66.4 kDa, pI = 4.7) and hemoglobin (64.5 kDa, pI- = 6.9) were often used as model foulants. The properties of the antifouling coating and of the final biosensing interfaces should not be affected by incubation with the sample. These properties include baseline electrochemical or optical characteristics, such as the peak current measured by voltammetry in the presence of a redox probe, or the SPR minimum and profile of the reflectivity curve in the absence of the target analyte. In addition, the affinity of the biosensor for the target analyte should not be affected by the sample matrix and should be recovered by appropriate surface regeneration procedures, in the case of devices used for multiple measurements. At the same time, the control interfaces should be insensitive to samples into which the analyte is added, at the maximum expected concentration levels. For samples containing baseline levels of the target analyte (e.g., a biomarker in serum), once the efficacy of the antifouling coating is confirmed, the tests with bioreceptor-functionalized interfaces (the final biosensor) are carried out by first pre-incubating the biosensor with the analyte so as to saturate the bioreceptor, before testing with actual samples, as illustrated in Figure 4A [93]. This strategy, widely used in studies proposing new antifouling coatings, ensures that the final biosensor has the appropriate antifouling capacity.
Under the second approach, biosensor developers have been focused more on increasing the device’s sensitivity, without studying NSA in particular. Fouling is generally addressed by some common strategies such as backfilling with alkanethiols (MCH mostly), blocking with BSA, etc. No data were provided in these research articles regarding measurements with control interfaces. Generally, hints regarding the potential contribution of NSA to the analytical signal can be obtained from the presented selectivity studies with potential interfering molecules. However, these might reflect mixed contributions due to the NSA and the bioreceptor’s cross-reactivity.
Regardless of the chosen approach, the ultimate proof that the NSA prevention strategy was effective comes from the parallel analysis of samples using the biosensor and current standard methods. An agreement between the two sets of results confirms the biosensor’s accuracy. Larger sample sets increase the reliability of such comparisons.
3. Solutions for Minimizing NSA in Electrochemical Biosensors
3.1. General Strategies to Address Fouling in EC Biosensors
EC biosensors have the potential to provide low-cost, fast and specific measurements for various applications. They cover a wide range of combinations of electrode materials and nanomaterial modifiers, bioreceptors and detection techniques, and, when properly developed, can be applied for measurement in complex media, including blood [8]. Not surprisingly, a large part of the research effort was dedicated to strategies for the prevention of fouling in biological fluids and in food [2,4,8,9,10,11,94,95].
An ideal coating for use in EC biosensors is uncharged under the measurement conditions, and has adequate hydrophilicity, low impedance and high loading capacity with bioreceptors.
In the following discussion, we will present some common and modern antifouling strategies applied to biosensors based on Au electrodes or modified with Au nanoparticles (AuNP). This choice of support material is motivated by the desire to highlight strategies that may also be applied in SPR and EC-SPR biosensors (which require a ~50 nm thick Au layer to function, and which are discussed in Section 4 and Section 5).
The main strategies for ensuring the EC biosensors’ resistance to NSA were to (i) limit the diffusion of fouling molecules by physical means, (ii) repel the foulants with NSA-resistant coatings, or (iii) use sacrificial, transient coatings on which NSA occurs but which dissolve in predetermined conditions, leaving the surface clean, allowing to perform accurate measurements of the target analyte (Figure 6). Most often, the strategy to prevent NSA combines several approaches, including, in addition to the main strategies, (i) sample pre-treatments, (ii) blocking the biosensor surface with proteins like BSA or casein or directly with solutions of non-fat milk powder [82], or (iii) the addition of BSA, milk powder or detergents, e.g., Tween-20, to the sample or buffer [82].
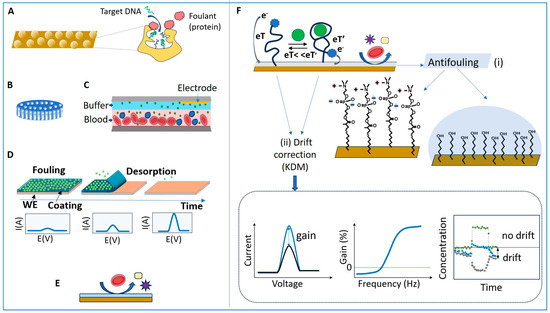
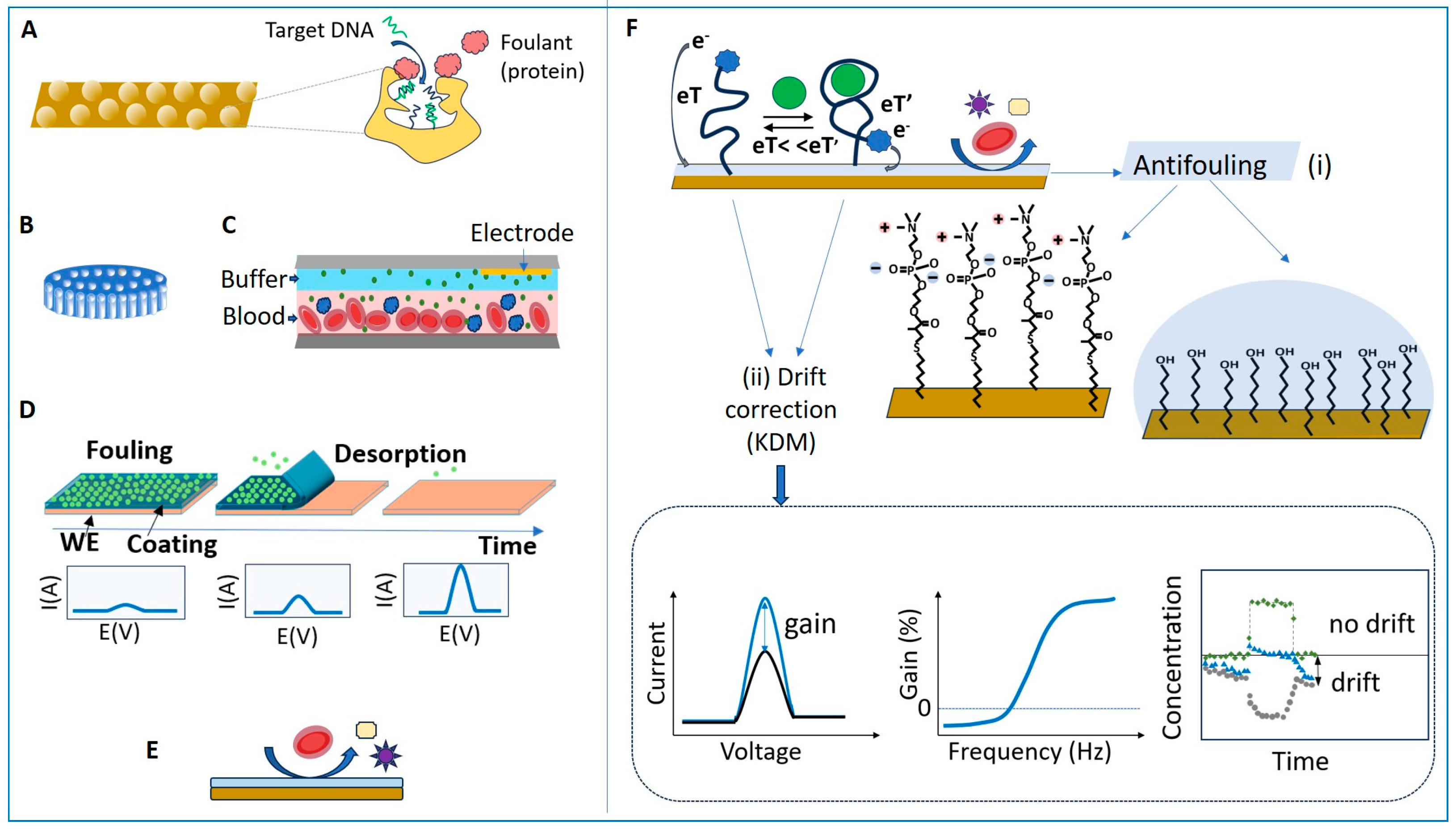
Figure 6.
Strategies to minimize NSA in EC biosensors using (A) nanoporous electrodes; (B) porous membranes; (C) liquid filters such as the continuous diffusion filter (CDF); (D) transient coatings; (E) antifouling coatings; (F) Minimizing the impact of fouling on E-AB biosensors by (i) using antifouling layers based on the SAM of phosphatidyl choline (PC)-ended-thiol or MCH and hydrogel overcoats and by (ii) using drift correction algorithms such as KDM. Lower right: variation in time of the square wave voltammetry (SWV) signal gain for the aptamer-bound state (blue) and for the unbound state (grey), when the sensor is exposed to a pulse of analyte. The signal drifts are synchronized, which enables to obtain a drift-corrected signal (green) by applying KDM. Redrawn in part from [96] (A) and [97] (C,F).
The physical restriction of the diffusion of interfering molecules towards the biosensor was achieved by structuring the biosensor interface to create nanopores of adequate size (Figure 6A) or by adding membranes or filters (Figure 6B,C). Nanoporous gold electrodes obtained by EC roughening are characterized by higher detection sensitivity and better adhesion of some antifouling coatings compared to planar electrodes [21], and, more importantly, have better resistance to NSA. Nanoporous interfaces act as diffusion filters, which minimize fouling by restricting the access of large proteins into small nanopores, while the transport of small molecules remains marginally affected (Figure 6A). For example, nanoporous Au electrodes with pore sizes lower than 50 nm preserved their electrochemical signal when exposed for 1 h to serum albumin (2 mg/ mL) and fibrinogen (1 mg/mL), while large signal suppression occurred for planar electrodes under the same conditions [98]. Bioreceptor probe density [99] and pore size [96] determine the overall detection performance of nanoporous biosensors. Electrodes with 25–30 nm pores were found to be ideal for the electrochemical detection of DNA hybridization in complex samples, reaching a balance between restricting the access of interfering compounds and enabling high sensitivity for the target analyte. These electrodes enabled accurate measurements of short oligonucleotides in 10% fetal bovine serum [96]. Notably, the passivation of the electrodes with MCH after their functionalization with capture DNA also contributed to this performance.
The addition of membranes and filters (Figure 6B,C) on top of the biosensor was particularly helpful for real-time monitoring in flowing blood, using E-AB biosensors. The in vivo monitoring of four drugs (doxorubicin, kanamycin, gentamicin, and tobramycin) in awake, freely moving rats was achieved with the E-AB biosensors coated with a 0.2 µm polysulfone membrane, which protected them from fouling by blood cells [100]. In the MEDIC platform, designed for monitoring drugs in real time via ex vivo measurements with an E-AB biosensor, fouling due to blood cells and proteins was prevented with a continuous-flow diffusion filter (CDF). The CDF consists of a stack of two laminar flows, blood and buffer, with flow rates adjusted so that the concentration of high-molecular-weight components reaching the biosensor surface is minimal compared to that of the small analyte doxorubicin [97].
Transient coatings (Figure 6D) of poly(meth)acrylate-based copolymers were adopted in EC biosensors as a strategy to delay fouling and to perform measurements at predetermined time intervals, in complex media such as blood, saliva and gastrointestinal fluids [26,101,102]. Polymers such as Eudragit® (from Evonik Nutrition & Care GMBH Darmstadt-Germany), used in the pharmaceutical development of oral solid dose drugs with time-controlled release, were used to design biosensor coatings whose dissolution is controlled by the type of polymer, film density and pH. Applications include edible enzymatic biosensors used for monitoring glucose in gastrointestinal fluids (resistant for up to 90 min at pH 1.5) [102] and a multi-electrode array used to measure glucose levels in blood and saliva over several hours, based on the delayed activation of individual biosensors [26]. The delayed biofouling of sensors overcoated with Eudragit-based polymers was also reported in the case of screen-printed Au electrodes, functionalized with SAMs of methylene blue (MB)-labeled probes responsive to pH and trypsin, when tested in Dulbecco’s modified Eagle medium (DMEM) containing 10% fetal bovine serum at 37 °C [101]. While promising for long-term monitoring in undiluted matrices, transient coatings have not yet been thoroughly investigated with EC biosensors based on Au electrodes.
The most widely used strategy for preventing NSA in complex samples relied on coating the EC biosensor with fouling-repellent layers (Figure 6E). A wide variety of such materials and coatings were studied, including PEG/OEG thiol SAM, alkylthiol SAM, HA, pHEMA, zwitterionic materials (such as thiols, polymers, peptides), porous films based on cBSA, and DNA (e.g., polyadenine)-based layers [1,8,9,12].
Various approaches have been adopted, depending on the measurement conditions (static or hydrodynamic, single-point measurement versus real-time monitoring) and on the reusability requirements of the biosensor. For real time monitoring in complex biological samples, not only the accuracy but also the biosensor’s stability during long operation periods had to be adequately addressed.
In the following we will discuss in more details a few of the successful strategies applied to measure blood or milk samples, with EC biosensors based on Au or AuNP-modified electrodes. These examples were selected from reports from the last 5 years, wherein NSA was addressed in great detail, and were chosen with the aim of emphasizing the variety of materials available, the manner by which NSA was evaluated, and the impressive antifouling performances derived. It is intended as a snapshot of a rich research area. There are many reports, including recent reviews [1,8,9,12], where interested readers can find detailed investigations of EC biosensors used for measuring various other types of complex samples, beside blood and milk.
3.2. Folding-Based Biosensors for Measurements in Undiluted Samples
Among the various biosensors, folding-based, E-AB biosensors are particularly promising for in vivo monitoring and for measuring in raw samples. They are impacted differently by NSA compared to other types of biosensors. The capabilities of E-AB biosensors (Figure 6F) were demonstrated with in vivo measurements in live animals, carried out with a temporal resolution of seconds and an operational stability of the electrodes of several hours [22].
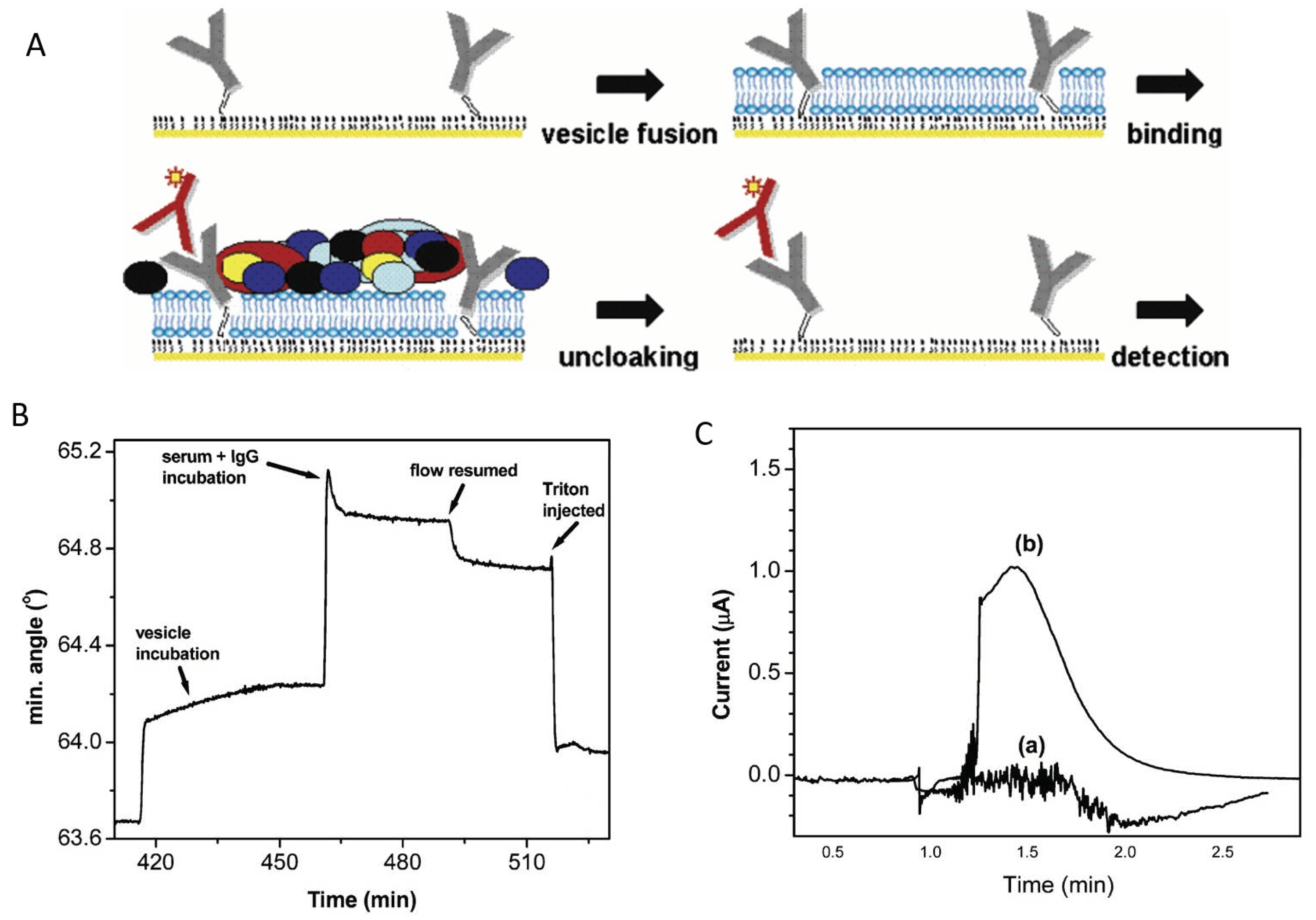
Folding-based biosensors (sometimes called “switches” or “nanoswitches”) contain a bioreceptor (DNA, aptamer, peptide) labeled with a signaling probe, which undergoes a drastic change in conformation upon binding its target. They are rapid, reagentless and reversible, and they can be miniaturized, which makes them attractive for real-time monitoring. In EC folding-based biosensors, the bioreceptor is typically labeled with an electroactive probe such as MB at one end and with a thiol at the other end, for attaching to the electrode surface. The working principle of the biosensor is based on a significant change in the distance and, consequently, in the rate of electron transfer between the bioreceptor label and the electrode when the specific binding of the target analyte occurs, i.e., similar to an ON/OFF switch. The change in bioreceptor conformation by which the electroactive label becomes close to the electrode requires significant energy. Weak interactions with non-specifically bound molecules cannot lead to similar EC outputs. Consequently, the impact of NSA on the biosensor’s output signal is minimized. Nonetheless, in complex media, fouling leads to significant drift of the EC signal and limits the operational time. Taking as a case study the operation of an E-AB for vancomycin in serum, at 37 °C for a week, Watkins et al. [21] emphasized that fouling occurs fast upon exposure to the sample, as the serum proteins first occupy the empty spaces on the electrode surface corresponding to defects in SAM. After the passivation and temporary stabilization of the surface, the serum proteins begin to rapidly displace the thiol molecules, and the quality of the antifouling coating decreases dramatically. The instability of the protective coating on the electrode surface may be amplified in time due to the operational conditions (such as repetitive scans in a potential range favoring the desorption of thiols). This leads to changes in the electrode coverage and properties, promoting further fouling and signal drift (as also shown in Figure 1A) [21].
Various solutions to limit fouling have been implemented to achieve the continuous operation of E-AB biosensors in undiluted media (e.g., blood) for several hours, and even up to several days [21]. These include (i) membranes [100], (ii) liquid diffusion filters [97], (iii) electrode coatings made of SAMs of MCH [100,103,104,105,106,107] or of biomimetic molecules with phosphatidylcholine end groups (Figure 6F, [91,97,107], and (iv) hydrogel overcoatings (Figure 6F [100,108,109]). In addition, optimized EC interrogation methods [110] intended to improve the stability of the sensing layer and drift correction procedures, such as “kinetic differential measurements” (KDM), contribute to minimizing the biosensor drift [22]. The principle of KDM (Figure 6F) exploits the differences in the rate of electron transfer from the redox label to the electrode between the target-bound and unbound state of the labeled aptamer. In the bound state, the electron transfer is very fast. In measurements using SWV, the peak current intensity increases as the redox probe is brought into the vicinity of the electrode, and the electron transfer to the adequately polarized electrode occurs at high speed. The binding of the target analyte is a fast, reversible process, and the corresponding signal gain can be measured when scanning the potential at high frequencies (e.g., 75 Hz [22]), in contrast to the unbound state. At low frequencies (e.g., 7.5 Hz, [22]), the signal corresponding to the unbound state can be measured. The drifts in the signal corresponding to the bound and unbound states are synchronized, and thus the signal of the bound state, measured at high frequency, can be corrected by considering the signal of the unbound state, measured at low frequency. By minimizing the sensor drift this way, the real-time pharmacokinetic monitoring of various drugs in living rats over several hours was successfully achieved.
Each of the above fouling-minimizing solutions has limitations. Interested readers can find details on the folding-based sensors, and their associated challenges and analytical opportunities, in several reviews [2,8,22,23]. Their wider application is limited by the low availability of adequate aptamers for a larger range of analytes [22] and by the challenges in ensuring long-term operational stability [23].
3.3. Antifouling Coatings Used in Electrochemical Biosensors
The most efficient NSA-resistant coatings used in EC biosensors for measuring raw, undiluted complex media include antifouling peptides, alkylthiol SAM or PC-ended SAM (functioning with E-AB sensors), nanocomposite films of cBSA and conductive nanomaterials, as well as zwitterionic materials. In particular, for biosensors based on DNA probes, to ensure resistance to fouling along with the adequate orientation and density of the DNA probe, successful antifouling coatings relied on SAMs of polyaromatic thiolated molecules (such as p-aminothiophenol and p-mercaptobenzoic acid), tetrahedral DNA nanostructures, or ternary layers including a thiolated DNA probe along, with (i) alkanethiols such as mercaptopropionic acid (MPA) and MCH, or (ii) short (three or six C atoms) alkanedithiols and MCH [2]. Table 1 summarizes a few of the successful antifouling coatings used in EC biosensors.

Table 1.
Antifouling strategies for EC biosensors.
3.3.1. Applications of Alkanethiol SAMs in Low Fouling Electrochemical Biosensors
SAMs of alkylthiols with hydroxyl end groups (e.g., MCH, 2-mercaptoethanol, 4-mercaptobutan-1-ol, MCU, etc.) have been widely used to prevent the fouling of EC Au-based biosensors. They are typically combined with carboxyl-ended thiols or thiolated bioreceptors to obtain mixed SAM coatings with optimized probe density and resistance to NSA.
Potential pulse-assisted deposition provides an easy approach to the fast preparation, i.e., in minutes, of efficient biosensing interfaces [114] based on the controlled immobilization of DNA [115], and of hydroxyl-ended alkylthiol SAMs with efficient antifouling protection [115]. The EC deposition of thiols is much faster compared to the classic chemisorption procedure, which involves incubation with a thiol solution for 30 min to 1 day. Moreover, the application of an external electric field can help to obtain defect-free SAMs [116], critical to the resistance to NSA.
MCH, along with BSA and PEG, is often taken as a reference for evaluating the resistance to NSA of EC biosensors. To study the effect of the head group of alkylthiol SAM used in E-AB biosensors operating in blood, urine and sweat [117], MCH was compared to uncharged OEG thiols, monocharged trimethylammonium chloride (AC) and potassium sulfopropylmethacrylate (SP) thiols, and zwitterionic phosphorylcholine (PC) and dimethylammoniopropane sulfonate (AP) thiols. The comparison criteria included wettability, antifouling, baseline stability, and the sensitivity and specificity of the obtained E-AB for the target analytes. The best stability in blood was achieved with PC and AP monolayers, while the best overall performance was achieved with SP and PC-coated sensors [117].
Despite the lower performance, MCH is widely used as a backfilling molecule in EC biosensors, due to the fact that it provides protection against NSA and at the same time forms layers with adequate conductivity for sensitive EC measurements. For example, MCH was used as a backfilling agent with thiolated aptamers to obtain biosensors that measured successfully over several hours in undiluted matrices, such as, e.g., cerebrospinal fluid [118] or serum [21]. Folding-based biosensors passivated with MCH were moreover applied for measuring melamine in flowing whole milk [103], cocaine in undiluted serum [119], interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α) in a microfluidic platform for monitoring the cytokine released from cells [106], doxorubicin, kanamycin, gentamycin, and tobramycin in the blood of awake rats, cocaine and doxorubicin in flowing blood [104], etc.
Nonetheless, the operational stability of the alkylthiol SAMs in undiluted matrices remains limited due to co-occurring fouling and layer desorption. The parameters of the EC measurement have to be optimized to prevent the desorption and oxidation of thiols [23]. Moreover, the inclusion of zwitterionic membranes or hydrogel coatings can add an additional protection against NSA and further improve the stability of the biosensors in serum [21]. The storage stability of alkylthiol-based SAM coatings has been rarely investigated and requires future studies.
3.3.2. Applications of BSA-Based Passivating Films in Low Fouling Electrochemical Biosensors
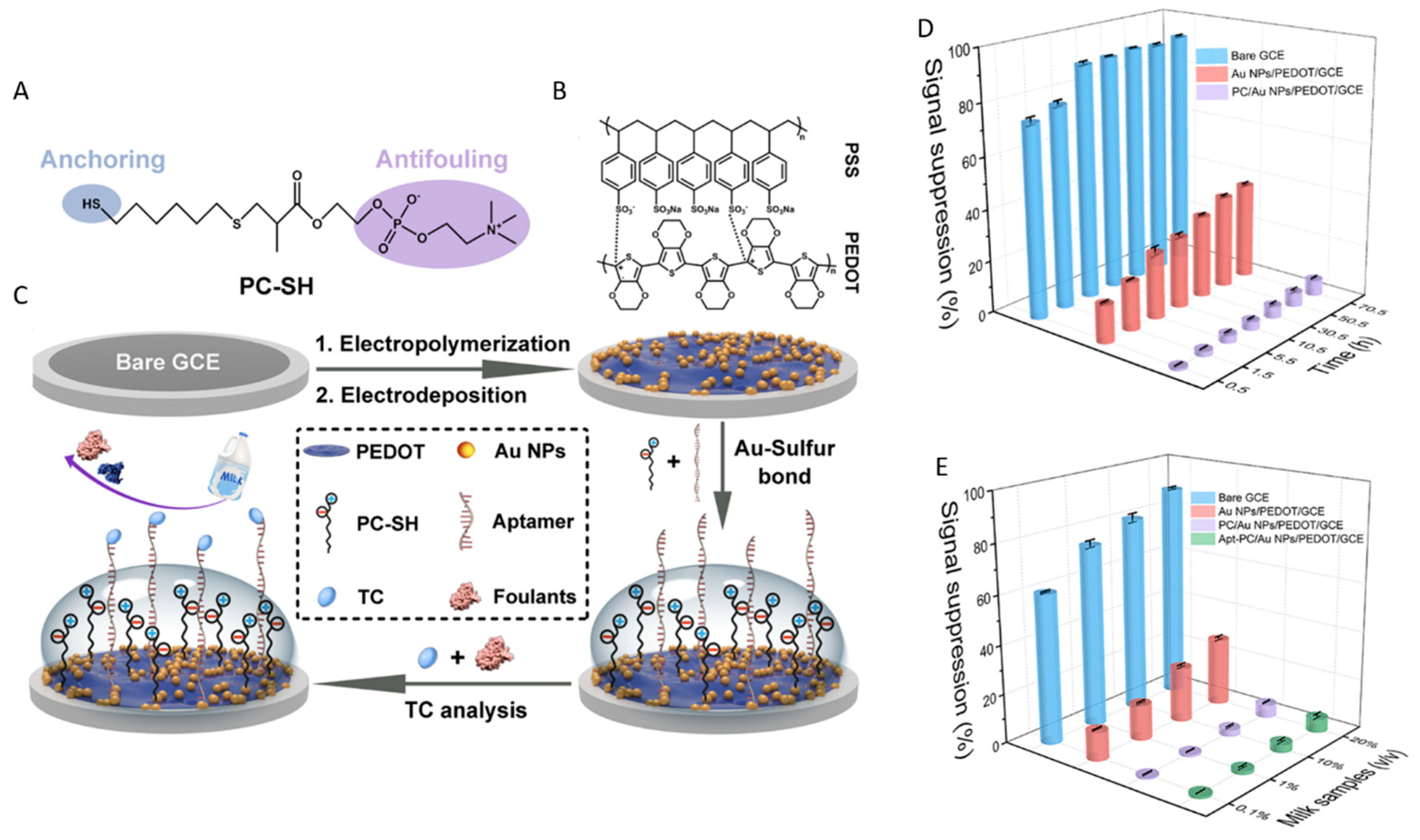
BSA is often used to prevent fouling in biosensors [6]. However, in EC devices, blocking with BSA to some extent also impedes the diffusion of small molecules to the sensor surface due to steric effects. Altogether, this reduces the sensitivity of measurements that rely on exogenous electroactive probes, such as ferrocyanide/ferricyanide, TMB, etc. Moreover, the protective effect of a BSA “blocking” monolayer against NSA can be limited in undiluted complex samples (e.g., serum). Better antifouling properties and stability, along with good conductivity, were achieved with porous, 3D coatings obtained by cross-linking BSA with glutaraldehyde and conductive nanomaterials such as AuNP, AuNW and CNT [83], carbon black [86], MXene [87], aminated reduced graphene oxide [84] or amino ferrocene-modified graphene nanosheets [85]. Besides demonstrating an impressive resistance to NSA in serum, urine or 1% BSA solutions, such coatings present rich functional groups (e.g., carboxyl, amine) and have a high loading capacity with bioreceptors. They are at the basis of sensitive aptamer- or antibody-based EC biosensors used for the detection of various analytes, including cortisol [86], sepsis biomarkers [84], interleukin 6 [83], interleukin-8 and vascular endothelial growth factor (VEGF) [85].
The nanocomposite of cBSA with AuNW (“BSA/AuNW/GA”) reported by del Rio et al. [83] had particularly good resistance to fouling from blood, i.e., a 10% decrease in the EC signal after a 1-month storage period in human plasma at 4 °C. By comparison, planar Au sensors coated with PEG-SAM, betaine-SAM or mixed betaine–PEG-SAM provided significantly lower current densities and lost their performance after 1 day in 1% BSA. The exceptional stability of the BSA/AuNW/GA was attributed to the nanoporous structure of the coating in which the nanowires were recessed by about 4 nm compared to the surface of the BSA film. The nanometer-sized holes acted as a sieve, impeding the diffusion of proteins and allowing small molecules, such as the redox probes used for the EC measurement, to access the NW surfaces. The BSA coating provided substantial functional groups to attach biorecognition elements, as demonstrated by the development of an immunosensor for the detection of interleukin 6 in plasma, showing good stability [83].
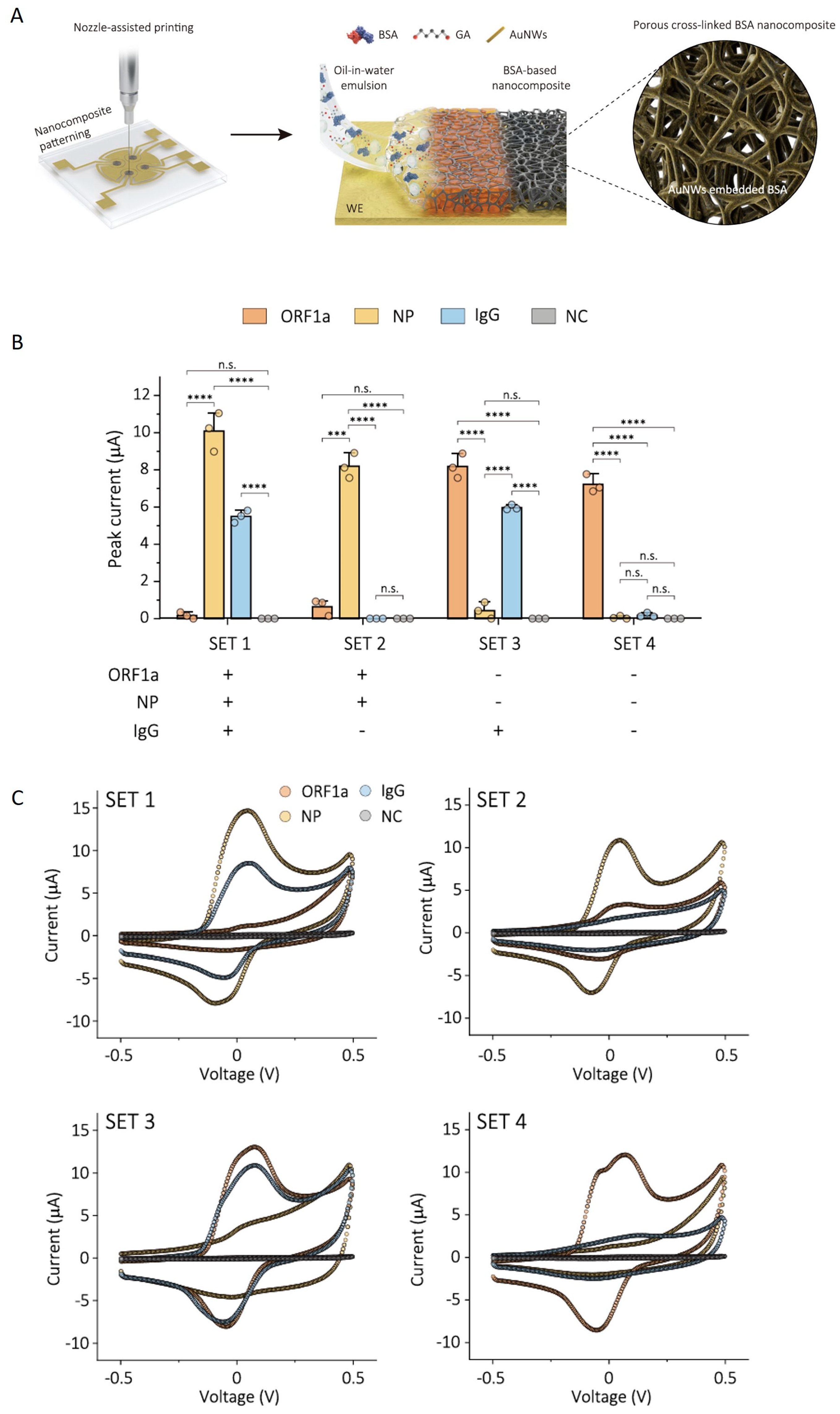
A further advance came with the use of 1 µm-thick nanoporous films of cross-linked BSA and AuNW obtained by the nozzle printing of oil in water emulsions (Figure 7A), which have increased sensitivity and stability compared to the ~10 nm-thick drop-casted coatings of the same material [82]. For example, the sensitivity of the biosensors for three target analytes related to infection with SARS-CoV-2 (the ORF1a gene, nucleocapsid protein and IgG antibody), obtained based on this cBSA nanocomposite, increased 3.75–17-fold compared to that of similar devices developed from 10 nm-thick drop-casted coatings [82].
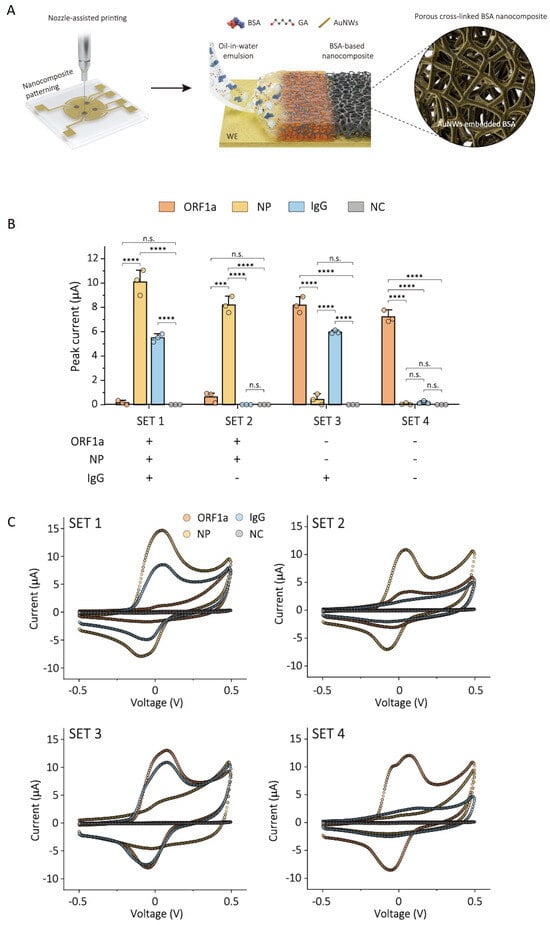
Figure 7.
Electrochemical sensing platform for the detection of SARS-CoV-2 virus, integrating four working electrodes coated with porous antifouling nanocomposite. (A) Illustration of the coating process leading to a thick, nanoporous film of cBSA with embedded AuNW (AuNW-cBSA). (B) Plot of the performance of the AuNW-cBSA sensor for the multiplexed detection of viral RNA (ORF1a), antigen (nucleocapsid protein, NP), and IgG antibody associated with SARS-CoV-2 infection, for four sets of combinations of COVID-19-positive and -negative NPS. NC represents the negative control. Data represented as mean values ± SD (n = 3 independent EC chips). Statistical significance was tested (*** p< 0.0001; **** p < 0.0001; two-tailed Student’s t-test; n.s.: not statistically significant). (C) Cyclic voltammograms recorded with the multiplexed sensors for SARS-CoV-2ORF1a, NP, IgG antibody, and NC. Reprinted from [82]. Creative Commons Attribution 4.0 International License.
The increased sensitivity was associated with the high conductivity and structure of the nanocomposite film, which includes macropores (averaging 1.123 μm, formed by solvent evaporation) as well as mesopores (averaging 9.53 nm, formed as a result of BSA cross-linking) [82]. The coating had excellent antifouling properties, proven by the preservation of the EC signal recorded for ferrocyanide/ferricyanide, after a 1-month storage period in serum, NPS and 1% BSA.
An important advantage of the nozzle printing deposition method is its suitability for localized deposition, which enables the user to address individual electrodes in a biosensor array. Both the individual and multiplexed detection of three biomarkers related to SARS-CoV-2, from NPS and serum, were demonstrated using a four-electrode array coated with the cBSA-AuNW thick film. Besides one electrode that was used as the control interface, the other three electrodes were functionalized with nucleic acid, capture antibody and an antigen, respectively. For the multiplexed detection of the three biomarkers, four sets of serum-spiked NPS samples were analyzed with the biosensor array (Figure 7B,C). The individual analysis of the ORF1a gene, nucleocapsid protein and IgG antibody with the biosensors was performed with a large set of clinical samples, including 60 NPS and 53 serum (positive and negative) samples. The comparison with standard RT-qPCR and ELISA methods revealed excellent specificities and sensitivities. It should be noted here that after the functionalization of the working electrodes with antigen and antibody, they were blocked with a solution of 5% non-fat dry milk in PBS containing 0.05% Tween 20. Moreover, the samples of NPS and serum for the individual analysis of nucleocapsid protein and IgG antibody were diluted 5 and 10 times, respectively, with a 2.5% non-fat dry milk solution. Consequently, the study of Lee et al. [82] illustrates how superior specificity and sensitivity were ensured by a combination of approaches to prevent and evaluate NSA, which included antifouling coatings, surface blocking after functionalization with bioreceptors, the addition of blocking agents to the sample or buffer, and the inclusion of a control electrode.
In another approach, a GCE electrode was modified with MXene, cBSA and polyethyleneimine (PEI). By coupling the negatively charged BSA with the positively charged PEI, the hydrophilicity and resistance to fouling in serum, at various salt concentrations and pH values, were significantly improved, compared to the BSA-based coating alone. The resistance to fouling in 20% serum, adjusted to various pH values, was best at pH 6 and 8, but decreased at acidic pH, i.e., the signal suppression rate was almost double at pH 2. The properties of the cBSA-PEI-MXene coating were demonstrated in a biosensor used for the detection of the ovarian cancer biomarker CA125 in human serum [120].
cBSA films have numerous advantages, including (i) the easiness of deposition by, e.g., drop-casting, (ii) compatibility with printing methods, (iii) the possibility of including other additives (e.g., PEI) to improve the resistance to NSA, (iv) the richness in functional groups facilitating the attachment of a high number of bioreceptor molecules, and (v) a demonstrated resistance to fouling in serum. However, as also shown by the study of Li et al. [120], these coatings may provide more limited antifouling protection in undiluted samples with different compositions and pH levels.
3.3.3. Applications of Zwitterionic Materials in Low Fouling Electrochemical Biosensors
Zwitterionic polymers and thiols provided efficient protection against NSA in EC biosensors measuring various types of samples, including blood and milk (Table 1). The coatings were attached to Au interfaces by (i) chemisorption [42,107,112], (ii) EC deposition, either directly [121] or after conjugation with monomers such as EDOT or pyrrole [122,123], or by (iii) mixing zwitterionic molecules with dopamine and carrying out the chemical polymerization of dopamine [67].
A zwitterionic copolymer called “Zwitter-repel”, with sulfobetaine, carboxylic, aldehyde, and thiol functional groups, was attached to Au electrodes by chemisorption, producing ∼16 nm thick coatings with excellent conductivity, hydrophilicity (contact angle: 15°) and antifouling properties, as well as versatile functionalization [112]. The coatings had good antifouling properties, diminishing the levels of adsorbed protein, after 1 h of incubation with human plasma spiked with 1% radiolabeled HSA-125I, to 38 ± 1 ng cm2 compared to 116 ± 21 ng/cm2 for bare Au [112]. Two biosensors were developed, achieving the detection of redox-labeled DNA in undiluted plasma down to 21 nM by SWV and the detection of SARS-CoV-2 pseudovirus down to 104 cp mL−1 in 50% saliva by EIS, respectively [121]. While good recoveries were noted for four samples of serum spiked with MB-labeled DNA, the accuracy of the biosensor remains to be demonstrated with larger sets of samples, by comparing with standard methods.
In another study, MPC was attached via a 1,6 hexanedithiol linker by chemisorption to Au NP on the surface of PEDOT and polystyrene sulfonate (PSS)-modified GCE [42] (Figure 8).
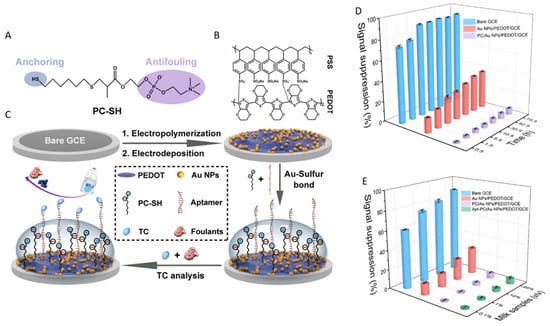
Figure 8.
(A) The structure of PC-SH (i.e., MPC conjugated to 1,6 hexanedithiol linker) including both surface anchoring and antifouling capabilities. (B) Chemical structure of PSS-doped PEDOT. (C) Graphical illustration of biosensor development steps. (D) Variation of the antifouling capacity of bare GCE, Au NPs/PEDOT/GCE, and PC/Au NPs/PEDOT/GCE exposed to 1% milk. (E) The resistance to NSA of bare GCE, Au NPs/PEDOT/GCE, and PC/Au NPs/PEDOT/GCE exposed for 30 min to 0.1%, 1%, 10%, and 20% milk. Reprinted from [42], with permission from Elsevier.
Along with the antifouling molecules, thiolated DNA aptamers for tetracycline were also immobilized on the sensing interface (see Figure 8C). Based on the average signal suppression of 6.1% when exposed to 1% milk for 70.5 h, the biosensor showed efficient antifouling protection and good stability [42]. Both the aptasensor and a control electrode were tested and showed similar fouling when incubated for 30 min with 0.1–20% milk (Figure 8E). The coatings’ resistance to NSA was confirmed by confocal microscopy tests, showing negligible fluorescence after 1 h incubation with 1.0 mg/mL FITC-labeled BSA. The Apt-PC/AuNPs/PEDOT/GCE biosensor had a linear range of 0.05–100.0 ng/mL, adequate for measuring tetracycline in milk samples diluted 1:100 with buffer. The biosensor’s accuracy was confirmed by both the good recoveries obtained for tetracycline-spiked samples and by comparing the results to those obtained in parallel by HPLC [42].
Monolayers with phosphatidylcholine end groups, mimicking the cell membrane, were moreover shown to efficiently prevent NSA in undiluted blood and perform better than MCH SAM, as discussed also in Section 3.3.1. When integrated into E-AB biosensors, such layers reduced the sensor drift from 70% (with MCH) to less than 10% over a period of 12 h, and enabled the accurate monitoring of doxorubicin in flowing blood [91]. There was also a higher signal gain in the presence of the target analyte for PC-ended layers compared to MCH and MCU [91].
The recently proposed biomimetic 3-aminopropyldimethylamine oxide (APDMAO)-based polymers and hybrid materials (e.g., with polydopamine) demonstrated very promising antifouling capacity in human serum. They were used as a coating in electrochemical biosensors, e.g., for the detection of alpha-fetoprotein [67].
3.3.4. Applications of Antifouling Peptides in Low Fouling Electrochemical Biosensors
Peptides have filled a variety of roles in EC biosensors, as a bioreceptor, enzymatic substrate or electrode modifier to add antifouling abilities to the biosensor interface [1]. When the aim was to ensure protection against NSA, the peptides were included not only as single sequences, but also (i) conjugated to bioreceptors such as DNA strands [44,124] or (ii) as multifunctional, complex structures playing simultaneous recognition, support, antifouling and even antibacterial roles [69,70,71,72,125].
The diversity of peptides used in EC biosensors, at least offering protection against NSA, and the complexity achieved in peptide structures are impressive [1]. There are U-shaped [70,126], cyclic [75], branched [127], Y-shaped, straight and loop-like peptides [73] and peptide nanoparticles [79]. Readers interested in understanding in more detail the EC peptide-based biosensors are referred to recent reviews [1,10].
Besides being used as part of multifunctional peptides, antifouling peptides have been used in conjunction with antibodies or aptamers to measure various biomarkers in blood or serum. For example, such biosensors were applied to analyze, e.g., aminopeptidase N and human hepatocellular carcinoma cells [125], N-gene (nucleocapsid phosphoprotein) of SARS-CoV-2 [74], T4 polynucleotide kinase [43], SARS-CoV-2 receptor-binding domain (RBD [75], PSA [41,71], breast cancer marker ErbB2 [128], alpha-fetoprotein (AFP) [126], extracellular signal-regulated kinase 2 (ERK2 [45], IgG [43,129] mucin-1 [124], PSA [130], CA-125 [44], as well as proteins in saliva [127] or small molecules, such as antibiotics, in milk [69,70] or other food matrices [72].
The following examples represent some detailed investigations of NSA-resistant, peptide-based biosensor coatings, as well as generic approaches to obtaining efficient biosensors based on antifouling peptides.
A branched peptide with antifouling properties, resistance to enzymatic hydrolysis, and HER2 recognition properties was immobilized on a GCE electrode that was previously modified with PEDOT, and coated with a self-assembled bilayer of distearoylphosphatidylethanolamine–PEG) (DSPE-PEG) [93]). The resulting biosensor had very good resistance to fouling, as demonstrated by extensive tests with undiluted serum, blood, sweat, milk, and concentrated solutions (20 mg/mL) of proteins (HSA, BSA, and Mb) and small molecules (UA, DA, and 5-HT). After 2 h of incubation in these matrices, the signal suppression rate measured by DPV for both the biosensor (HER2/DSPE-PEG/PEDOT/GCE) and control (DSPE-PEG/PEDOT/GCE) interfaces was lower than 6%, as indicated by the plots provided by the authors. Tests with small molecules at high concentrations and with several biological matrices, while very relevant, are rather rare in the biosensor literature. The resistance to NSA was moreover proven by the lack of fluorescence in the images of the biosensor and control interfaces after incubation with 0.2 mg/mL FITC-BSA for 12 h. The sensitivity of the biosensor for the detection of HER2 in fetal bovine serum (FBS) was very similar to that in PBS buffer. When incubated in undiluted human serum, the signal suppression rate progressively increased, surpassing 10% after ~5 days and reaching 17.7% after 20 days. The signal suppression rate after 1 h of incubation (same duration as used in the actual measurements) was ~3%, as estimated from the plots provided by the authors. The peptide’s resistance to enzymatic hydrolysis, ensured by including in its structure D- instead of L-amino acids, was confirmed by tests with carboxypeptidase Y. Seven undiluted clinical serum samples were analyzed, and the results obtained with the biosensor are in agreement with those derived from the established ELISA approach.
Altogether, this report provides an excellent illustration of a thorough investigation of antifouling capabilities that could serve as model for other biosensor developers. While the investigations were performed with GCE electrodes, the authors have shown that DSPE-PEG bilayers can also be deposited on Au interfaces, imparting a hydrophilic character (contact angle: 14.3 ± 2.1°) that becomes even more pronounced (contact angle: 7.2 ± 1.4°) after attaching the peptide. Importantly, the combination of peptide and DSPE-PEG also improved the resistance to NSA, as verified by the low signal suppression rate measured by EIS after 1 h of incubation in FBS, i.e., 1.2% compared to 7.15%, 5.3% and 619.1% for Pep/Au, DSPE-PEG/Au and bare Au, respectively. It is therefore reasonable to anticipate that such similarly effective antifouling biosensors will be developed with Au electrodes in the future.
Another interesting development concerning peptides refers to their conjugation with DNA by click chemistry, which facilitates the design of universal strategies for surface functionalization for obtaining low-fouling biosensors. In a representative example, a peptide with the ability to recognize IgG and antifouling characteristics was conjugated with a DNA sequence of polyadenine–polythymine (“PoliA15-polyT5”) [43]. The immobilization of the peptide–DNA conjugate on the AuNP-modified electrode surface relied on the strong adsorption of polyadenine on Au (Figure 9A,B). The PolyA15-polyT5-peptide/AuNPs/PEDOT/GCE-coated interface was hydrophilic (contact angle: 16.18°) and had good resistance to NSA, as indicated by the negligible fluorescence of surfaces incubated for 2 h with 0.2 mg/⋅mL FITC-BSA. Moreover, the biosensor had improved antifouling properties compared to control interfaces containing exclusively the peptide or the DNA, i.e., 8.5% signal suppression after 30 min incubation with undiluted human serum, compared to ~11% (for Pep/AuNP/PEDOT/GCE) and ~21% (DNA/AuNP/PEDOT/GCE), as estimated from the plots provided by the authors. While specific information on sample preparation was not provided by the authors, to bring the serum samples into the linear range of the biosensor, the samples had to be diluted at least 1000 times. Given the biosensor’s resistance to NSA from undiluted serum and the high dilution factors involved, the biosensor’s accuracy was not affected by fouling. This was demonstrated by spiking experiments and comparison with the standard immunoturbidimetry method [43].
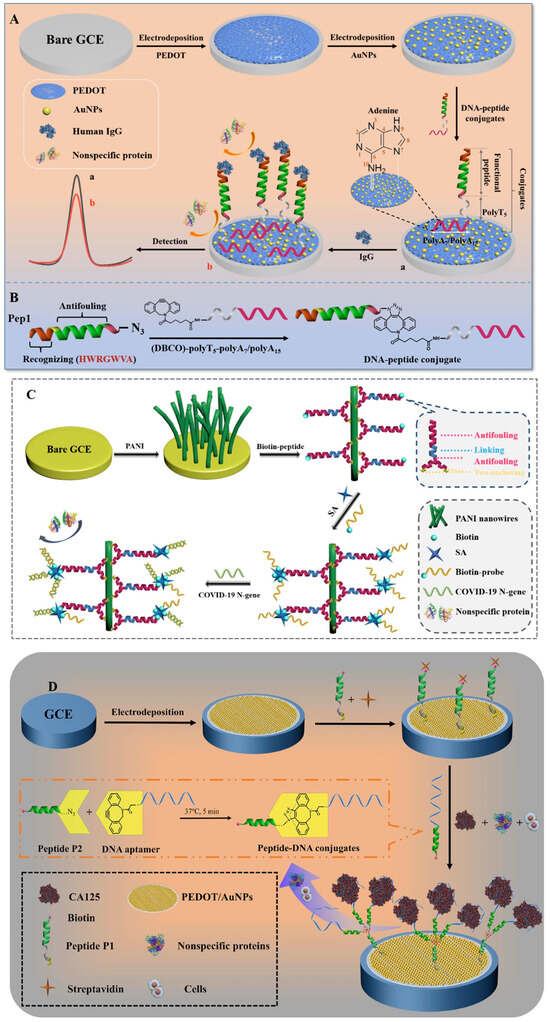
Figure 9.
(A) Graphical representation of the steps of development of the biosensor for the detection of IgG. (B) The synthesis of DNA–peptide conjugates used in the IgG biosensor by click chemistry. (C) Schematic representation of the fabrication process of the genosensor for the detection of COVID-19 infection, based on DNA–peptide conjugates obtained by avidin–neutravidin affinity. (D) Graphical illustration of the development of the biosensor for the detection of CA125 based on DNA aptamer–peptide conjugates obtained by click chemistry and attached to peptide-coated electrodes by streptavidin–biotin affinity. Reprinted from [43] (A,B), [74] (C) and [44] (D), with permission.
Alternative approaches for conjugating DNA bioreceptors to antifouling peptides relied on biotin–avidin affinity ([74], Figure 9C) or click chemistry ([44], Figure 9D). To assemble a genosensor for the detection of the SARS-CoV-2 N-gene, a GCE was modified with electropolymerized polyaniline, onto which a layer of Y-shaped peptide was attached. The peptide had two anchoring points for binding to the surface and terminal biotin groups [74]. By further modification with streptavidin and a biotinylated DNA probe specific for the SARS-CoV-2 N gene, a peptide–DNA conjugate was obtained (Figure 9C). The conjugate had antifouling properties given by the peptide and a specific recognition capacity due to the DNA probe.
In the biosensor used for the detection of CA125 [44], an antifouling peptide (“P2”, Figure 9D), with the sequence QEQKQEK, labeled with biotin at the Q end and with azide at the K end, was “clicked” to a DNA aptamer for CA125, tagged with 5′-dibenzo cyclooctyne (DBCO) at its 5’ end. Meanwhile, the GCE surface (coated with PEDOT and AuNP) was modified with a 12-amino acid antifouling and biotinylated peptide (“P1”), and streptavidin was subsequently attached. Thanks to its biotin label, the resulting aptamer-peptide conjugate was strongly attached to the sensor surface by biotin–streptavidin affinity. The aptasensor obtained by this design resisted the NSA from 100% human serum, sweat and urine. The ability to resist fouling was attributed to the hydrophilicity of the sensing interface (contact angle: 12.2°) combined with the electrical neutrality of the peptides, which discouraged hydrophobic and electrostatic interactions altogether. Additional measurements by fluorescence microscopy after challenging the aptasensor with MCF-7 cells and with a DNA sequence labeled with the fluorescent dye Cyanine 5 (Cy5) confirmed its high capacity to resist NSA, compared to the sensor without the peptides and aptamer [44]. The antifouling layer also had good stability, as emphasized by the negligible decrease in the DPV signal after 48 h in 20% human serum. Finally, the aptasensor provided accurate measurements of CA 125, as demonstrated by the analysis of five samples of human serum, by comparison with the standard electrochemiluminescence test implemented in the hospital [44].
By changing the peptide and DNA probe and exploiting the conjugation by click chemistry and biotin–neutravidin affinity, various sensing configurations can be achieved. While the above examples and the data in Table 1 emphasize the tremendous potential of antifouling peptides in biosensors, in practice, this should be balanced against the necessity of performing chemical synthesis (with associated costs) and the low conductivity of peptides.
3.3.5. Applications of Other Antifouling Coatings in Low Fouling Electrochemical Biosensors
Polyadenine coatings had better resistance to fouling from clinical plasma samples, compared to MCH or BSA-coated coatings, and were integrated in a peptide-based biosensor for the detection of rituximab in the plasma of patients suffering from lymphoma [113]. The biosensor reached a detection limit of 35.26 ng/mL and provided accurate results, confirmed with a commercial ELISA kit.
Conductive coatings inspired by cell membranes have also been proposed to minimize NSA in EC biosensors. To simulate the architecture of the cell membrane, Huang et al. [92] modified an electrode with AuNPs and a SAM of SH-PEG-cholesterol, onto which they attached a phospholipids layer and 2-MPC ampholytes. While the base layer provided conductivity, the interface’s hydrophilicity increased upon functionalization with phospholipids and ampholytes (contact angle: 27.4°). The hydration layer created at the interface between the sensor and the sample solution ensured high resistance to NSA. The antifouling capacity was demonstrated with biological fluids (whole blood, serum and urine), and was an important element in the development of accurate aptasensors for the detection of thrombin in serum or cocaine in blood. The analysis of a set of clinical samples provided results in agreement with those obtained by the corresponding standard methods (ELISA and LC/MS, respectively) [92].
Hydrogels obtained from different materials have been used as the main protective layer or as an overcoat to increase the stability of EC biosensors in complex media [108,131]. Among the most remarkable endeavors, Chan et al. [108] reported the high-throughput evaluation of a library of 172 polyacrylamide copolymer hydrogels, to be used as an overcoat for E-AB biosensors passivated with MCH. To account for variations in the origin of biological samples, the assessment of the coatings was based on platelet assays, performed by incubation in 50% fetal bovine serum for 24 h at 37 °C, followed by 1 h in platelet-rich rat plasma at 37 °C. Moreover, the detailed study included in vitro forced degradation studies to determine the best hydrogel (F50-C50) and analysis by machine learning to find correlations between the properties of the used monomers and the resistance to NSA of the resulting hydrogels. The in vitro studies emphasized reduced platelet adhesion on hydrogel-coated interfaces compared to control (PEG-coated) or bare Au, in both stationary conditions and in flowing blood. The hydrogel-coated biosensor was used for the real time monitoring of kanamycin in living rats. It was shown that in vivo, although performing much better than PEG-coated sensors, the biosensors protected with F50-C50 copolymer lost on average 33.8% of the signal over 200 min of operation [108]. While the study highlights the great challenges faced by biosensors used in vivo, this type of systematic approach is expected to lead to the discovery of more efficient coatings.
As a corollary of the antifouling strategies described above for EC biosensors, it should be emphasized that often, several materials are combined to obtain the necessary protection against NSA (e.g., PEG and antifouling peptides [129]), and additional measures are taken to ensure accurate measurements, e.g., sample pre-treatment, supplementary blocking of the interface, or over-coating the interface.
Highly sensitive detection with EC biosensors requires sensing interfaces with low impedance. Consequently, conductive polymers and nanomaterials such as PEDOT, polyaniline nanowires, AuNW, reduced graphene oxide, etc., were integrated into the antifouling coatings based on peptides, BSA or other low-electrical conductivity materials [79,82,83,84,131]. Such combinations lead sometimes to unexpected benefits in terms of protection to NSA. For example, the tridimensional porous structure of cBSA-AuNW or cBSA-reduced graphene oxide coatings acted as a diffusion filter to repel large foulant proteins [82,83,84], in addition to the expected properties of antifouling, good conductivity and high loading capacity.
3.4. Challenges in the Development of Low Fouling Electrochemical Biosensors
The stability of some of the EC biosensors summarized in Table 1 was found to be adequate for 5–12 days when stored in buffer at 4 °C [42,73], and even up to 1 month when stored in 1% BSA at 4 °C [83]. When stored in real matrices, there was a progressive degradation in signal due to fouling.
Determining the time interval after which fouling significantly affects the accuracy of the biosensor from the data presented in the articles is not straightforward. First of all, in these studies, fouling was not routinely evaluated under the same conditions of time duration, sample dilution and temperature as those used for the actual quantitative determination of analytes. In many studies, the analytical signal is represented by the change in signal measured by the same procedure as the one used for fouling, e.g., by DPV, using ferricyanide/ferrocyanide, before and after incubation with the sample. Signal suppression under 10% is often presented as proof of insignificant fouling; however, as inferred from the data presented, the linear range of the biosensor corresponds to comparable relative changes in the biosensor signal (e.g., 4% and less than 15% for the lower and upper limits of the linear range of the biosensor in [42]). In consequence, the ultimate proof that the biosensor’s accuracy is not affected by fouling when analyzing real samples comes from the comparison with standard methods. However, with few notable exceptions (e.g., [82]), the set of analyzed samples was limited to five to seven (Table 1). Promising NSA-resistant biosensors should be evaluated with larger sample sets.
Another challenge for the future concerns the storage of the EC biosensors with antifouling coatings over longer periods of time, in a dry state. While recent literature lacks specific data [10], this aspect is important for the future commercialization of the biosensors. Moreover, the regeneration of the biosensor is rarely addressed and has shown a low number of potential re-uses (e.g., seven tests [73]). Given the complexity of preparation of some antifouling coatings of disposable biosensors, adequate consideration should be given to sustainability and cost benefits.
The recent studies summarized above reveal great potential for more efficient antifouling solutions for EC biosensors. First of all, the design and a deeper understanding of the features determining good resistance to NSA can be inferred from molecular simulations, high-throughput screening strategies and data analysis by machine learning. Also, while earlier studies concentrated mostly on proteins, some recent studies included small molecules at large concentrations, in addition to several proteins and real matrices [93]. Another encouraging trend is the careful characterization, in studies reporting new antifouling layers, of the effect on the resistance to NSA of (i) functionalization with the bioreceptor and (ii) real samples with different dilution factors, over short and longer times.
4. Solutions to Minimize NSA in SPR Biosensors
4.1. Surface Sensitivity in SPR Biosensing
SPR biosensing is representative of a powerful class of surface-sensitive techniques that enable the trace and ultra-trace detection of various analytes through affinity pairing [30,33,34].
SPR enables to evaluate, in real time, the biosensor development steps (Figure 10). The amount of mass collected at the sensing interface may be estimated from the magnitude of the resulting change in the SPR signal, or calculated based on calibration curves. Alternatively, the whole characteristic SPR intensity versus incidence angle curve (the reflectivity curve) can be analyzed. This provides a wealth of information about molecular changes occurring at the surface related to the accumulation of compounds over the surface or the degradation of the sensor matrix, which are not effectively revealed by a change of the position of the SPR dip [32,132]. Since the position (i.e., the SPR angle) and the shape of the SPR dip can be accurately described by theory (e.g., Transfer Matrix approaches [133,134,135]), this provides the ability to assess and optimize the sensor structure, including the assessment of antifouling stability.
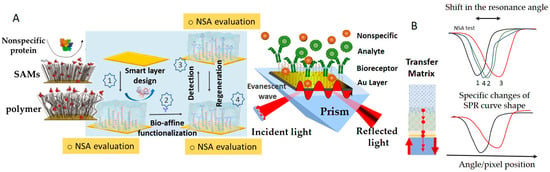
Figure 10.
(A) Graphical representation of the development/evaluation steps of the SPR biosensor with smart layer design and Kretschmann configuration. (B) Shifts in the resonance angle versus full dip assessment using Transfer Matrix approaches for quantitative SPR assays and NSA control. Numbers associated to the curves with different colors correspond to steps indicated in (A).
Surface regeneration is an important element in achieving cost- and labor-effective biosensors. As shown in Olaru et al. [132], NSA can induce the decline of the sensor surface as a function of the number of experimental cycles. While the method was here tested on L1 SPR sensor chips, its amenability to assess the quality of other types of SPR chips is advocated.
4.2. Smart Layers in the Design of SPR Surfaces
The NSA of sample components at the functional interface of the SPR biosensors is a significant barrier to the full implementation of SPR technology in the analysis of complex media, such as blood samples or, alternatively, milk. Smart layers coating the metal surface, resistant to fouling and to which bioreceptors can be immobilized in a controlled, oriented and optimized manner, represent a critical feature of SPR biosensors operating in complex media [15]. Many state-of-the-art strategies of SPR biosensors involve efficient ways to prevent biofouling [14]. Antifouling strategies implemented in SPR sensing in real samples are summarized in Table 2.

Table 2.
Smart layers and the performance of SPR biosensors.
Typical linker anchors used in SPR coatings include alkanethiolate SAMs, carboxymethylated dextran, and alkoxysilane SAMs that are made compatible with subsequent ligand random or oriented immobilization avenues, e.g., EDC/NHS, aldehyde and epoxy, maleimide reactions or biotin–streptavidin, click chemistry, His-tag and A/G protein interactions [30].
In terms of the material properties of these “smart layers”, several types of compounds were reported to show good anti-fouling properties—materials containing hydrophilic functional groups (PEG, zwitterionic compounds, polysaccharides, polymer brush surfaces, polyelectrolyte multilayers) [143], DNA nanostructures, biomimicking materials [15], SAM coatings [144] and nanomaterials [61]. The material’s ability to be functionalized with different reactive groups able to attach a ligand is a prerequisite. As shown for PEG, the optimization of molecular weight, concentration and chain length is also needed to achieve appropriate surface densities of the grafted layer [145].
Considering the diversity in the samples’ compositions and pH levels, the surface coating has to be adapted for each application [80]. There is no universal solution to achieve the efficient minimization of NSA of proteins and other components from real samples. This aspect is widely depicted in application-oriented reviews that deal with ways to prevent biofouling, specifically for SPR biosensors used in clinical analysis [3,14,142], cancer detection [146], virus detection [147] or food safety [95,142].
These recent reviews confirm that smart layer design is essential to achieving advancements in SPR biosensing technology that lead towards novel multiplexing concepts, continuous monitoring and in vivo sensing [15]. To this end, suitable protocols for evaluating the antifouling performance, as well as the changes in the resistance to NSA after functionalization, are prerequisites [148]. When reacting with a ligand, the effect exerted by surface functionalization on the resistance to fouling depends on both the type of coatings and the nature of the ligand [52]. As emphasized by the thorough study of Vaisocherova et al. [52], an efficient coating should enable the immobilization of adequate levels of bioreceptors, and at the same time preserve good resistance to NSA in its final sensing configuration, i.e., after functionalization [52].
For SPR assays, NSA levels of less than a few ng/cm2 are typically used as a quantitative indicator to designate coatings with ultralow fouling. In most SPR biosensors, the verification of antifouling properties of the “smart” coatings following their modification with bioreceptors as proposed by Vaisocherova back in 2008 [149] is seldom reported. This happens despite numerous studies cautioning on the significant effects of particular functionalization on a biosensor’s resistance to the NSA of the components of blood plasma [38]. In biosensor studies, BSA is typically used as a proxy for protein foulants [150]. Low adsorption must be demonstrated at high concentrations of interfering proteins, e.g., BSA at up to 50 mg/mL.
Notably, while the SPR biosensors-based literature is vast, there are only a handful of examples of “smart layers” appropriate for the design of SPR sensors that work in undiluted biological samples that provide quantitative estimations of the antifouling effectiveness in every stage of biosensor development (including after their modification with bioreceptors). This review aims to present these.
4.2.1. Applications of DNA-Based Coatings (Thiol-Ended DNA Tetrahedrons and Polyadenine) in Low Fouling SPR Biosensors
A “smart layer” was obtained by incubating a gold interface with thiol-ended DNA tetrahedron probes (to covalently attach them by chemisorption), followed by backfilling with MCH [137]. The resulting layer prevented fouling from proteins (hemoglobin, at 1 mg/mL and HSA at 48 mg/mL), complex samples (100% serum, 100% plasma, 9.85 × 108 red blood cells/mL, 5% whole blood) and cell lysate. In addition, it resisted the NSA of Au NPs that were used in a catalytic growth-based amplification mechanism. The DNA tetrahedron/MCH coating was compared to the classical ssDNA/MCH approach in a biosensor for the detection of miRNA. A high antifouling ability was revealed for the biosensor exposed to different samples, i.e., adsorption levels less than 8 ng/cm2, compared to 41.5–150.3 ng/cm2 for ssDNA/MCH [137]. Additionally, the results of the analysis of miRNA from cancer cells are in accord with those from qRT-PCR [137], confirming the accuracy of the DNA tetrahedron- based biosensor
Polyadenine-conjugated bioreceptors offer a convenient solution for the simple development of antifouling biosensors. Huertas et al. [138] compared the antifouling and biosensor performances when coating the sensor interface with polyadenine-conjugated antibodies or DNA with alternative approaches, whereby the bioreceptors were either (i) covalently immobilized based on SAMs of mixed thiol, (ii) physically adsorbed (i.e., the antibody for CRP), or (iii) chemisorbed (DNA probe specific for Fas567 gene mRNA). Polyadenine-conjugated bioreceptors enabled the oriented immobilization of bioreceptors, optimum biosensor sensitivity, and the highest antifouling resistance (to 100% serum). Compared to thiol-SAMs with covalently immobilized antibodies and DNA, the NSA from serum decreased by 20.5% and 40.1%, respectively. The polyadenine-based coatings were also stable during surface regeneration. Thus, the biosensor for CRP preserved 62% of the initial signal after 15 regeneration cycles, while the biosensor for Fas567 showed less than 3% variation in the signal after 25 hybridization/regeneration cycles.
4.2.2. Applications of Zwitterionic Compounds in Low Fouling SPR Biosensors
The antifouling performance of zwitterionic materials is well demonstrated [60,151]. Polymeric zwitterionic brushes with thicknesses ranging from 20 to 40 nm, adequate for use as coatings for SPR biosensors, significantly reduce the adhesion of proteins and cell attachment [60,152].
Poly(carboxybetaine acrylamide) (pCBAA) brushes prepared by atom transfer radical polymerization (ATRP) were coated on a Au interface as a step towards an SPR biosensor for the measurement of miRNAs in erythrocyte lysates [153]. The 40 nm thick polymer brush layer was suitable for the immobilization of ~9.8 × 1012 oligonucleotide probes/cm2, and has shown ultralow fouling (<2 ng/cm2) when exposed to erythrocyte lysate samples. High sensitivity (i.e., detection limits of less than 0.5 pM miRNA without the need for miRNA extraction) was reached by integrating this coated interface in a sandwich assay format. To this end, besides the capture oligonucleotide immobilized on the sensor surface, the detection system includes biotinylated oligonucleotides complementary to the target miRNA and streptavidin-modified gold nanoparticles. This rather complex experimental protocol is important since the sandwich assay mixes different materials and interactions, and the careful evaluation of each experimental step is of paramount importance.
An effective coating used as the basis of an biosensor for miRNA detection in whole blood plasma was developed, consisting of a copolymer of carboxybetaine methacrylamide (CBMAA) and N(2hydroxypropyl) methacrylamide (HPMAA), with 15% CBMAA [38]. Upon functionalization with a specific DNA probe by carbodiimide chemistry followed by the deactivation of residual NHS esters with glycine, the coating showed an improved antifouling capacity and sensitivity for the detection of miRNA compared to similar biosensors that relied on a polymer of CBMAA and OEG SAM. The deactivation of residual NHS esters with glycine was essential for recovering the resistance to NSA of the copolymer brush after the steps of activation and attachment of bioreceptors. [38].
A significant advantage of zwitterionic materials for SPR smart sensor development is the possibility to control their synthesis, since alterations in the compositions and chain lengths enable the tuning of antifouling performance [151]. Moreover, hybrid materials, e.g., with peptides and polymers, and composite layers including several types of zwitterionic polymers, can provide improved performance in SPR biosensors.
4.2.3. Applications of Other Antifouling Coatings in Low Fouling SPR Biosensors
Various materials, including lipid vesicles, hydrophobic materials and polymer–peptide hybrids with opposite charges, were recently explored to prevent fouling in SPR biosensors. More recently, McKeating et al. proposed a lipid layer of positively charged ethylphosphocholine (EPC) vesicles over interfaces functionalized with protein A and 3-mecapto-1-propanol (MPO,) with net negative charge, as alternative coatings for SPR biosensors [154]. Following incubation with undiluted serum or plasma, the adsorbed foulants were removed by simply rinsing with a buffer. Proof-of-principle demonstrations were provided for the detection of IgG and cholera toxin in undiluted serum with biosensors incorporating EPC/Protein A-MPO layers [154]. A follow-up study revealed the importance of having a high-density base layer of protein A, in order to provide strong electrostatic interactions with EPC and lipids and promote their tight attachment. A tightly bound, rigid lipid layer was associated with optimum resistance to fouling in undiluted serum [155].
Bellassai et al. [140] developed a coating with mixed positive and negative charges that drastically reduced the fouling from 10% diluted serum and milk samples in an SPRI setup. Au surfaces were modified with poly-L-lysine (PLL), to which the CEEEEE peptide, with cysteine at its NH2 end, was attached via a maleimide linker. First, the contributions of individual components of the coating to the prevention of fouling were thoroughly investigated. The covalently attached peptide reduced the amounts of adsorbed material from serum from 381 ± 31 ng/cm2 to 46 ± 34 ng/cm2. It provided improved protection compared to simply adsorbed peptide and PNA or a basic PLL layer (535 ± 17 ng/cm2). The modification with PNA did not affect the resistance to fouling. In a later study, the coverage with a specific aptamer was optimized for the detection of lysozyme in diluted milk [139]. Aside from the aptasensor, control interfaces were also evaluated. These were functionalized with a DNA sequence of the same length and similar percentages of adenine, thymine, cytosine and guanine in its composition as the lysozyme aptamer. Studies with several proteins (myoglobin, cytochrome C and BSA, at 1 µg/mL and 10 µg/mL) and with lysozyme-spiked milk or serum emphasized a significant matrix effect. In consequence, the standard addition method was applied, instead of external calibration to calculate the amounts of lysozyme in spiked samples. However, the accuracy of the biosensor remains to be confirmed by analyzing a larger set of samples and by comparing the results with those provided by standard analytical methods.
A recent review [156] summarized the characteristics of plasmonic biosensors for the detection of autoimmune diseases. It emphasized biosensors used for measuring antibodies, cytokines and miRNA in undiluted (rarely) or diluted (1:10 to 1:8000) serum and urine. Among them, the biosensor operating in undiluted serum developed by Malinick et al. [141] detected antibodies against gangliosides, relevant for multiple sclerosis. This was achieved by a combination of (i) a smart designed interface, (ii) a surface blocking step and (iii) the implementation of machine learning-assisted data interpretation. An almost superhydrophobic layer was obtained by coating the gold interface with a 1–3 nm layer of silica, followed by functionalization with 1H,1H,2H,2H-perfluorodecyltrichlorosilane. Gangliosides were strongly adsorbed to the surface to obtain a gangliosides array for SPRI. The antifouling properties of the biosensor arrays were attributed to the hydrophobicity of the undercoating and the selectivity of the sialic groups in the recognition site of the gangliosides. The NSA was further minimized by injecting 10% serum before the actual undiluted serum sample. By analyzing both the binding kinetics and the endpoint data using machine learning tools such as neural networks and k-nearest neighbor, it was possible to account for cross-reactivity, and sensitively determine specific interactions between gangliosides and specific antibodies.
Finally, an alternative approach that circumvents altogether the need for antifouling coatings used appropriately functionalized magnetic particles to specifically capture p.G13D-mutated DNA from blood offline. Afterwards, these were resuspended in buffer and analyzed by SPRI with a PNA-functionalized biosensor. The analysis had high sensitivity (DL: 300 copies/mL) [157], showing promise for cancer diagnostics.
4.3. SPR Sensors to Work in Undiluted Food Samples
Examples of “smart layers” which facilitate SPR sensors that work in undiluted food (e.g., milk, juices) samples are important considering the complex, varied compositions of real food samples. These matrices contain carbohydrates and fat, besides important concentrations of proteins. Moreover, some of them have acidic pH levels. SPR biosensors, with a ~10.5 nm layer of HA covalently grafted onto a Au surface [80], have shown resistance to fouling in undiluted milk and juice samples. The coating induced high hydrophilicity (contact angle: 12°), and resisted the adsorption of foulants such as soybean milk (~10 mg/mL protein concentration; 0.6 ng/cm2 adsorption,), undiluted cow milk (~30 mg/mL protein, 9.8 ng/cm2), and orange juice (~10 mg/mL protein concentration, 16.1 ng/cm2). The importance of this study is manifold, since it emphasizes (i) different NSA behaviors in real samples and in protein control solutions, (ii) the effects of ligand functionalization on NSA and (iii) the non-universality of NSA solutions. More specifically, the NSA in real samples was higher than for single-protein solutions, since the analyzed real food samples contain carbohydrates and fat and have different pH levels. The ability to resist NSA was also examined after functionalization with the antiBSA antibody. Somewhat increased adsorption levels were measured for cow milk (17 ng/cm2) and soybean milk (2.5 ng/cm2), compared to those before the functionalization. However, a striking effect was observed with 100% blood serum. A level of ~60 ng/cm2 adsorption was measured—much higher compared to other coatings. This confirms that the surface coating has to be adapted for each application.
The non-universality of NSA solutions is moreover well exemplified by the series of studies on zwitterionic polymers by Vaisocherova et al. [54,158,159,160]. Functionalization with antibodies, streptavidin and oligonucleotides had contrasting effects on different coatings. It did not substantially affect the ultra-low fouling properties of zwitterionic coatings (plasma fouling of ~20 ng/cm2) or of SAMs, while for methacrylate layers, resistance to fouling is completely lost after the activation of hydroxyl groups. The effects of analyte size on the biorecognition activities of functionalized coatings were also investigated. The best performance in terms of overall fouling resistance and biorecognition ability was provided by zwitterionic coatings (pCBAA). All three types of coated interfaces enabled a good loading capacity, as judged by surface density levels of 200–400 ng/cm2 for covalently immobilized antibodies for human chorionic gonadotropin, S. typhimurium and E. coli O157:H7. Although the antibody immobilization levels were similar, the detection limit for E. coli in milk was 6 × 104cfu/mL—an order of magnitude better than those of other surfaces [52]. Notably, the net responses for E. coli, used for the calibration in undiluted milk, were obtained after considering the signal measured with a “reference” surface, functionalized with antibodies specific for S. typhimurium.
The main conclusion based on the information presented in this section is that fouling-resistant coatings, onto which bioreceptors can be immobilized in a controlled, oriented and optimized manner, represent a critical feature of SPR biosensors operating in complex media.
When designing the smart SPR surfaces, appropriate considerations should be made of process compatibility (linker, affine and NSA-resistant coating) and surface treatment prerequisites (e.g., the antifouling properties of mixed SAMs can be drastically affected by the cleaning procedure). Since the effect exerted by surface functionalization on the resistance to fouling depends on both the type of coatings and the nature of the ligand [148], suitable protocols for evaluating the antifouling performance, as well as the changes in the resistance to NSA after functionalization, are prerequisites.
5. Solutions for Minimizing NSA in Coupled EC-SPR Biosensors
5.1. Coupled EC-SPR Biosensors
Multimodal biosensors surpass the limitations of devices based on single detection methods [161,162,163] by (i) enlarging the detection ranges [164], (ii) offering more accurate results [165] and (iii) providing complementary information on the biorecognition event and non-specific phenomena occurring at the biosensor interface [25]. They also allow us to reach high signal-to-noise ratios in imaging, with numerous applications in various fields [166].
The combination of EC techniques with SPR in particular enables us to obtain detailed information regarding the redox processes at electrodes, among other things. Applications include monitoring electropolymerization processes in real time, evaluating the coating thickness, studying the reorganization of proteins or SAM under an applied electrical field, etc. Moreover, EC measurements at the SPR–gold surface enhanced the magnitude of the analytical signal, allowing us to determine lower concentrations of analytes compared to SPR alone and eliminating the need for additional signal amplification steps [20,167]. In parallel, the EC deposition of the bioreceptor and antifouling layer enabled the optimization of biosensor development and created the premises for sensitive detection by SPR [168]. Another advantage of in situ combined EC and SPR detection lies in the possibility for the multiparametric evaluation of biomimetic membranes [169] and cells, as well as monitoring in real time the cellular response to various stimuli, including drugs [170], toxins [171] and amyloid fibrils [172]. Both non-faradaic and faradaic approaches were developed.
Aside from approaches wherein the EC mode and SPR were used as independent detection modes, the coupled electro-optical approaches where the SPR signal is modulated by an AC electrical stimulus brought additional advantages in terms of sensitivity and accuracy, minimizing the influence of NSA. The electrical stimulus consists typically of an applied potential, a voltammetry scan, or a combination of potential steps and voltammetry. Both faradaic and non-faradaic processes have been exploited in coupled EC-SPR methods. The SPR signal modulated by the application of the electrical stimulus was either directly used as analytical signal, or further interpreted to derive an electrical parameter that was used for quantitative evaluations and imaging purposes, as explained further below.
Most often, in coupled EC-SPR, the parameter used to express the SPR signal is the shift in minimum SPR angle. When an EC input is applied, the angular shift amplitude, Δθ, depends on (i) the binding of electrochemically active molecules at the biosensor interface, (ii) the EC deposition of additional material at the electrode interface, (iii) changes in the refractive index in the vicinity of the electrode that are induced by EC transformations, and (iii) changes in the dielectric properties of the electrode coating. The quantitative relations between the angular shift Δθ and the EC parameters (electrode potential, double layer charging current density, and faradaic current) were synthetically presented in the review of Ribeiro et al. [20] and are described in several works [173,174,175,176]. Coupling electrochemistry with SPR imaging (SPRi) [20] allowed the obtaining of submicron-resolution images representing local variations in electrochemical properties. This high sensitivity approach has analytical applications in measurements with microarrays [177]. It is important to point out that excellent spatial resolution is conferred by the optical method (i.e., SPRi) to the EC method (e.g., EIS), while also maintaining the excellent temporal resolution of the EC method.
A detailed image of the potential utility of combined EC and SPR methods can be gathered from recent reviews, including [18,19,20,178,179].
The setup for combined EC-SPR methods is not complicated, in principle [20,25]. Thin Au-coated interfaces serve both as SPR chips and working electrodes, and are integrated in an EC cell, either an open- or a microfluidic cell, as shown in Figure 11.
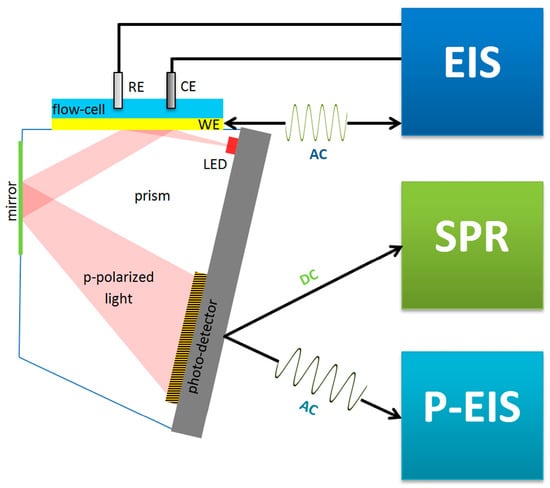
Figure 11.
A schematic representation of an experimental setup used for coupled EC-SPR measurements, exemplified for the case of P-EIS testing. Details are given in the text. Reprinted from [25] with permission.
Interrogating a biosensing interface with both EC methods and SPR-based methods is, however, a far from simple and easy task. There are several limitations regarding the stability of the electrodes based on thin Au layers and the low commercial availability of dedicated equipment and required accessories. In order to be compatible with both EC and SPR methods, the substrates must be a thin semi-transparent metal layer deposited onto an optically transparent substrate, typically a 50 nm-thick Au layer deposited on glass. Such thin Au layers are not very stable electrodes, e.g., they degrade in hexacyanoferrate solutions and under the effect of EIS-specific potential perturbations both when unmodified [180] and when modified with SAMs made of different alkanethiols [181]. Moreover, very few commercially available products sustain synchronized EC and SPR-based measurements, e.g., the relatively new line of products by Biosensing Instrument, Tempe, AZ, USA. As a consequence, researchers exploring the use of such dual measurements find themselves forced to build their own measuring chambers and integrate microfluidic elements to facilitate the exposure of the biosensing interface to several different solutions. The development of dedicated software for instrument control, data acquisition and analysis is also required. The interdisciplinary know-how needed to perfect a setup for combining EC and SPR analysis has limited the development of EC-SPR biosensors.
An important challenge for EC-SPR biosensors refers to the functionalization of the sensing interface, which has to match requirements of both adequate conductivity for electrochemical tests and small coating thickness for sensitive SPR measurements. This adds to the “basic” biosensor specifications of antifouling properties and high capacity for loading with bioreceptors. The inclusion of nanomaterials in the biosensor coating layer can provide a good solution to embed the desired characteristics, along with versatility in further functionalization. The surface regeneration of EC-SPR biosensors might prove difficult in some cases [182], although procedures used typically with SPR sensors were adequate in others [20]. Some authors suggested the use of EC-SPR biosensors as disposable devices [20], which will definitely influence future designs, particularly when also considering sustainability issues.
5.2. Minimizing NSA in Coupled EC-SPR Biosensors
In the EC-SPR biosensors, the optical signal originates in part from the application of an electrical potential. EC-SPR biosensors rely on both non-faradaic and faradaic processes, and include both (i) those where the analytical signal is based on an electrical parameter (e.g., admittance), derived from the SPR signal (P-EIS and P-EIM), and (ii) those where the analytical signal is given directly by the optical SPR parameters. To these, several studies can be added on coupled EC-SPR, wherein EC and SPR detection modes, integrated on the same platform, are independently used with a biosensor. While the parallel study of NSA via EC and SPR increases the chances of finding adequate antifouling layers using coupled EC-SPR, due to the complementarity of the EC-SPR signals [25], the true impact of fouling cannot be predicted by investigations using separate techniques. In fact, some EC-SPR biosensor methods have shown a lack of sensitivity to NSA, related to the way in which the analytical signal is derived [183,184,185].
Compared to the variety of approaches for minimizing NSA in EC or SPR biosensors, very few strategies have been investigated with coupled EC-SPR methods (Table 3). Table 3 summarizes representative examples of such biosensors, for which specific information is provided on the NSA evaluation and prevention strategy. Analytes of all sizes, including ions, small molecules, protein biomarkers, antibodies and DNA sequences, were determined with these biosensors. Moreover, the information presented highlights a variety of detection approaches based on both faradaic and non-faradaic processes.

Table 3.
Antifouling strategies used for coupled EC-SPR biosensors.
The existence of a low number of EC-SPR biosensors is likely due to (i) the great challenges associated with developing interfaces that function properly as part of both detection techniques, and (ii) the increased complexity of the experimental setup for such coupled EC and optical approaches. The main antifouling measures integrated with these coupled detection methods rely mostly on coating the gold interfaces with alkanethiol SAMs [182,185,188], the addition of a removable “cloaking membrane” [27], or the coating of the interface with cross-linked BSA films [25]. Additional measures to prevent the non-specific binding of serum proteins involved blocking the sensing interface with blank serum, or including Tween, BSA and high concentrations of salt in the buffer used in the measurements and for sample dilution [188]. A handful of studies focused in more detail on differentiating between the non-specific and specific binding effects. Therefore, in presenting the available information below, we hope to encourage further research in this field.
5.2.1. NSA in EC-SPR Biosensors Exploiting Non-Faradaic Processes
Non-faradaic processes were exploited in both plasmonic-based electrochemical impedance spectroscopy (P-EIS) and plasmonic-based electrochemical impedance microscopy (P-EIM). P-EIM is a method by which the local electrochemical impedance is evaluated and visualized by SPRi—thus, with high spatial resolution. Leveraging this capability, the method was used in several studies of cellular processes for measuring binding kinetics and affinity, and for the detection of analytes with microarrays [183,184,189,190].
An important outcome of research works on P-EIS and P-EIM, investigating both large (proteins) and small molecules (drugs) binding to the sensing interfaces, was that these methods were properly developed in order to eliminate non-specific binding.
A P-EIS study from 2012 evaluated the molecular binding to an IgG-functionalized sensing interface [186]. Specifically, a Au interface was functionalized with a 1:1 mixture of 6-mercaptohexanoic acid and 3-mercapto-1-propanol solution, to which IgG was subsequently immobilized by carbodiimide chemistry. The residual activated groups on the chip surface were blocked with ethanolamine. The functionalized interface was tested with solutions of a very different refractive index (NaCl and glucose), with a fouling protein (BSA) and the specific target analyte anti IgG. By comparing the binding profiles obtained by SPR and P-EIS, the authors concluded that SPR was more sensitive to NSA than P-EIS (Figure 12). As observed in the reflectivity plot in SPR, the binding and dissociation of BSA were slower than for NaCl or glucose. When comparing the dissociation profiles of BSA and IgG, it is obvious that with enough washing (e.g., at 200 s total analysis time), the contribution of residual signal given by BSA to the measured signal is minimal. At the same time, there is still a strong specific signal due to the affinity binding between IgG and anti IgG.
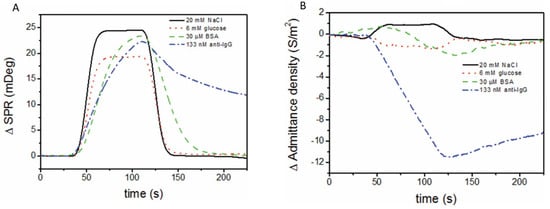
Figure 12.
(A) SPR and (B) admittance density responses recorded with the P-EIS and IgG-based biosensor for solutions of NaCl, glucose, BSA and anti-IgG prepared in PBS buffer. The flow rate was 60 μL/min. A 100 Hz, 200 mVpp potential modulation with 120 mV DC bias was applied. Reproduced from [186] with permission.
The concentration of BSA solution used in the test (30 µM) is somewhat low compared, for example, to the albumin concentration in human serum (~0.6 mM). This would imply that actual serum samples analyzed with this device will have to be diluted by at least 20 times. Therefore, the suitability of the developed sensing interface for measurements in clinical samples remains somewhat limited. At the same time, when examining the P-EIS output expressed as the variation of the admittance of the IgG-modified surface (Figure 12B), it is obvious that both for the association and dissociation phases, there is a much higher ratio between the specific signal (for anti IgG) and the non-specific one (for BSA, NaCl and glucose) compared to SPR (Figure 12A). Therefore, the specific binding is more sensitively monitored by P-EIS than by SPR.
The study was centered on comparing SPR and P-EIS with respect to measuring the binding affinities for the IgG–anti IgG, interactions and no real samples were investigated. The method was later used to emphasize the specific effects of K+ cations on stabilizing a G quadruplex, versus other cations such as Na+ and Mg2+. The changes in the admittance amplitude measured by P-EIS mirrored the profile observed by SPR [187].
In a further advance of the method, in 2014, Liang et al. [183] proposed the use of P-EIM for measuring the binding kinetics of small molecules to a protein microarray. The array was obtained by functionalizing the interface of Au with SAM of mixed PEG-containing dithiols, with carboxyl and hydroxyl end groups. Four proteins (Abl, Abl Y253F, p38-α, and myelin basic protein) were printed and covalently coupled by carbodiimide chemistry to the EDC/NHS-activated, thiol-coated interface (Figure 13).
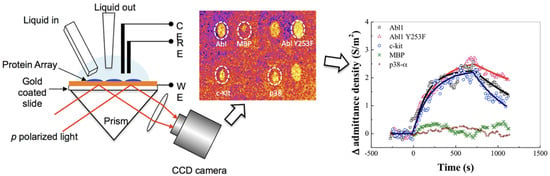
Figure 13.
(Left) Schematic illustration of P-EIM experimental setup. An AC potential modulation is applied to the gold-coated “sensor chip” (WE) via a potentiostat. A p-polarized collimated point source red LED light is directed to the sensor chip via a triangle prism at the SPR resonance angle, and the reflected light is recorded via a CCD camera through a zoom lens. The DC component of the image is the conventional SPR image, and the AC component is converted to an admittance image via real-time data processing. (Center) The admittance amplitude image showing an area on a gold sensor chip printed with proteins. The image size is 3.63 × 2.27 mm. The responses for each protein spot are reference-corrected. (Right) Admittance responses for the interaction of imatinib (0.2 μM) with the proteins in the sensor array. Solid lines are kinetic fitting curves. Reproduced from [183] with permission.
To demonstrate the accuracy of P-EIM measurements, the authors applied them for several test cases including known interactions between small molecules and proteins. Specifically, the authors studied the binding of imatinib to various proteins including the specific ligand Abl1, and the interaction between the SB202190a (a small molecule) and several proteins including p38-α, which is inhibited by SB202190a. The results of the binding affinity measurements confirm the small molecule–protein interactions that were revealed beforehand by competitive binding assays (i.e., imatinib—ABL1, KD = 3.4 × 10−8 M and SB202190a-p38-α, KD = 4.3 × 10−8 M) and emphasized new, weaker interaction, e.g., the binding of SB202190a to ABL1 (KD = 1.6 × 10−5 M). Moreover, the P-EIM platform was also applied to study the influence of Mg2+ ions on the binding between imatinib and Abl1, as well as the competitive inhibition of imatinib–Abl1 binding by AMPPNP.
The study concluded that P-EIM is not affected by the non-specific binding of small molecules, while showing high sensitivity to specific interactions. The explanation for the insensitivity to NSA stems from the principle of the P-EIM method. The resonant SPR angle depends on the surface charge density of the SPR interface, in addition to the refractive index and the thickness of the layer at the metal–solution interface. In P-EIM, there is no redox probe involved, and an AC potential is applied to the sensing interface, as in non-faradaic EIS. The result is that the intensity of the SPR images oscillates with the applied potential. The SPR image can be separated into the DC part (the SPR image that would be normally recorded in the absence of the AC potential) and the AC part, i.e., the P-EIM signal. The P-EIM signal is directly correlated with the capacitance of the interface. The authors argued that weakly and non-specifically bound molecules (bigger or smaller) have no significant impact on the interface capacitance since they neither modify the charge or conformation of the proteins immobilized on the interface, nor have a significant charge-countering effect on the ions in the bulk solution. However, it is important to note that the images of the protein spots after the interaction with the small drug and inhibitor molecules were reference-corrected. In imaging-based approaches, one can evaluate the NSA by analyzing intensity changes occurring during sensing experiments outside the sensing spots. By subtracting the intensity of a background spot from the intensity of a sensing spot, the impact of NSA on the analytical signal can be minimized. Moreover, the lack of NSA was observed in pure solutions, to which the PEG-based SAM coating was resistant. Larger studies considering other small molecules, in wider concentration ranges or in real samples, could help probe the limitations of the proposed approach with regard to NSA.
This label-free method can be used for kinetics measurements to emphasize even weak interactions, which cannot be detected by endpoint assays, and is moreover adequate for high-throughput determinations with protein microarrays. In the work described above, the advantage of P-EIM compared to conventional SPR imaging, with regard to sensitively monitoring the binding of small molecules to their ligands in a protein microarray, comes from a combination of factors: (i) the fact that the P-EIM signal is proportional to the area occupied by the bound molecules, instead of their mass (as in SPRi) and (ii) the low influences of NSA and the refractive index of the bulk solution.
A confirmation of the sensitivity of P-EIS to the binding of small molecules was offered by the study of Polonschii et al. [25]. The researchers exploited the non-faradaic processes to derive P-EIS, along with SPR and EIS signals obtained with a Au interface functionalized with a cBSA film. IgG was covalently attached to the cBSA interface, and the effects on this interface of biotin, anti IgG and diluted serum were tested (Figure 14). Contrary to the findings of Lu et al. [186], this study indicated that SPR mostly emphasized the specific binding of anti IgG to IgG (Figure 14B,D). At the same time, P-EIS mirrored the EIS signal and revealed non-specific binding phenomena, where the binding of the small molecule biotin (244.3 Da, at 4 mM) produced a larger relative variation in the signal recorded with cBSA interfaces than the binding of the 150 kDa anti IgG (at 80 nM), averaging >28% and 13%, respectively (blue bars in Figure 14A,C).
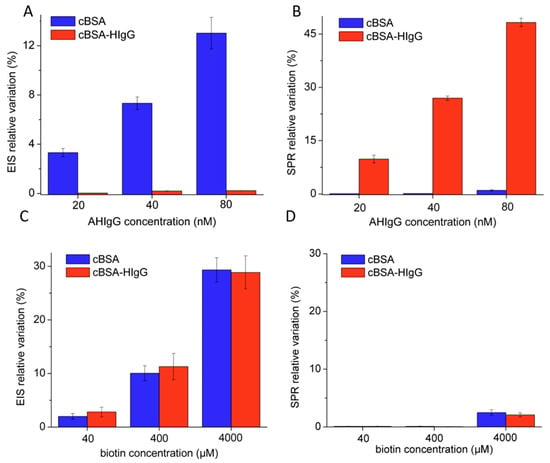
Figure 14.
(A) The dependence of the relative variations in the EIS impedance magnitude at 3.5 Hz (A) and the SPR response (B) on anti-human-IgG concentration upon successive injections of increasing concentrations (20 nM, 40 nM, and 80 nM) on the cBSA surface and cBSA-HIgG-modified surface. The dependence of the relative variations in the EIS impedance magnitude at 3.5 Hz (C) and the SPR response (D) on biotin concentration upon successive injections of increasing concentrations (40 μM, 400 μM, and 4 mM). Reproduced from [25], with permission.
The setups and experimental conditions used in the reports of Lu et al. [186] and Polonschii et al. [25] were different, with a major difference regarding the coating layer, i.e., the planar, thin thiol-based coating [186] versus the tridimensional film of cross-linked BSA [25]. The authors explained that the amplified P-EIS signal related to the small molecule biotin, compared to large molecules such as anti IgG, is related to the nature of the cBSA film, as the small molecules can diffuse into the 3D layer and reach the Au interface through defects in the layer. Large molecules cannot access the surface as they cannot enter the pinholes in the BSA layer. The P-EIS signal is very sensitive to phenomena in the close vicinity of the SPR interface, and much less affected by events farther from the surface. Moreover, the P-EIS signal is linked to capacitive currents originating from the movement of electrolyte ions through pinholes in the BSA film towards the electrode surface. It also depends on the reorientation of solvent dipoles and adsorption/desorption phenomena at the interface between the Au electrode and the solution. Consequently, the modulation of the capacitive current by small molecules has a dramatic effect on the P-EIS signal. Polonschii et al. emphasized the potential of their approach to highlight the NSA due to small weight components in complex samples, such as diluted serum. Most often, when dealing with serum and blood, blood cells and large-sized proteins were exclusively targeted in NSA studies as the main contributors to fouling. Small-sized sample components were very rarely considered. In consequence, P-EIS, with its sensitivity to small molecules, can assist detailed assessments, and deepen our understanding of individual contributions to NSA in complex samples.
From the information available so far, it is clear that P-EIS and SPR offer complementary information with regard to specific and non-specific binding, and can be used for evaluating the antifouling efficiency of various coatings. More data are needed, with other types of coatings, affinity ligands, and foulants of various sizes and charges, to obtain a full picture of the potential and limitations of the non-faradaic P-EIS.
In addition to the study of biomolecular interactions, P-EIS/P-EIM were particularly useful in studying cells and cellular events triggered by different stimuli. Applications included the high-resolution monitoring of electrical activities in single neurons [189], or the activation of G-protein-coupled receptors in cells induced by histamine [190,191]. In these studies, the sensing surfaces were coated with poly(lysine), and the composition of the medium was controlled. The spatial and temporal resolution achieved was unparalleled, and makes the coupled electro-optical methods particularly attractive for further applications in cell imaging.
5.2.2. NSA in EC-SPR Biosensors Exploiting Faradaic Processes
Faradaic processes have also been exploited in conjunction with SPR for characterizations, quantitative measurements, and the investigation of NSA phenomena in biosensors [18,20].
Most strategies to prevent fouling in faradaic EC-SPR biosensors were designed to avoid the adherence of non-target analytes and sample components to the biosensor surface. In contrast, the “cloaking membrane” approach [27] relies on the adsorption of foulants onto a lipidic bilayer coating the biosensor, while the target analyte binds to the immobilized bioreceptor. The lipidic bilayer is then removed by washing with a surfactant, leaving the surface free of interferences (Figure 15 [27]).
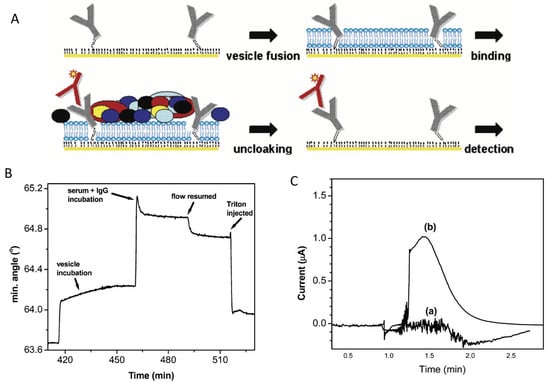
Figure 15.
(A) Graphical illustration of the cloaking membrane approach for detection of proteins in serum samples. (B) SPR sensorgram showing the membrane cloaking process with nanoparticle-conjugated anti-rabbit IgG spiked in serum. (C) Flow injection response of TMB for in situ EC-SPR analysis with no HRP (curve a) and immobilized HRP (curve b). Reproduced from [27] with permission.
The cloaking membrane approach described by Philips et al. [27] was successfully used for measurements in undiluted serum. To carry out the measurements, Au interfaces were modified with alkanethiol SAMs, onto which IgG was covalently attached. A coating of lipid PC/DOPC+ vesicles was then applied. SPR was used in following the steps of bioreceptor immobilization, coating assembly, washing with surfactant (Triton X-100) and testing with serum. After the washing step, the surface was used for electrochemical measurements. Anti IgG, labeled with HRP, was detected in situ by amperometry. The SPR biosensor, functioning as a working electrode in a flow cell, was polarized at 0.175 V vs. Ag/AgCl to measure the oxidation signal of TMB, the enzymatic substrate of HRP.
The detection limit for anti IgG was lower than 5 fM, which underlines the potential of the cloaking membrane approach for measuring small concentrations of analytes directly in the biological samples. A disadvantage of this method is that the coating with lipid vesicles has to be performed before incubation with the sample. Combined with washing with a surfactant, these steps add complexity, and make the approach appropriate for research laboratory implementation only.
An alternative strategy to carry out accurate measurements in blood using EC-SPR biosensors has been described, which relied on “nanoswitches” and offered a solution to the selective detection of nucleotides [185]. Nanoswitches are DNA sequences, labeled with an electroactive probe at one end, which undergo dramatic changes in conformation upon binding their target analyte. Differently from the structure-switching aptamers used in E-AB biosensors (described in Section 3.2), the nanoswitches used by Dallaire et al. are stem–loop DNA sequences wherein hybridization with the complementary target nucleotide induces stem opening, increasing the distance between the electroactive tag and the electrode. Thus, in the analyte-bound state, the probability of electron transfer to the electrode is minimized. The conformation change has high energy requirements and is specifically associated with the binding of the target analyte; hence, the specific binding event acts as a switch. Dallaire et al. [185] used a stem–loop DNA sequences complementary to a gene in Mycobacterium tuberculosis. The oligonucleotides were labeled with MB at the 3’ end and had a 5’ terminal thiol group, which facilitated their attachment to the Au SPR interface by chemisorption. Backfilling with MCH ensured that any defects in the formed SAM were covered, so as to prevent NSA, and that the nanoswitches on the sensing interface had a proper orientation. The SPR interface was used as a working electrode in the EC-SPR setup (Figure 16). An electrochemical input was applied—either a fixed potential (−0.275 V) or a cyclic voltammetry scan—over which an AC potential of 50 mV amplitude was imposed. This served to trigger the oxidation/reduction of MB and a measurable change in the SPR signal.
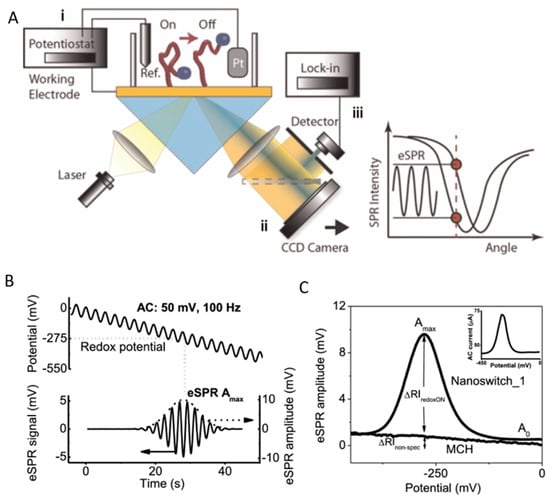
Figure 16.
(A) Representation of the nanoswitches signaling mechanism. A potentiostat is used to measure electrochemical voltammograms (i) and apply the voltage on the gold surface. The light containing plasmonic information is collected in two ways. First, a collimated beam is detected on a CCD camera to obtain the full angular/intensity dependency used in conventional angular SPR sensing (ii). Second, light from a fixed angle is sent on a photodetector. The average intensity measured by this Si detector is the SPR curve intensity at fixed angle (DC component). The detector is also coupled to a lock-in amplifier to obtain the EC-SPR signal (AC component) (iii). The inset graph shows the relation between the full SPR curve and the EC-SPR signal as the refractive index is modulated. (B) Schematic representation of the EC-SPR AC voltammetry method showing the variation in time of the applied potential and the resulting EC-SPR signal. (C) EC-SPR AC voltammograms measured for a Nanoswitch_1-modified surface and a MCH-modified surface. Inset: the EC (only) voltammogram with the Nanoswitch_1 modified surface. Reproduced from [185] with permission.
In the initial state (Figure 16), as the MB probe is close to the electrode, the electron transfer is fast, and the differences in the refractive indexes of the oxidized and reduced forms of MB cause a variation in the SPR signal. Upon hybridizing with the target DNA sequence, the stem–loop capture probe suffers a large change in conformation; the loop is opened and the MB tag gets away from the electrode. In consequence, the variations in the SPR signal associated with the oxidation of MB are diminished. Since the conformational change is related to the specific hybridization of the probe with the complementary DNA sequence, the output SPR signal is correlated with the concentration of target DNA. Tests with control interfaces lacking the nanoswitches and measurements with mismatched DNA sequences have proven that the output signal is not affected by NSA. The binding kinetics for the target sequence and the changes of the EC-SPR signal measured in blood and buffer were very similar, showing no influence of non-specific binding and the possibility of using the EC-SPR method to analyze complex samples [185]. One disadvantage of the proposed method is that it requires calibration before the tests in buffer and blood, respectively, due to the large differences in the refractive indexes of the two media. The authors have shown the possibility of applying the EC-SPR approach, with some adaptations for multiplexed detection with two nanoswitches, using planar three-electrode systems, towards the development of microarrays [185].
The use of MB as an electroactive label in EC-SPR biosensors has not been restricted to oligonucleotide probes; for example, Qatamin et al. [192] described a sandwich immunoassay for the detection of the hemagglutinin protein from the H5N1 avian influenza A virus, involving the use of a secondary antibody labeled with MB. The optical signal, i.e., the variation in the refractive index at the biosensor interface, was modulated by the electrochemical activity of MB and the applied potential.
Exogeneous probes, [Fe(CN)6]3−/4− in particular, have also been employed in coupled or parallel EC and SPR studies with biosensors.
Ribeiro et al. demonstrated a significant improvement in sensitivity for the detection of miRNA when using EC-SPR compared to SPR alone [182].
Specifically, the linear range achieved with the DNA sensor changed from 1 × 10−9–1.5 × 10−7 M to 1 × 10−15 M–1 × 10−10 M miRNA-145 (Figure 17). In this approach, the EC-SPR was used for signal amplification after the hybridization step, which was followed exclusively by SPR (Figure 17). Similarly to electrochemical DNA biosensors using [Fe(CN)6]3−/4−, the approach exploited the steric hindrances-inducing effect of the biorecognition event (hybridization) on the diffusion of the redox probe towards the electrode. Ribeiro et al. measured the shift in the SPR angle resulting from the differences in the refractive indexes of the oxidized and reduced redox probes at the biosensor interface. To maximize the signal, a positive potential was applied to attract and oxidize the probe at the electrode, followed by sweeping to a negative potential, whereby the probe− was reduced and repelled from the surface.
Figure 17.
(A) Principle of the EC-SPR detection of miRNA-145. (B) a: Real-time monitoring of the interaction between immobilized RNA strands on SPR gold substrates and miRNA-145 in the range 1.0 fM–150 nM by SPR (left), followed by EC-SPR measurement in the presence of ferro/ferricyanide redox probe (right). Line 1: baseline in buffer. Line 2: monitoring the interaction between surface-immobilized RNA and miRNA-145. Line 3: washing with buffer. Dashed line: injection of buffer without miRNA-145. Line 4: combined EC-SPR after injection of 5 mM ferro/ferricyanide. Line 5: washing with buffer. (b,c) Graphical representations of the total angle variation as a function of the logarithm of the miRNA-145 concentration obtained under conditions of hybridization equilibrium by SPR (b) and combined EC-SPR (c) Reproduced from [182] with permission.
As illustrated in Figure 17B, increasing variation in the SPR angle with higher (nM) concentrations of miRNA-145 were measured based solely on SPR detection. In contrast, the introduction of the EC-SPR step allows us to observe changes in the SPR angle at much lower analyte levels. The signal decreases with analyte concentration and reaches a plateau at nM levels. In consequence, this approach enables a simple signal amplification strategy that does not require the use of costly labeled probes.
The same group demonstrated the use of an electrochemistry-assisted SPR immunosensor for the analysis of the cancer biomarker CA 15–3 [188] based on the change of the SPR signal triggered by a square wave voltammetry scan in the presence of [Fe(CN)6]3−/4−. EC measurements at the SPR gold surface enabled an enhancement in the magnitude of the analytical signal, and allowed us to determine lower concentrations of CA 15–3 (DL of 0.098 U/mL compared to 20 U/mL for SPR alone). To protect the biosensor against NSA and allow sensitive EC detection, in both studies, the interfaces were coated with short thiols. Short thiols can be expected to increase the resistance of the interface to NSA without completely suppressing the faradaic EC processes contributing to the analytical signal. Serum samples were successfully analyzed; however additional measures were taken to avoid interferences due to NSA. In the first study [182], the synthetic human serum was filtered through a 10 kDa cutoff membrane and diluted 15 times with buffer. In the second work, the human serum was diluted 100 times in phosphate buffer containing Tween 20 detergent, BSA and NaCl [188]. In addition, after attaching the antibody onto the sensing interface, the interface was “blocked” by performing two successive injections of diluted serum. These details illustrate the amplitude of the NSA problem and the limitations of the short thiols used to coat the sensing interfaces.
5.3. Challenges in Low Fouling EC-SPR Biosensors
The electrified biointerface characteristic for coupled EC-SPR sensing is impacted by NSA in a different manner compared to either EC or SPR biosensors, considering that the analytical signal is extracted from the change in the optical signal, triggered by the application of an electrical field. While the studies mentioned in Section 5.2 show new opportunities for accurate quantitative detection, the coatings of short thiols or BSA used in EC-SPR biosensors so far may not be appropriate over the long measuring times required for complex samples. Finding a suitable antifouling coating might compromise the sensitivity of EC-SPR compared to individual sensors operating in the same media [18]. Nonetheless, to solve this issue, a whole range of materials (discussed in Section 3 and Section 4) could be tested.
Many biosensor studies targeting quantitative measurements, including those on EC-SPR, have focused on reaching high sensitivity, while the problem of NSA has not been investigated in enough detail. Often, the provided plots of biosensor selectivity give an overview of interferences originating from both NSA and bioreceptor cross-reactivity. Signals obtained for non-target molecules higher than the 10% displayed by the analyte of interest are sometimes observed, thus non-specific effects can be considered as significant [168,193]. The implementation of a unitary approach to evaluate the extent of NSA and establish acceptable thresholds will advance the field and allow a more objective overview of the research data.
6. Conclusions and Perspectives
EC-SPR biosensors offer exciting analytical opportunities with regard to the detection of small molecules, high-throughput analysis with arrays of biorecognition elements, monitoring a wider range of affinity interactions, and monitoring cell events. Coupled EC-SPR methods used with biosensors have clear advantages over the individual detection methods. These are typically related to the sensitivity of quantitative measurements and to the spatial resolution in imaging applications. This is due to the unique mechanisms required to obtain the analytical signal, enabled by the electro-optical methods, which alleviated the NSA and enhanced biosensor output. Non-faradaic P-EIS and P-EIM methods have advanced the study of binding interactions by providing complementary information, enlarging the detection range of biomolecules and allowing the multiparametric evaluation of cellular processes. The integration of structure-switching bioreceptors within EC-SPR devices offered a new way to separate the specific from non-specific responses. Additionally, the EC triggering of large optical shifts at the biosensor–sample interface using electroactive probes led to simple strategies to amplify the SPR signal.
The data summarized in this review show that the field of using EC-SPR biosensors for the analysis of real samples is underexplored. Much of the effort so far has been put into the development of new analytical approaches, exploiting both non-faradaic and faradaic processes.
In this context, to prevent fouling, several basic strategies, typically used with SPR or EC sensors, were adopted. Due to the small number of studies on EC-SPR biosensors, as well as the diversity of approaches and experimental conditions, it is difficult to compare the experimental data. In parallel, the research into antifouling materials adapted for individual detection methods has been very dynamic. Significant advancements have been registered for zwitterionic polymers and antifouling peptides related to their stability in operating conditions, their versatile functionalization and their loading capacity with bioreceptors. Coatings performing well with SPR, such as those based on PEG-derivatives or cBSA films, were adapted for EC measurement by incorporating conductive nanomaterials. Their evaluation and optimization for coupled EC-SPR methods, however, is still awaited. It is foreseeable that the NSA issue in EC-SPR biosensors will be addressed with a whole range of new solutions.
Notably, the use of molecular simulations and machine learning tools has accelerated the development of new antifouling materials, such as copolymer hydrogels for in vivo devices [108]. It can be anticipated that this trend will continue in the following years, given the increased implementation of machine learning approaches in all research fields. The fast-accumulating knowledge on the degradation mechanism of antifouling coatings upon prolonged exposure to complex media [21] and on the storage stability of the biosensors operating in such media [194] is very encouraging in relation to envisaging the translation of some of these analytical tools, such as E-AB biosensors, into commercial devices in the short term [21]. The progress made on in vivo biosensor measurements in the blood of animals, and biosensor measurements in the interstitial fluid in the human body [195], provides a solid motivation for solving the fouling issue in a manner appropriate for commercially available devices, and approved by regulatory authorities.
Profiting from these advances, the integration of redox nanoswitches and structure-switching aptamers with EC-SPR analytical devices such as the eNanoSPR based on Au nanohole arrays [184] appears very promising for achieving interference-free measurements. Despite limitations related to the availability of suitable bioreceptors with adequate selectivity and dramatic conformational changes in the presence of targeted molecules, there is good potential for widening the EC-SPR studies to new analytes.
Nonetheless, to advance towards commercial biosensors, “standardized” ways to evaluate fouling are needed so as to enable accurate comparisons and evaluations of various materials. The responses to the pH and salt of new materials, and the loading capacity/functionalization, have to be characterized. Moreover, scalable functionalization strategies with antifouling coatings should be developed for manufacturing.
The “universal” functionalization protocols with polyadenine–polythymine-linked bioreceptors and antifouling peptides–DNA conjugates have the potential to provide adequate protection against fouling, along with bioreceptor attachment, for EC-SPR detection modes. This promising direction remains to be investigated in the future.
By presenting the available information on the use of biosensors based on EC-SPR detection for the analysis of real samples, along with the recent progress made in antifouling coatings for EC and SPR applications, we hope to encourage further research into EC-SPR biosensors.
Author Contributions
Conceptualization, A.V., S.G., C.P. and M.G.; methodology, A.V.; writing—original draft preparation, A.V., S.G., M.G., C.P., R.M.B. and S.D.; writing—review and editing, A.V., S.G., M.G., E.G and J.-L.M.; funding acquisition, E.G., J.-L.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by The Romanian Ministry for Education and Research, grant PNRR-III-C9-2023-I8, contract CF129-31.07.2023.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created in this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Campuzano, S.; Pedrero, M.; Barderas, R.; Pingarrón, J.M. Breaking barriers in electrochemical biosensing using bioinspired peptide and phage probes. Anal. Bioanal. Chem. 2024, 416, 7225–7247. [Google Scholar] [CrossRef] [PubMed]
- Campuzano, S.; Pedrero, M.; Gamella, M.; Serafín, V.; Yáñez-Sedeño, P.; Pingarrón, J.M. Beyond Sensitive and Selective Electrochemical Biosensors: Towards Continuous, Real-Time, Antibiofouling and Calibration-Free Devices. Sensors 2020, 20, 3376. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, G.; Hein, R.; Liu, N.; Luo, X.; Davis, J.J. Antifouling Strategies for Selective In Vitro and In Vivo Sensing. Chem. Rev. 2020, 120, 3852–3889. [Google Scholar] [CrossRef]
- Gu, Y.; Li, Y.; Wu, Q.; Wu, Z.; Sun, L.; Shang, Y.; Zhuang, Y.; Fan, X.; Yi, L.; Wang, S. Chemical antifouling strategies in sensors for food analysis: A review. Compr. Rev. Food Sci. Food Saf. 2023, 22, 4074–4106. [Google Scholar] [CrossRef]
- Lichtenberg, J.Y.; Ling, Y.; Kim, S. Non-Specific Adsorption Reduction Methods in Biosensing. Sensors 2019, 19, 2488. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudpour, M.; Jouyban, A.; Soleymani, J.; Rahimi, M. Rational design of smart nano-platforms based on antifouling-nanomaterials toward multifunctional bioanalysis. Adv. Colloid Interface Sci. 2022, 302, 102637. [Google Scholar] [CrossRef]
- Contreras-Naranjo, J.E.; Aguilar, O. Suppressing Non-Specific Binding of Proteins onto Electrode Surfaces in the Development of Electrochemical Immunosensors. Biosensors 2019, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, H.; Zhu, M.; Kuang, Z.; Li, X.; Xu, F.; Miao, S.; Zhang, Z.; Lou, X.; Li, H.; et al. Electrochemical Biosensors for Whole Blood Analysis: Recent Progress, Challenges, and Future Perspectives. Chem. Rev. 2023, 123, 7953–8039. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.J.; Han, M.; Desroches, P.E.; Manasa, C.S.; Dennaoui, J.; Quigley, A.F.; Kapsa, R.M.I.; Moulton, S.E.; Guijt, R.M.; Greene, G.W.; et al. Antifouling Strategies for Electrochemical Biosensing: Mechanisms and Performance toward Point of Care Based Diagnostic Applications. ACS Sens. 2021, 6, 1482–1507. [Google Scholar] [CrossRef]
- Saxena, S.; Sen, P.; Soleymani, L.; Hoare, T. Anti-Fouling Polymer or Peptide-Modified Electrochemical Biosensors for Improved Biosensing in Complex Media. Adv. Sens. Res. 2024, 3, 2300170. [Google Scholar] [CrossRef]
- Song, Z.; Han, R.; Yu, K.; Li, R.; Luo, X. Antifouling strategies for electrochemical sensing in complex biological media. Microchim. Acta 2024, 191, 138. [Google Scholar] [CrossRef] [PubMed]
- Szunerits, S.; Pagneux, Q.; M’Barek, Y.B.; Vassal, S.; Boukherroub, R. Do not let electrode fouling be the enemy of bioanalysis. Bioelectrochemistry 2023, 153, 108479. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Li, X.; Zhu, B.; Su, B. An Overview of Antifouling Strategies for Electrochemical Analysis. Electroanalysis 2022, 34, 966–975. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, Y.; Zhang, G.-J. State-of-the-art strategies of surface plasmon resonance biosensors in clinical analysis: A comprehensive review. Coord. Chem. Rev. 2024, 520, 216149. [Google Scholar] [CrossRef]
- Qu, J.-H.; Dillen, A.; Saeys, W.; Lammertyn, J.; Spasic, D. Advancements in SPR biosensing technology: An overview of recent trends in smart layers design, multiplexing concepts, continuous monitoring and in vivo sensing. Anal. Chim. Acta 2020, 1104, 10–27. [Google Scholar] [CrossRef] [PubMed]
- D’Agata, R.; Bellassai, N.; Spoto, G. Exploiting the design of surface plasmon resonance interfaces for better diagnostics: A perspective review. Talanta 2024, 266, 125033. [Google Scholar] [CrossRef]
- Jungnickel, R.; Balasubramanian, K. Electrochemistry-coupled surface plasmon resonance on 2D materials for analysis at solid–liquid interfaces. Curr. Opin. Electrochem. 2025, 50, 101634. [Google Scholar] [CrossRef]
- Hemmerová, E.; Homola, J. Combining plasmonic and electrochemical biosensing methods. Biosens. Bioelectron. 2024, 251, 116098. [Google Scholar] [CrossRef]
- Geilfuss, D.; Boukherroub, R.; Dostalek, J.; Knoll, W.; Masson, J.-F.; Baeumner, A.; Szunerits, S. Can classical surface plasmon resonance advance via the coupling to other analytical approaches? Front. Anal. Sci. 2022, 2, 1091869. [Google Scholar] [CrossRef]
- Ribeiro, J.A.; Sales, M.G.F.; Pereira, C.M. Electrochemistry combined-surface plasmon resonance biosensors: A review. TrAC Trends Anal. Chem. 2022, 157, 116766. [Google Scholar] [CrossRef]
- Watkins, Z.; Karajic, A.; Young, T.; White, R.; Heikenfeld, J. Week-Long Operation of Electrochemical Aptamer Sensors: New Insights into Self-Assembled Monolayer Degradation Mechanisms and Solutions for Stability in Serum at Body Temperature. ACS Sens. 2023, 8, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Downs, A.M.; Plaxco, K.W. Real-Time, In Vivo Molecular Monitoring Using Electrochemical Aptamer Based Sensors: Opportunities and Challenges. ACS Sens. 2022, 7, 2823–2832. [Google Scholar] [CrossRef] [PubMed]
- Shaver, A.; Arroyo-Currás, N. The challenge of long-term stability for nucleic acid-based electrochemical sensors. Curr. Opin. Electrochem. 2022, 32, 100902. [Google Scholar] [CrossRef] [PubMed]
- Leung, K.K.; Downs, A.M.; Ortega, G.; Kurnik, M.; Plaxco, K.W. Elucidating the Mechanisms Underlying the Signal Drift of Electrochemical Aptamer-Based Sensors in Whole Blood. ACS Sens. 2021, 6, 3340–3347. [Google Scholar] [CrossRef]
- Polonschii, C.; David, S.; Gaspar, S.; Gheorghiu, M.; Rosu-Hamzescu, M.; Gheorghiu, E. Complementarity of EIS and SPR to Reveal Specific and Nonspecific Binding When Interrogating a Model Bioaffinity Sensor; Perspective Offered by Plasmonic Based EIS. Anal. Chem. 2014, 86, 8553–8562. [Google Scholar] [CrossRef]
- Ruiz-Valdepeñas Montiel, V.; Sempionatto, J.R.; Esteban-Fernández de Ávila, B.; Whitworth, A.; Campuzano, S.; Pingarrón, J.M.; Wang, J. Delayed Sensor Activation Based on Transient Coatings: Biofouling Protection in Complex Biofluids. J. Am. Chem. Soc. 2018, 140, 14050–14053. [Google Scholar] [CrossRef]
- Phillips, K.S.; Han, J.H.; Cheng, Q. Development of a “Membrane Cloaking” Method for Amperometric Enzyme Immunoassay and Surface Plasmon Resonance Analysis of Proteins in Serum Samples. Anal. Chem. 2007, 79, 899–907. [Google Scholar] [CrossRef]
- Jeon, S.I.; Lee, J.H.; Andrade, J.D.; De Gennes, P.G. Protein—Surface interactions in the presence of polyethylene oxide: I. Simplified theory. J. Colloid Interface Sci. 1991, 142, 149–158. [Google Scholar] [CrossRef]
- Liu, B.; Liu, X.; Shi, S.; Huang, R.; Su, R.; Qi, W.; He, Z. Design and mechanisms of antifouling materials for surface plasmon resonance sensors. Acta Biomater. 2016, 40, 100–118. [Google Scholar] [CrossRef]
- Topor, C.-V.; Puiu, M.; Bala, C. Strategies for Surface Design in Surface Plasmon Resonance (SPR) Sensing. Biosensors 2023, 13, 465. [Google Scholar] [CrossRef]
- Nangare, S.N.; Patil, P.O. Affinity-Based Nanoarchitectured Biotransducer for Sensitivity Enhancement of Surface Plasmon Resonance Sensors for In Vitro Diagnosis: A Review. ACS Biomater. Sci. Eng. 2021, 7, 2–30. [Google Scholar] [CrossRef]
- David, S.; Gheorghiu, M.; Daakour, S.; Munteanu, R.-E.; Polonschii, C.; Gáspár, S.; Barboiu, M.; Gheorghiu, E. Real Time SPR Assessment of the Structural Changes of Adaptive Dynamic Constitutional Frameworks as a New Route for Sensing. Materials 2022, 15, 483. [Google Scholar] [CrossRef] [PubMed]
- Kurt, H.; Pishva, P.; Pehlivan, Z.S.; Arsoy, E.G.; Saleem, Q.; Bayazıt, M.K.; Yüce, M. Nanoplasmonic biosensors: Theory, structure, design, and review of recent applications. Anal. Chim. Acta 2021, 1185, 338842. [Google Scholar] [CrossRef]
- Wang, H.; Wang, T.; Yuan, X.; Wang, Y.; Yue, X.; Wang, L.; Zhang, J.; Wang, J. Plasmonic Nanostructure Biosensors: A Review. Sensors 2023, 23, 8156. [Google Scholar] [CrossRef]
- Chen, S.; Cao, Z.; Jiang, S. Ultra-low fouling peptide surfaces derived from natural amino acids. Biomaterials 2009, 30, 5892–5896. [Google Scholar] [CrossRef] [PubMed]
- Mauriz, E. Low-Fouling Substrates for Plasmonic Sensing of Circulating Biomarkers in Biological Fluids. Biosensors 2020, 10, 63. [Google Scholar] [CrossRef] [PubMed]
- Nurrohman, D.T.; Chiu, N.-F.; Hsiao, Y.-S.; Lai, Y.-J.; Nanda, H.S. Advances in Nanoplasmonic Biosensors: Optimizing Performance for Exosome Detection Applications. Biosensors 2024, 14, 307. [Google Scholar] [CrossRef]
- Lísalová, H.; Brynda, E.; Houska, M.; Víšová, I.; Mrkvová, K.; Song, X.C.; Gedeonová, E.; Surman, F.; Riedel, T.; Pop-Georgievski, O.; et al. Ultralow-Fouling Behavior of Biorecognition Coatings Based on Carboxy-Functional Brushes of Zwitterionic Homo- and Copolymers in Blood Plasma: Functionalization Matters. Anal. Chem. 2017, 89, 3524–3531. [Google Scholar] [CrossRef]
- Wang, Y.-M.; Kálosi, A.; Halahovets, Y.; Romanenko, I.; Slabý, J.; Homola, J.; Svoboda, J.; de los Santos Pereira, A.; Pop-Georgievski, O. Grafting density and antifouling properties of poly[N-(2-hydroxypropyl) methacrylamide] brushes prepared by “grafting to” and “grafting from”. Polym. Chem. 2022, 13, 3815–3826. [Google Scholar] [CrossRef]
- Blaszykowski, C.; Sheikh, S.; Thompson, M. A survey of state-of-the-art surface chemistries to minimize fouling from human and animal biofluids. Biomater. Sci. 2015, 3, 1335–1370. [Google Scholar] [CrossRef]
- Du, Q.; Wang, W.; Zeng, X.; Luo, X. Antifouling zwitterionic peptide hydrogel based electrochemical biosensor for reliable detection of prostate specific antigen in human serum. Anal. Chim. Acta 2023, 1239, 340674. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zhang, C.; Yi, L.; Fan, X.; Gu, Y.; Wang, S. Biomimetic phosphorylcholine-based antifouling electrochemical biosensor for tetracycline analysis in milk. Sens. Actuators B Chem. 2024, 419, 136411. [Google Scholar] [CrossRef]
- Song, Z.; Li, R.; Yang, X.; Zhang, Z.; Luo, X. Functional DNA-peptide conjugates with enhanced antifouling capabilities for electrochemical detection of proteins in complex human serum. Sens. Actuators B Chem. 2022, 367, 132110. [Google Scholar] [CrossRef]
- Chen, M.; Song, Z.; Yang, X.; Song, Z.; Luo, X. Antifouling peptides combined with recognizing DNA probes for ultralow fouling electrochemical detection of cancer biomarkers in human bodily fluids. Biosens. Bioelectron. 2022, 206, 114162. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, X.; Shi, M.; Yu, X.; Luo, X. Antifouling electrochemical biosensors based on mussel-inspired poly(norepinephrine) and functional peptides for the detection of extracellular signal-regulated kinase 2 in serum. Sens. Actuators B Chem. 2025, 425, 136964. [Google Scholar] [CrossRef]
- Hedayati, M.; Marruecos, D.F.; Krapf, D.; Kaar, J.L.; Kipper, M.J. Protein adsorption measurements on low fouling and ultralow fouling surfaces: A critical comparison of surface characterization techniques. Acta Biomater. 2020, 102, 169–180. [Google Scholar] [CrossRef]
- Rodahl, M.; Kasemo, B. A simple setup to simultaneously measure the resonant frequency and the absolute dissipation factor of a quartz crystal microbalance. Rev. Sci. Instrum. 1996, 67, 3238–3241. [Google Scholar] [CrossRef]
- Regev, C.; Jiang, Z.; Kasher, R.; Miller, Y. Critical surface density of zwitterionic polymer chains affect antifouling properties. Appl. Surf. Sci. 2022, 596, 153652. [Google Scholar] [CrossRef]
- Xia, Y.; Adibnia, V.; Huang, R.; Murschel, F.; Faivre, J.; Xie, G.; Olszewski, M.; De Crescenzo, G.; Qi, W.; He, Z.; et al. Biomimetic Bottlebrush Polymer Coatings for Fabrication of Ultralow Fouling Surfaces. Angew. Chem. Int. Ed. 2019, 58, 1308–1314. [Google Scholar] [CrossRef]
- Forinová, M.; Pilipenco, A.; Lynn, N.S.; Obořilová, R.; Šimečková, H.; Vrabcová, M.; Spasovová, M.; Jack, R.; Horák, P.; Houska, M.; et al. A reusable QCM biosensor with stable antifouling nano-coating for on-site reagent-free rapid detection of E. coli O157:H7 in food products. Food Control 2024, 165, 110695. [Google Scholar] [CrossRef]
- Spagnolo, S.; Davoudian, K.; De La Franier, B.; Hianik, T.; Thompson, M. Staphylococcus aureus Detection in Milk Using a Thickness Shear Mode Acoustic Aptasensor with an Antifouling Probe Linker. Biosensors 2023, 13, 614. [Google Scholar] [CrossRef]
- Vaisocherová, H.; Ševců, V.; Adam, P.; Špačková, B.; Hegnerová, K.; de los Santos Pereira, A.; Rodriguez-Emmenegger, C.; Riedel, T.; Houska, M.; Brynda, E.; et al. Functionalized ultra-low fouling carboxy- and hydroxy-functional surface platforms: Functionalization capacity, biorecognition capability and resistance to fouling from undiluted biological media. Biosens. Bioelectron. 2014, 51, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Lin, W.; Wang, Z.; Chen, S.; Chang, Y. Investigation of the Hydration of Nonfouling Material Poly(sulfobetaine methacrylate) by Low-Field Nuclear Magnetic Resonance. Langmuir 2012, 28, 7436–7441. [Google Scholar] [CrossRef]
- Vrabcová, M.; Spasovová, M.; Houska, M.; Mrkvová, K.; Lynn, N.S.; Fekete, L.; Romanyuk, O.; Dejneka, A.; Vaisocherová-Lísalová, H. Long-term stability of antifouling poly(carboxybetaine acrylamide) brush coatings. Prog. Org. Coat. 2024, 188, 108187. [Google Scholar] [CrossRef]
- Han, M.; Hlaing, M.M.; Stoddart, P.R.; Greene, G.W. Self-Assembled Lubricin (PRG-4)-Based Biomimetic Surface-Enhanced Raman Scattering Sensor for Direct Droplet Detection of Melamine in Undiluted Milk. Biosensors 2024, 14, 591. [Google Scholar] [CrossRef] [PubMed]
- Leng, C.; Sun, S.; Zhang, K.; Jiang, S.; Chen, Z. Molecular level studies on interfacial hydration of zwitterionic and other antifouling polymers in situ. Acta Biomater. 2016, 40, 6–15. [Google Scholar] [CrossRef]
- Breault-Turcot, J.; Chaurand, P.; Masson, J.-F. Unravelling Nonspecific Adsorption of Complex Protein Mixture on Surfaces with SPR and MS. Anal. Chem. 2014, 86, 9612–9619. [Google Scholar] [CrossRef]
- Li, C.; Xia, Y.; Liu, C.; Huang, R.; Qi, W.; He, Z.; Su, R. Lubricin-Inspired Loop Zwitterionic Peptide for Fabrication of Superior Antifouling Surfaces. ACS Appl. Mater. Interfaces 2021, 13, 41978–41986. [Google Scholar] [CrossRef]
- Sun, S.; Chen, J. Recent Advances in Hydrogel-Based Biosensors for Cancer Detection. ACS Appl. Mater. Interfaces 2024, 16, 46988–47002. [Google Scholar] [CrossRef]
- Zhang, C.; Huang, H.; Wang, X.; Zhang, Y.; Sun, W.; Liu, Q.; Zhou, X.; Xu, W.; Luo, Y.; Huang, K.; et al. Zwitterions modified biosensors improve detection performance in complex food matrices. Trends Food Sci. Technol. 2024, 145, 104374. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, R.; Liu, K.; Zhang, Y.; Shi, X.; Sand, W.; Hou, B. Application of nanomaterials in antifouling: A review. Nano Mater. Sci. 2024, 6, 672–700. [Google Scholar] [CrossRef]
- Wu, J.; Zhao, C.; Lin, W.; Hu, R.; Wang, Q.; Chen, H.; Li, L.; Chen, S.; Zheng, J. Binding characteristics between polyethylene glycol (PEG) and proteins in aqueous solution. J. Mater. Chem. B 2014, 2, 2983–2992. [Google Scholar] [CrossRef]
- Zheng, J.; Li, L.; Tsao, H.-K.; Sheng, Y.-J.; Chen, S.; Jiang, S. Strong Repulsive Forces between Protein and Oligo (Ethylene Glycol) Self-Assembled Monolayers: A Molecular Simulation Study. Biophys. J. 2005, 89, 158–166. [Google Scholar] [CrossRef]
- Shaver, A.; Curtis, S.D.; Arroyo-Currás, N. Alkanethiol Monolayer End Groups Affect the Long-Term Operational Stability and Signaling of Electrochemical, Aptamer-Based Sensors in Biological Fluids. ACS Appl. Mater. Interfaces 2020, 12, 11214–11223. [Google Scholar] [CrossRef] [PubMed]
- Ge, D.; Wang, X.; Williams, K.; Levicky, R. Thermostable DNA Immobilization and Temperature Effects on Surface Hybridization. Langmuir 2012, 28, 8446–8455. [Google Scholar] [CrossRef]
- Phares, N.; White, R.J.; Plaxco, K.W. Improving the Stability and Sensing of Electrochemical Biosensors by Employing Trithiol-Anchoring Groups in a Six-Carbon Self-Assembled Monolayer. Anal. Chem. 2009, 81, 1095–1100. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Zhang, M.; Ding, M.; Ji, X.; Song, X.; Ding, C. Ultrasensitive Electrochemical Biosensor Based on Efficient PDA-APDMAO Antifouling Interface and Dual-Signal Ratio Strategy for Trace Detection of Alpha-Fetoprotein in Human Serum. Anal. Chem. 2024, 96, 14108–14115. [Google Scholar] [CrossRef]
- Li, B.; Jain, P.; Ma, J.; Smith, J.K.; Yuan, Z.; Hung, H.-C.; He, Y.; Lin, X.; Wu, K.; Pfaendtner, J.; et al. Trimethylamine N-oxide–derived zwitterionic polymers: A new class of ultralow fouling bioinspired materials. Sci. Adv. 2019, 5, eaaw9562. [Google Scholar] [CrossRef]
- Li, Y.; Pu, X.; Niu, M.; Fan, X.; Ding, Y.; Ma, W.; Gu, Y. Engineering an antifouling electrochemical aptasensor based on a designed zwitterionic peptide for tetracycline detection in milk. Food Control 2023, 153, 109929. [Google Scholar] [CrossRef]
- Li, Y.; Pu, X.; Ding, Y.; Yi, L.; Yang, Y.; Gu, Y.; Wang, S. An antifouling electrochemical sensor based on a U-shaped four-in-one peptide and poly(3,4-ethylenedioxythiophene) for vancomycin detection in fresh goat milk. Food Chem. 2025, 463, 141056. [Google Scholar] [CrossRef]
- Hui, N.; Wang, J.; Wang, D.; Wang, P.; Luo, X.; Lv, S. An ultrasensitive biosensor for prostate specific antigen detection in complex serum based on functional signal amplifier and designed peptides with both antifouling and recognizing capabilities. Biosens. Bioelectron. 2022, 200, 113921. [Google Scholar] [CrossRef]
- Li, Y.; Pu, X.; Ge, K.; Yi, L.; Ren, D.; Gu, Y.; Wang, S. Engineering an Antifouling Electrochemical Sensing Platform Based on an All-in-One Peptide and a Hierarchical β-Bi2O3–Au Microsphere for Vancomycin Detection in Food. J. Agric. Food Chem. 2023, 71, 19866–19878. [Google Scholar] [CrossRef] [PubMed]
- Pu, X.; Hu, Y.; Niu, M.; Liu, H.; Li, C.; Ma, W.; Gu, Y. An antifouling electrochemical aptasensor based on a Y-shaped peptide for tetracycline detection in milk. J. Food Compos. Anal. 2024, 132, 106349. [Google Scholar] [CrossRef]
- Song, Z.; Ma, Y.; Chen, M.; Ambrosi, A.; Ding, C.; Luo, X. Electrochemical Biosensor with Enhanced Antifouling Capability for COVID-19 Nucleic Acid Detection in Complex Biological Media. Anal. Chem. 2021, 93, 5963–5971. [Google Scholar] [CrossRef]
- Han, R.; Hou, W.; Li, Y.; Chen, M.; Ding, C.; Luo, X. The design of anti-fouling and anti-hydrolysis cyclic peptides for accurate electrochemical antigen testing in human blood. Sens. Diagn. 2023, 2, 382–389. [Google Scholar] [CrossRef]
- Yang, X.; Chen, M.; Zhang, Z.; Li, Y.; Wang, P.; Luo, X.; Lv, S. Alpha-aminoisobutyric acid incorporated peptides for the construction of electrochemical biosensors with high stability and low fouling in serum. Anal. Chim. Acta 2023, 1238, 340646. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Qiao, X.; Chen, M.; Li, Y.; Wang, X.; Xu, Z.; Wu, Y.; Luo, X. d-Amino Acid-Based Antifouling Peptides for the Construction of Electrochemical Biosensors Capable of Assaying Proteins in Serum with Enhanced Stability. ACS Sens. 2022, 7, 1740–1746. [Google Scholar] [CrossRef] [PubMed]
- Gentilucci, L.; Marco, R.; Cerisoli, L. Chemical Modifications Designed to Improve Peptide Stability: Incorporation of Non-Natural Amino Acids, Pseudo-Peptide Bonds, and Cyclization. Curr. Pharm. Des. 2010, 16, 3185–3203. [Google Scholar] [CrossRef]
- Song, Z.; Li, Y.; Li, R.; Fan, G.-C.; Luo, X. Robust Electrochemical Biosensors Based on Antifouling Peptide Nanoparticles for Protein Quantification in Complex Biofluids. ACS Sens. 2024, 9, 1525–1532. [Google Scholar] [CrossRef]
- Liu, X.; Huang, R.; Su, R.; Qi, W.; Wang, L.; He, Z. Grafting Hyaluronic Acid onto Gold Surface to Achieve Low Protein Fouling in Surface Plasmon Resonance Biosensors. ACS Appl. Mater. Interfaces 2014, 6, 13034–13042. [Google Scholar] [CrossRef]
- Polonschii, C.; David, S.; Tombelli, S.; Mascini, M.; Gheorghiu, M. A novel low-cost and easy to develop functionalization platform. Case study: Aptamer-based detection of thrombin by surface plasmon resonance. Talanta 2010, 80, 2157–2164. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-C.; Kim, S.Y.; Song, J.; Jang, H.; Kim, M.; Kim, H.; Choi, S.Q.; Kim, S.; Jolly, P.; Kang, T.; et al. Micrometer-thick and porous nanocomposite coating for electrochemical sensors with exceptional antifouling and electroconducting properties. Nat. Commun. 2024, 15, 711. [Google Scholar] [CrossRef]
- Sabaté del Río, J.; Henry, O.Y.F.; Jolly, P.; Ingber, D.E. An antifouling coating that enables affinity-based electrochemical biosensing in complex biological fluids. Nat. Nanotechnol. 2019, 14, 1143–1149. [Google Scholar] [CrossRef] [PubMed]
- Zupančič, U.; Jolly, P.; Estrela, P.; Moschou, D.; Ingber, D.E. Electrochemical Biosensors: Graphene Enabled Low-Noise Surface Chemistry for Multiplexed Sepsis Biomarker Detection in Whole Blood (Adv. Funct. Mater. 16/2021). Adv. Funct. Mater. 2021, 31, 2170107. [Google Scholar] [CrossRef]
- Bharti, A.M.; Rakesh Kumar, R.K.; Chuang, C.-H.; Shaikh, M.O. Universal nanocomposite coating with antifouling and redox capabilities for electrochemical affinity biosensing in complex biological fluids. Nanoscale Horiz. 2024, 9, 843–852. [Google Scholar] [CrossRef]
- Yu, X.; Meng, W.; Li, Y.; Luo, X. A low-fouling electrochemical biosensor based on BSA hydrogel doped with carbon black for the detection of cortisol in human serum. Anal. Chim. Acta 2024, 1307, 342645. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Hou, Q.; Wang, P.; Ding, C.; Lv, S. Antifouling Electrochemiluminescence Biosensor Based on Bovine Serum Albumin Hydrogel for the Accurate Detection of p53 Gene in Human Serum. ACS Appl. Mater. Interfaces 2023, 15, 44322–44330. [Google Scholar] [CrossRef]
- Kimura-Suda, H.; Petrovykh, D.Y.; Tarlov, M.J.; Whitman, L.J. Base-Dependent Competitive Adsorption of Single-Stranded DNA on Gold. J. Am. Chem. Soc. 2003, 125, 9014–9015. [Google Scholar] [CrossRef]
- Opdahl, A.; Petrovykh, D.Y.; Kimura-Suda, H.; Tarlov, M.J.; Whitman, L.J. Independent control of grafting density and conformation of single-stranded DNA brushes. Proc. Natl. Acad. Sci. USA 2007, 104, 9–14. [Google Scholar] [CrossRef]
- Schreiner, S.M.; Shudy, D.F.; Hatch, A.L.; Opdahl, A.; Whitman, L.J.; Petrovykh, D.Y. Controlled and Efficient Hybridization Achieved with DNA Probes Immobilized Solely through Preferential DNA-Substrate Interactions. Anal. Chem. 2010, 82, 2803–2810. [Google Scholar] [CrossRef]
- Li, H.; Dauphin-Ducharme, P.; Arroyo-Currás, N.; Tran, C.H.; Vieira, P.A.; Li, S.; Shin, C.; Somerson, J.; Kippin, T.E.; Plaxco, K.W. A Biomimetic Phosphatidylcholine-Terminated Monolayer Greatly Improves the In Vivo Performance of Electrochemical Aptamer-Based Sensors. Angew. Chem. Int. Ed. 2017, 56, 7492–7495. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wu, H.; Xie, N.; Zhang, X.; Zou, Z.; Deng, M.; Cheng, W.; Guo, X.; Ding, S.; Guo, B. Conductive Antifouling Sensing Coating: A Bionic Design Inspired by Natural Cell Membrane. Adv. Healthc. Mater. 2023, 12, 2202790. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Han, R.; Feng, J.; Li, J.; Luo, X. Phospholipid Bilayer Integrated with Multifunctional Peptide for Ultralow-Fouling Electrochemical Detection of HER2 in Human Serum. Anal. Chem. 2024, 96, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Han, R.; Li, Q.; Han, Y.; Luo, X. Electrochemical Biosensors Capable of Detecting Biomarkers in Human Serum with Unique Long-Term Antifouling Abilities Based on Designed Multifunctional Peptides. Anal. Chem. 2020, 92, 7186–7193. [Google Scholar] [CrossRef]
- Abedi-Firoozjah, R.; Alizadeh-Sani, M.; Zare, L.; Rostami, O.; Azimi Salim, S.; Assadpour, E.; Azizi-Lalabadi, M.; Zhang, F.; Lin, X.; Jafari, S.M. State-of-the-art nanosensors and kits for the detection of antibiotic residues in milk and dairy products. Adv. Colloid Interface Sci. 2024, 328, 103164. [Google Scholar] [CrossRef]
- Matharu, Z.; Daggumati, P.; Wang, L.; Dorofeeva, T.S.; Li, Z.; Seker, E. Nanoporous-Gold-Based Electrode Morphology Libraries for Investigating Structure–Property Relationships in Nucleic Acid Based Electrochemical Biosensors. ACS Appl. Mater. Interfaces 2017, 9, 12959–12966. [Google Scholar] [CrossRef]
- Ferguson, B.S.; Hoggarth, D.A.; Maliniak, D.; Ploense, K.; White, R.J.; Woodward, N.; Hsieh, K.; Bonham, A.J.; Eisenstein, M.; Kippin, T.E.; et al. Real-Time, Aptamer-Based Tracking of Circulating Therapeutic Agents in Living Animals. Sci. Transl. Med. 2013, 5, 213ra165. [Google Scholar] [CrossRef]
- Patel, J.; Radhakrishnan, L.; Zhao, B.; Uppalapati, B.; Daniels, R.C.; Ward, K.R.; Collinson, M.M. Electrochemical Properties of Nanostructured Porous Gold Electrodes in Biofouling Solutions. Anal. Chem. 2013, 85, 11610–11618. [Google Scholar] [CrossRef]
- Osman, E.; Sakib, S.; Maclachlan, R.; Saxena, S.; Akhlaghi, A.A.; Adhikari, B.R.; Zhang, Z.; Li, Y.; Soleymani, L. A Comparison of DNA–DNA Hybridization Kinetics in Complex Media on Planar and Nanostructured Electrodes. ACS Sens. 2024, 9, 4599–4607. [Google Scholar] [CrossRef]
- Arroyo-Currás, N.; Somerson, J.; Vieira, P.A.; Ploense, K.L.; Kippin, T.E.; Plaxco, K.W. Real-time measurement of small molecules directly in awake, ambulatory animals. Proc. Natl. Acad. Sci. USA 2017, 114, 645–650. [Google Scholar] [CrossRef]
- Uçar, A.; González-Fernández, E.; Staderini, M.; Murray, A.F.; Mount, A.R.; Bradley, M. pH-Activated Dissolvable Polymeric Coatings to Reduce Biofouling on Electrochemical Sensors. J. Funct. Biomater. 2023, 14, 329. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Valdepeñas Montiel, V.; Sempionatto, J.R.; Campuzano, S.; Pingarrón, J.M.; Esteban Fernández de Ávila, B.; Wang, J. Direct electrochemical biosensing in gastrointestinal fluids. Anal. Bioanal. Chem. 2019, 411, 4597–4604. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Somerson, J.; Xia, F.; Plaxco, K.W. Electrochemical DNA-Based Sensors for Molecular Quality Control: Continuous, Real-Time Melamine Detection in Flowing Whole Milk. Anal. Chem. 2018, 90, 10641–10645. [Google Scholar] [CrossRef]
- Li, H.; Dauphin-Ducharme, P.; Ortega, G.; Plaxco, K.W. Calibration-Free Electrochemical Biosensors Supporting Accurate Molecular Measurements Directly in Undiluted Whole Blood. J. Am. Chem. Soc. 2017, 139, 11207–11213. [Google Scholar] [CrossRef]
- Idili, A.; Parolo, C.; Ortega, G.; Plaxco, K.W. Calibration-Free Measurement of Phenylalanine Levels in the Blood Using an Electrochemical Aptamer-Based Sensor Suitable for Point-of-Care Applications. ACS Sens. 2019, 4, 3227–3233. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Matharu, Z.; Rahimian, A.; Revzin, A. Detecting multiple cell-secreted cytokines from the same aptamer-functionalized electrode. Biosens. Bioelectron. 2015, 64, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, S.; Dai, J.; Li, C.; Zhu, M.; Li, H.; Lou, X.; Xia, F.; Plaxco, K.W. High frequency, calibration-free molecular measurements in situ in the living body. Chem. Sci. 2019, 10, 10843–10848. [Google Scholar] [CrossRef]
- Chan, D.; Chien, J.-C.; Axpe, E.; Blankemeier, L.; Baker, S.W.; Swaminathan, S.; Piunova, V.A.; Zubarev, D.Y.; Maikawa, C.L.; Grosskopf, A.K.; et al. Combinatorial Polyacrylamide Hydrogels for Preventing Biofouling on Implantable Biosensors. Adv. Mater. 2022, 34, 2109764. [Google Scholar] [CrossRef]
- Santos-Cancel, M.; White, R.J. Collagen Membranes with Ribonuclease Inhibitors for Long-Term Stability of Electrochemical Aptamer-Based Sensors Employing RNA. Anal. Chem. 2017, 89, 5598–5604. [Google Scholar] [CrossRef]
- Pellitero, M.A.; Curtis, S.D.; Arroyo-Currás, N. Interrogation of Electrochemical Aptamer-Based Sensors via Peak-to-Peak Separation in Cyclic Voltammetry Improves the Temporal Stability and Batch-to-Batch Variability in Biological Fluids. ACS Sens. 2021, 6, 1199–1207. [Google Scholar] [CrossRef]
- Kourti, D.; Geka, G.; Nemtsov, L.; Ahmadi, S.; Economou, A.; Thompson, M. Electrochemical Aptasensor with Antifouling Properties for Label-Free Detection of Oxytetracycline. Sensors 2024, 24, 5488. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S.; Lu, Y.; Zhang, Z.; Li, Y.; Soleymani, L.; Hoare, T. Zwitter-repel: An anti-fouling coating promoting electrochemical biosensing in biological fluids. Chem. Eng. J. 2024, 495, 153522. [Google Scholar] [CrossRef]
- Huang, S.; Tang, R.; Zhang, T.; Zhao, J.; Jiang, Z.; Wang, Q. Anti-fouling poly adenine coating combined with highly specific CD20 epitope mimetic peptide for rituximab detection in clinical patients’ plasma. Biosens. Bioelectron. 2021, 171, 112678. [Google Scholar] [CrossRef]
- Capatina, D.; Lupoi, T.; Feier, B.; Blidar, A.; Hosu, O.; Tertis, M.; Olah, D.; Cristea, C.; Oprean, R. Label-Free Electrochemical Aptasensor for the Detection of the 3-O-C12-HSL Quorum-Sensing Molecule in Pseudomonas aeruginosa. Biosensors 2022, 12, 440. [Google Scholar] [CrossRef]
- Jambrec, D.; Conzuelo, F.; Zhao, B.; Schuhmann, W. Potential-pulse assisted thiol chemisorption minimizes non-specific adsorptions in DNA assays. Electrochim. Acta 2018, 276, 233–239. [Google Scholar] [CrossRef]
- Bhadra, P.; Siu, S.W.I. Effect of Concentration, Chain Length, Hydrophobicity, and an External Electric Field on the Growth of Mixed Alkanethiol Self-Assembled Monolayers: A Molecular Dynamics Study. Langmuir 2021, 37, 1913–1924. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, Y.; Zhang, Z.; Wang, Y.; Li, H.; Xia, F. Exploring End-Group Effect of Alkanethiol Self-Assembled Monolayers on Electrochemical Aptamer-Based Sensors in Biological Fluids. Anal. Chem. 2021, 93, 5849–5855. [Google Scholar] [CrossRef]
- Gerson, J.; Erdal, M.K.; McDonough, M.H.; Ploense, K.L.; Dauphin-Ducharme, P.; Honeywell, K.M.; Leung, K.K.; Arroyo-Curras, N.; Gibson, J.M.; Emmons, N.A.; et al. High-precision monitoring of and feedback control over drug concentrations in the brains of freely moving rats. Sci. Adv. 2023, 9, eadg3254. [Google Scholar] [CrossRef]
- Swensen, J.S.; Xiao, Y.; Ferguson, B.S.; Lubin, A.A.; Lai, R.Y.; Heeger, A.J.; Plaxco, K.W.; Soh, H.T. Continuous, Real-Time Monitoring of Cocaine in Undiluted Blood Serum via a Microfluidic, Electrochemical Aptamer-Based Sensor. J. Am. Chem. Soc. 2009, 131, 4262–4266. [Google Scholar] [CrossRef]
- Li, R.; Yu, K.; Fan, G.-C.; Song, Z.; Luo, X. Polyethyleneimine cross-linked BSA with enhanced antifouling capability for electrochemical detection of cancer antigen 125 in complex biofluids. Sens. Actuators B Chem. 2024, 408, 135520. [Google Scholar] [CrossRef]
- Pillai, R.G.; Azyat, K.; Chan, N.W.C.; Jemere, A.B. Rapid assembly of mixed thiols for toll-like receptor-based electrochemical pathogen sensing. Anal. Methods 2024, 16, 7021–7032. [Google Scholar] [CrossRef]
- Kilic, T.; Gessner, I.; Cho, Y.K.; Jeong, N.; Quintana, J.; Weissleder, R.; Lee, H. Zwitterionic Polymer Electroplating Facilitates the Preparation of Electrode Surfaces for Biosensing. Adv. Mater. 2022, 34, 2107892. [Google Scholar] [CrossRef]
- Goda, T.; Miyahara, Y. Electrodeposition of Zwitterionic PEDOT Films for Conducting and Antifouling Surfaces. Langmuir 2019, 35, 1126–1133. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, Z.; Song, Z.-L.; Fan, G.-C.; Luo, X. Click Conjugation of Zwitterionic Peptide with DNA Strand: An Efficient Antifouling Strategy for Versatile Photoelectrochemical Aptasensor. Anal. Chem. 2024, 96, 3233–3242. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Chen, M.; Ding, C.; Luo, X. Designed Three-in-One Peptides with Anchoring, Antifouling, and Recognizing Capabilities for Highly Sensitive and Low-Fouling Electrochemical Sensing in Complex Biological Media. Anal. Chem. 2020, 92, 5795–5802. [Google Scholar] [CrossRef]
- Li, Y.; Wei, Z.; Guo, S.; Zhan, Y.; Fan, G.-C.; Luo, X. Design of U-shaped peptides with long-lasting antifouling efficacy: Toward a feasible electrochemical aptasensor for robust detection in human serum. Anal. Chim. Acta 2024, 1318, 342953. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Han, R.; Yu, X.; Jiang, L.; Luo, X. A low-fouling electrochemical biosensor based on multifunction branched peptides with antifouling, antibacterial and recognizing sequences for protein detection in saliva. Sens. Actuators B Chem. 2024, 405, 135322. [Google Scholar] [CrossRef]
- Han, R.; Li, Y.; Zhang, Y.; Wang, P.; Ding, C.; Luo, X.; Lv, S. An electrochemical biosensor with enhanced antifouling properties enabled by peptide self-assembly via robust Pt-S interactions. Sens. Actuators B Chem. 2024, 418, 136321. [Google Scholar] [CrossRef]
- Shi, M.; Li, Y.; Wang, W.; Han, R.; Luo, X. A Super-Antifouling Electrochemical Biosensor for Protein Detection in Complex Biofluids Based on PEGylated Multifunctional Peptide. ACS Sens. 2024, 9, 2956–2963. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, X.; Ding, C.; Luo, X. Ratiometric Antifouling Electrochemical Biosensors Based on Multifunctional Peptides and MXene Loaded with Au Nanoparticles and Methylene Blue. ACS Appl. Mater. Interfaces 2021, 13, 20388–20396. [Google Scholar] [CrossRef]
- Wang, W.; Han, R.; Chen, M.; Luo, X. Antifouling Peptide Hydrogel Based Electrochemical Biosensors for Highly Sensitive Detection of Cancer Biomarker HER2 in Human Serum. Anal. Chem. 2021, 93, 7355–7361. [Google Scholar] [CrossRef]
- Olaru, A.; Gheorghiu, M.; David, S.; Polonschii, C.; Gheorghiu, E. Quality assessment of SPR sensor chips; case study on L1 chips. Biosens. Bioelectron. 2013, 45, 77–81. [Google Scholar] [CrossRef]
- Olaru, A.; Gheorghiu, M.; David, S.; Wohland, T.; Gheorghiu, E. Assessment of the Multiphase Interaction between a Membrane Disrupting Peptide and a Lipid Membrane. J. Phys. Chem. B 2009, 113, 14369–14380. [Google Scholar] [CrossRef] [PubMed]
- Rumi, R.B.; Paul, A.K.; Alyami, S.A.; Moni, M.A. Multi-Disease Detection Using a Prism-Based Surface Plasmon Resonance Sensor: A TMM and FEM Approach. IEEE Trans. NanoBioscience 2024, 23, 51–62. [Google Scholar] [CrossRef]
- Srivastava, S.; Singh, S.; Mishra, A.C.; Lohia, P.; Dwivedi, D.K. Numerical Study of Titanium Dioxide and MXene Nanomaterial-Based Surface Plasmon Resonance Biosensor for Virus SARS-CoV-2 Detection. Plasmonics 2023, 18, 1477–1488. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.-N.; Sun, F.; Hung, H.-C.; Jain, P.; Sinclair, A.; Zhang, P.; Bai, T.; Chang, Y.; Wen, T.-C.; Yu, Q.; et al. Ultra-low fouling and high antibody loading zwitterionic hydrogel coatings for sensing and detection in complex media. Acta Biomater. 2016, 40, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Nie, W.; Wang, Q.; Zou, L.; Zheng, Y.; Liu, X.; Yang, X.; Wang, K. Low-Fouling Surface Plasmon Resonance Sensor for Highly Sensitive Detection of MicroRNA in a Complex Matrix Based on the DNA Tetrahedron. Anal. Chem. 2018, 90, 12584–12591. [Google Scholar] [CrossRef] [PubMed]
- Huertas, C.S.; Soler, M.; Estevez, M.C.; Lechuga, L.M. One-Step Immobilization of Antibodies and DNA on Gold Sensor Surfaces via a Poly-Adenine Oligonucleotide Approach. Anal. Chem. 2020, 92, 12596–12604. [Google Scholar] [CrossRef]
- Bellassai, N.; D’Agata, R.; Spoto, G. Plasmonic aptasensor with antifouling dual-functional surface layer for lysozyme detection in food. Anal. Chim. Acta 2023, 1283, 341979. [Google Scholar] [CrossRef]
- Bellassai, N.; D’Agata, R.; Marti, A.; Rozzi, A.; Volpi, S.; Allegretti, M.; Corradini, R.; Giacomini, P.; Huskens, J.; Spoto, G. Detection of Tumor DNA in Human Plasma with a Functional PLL-Based Surface Layer and Plasmonic Biosensing. ACS Sens. 2021, 6, 2307–2319. [Google Scholar] [CrossRef]
- Malinick, A.S.; Stuart, D.D.; Lambert, A.S.; Cheng, Q. Surface plasmon resonance imaging (SPRi) in combination with machine learning for microarray analysis of multiple sclerosis biomarkers in whole serum. Biosens. Bioelectron. X 2022, 10, 100127. [Google Scholar] [CrossRef]
- D’Agata, R.; Bellassai, N.; Giuffrida, M.C.; Aura, A.M.; Petri, C.; Kögler, P.; Vecchio, G.; Jonas, U.; Spoto, G. A new ultralow fouling surface for the analysis of human plasma samples with surface plasmon resonance. Talanta 2021, 221, 121483. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, H.; Yu, B.; Wang, S.; Shen, Y.; Cong, H. Review of the research on anti-protein fouling coatings materials. Prog. Org. Coat. 2020, 147, 105860. [Google Scholar] [CrossRef]
- Choi, Y.; Tran, H.-V.; Lee, T.R. Self-Assembled Monolayer Coatings on Gold and Silica Surfaces for Antifouling Applications: A Review. Coatings 2022, 12, 1462. [Google Scholar] [CrossRef]
- Emilsson, G.; Schoch, R.L.; Feuz, L.; Höök, F.; Lim, R.Y.H.; Dahlin, A.B. Strongly Stretched Protein Resistant Poly(ethylene glycol) Brushes Prepared by Grafting-To. ACS Appl. Mater. Interfaces 2015, 7, 7505–7515. [Google Scholar] [CrossRef]
- Sun, R.; Ma, H.; Wang, H.; Wang, Y.; Zhang, L.; Zhang, X.; Sun, H.; Zheng, H.; Guo, J.; Guo, N.; et al. A multifunctional nanoplatform for dual-readout visual detection and real-time inactivation of Staphylococcus aureus with nanozymes and Au nanorods. Food Biosci. 2024, 60, 104453. [Google Scholar] [CrossRef]
- Ribeiro, B.V.; Cordeiro, T.A.R.; Oliveira e Freitas, G.R.; Ferreira, L.F.; Franco, D.L. Biosensors for the detection of respiratory viruses: A review. Talanta Open 2020, 2, 100007. [Google Scholar] [CrossRef]
- Víšová, I.; Houska, M.; Vaisocherová-Lísalová, H. Biorecognition antifouling coatings in complex biological fluids: A review of functionalization aspects. Analyst 2022, 147, 2597–2614. [Google Scholar] [CrossRef]
- Vaisocherová, H.; Yang, W.; Zhang, Z.; Cao, Z.; Cheng, G.; Piliarik, M.; Homola, J.; Jiang, S. Ultralow Fouling and Functionalizable Surface Chemistry Based on a Zwitterionic Polymer Enabling Sensitive and Specific Protein Detection in Undiluted Blood Plasma. Anal. Chem. 2008, 80, 7894–7901. [Google Scholar] [CrossRef]
- Su, H.; Li, S.; Kerman, K. Novel thiolated-PEG linker molecule for biosensor development on gold surfaces. Biosens. Bioelectron. 2019, 141, 111477. [Google Scholar] [CrossRef]
- Li, Q.; Wen, C.; Yang, J.; Zhou, X.; Zhu, Y.; Zheng, J.; Cheng, G.; Bai, J.; Xu, T.; Ji, J.; et al. Zwitterionic Biomaterials. Chem. Rev. 2022, 122, 17073–17154. [Google Scholar] [CrossRef]
- Zhang, D.; Ren, B.; Zhang, Y.; Liu, Y.; Chen, H.; Xiao, S.; Chang, Y.; Yang, J.; Zheng, J. Micro- and macroscopically structured zwitterionic polymers with ultralow fouling property. J. Colloid Interface Sci. 2020, 578, 242–253. [Google Scholar] [CrossRef]
- Vaisocherová, H.; Šípová, H.; Víšová, I.; Bocková, M.; Špringer, T.; Laura Ermini, M.; Song, X.; Krejčík, Z.; Chrastinová, L.; Pastva, O.; et al. Rapid and sensitive detection of multiple microRNAs in cell lysate by low-fouling surface plasmon resonance biosensor. Biosens. Bioelectron. 2015, 70, 226–231. [Google Scholar] [CrossRef] [PubMed]
- McKeating, K.S.; Hinman, S.S.; Rais, N.A.; Zhou, Z.; Cheng, Q. Antifouling Lipid Membranes over Protein A for Orientation-Controlled Immunosensing in Undiluted Serum and Plasma. ACS Sens. 2019, 4, 1774–1782. [Google Scholar] [CrossRef]
- Stuart, D.D.; Pike, C.D.; Malinick, A.S.; Cheng, Q. Characterization of a Charged Biomimetic Lipid Membrane for Unique Antifouling Effects against Clinically Relevant Matrices in Biosensing. ACS Appl. Mater. Interfaces 2024, 16, 56438–56447. [Google Scholar] [CrossRef] [PubMed]
- Giarola, J.F.; Estevez, M.C.; Lechuga, L.M. Plasmonic biosensors: Towards fully operative detection platforms for biomedical application and its potential for the diagnosis of autoimmune diseases. TrAC Trends Anal. Chem. 2024, 176, 117763. [Google Scholar] [CrossRef]
- Bellassai, N.; D’Agata, R.; Giordani, E.; Ziccheddu, G.; Corradini, R.; Spoto, G. A novel method for detecting genetic biomarkers in blood-based liquid biopsies using surface plasmon resonance imaging and magnetic beads shows promise in cancer diagnosis and monitoring. Talanta 2025, 286, 127543. [Google Scholar] [CrossRef] [PubMed]
- Vaisocherová-Lísalová, H.; Surman, F.; Víšová, I.; Vala, M.; Špringer, T.; Ermini, M.L.; Šípová, H.; Šedivák, P.; Houska, M.; Riedel, T.; et al. Copolymer Brush-Based Ultralow-Fouling Biorecognition Surface Platform for Food Safety. Anal. Chem. 2016, 88, 10533–10539. [Google Scholar] [CrossRef]
- Vaisocherová-Lísalová, H.; Víšová, I.; Ermini, M.L.; Špringer, T.; Song, X.C.; Mrázek, J.; Lamačová, J.; Scott Lynn, N.; Šedivák, P.; Homola, J. Low-fouling surface plasmon resonance biosensor for multi-step detection of foodborne bacterial pathogens in complex food samples. Biosens. Bioelectron. 2016, 80, 84–90. [Google Scholar] [CrossRef]
- Víšová, I.; Vrabcová, M.; Forinová, M.; Zhigunová, Y.; Mironov, V.; Houska, M.; Bittrich, E.; Eichhorn, K.-J.; Hashim, H.; Schovánek, P.; et al. Surface Preconditioning Influences the Antifouling Capabilities of Zwitterionic and Nonionic Polymer Brushes. Langmuir 2020, 36, 8485–8493. [Google Scholar] [CrossRef]
- Hsu, C.-Y.; Saleh, R.O.; Pallathadka, H.; Kumar, A.; Mansouri, S.; Bhupathi, P.; Jasim Ali, S.H.; Al-Mashhadani, Z.I.; Alzubaidi, L.H.; Hizam, M.M. Advances in electrochemical-optical dual-mode biosensors for detection of environmental pathogens. Anal. Methods 2024, 16, 1306–1322. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wu, D.; Xiao, S.; Yin, Y.; Li, L.; Wang, J.; Wu, Y.; Qiu, Y.; Dong, Y. Dual-mode aptasensors with cross validation capacity for reliability enhancement and analytical assurance. TrAC Trends Anal. Chem. 2024, 177, 117755. [Google Scholar] [CrossRef]
- Li, T.; Zhang, J.; Bu, P.; Wu, H.; Guo, J.; Guo, J. Multi-modal nanoprobe-enabled biosensing platforms: A critical review. Nanoscale 2024, 16, 3784–3816. [Google Scholar] [CrossRef]
- Liu, S.; Shi, J.; Lin, Y.; Luo, H.; Wu, Y.; Yan, J.; Tan, X.; Huang, K.-J. A sandwich-type dual-mode biosensor based on graphdiyne and DNA nanoframework for ultra-sensitive detection of CD142 gene. Biosens. Bioelectron. 2024, 248, 115962. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Zhao, L.; Wang, T.; Chen, Y.; Hao, W.; Zhang, Z.; Hao, Y.; Zhang, C.; Wei, X.; Zhang, Y.; et al. Dual-Mode Ratiometric Electrochemical and Turn-On Fluorescent Detection of Butyrylcholinesterase Utilizing a Single Probe for the Diagnosis of Alzheimer’s Disease. Anal. Chem. 2023, 95, 8340–8347. [Google Scholar] [CrossRef]
- Polonschii, C.; Gheorghiu, M.; David, S.; Gaspar, S.; Melinte, S.; Majeed, H.; Kandel, M.; Popescu, G.; Gheorghiu, E. High-resolution impedance mapping using electrically activated quantitative phase imaging. Light: Sci. Appl. 2021, 10, 20. [Google Scholar] [CrossRef]
- Ma, S.-C.; Gupta, R.; Ondevilla, N.A.P.; Barman, K.; Lee, L.-Y.; Chang, H.-C.; Huang, J.-J. Voltage-modulated surface plasmon resonance biosensors integrated with gold nanohole arrays. Biomed. Opt. Express 2023, 14, 182–193. [Google Scholar] [CrossRef]
- Blidar, A.; Feier, B.; Tertis, M.; Galatus, R.; Cristea, C. Electrochemical surface plasmon resonance (EC-SPR) aptasensor for ampicillin detection. Anal. Bioanal. Chem. 2019, 411, 1053–1065. [Google Scholar] [CrossRef]
- Smeazzetto, S.; Sacconi, A.; Schwan, A.L.; Margheri, G.; Tadini-Buoninsegni, F. Binding of a Monoclonal Antibody to the Phospholamban Cytoplasmic Domain Interferes with the Channel Activity of Phospholamban Reconstituted in a Tethered Bilayer Lipid Membrane. Langmuir 2014, 30, 10384–10388. [Google Scholar] [CrossRef]
- Wu, C.; Rehman, F.u.; Li, J.; Ye, J.; Zhang, Y.; Su, M.; Jiang, H.; Wang, X. Real-Time Evaluation of Live Cancer Cells by an in Situ Surface Plasmon Resonance and Electrochemical Study. ACS Appl. Mater. Interfaces 2015, 7, 24848–24854. [Google Scholar] [CrossRef]
- Michaelis, S.; Wegener, J.; Robelek, R. Label-free monitoring of cell-based assays: Combining impedance analysis with SPR for multiparametric cell profiling. Biosens. Bioelectron. 2013, 49, 63–70. [Google Scholar] [CrossRef]
- Gheorghiu, M.; David, S.; Polonschii, C.; Olaru, A.; Gaspar, S.; Bajenaru, O.; Popescu, B.O.; Gheorghiu, E. Label free sensing platform for amyloid fibrils effect on living cells. Biosens. Bioelectron. 2014, 52, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Boussaad, S.; Wong, S.; Tao, N.J. High-sensitivity Stark spectroscopy obtained by surface plasmon resonance measurement. Anal. Chem. 2000, 72, 4003–4008. [Google Scholar] [CrossRef]
- Garland, J.E.; Assiongbon, K.A.; Pettit, C.M.; Roy, D. Surface plasmon resonance transients at an electrochemical interface: Time resolved measurements using a bicell photodiode. Anal. Chim. Acta 2003, 475, 47–58. [Google Scholar] [CrossRef]
- Foley, K.J.; Shan, X.; Tao, N.J. Surface Impedance Imaging Technique. Anal. Chem. 2008, 80, 5146–5151. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Huang, X.; Shan, X.; Foley, K.J.; Tao, N. Electrochemical Surface Plasmon Resonance: Basic Formalism and Experimental Validation. Anal. Chem. 2010, 82, 935–941. [Google Scholar] [CrossRef]
- Patskovsky, S.; Latendresse, V.; Dallaire, A.M.; Doré-Mathieu, L.; Meunier, M. Combined surface plasmon resonance and impedance spectroscopy systems for biosensing. Analyst 2014, 139, 596–602. [Google Scholar] [CrossRef]
- Chen, T.; Xin, J.; Chang, S.J.; Chen, C.-J.; Liu, J.-T. Surface Plasmon Resonance (SPR) Combined Technology: A Powerful Tool for Investigating Interface Phenomena. Adv. Mater. Interfaces 2023, 10, 2202202. [Google Scholar] [CrossRef]
- Chieng, A.; Chiang, M.; Triloges, K.; Chang, M.; Wang, Y. Recent progress in the studies of electrochemical interfaces by surface plasmon resonance spectroscopy and microscopy. Curr. Opin. Electrochem. 2019, 13, 94–99. [Google Scholar] [CrossRef]
- Lazar, J.; Schnelting, C.; Slavcheva, E.; Schnakenberg, U. Hampering of the Stability of Gold Electrodes by Ferri-/Ferrocyanide Redox Couple Electrolytes during Electrochemical Impedance Spectroscopy. Anal. Chem. 2016, 88, 682–687. [Google Scholar] [CrossRef]
- Dijksma, M.; Boukamp, B.A.; Kamp, B.; van Bennekom, W.P. Effect of Hexacyanoferrate(II/III) on Self-Assembled Monolayers of Thioctic Acid and 11-Mercaptoundecanoic Acid on Gold. Langmuir 2002, 18, 3105–3112. [Google Scholar] [CrossRef]
- Ribeiro, J.A.; Sales, M.G.F.; Pereira, C.M. Electrochemistry-assisted surface plasmon resonance detection of miRNA-145 at femtomolar level. Sens. Actuators B Chem. 2020, 316, 128129. [Google Scholar] [CrossRef]
- Liang, W.; Wang, S.; Festa, F.; Wiktor, P.; Wang, W.; Magee, M.; LaBaer, J.; Tao, N. Measurement of Small Molecule Binding Kinetics on a Protein Microarray by Plasmonic-Based Electrochemical Impedance Imaging. Anal. Chem. 2014, 86, 9860–9865. [Google Scholar] [CrossRef] [PubMed]
- Patskovsky, S.; Dallaire, A.-M.; Blanchard-Dionne, A.-P.; Vallée-Bélisle, A.; Meunier, M. Electrochemical structure-switching sensing using nanoplasmonic devices. Ann. Phys. 2015, 527, 806–813. [Google Scholar] [CrossRef]
- Dallaire, A.-M.; Patskovsky, S.; Vallée-Bélisle, A.; Meunier, M. Electrochemical plasmonic sensing system for highly selective multiplexed detection of biomolecules based on redox nanoswitches. Biosens. Bioelectron. 2015, 71, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Wang, W.; Wang, S.; Shan, X.; Li, J.; Tao, N. Plasmonic-Based Electrochemical Impedance Spectroscopy: Application to Molecular Binding. Anal. Chem. 2012, 84, 327–333. [Google Scholar] [CrossRef]
- Lu, J.; Li, J. Monitoring DNA conformation and charge regulations by plasmonic-based electrochemical impedance platform. Electrochem. Commun. 2014, 45, 5–8. [Google Scholar] [CrossRef]
- Ribeiro, J.A.; Sales, M.G.F.; Pereira, C.M. Electrochemistry-Assisted Surface Plasmon Resonance Biosensor for Detection of CA 15–3. Anal. Chem. 2021, 93, 7815–7824. [Google Scholar] [CrossRef]
- Liu, X.-W.; Yang, Y.; Wang, W.; Wang, S.; Gao, M.; Wu, J.; Tao, N. Plasmonic-Based Electrochemical Impedance Imaging of Electrical Activities in Single Cells. Angew. Chem. Int. Ed. 2017, 56, 8855–8859. [Google Scholar] [CrossRef]
- Lu, J.; Yang, Y.; Wang, W.; Li, J.; Tao, N.; Wang, S. Label-Free Imaging of Histamine Mediated G Protein-Coupled Receptors Activation in Live Cells. Anal. Chem. 2016, 88, 11498–11503. [Google Scholar] [CrossRef]
- Lu, J.; Li, J. Label-Free Imaging of Dynamic and Transient Calcium Signaling in Single Cells. Angew. Chem. Int. Ed. 2015, 54, 13576–13580. [Google Scholar] [CrossRef] [PubMed]
- Qatamin, A.H.; Ghithan, J.H.; Moreno, M.; Nunn, B.M.; Jones, K.B.; Zamborini, F.P.; Keynton, R.S.; O’Toole, M.G.; Mendes, S.B. Detection of influenza virus by electrochemical surface plasmon resonance under potential modulation. Appl. Opt. 2019, 58, 2839–2844. [Google Scholar] [CrossRef] [PubMed]
- Lenyk, B.; Figueroa-Miranda, G.; Pavlushko, I.; Lo, Y.; Tanner, J.A.; Offenhäusser, A.; Mayer, D. Dual-Transducer Malaria Aptasensor Combining Electrochemical Impedance and Surface Plasmon Polariton Detection on Gold Nanohole Arrays. ChemElectroChem 2020, 7, 4594–4600. [Google Scholar] [CrossRef]
- Chung, J.; Billante, A.; Flatebo, C.; Leung, K.K.; Gerson, J.; Emmons, N.; Kippin, T.E.; Sepunaru, L.; Plaxco, K.W. Effects of storage conditions on the performance of an electrochemical aptamer-based sensor. Sens. Diagn. 2024, 3, 1044–1050. [Google Scholar] [CrossRef] [PubMed]
- Mishi, R.D.; Stokes, M.A.; Campbell, C.A.; Plaxco, K.W.; Stocker, S.L. Real-Time Monitoring of Antibiotics in the Critically Ill Using Biosensors. Antibiotics 2023, 12, 1478. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).