Proving the Formation of Carbonic Acid Hemiesters Using Self-Assembled Monolayers and Electrochemistry
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Modification of the Gold Electrode with Mercaptoethanol
2.3. Electrochemical Measurements
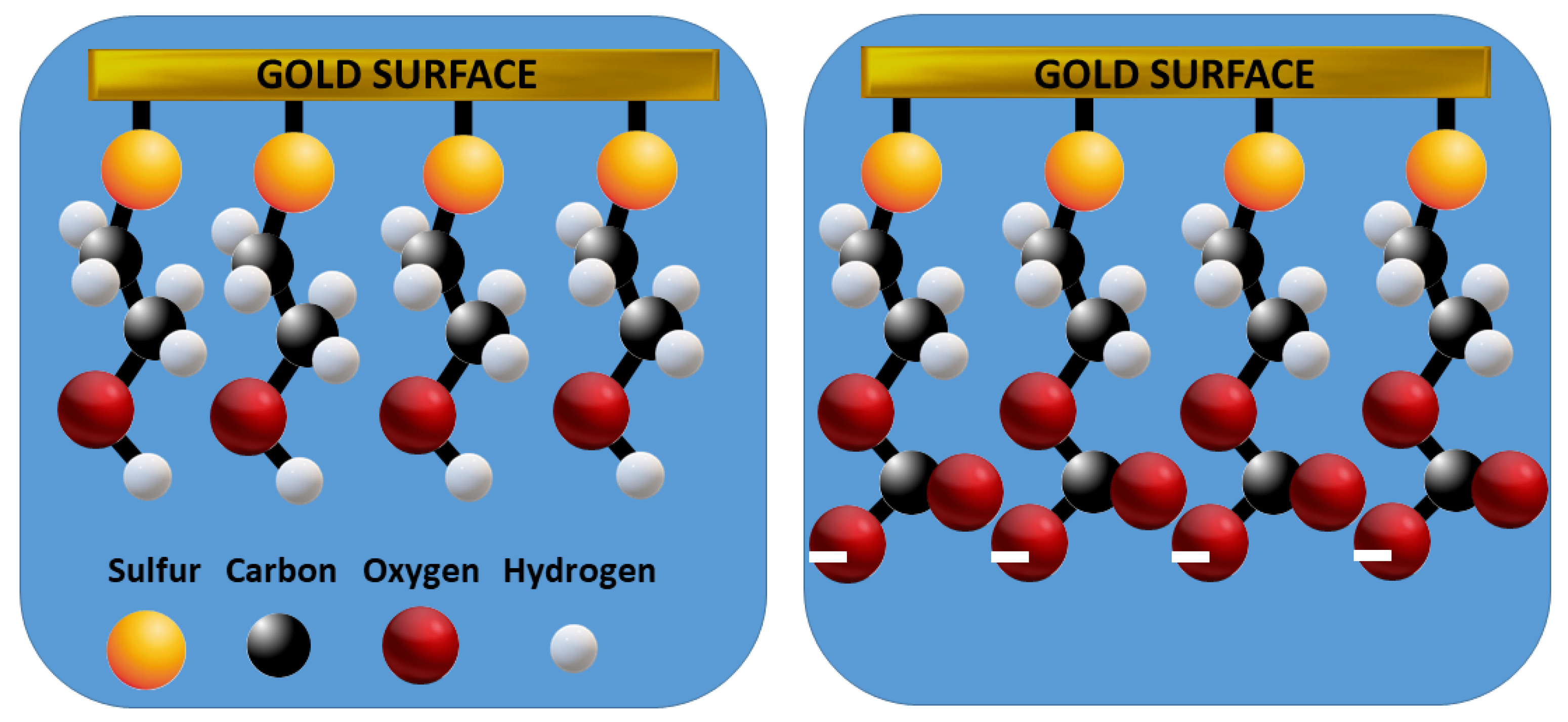
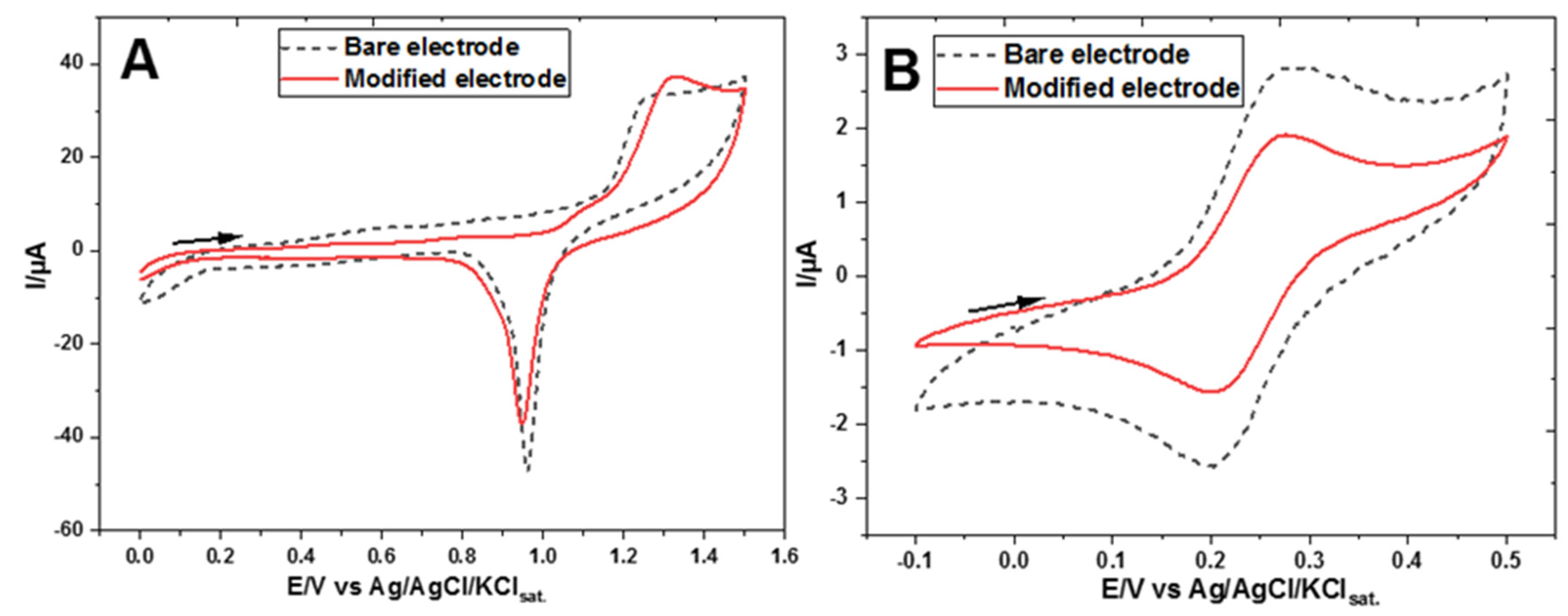
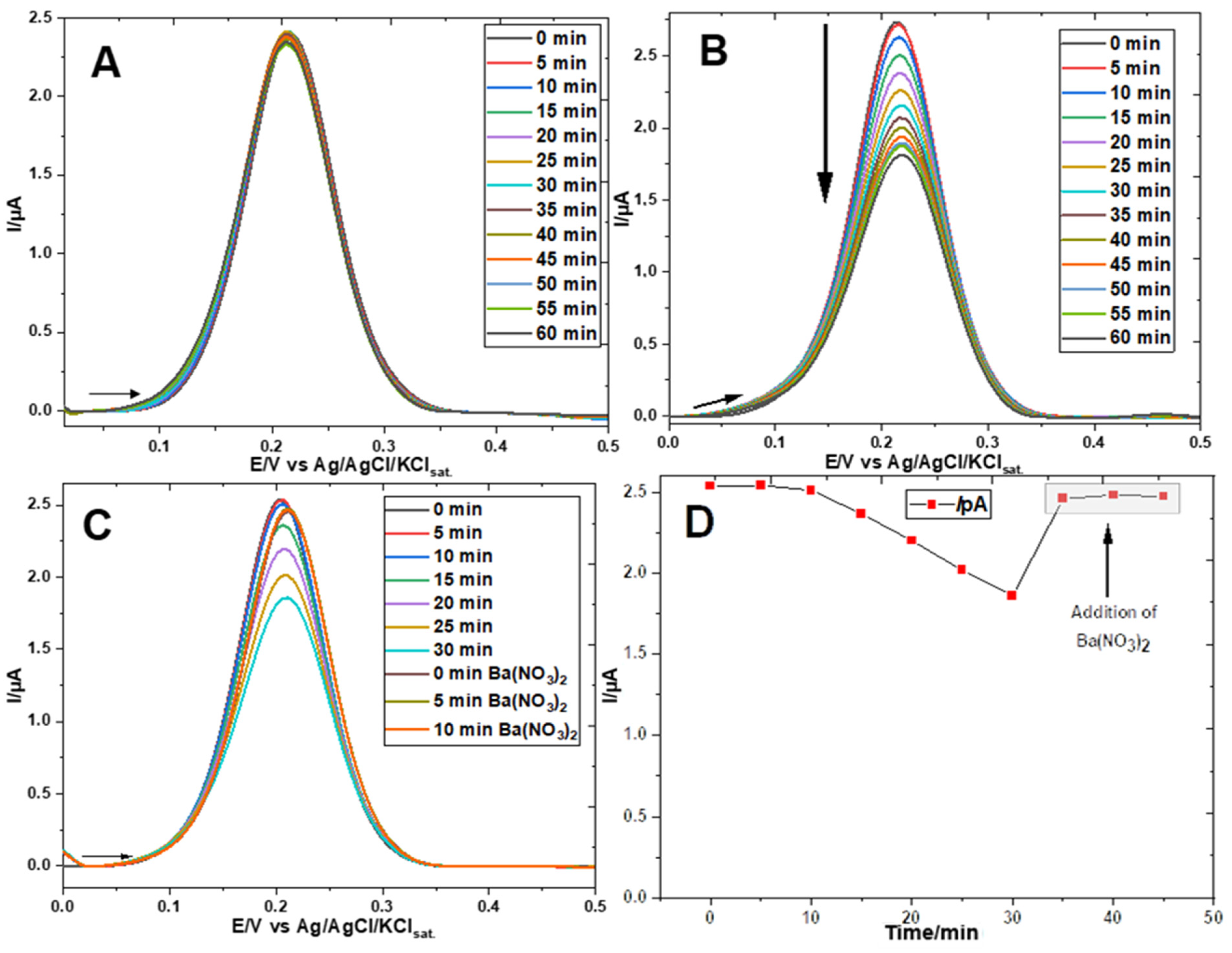
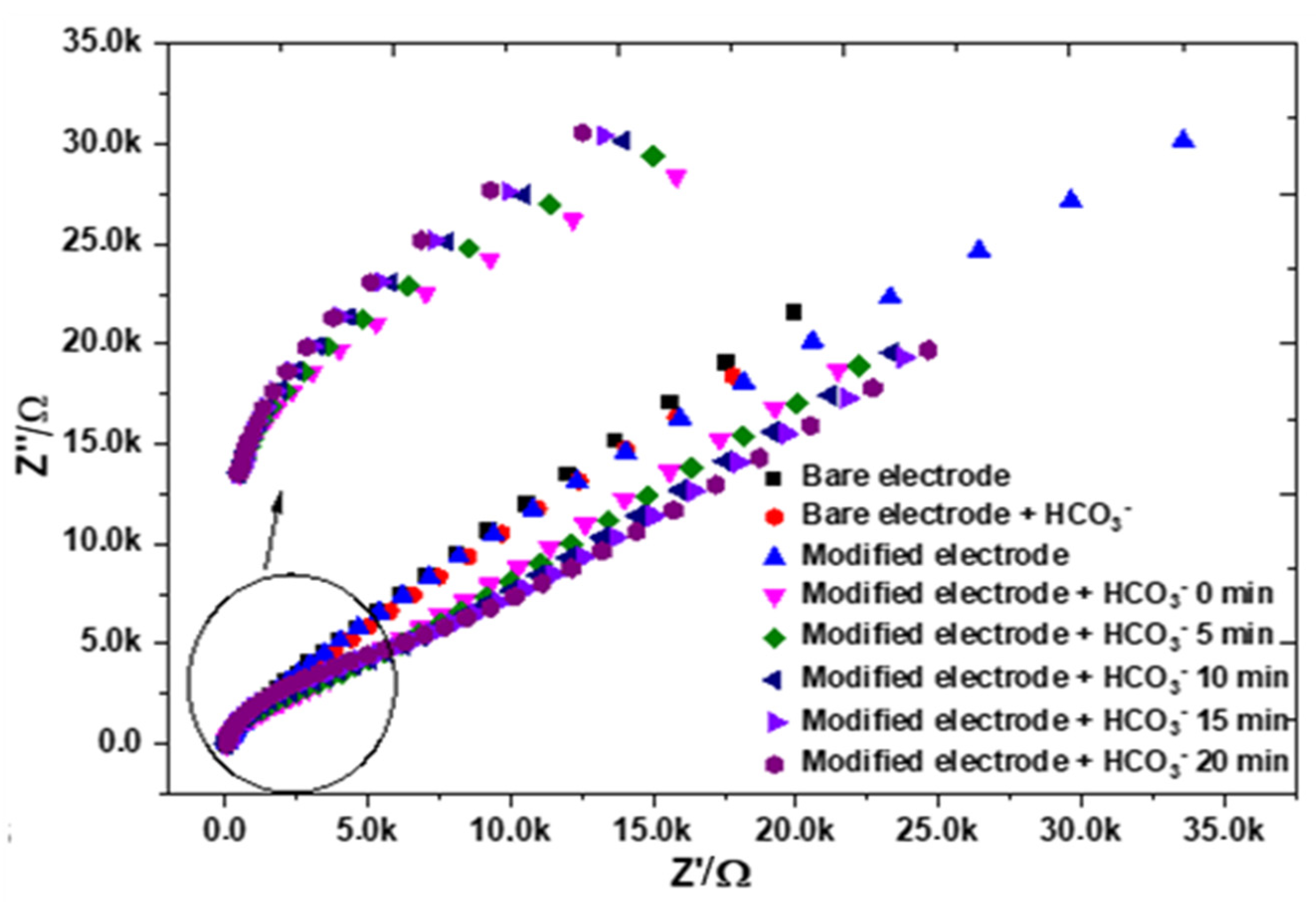
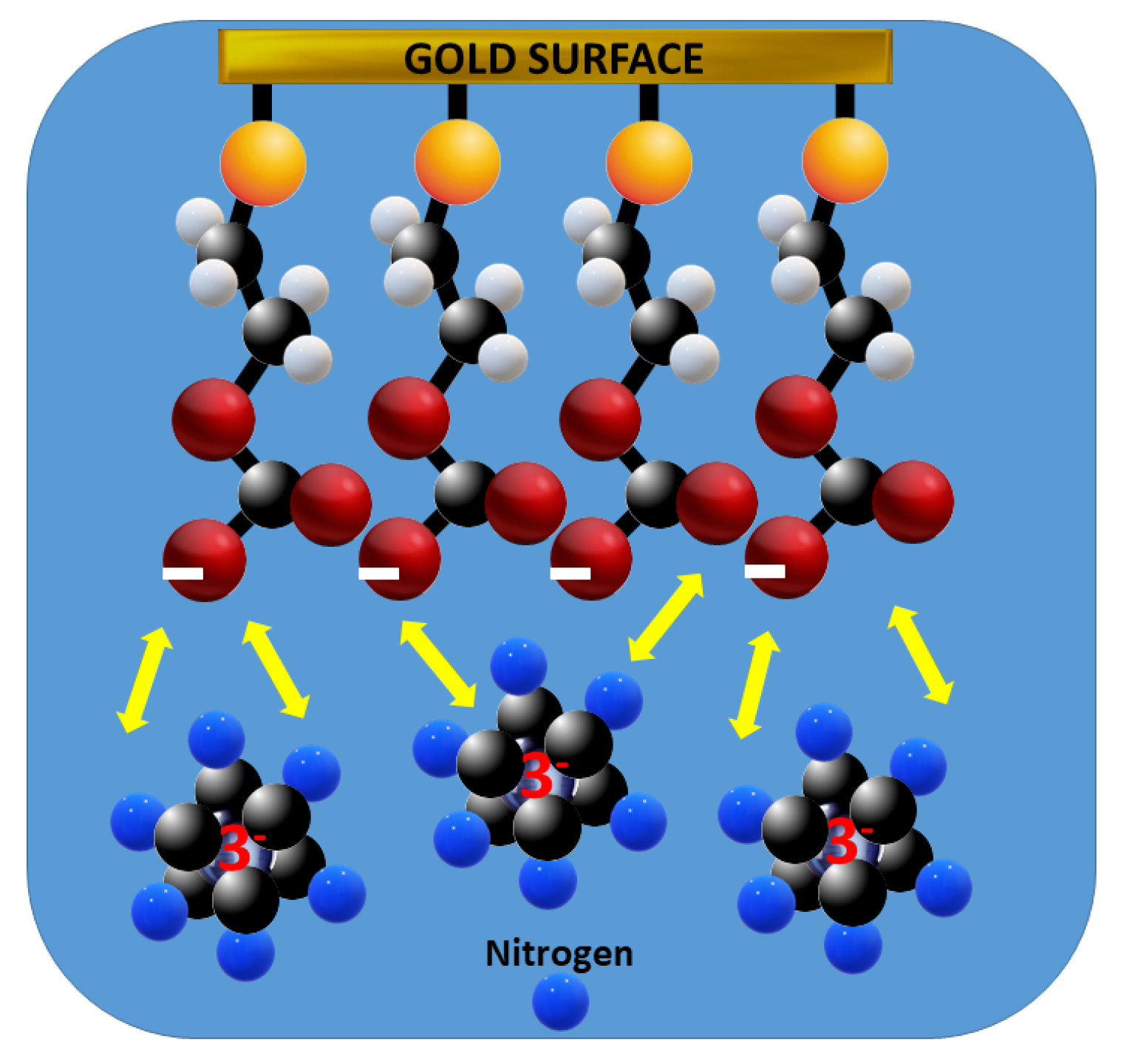
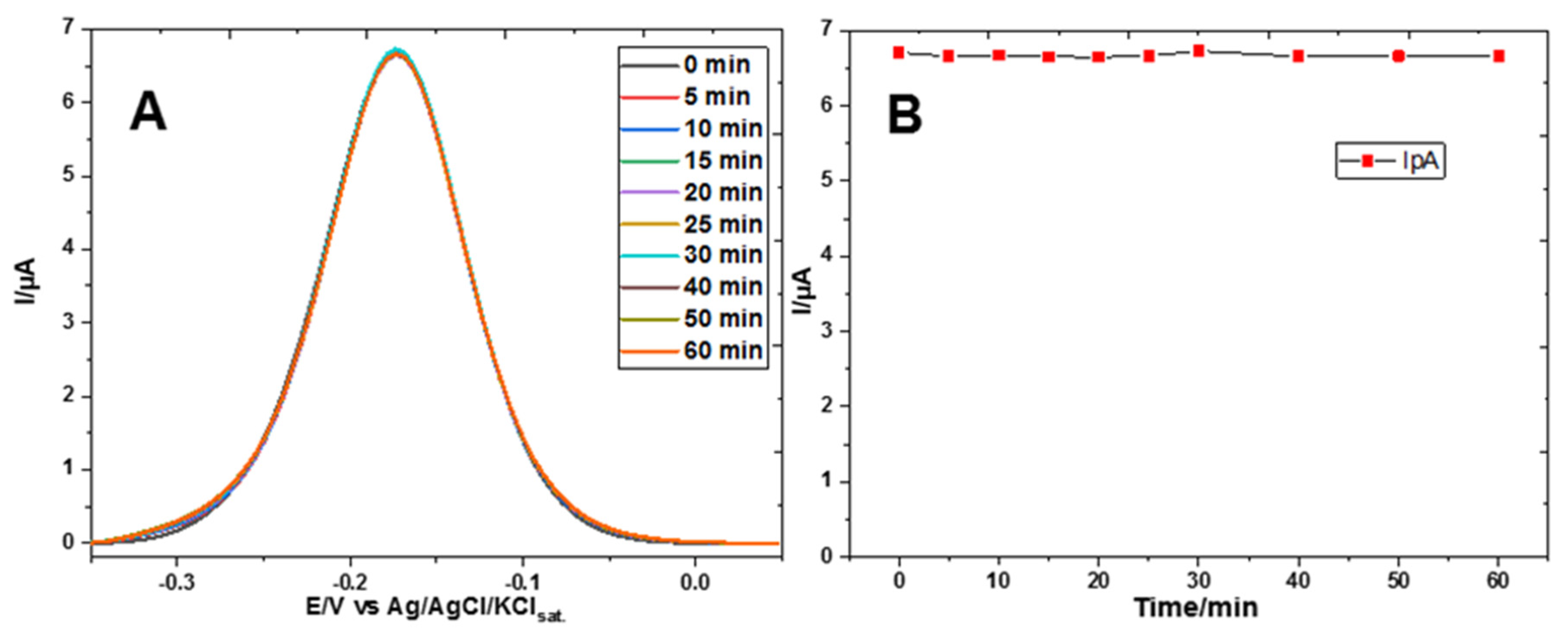
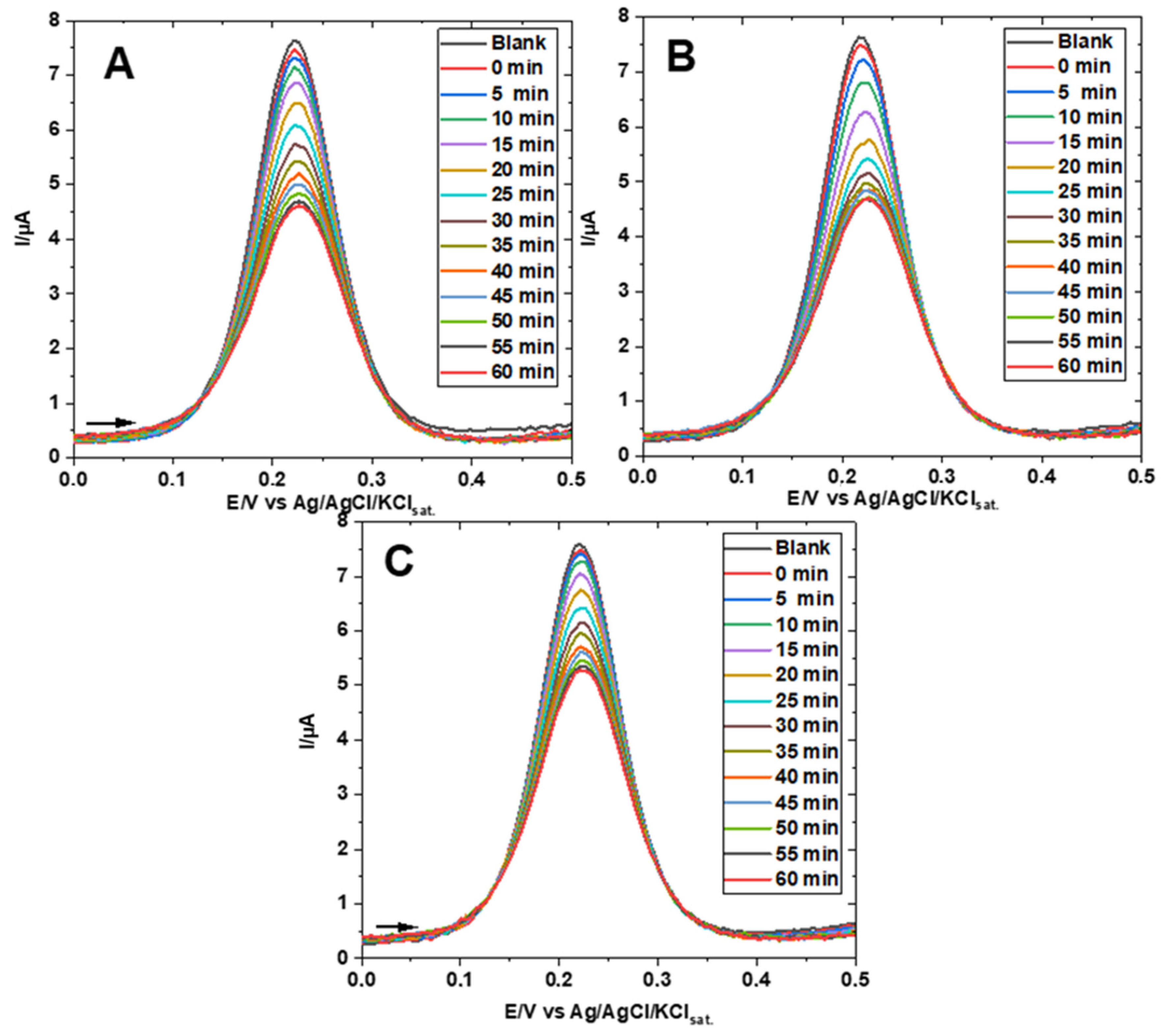
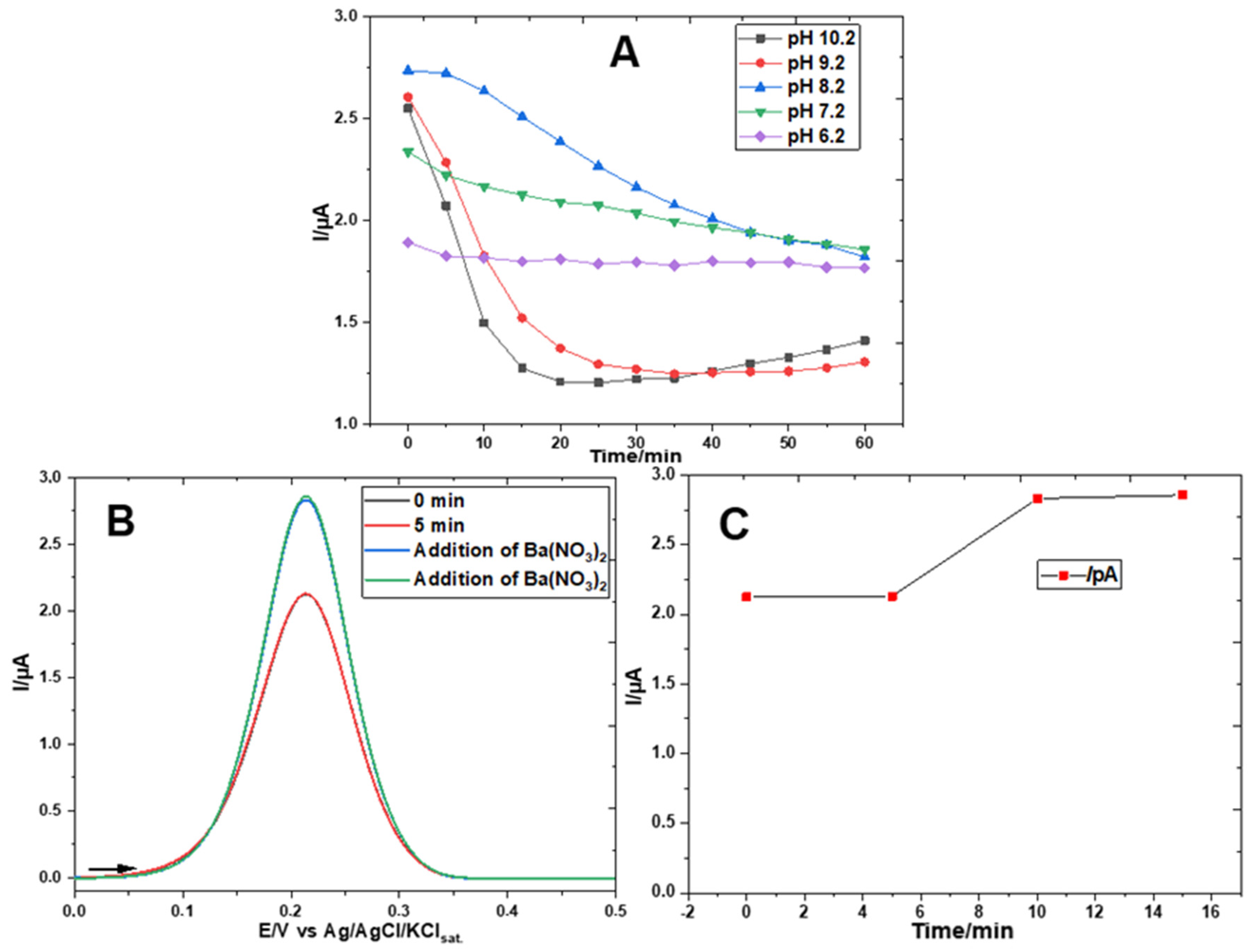
3. Results
Electrochemical Characterization of a Gold Electrode Modified with 2-Mercaptoethanol
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- dos Santos, V.B.; Vidal, D.T.; Francisco, K.J.; Ducati, L.C.; do Lago, C.L. Formation of isomers of anionic hemiesters of sugars and carbonic acid in aqueous medium. Carbohydr. Res. 2016, 428, 18–22. [Google Scholar] [CrossRef]
- Vidal, D.T.R.; Nogueira, T.; Saito, R.M.; do Lago, C.L. Investigating the formation and the properties of monoalkyl carbonates in aqueous medium using capillary electrophoresis with capacitively coupled contactless conductivity detection. Electrophoresis 2011, 32, 850–856. [Google Scholar] [CrossRef]
- Dumas, J.B.; Peligot, E. Sur le Carbrbovinate de Potasse. Compte Rendu 1837, 4, 563–565. [Google Scholar]
- Habermann, J. Über die Elektrolyse organischer Substanzen. Monatshefte Chem. Verwandte Teile Anderer Wiss. 1886, 7, 529–551. [Google Scholar] [CrossRef]
- Hempel, W.; Seidel, J. Über verbindungen des kohlendioxyds mit wasser, aethyläther und alkoholen. Berichte Der Dtsch. Chem. Ges. 1898, 31, 2997–3001. [Google Scholar] [CrossRef]
- Siegfried, M.; Howwjanz, S. On the bond of carbonic acid by alcohols, sugars and oxy acids. HS Z Physiol. Chem. 1909, 59, 376–404. [Google Scholar] [CrossRef][Green Version]
- do Lago, C.L.; Vidal, D.T.; Rossi, M.R.; Hotta, G.M.; da Costa, E.T. On the formation of carbonate adducts of fatty alcohols, sterols, and sugars in biological conditions. Electrophoresis 2012, 33, 2102–2111. [Google Scholar] [CrossRef]
- Cieslarova, Z.; dos Santos, V.B.; do Lago, C.L. Both carbamates and monoalkyl carbonates are involved in carbon dioxide capture by alkanolamines. Int. J. Greenh. Gas Control 2018, 76, 142–149. [Google Scholar] [CrossRef]
- Behrens, R.; von Harbou, E.; Thiel, W.R.; Böttinger, W.; Ingram, T.; Sieder, G.; Hasse, H. Monoalkylcarbonate formation in methyldiethanolamine–H2O–CO2. Ind. Eng. Chem. Res. 2017, 56, 9006–9015. [Google Scholar] [CrossRef]
- Behrens, R.; Kessler, E.; Münnemann, K.; Hasse, H.; von Harbou, E. Monoalkylcarbonate formation in the system monoethanolamine–water–carbon dioxide. Fluid Phase Equilibria 2019, 486, 98–105. [Google Scholar] [CrossRef]
- Rossi, M.R.; Vidal, D.T.R.; do Lago, C.L. Monoalkyl carbonates in carbonated alcoholic beverages. Food Chem. 2012, 133, 352–357. [Google Scholar] [CrossRef]
- do Lago, C.L.; Francisco, K.J.M.; Daniel, D.; Vidal, D.T.R.; dos Santos, V.B. A capillary electrophoresis/tandem mass spectrometry approach for the determination of monoalkyl carbonates. RSC Adv. 2014, 4, 19674–19679. [Google Scholar] [CrossRef]
- Bernard, J.; Köck, E.M.; Huber, R.G.; Liedl, K.R.; Call, L.; Schlögl, R.; Grothe, H.; Loerting, T. Carbonic acid monoethyl ester as a pure solid and its conformational isomerism in the gas-phase. RSC Adv. 2017, 7, 22222–22233. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Kaur, N.; Comini, E. The role of self-assembled monolayers in electronic devices. J. Mater. Chem. C 2020, 8, 3938–3955. [Google Scholar] [CrossRef]
- Mirsky, V.M. New electroanalytical applications of self-assembled monolayers. TrAC Trends Anal. Chem. 2002, 21, 439–450. [Google Scholar] [CrossRef]
- Casalini, S.; Bortolotti, C.A.; Leonardi, F.; Biscarini, F. Self-assembled monolayers in organic electronics. Chem. Soc. Rev. 2017, 46, 40–71. [Google Scholar] [CrossRef]
- Nicosia, C.; Huskens, J. Reactive self-assembled monolayers: From surface functionalization to gradient formation. Mater. Horiz. 2014, 1, 32–45. [Google Scholar] [CrossRef]
- Kudelski, A. Chemisorption of 2-mercaptoethanol on silver, copper, and gold: Direct Raman evidence of acid-induced changes in adsorption/desorption equilibria. Langmuir 2003, 19, 3805–3813. [Google Scholar] [CrossRef]
- Paik, W.K.; Han, S.; Shin, W.; Kim, Y. Adsorption of carboxylic acids on gold by anodic reaction. Langmuir 2003, 19, 4211–4216. [Google Scholar] [CrossRef]
- Weissham, D.E.; Walczak, M.M.; Porter, M.D. Electrochemically induced transformations of monolayers formed by self-assembly of mercaptoethanol at gold. Langmuir 1993, 9, 323–329. [Google Scholar] [CrossRef]
- Wang, W.; Lee, T.; Reed, M.A. Mechanism of electron conduction in self-assembled alkanethiol monolayer devices. Phys. Rev. 2003, 68, 035416. [Google Scholar] [CrossRef]
- Busenberg, E.; Plummer, L.N. The solubility of BaCO3 (cr)(witherite) in CO2-H2O solutions between 0 and 90 °C, evaluation of the association constants of BaHCO3+ (aq) and BaCO3 (aq) between 5 and 80 °C, and a preliminary evaluation of the thermodynamic properties of Ba2+ (aq). Geochim. Cosmochim. Acta 1986, 50, 2225–2233. [Google Scholar] [CrossRef]
- Lazanas, A.C.; Prodromidis, M.I. Electrochemical Impedance Spectroscopy—A Tutorial. ACS Meas. Sci. Au 2023, 3, 162–193. [Google Scholar] [CrossRef]
- Laschuk, N.O.; Easton, E.B.; Zenkina, O.V. Reducing the resistance for the use of electrochemical impedance spectroscopy analysis in materials chemistry. RSC Adv. 2021, 11, 27925. [Google Scholar] [CrossRef]
- Jordan, J.; Ewing, G.J. The Protonation of Hexacyanoferrates. Inorg. Chem. 1962, 1, 587–591. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, B.G.; Carli, F.P.; do Lago, C.L.; Gutz, I.G.R.; Angnes, L. Proving the Formation of Carbonic Acid Hemiesters Using Self-Assembled Monolayers and Electrochemistry. Chemosensors 2025, 13, 93. https://doi.org/10.3390/chemosensors13030093
Santos BG, Carli FP, do Lago CL, Gutz IGR, Angnes L. Proving the Formation of Carbonic Acid Hemiesters Using Self-Assembled Monolayers and Electrochemistry. Chemosensors. 2025; 13(3):93. https://doi.org/10.3390/chemosensors13030093
Chicago/Turabian StyleSantos, Berlane G., Fernanda P. Carli, Claudimir L. do Lago, Ivano G. R. Gutz, and Lúcio Angnes. 2025. "Proving the Formation of Carbonic Acid Hemiesters Using Self-Assembled Monolayers and Electrochemistry" Chemosensors 13, no. 3: 93. https://doi.org/10.3390/chemosensors13030093
APA StyleSantos, B. G., Carli, F. P., do Lago, C. L., Gutz, I. G. R., & Angnes, L. (2025). Proving the Formation of Carbonic Acid Hemiesters Using Self-Assembled Monolayers and Electrochemistry. Chemosensors, 13(3), 93. https://doi.org/10.3390/chemosensors13030093






