Pushing Peak Shapes to Perfection by High-Temperature Focus GC-IMS
Abstract
:1. Introduction
- wf_10 = width of the front half at 10% of the peak height;
- wt_10 = width of the back half at 10% of the peak height.
2. Materials and Methods
2.1. Reagents and Samples
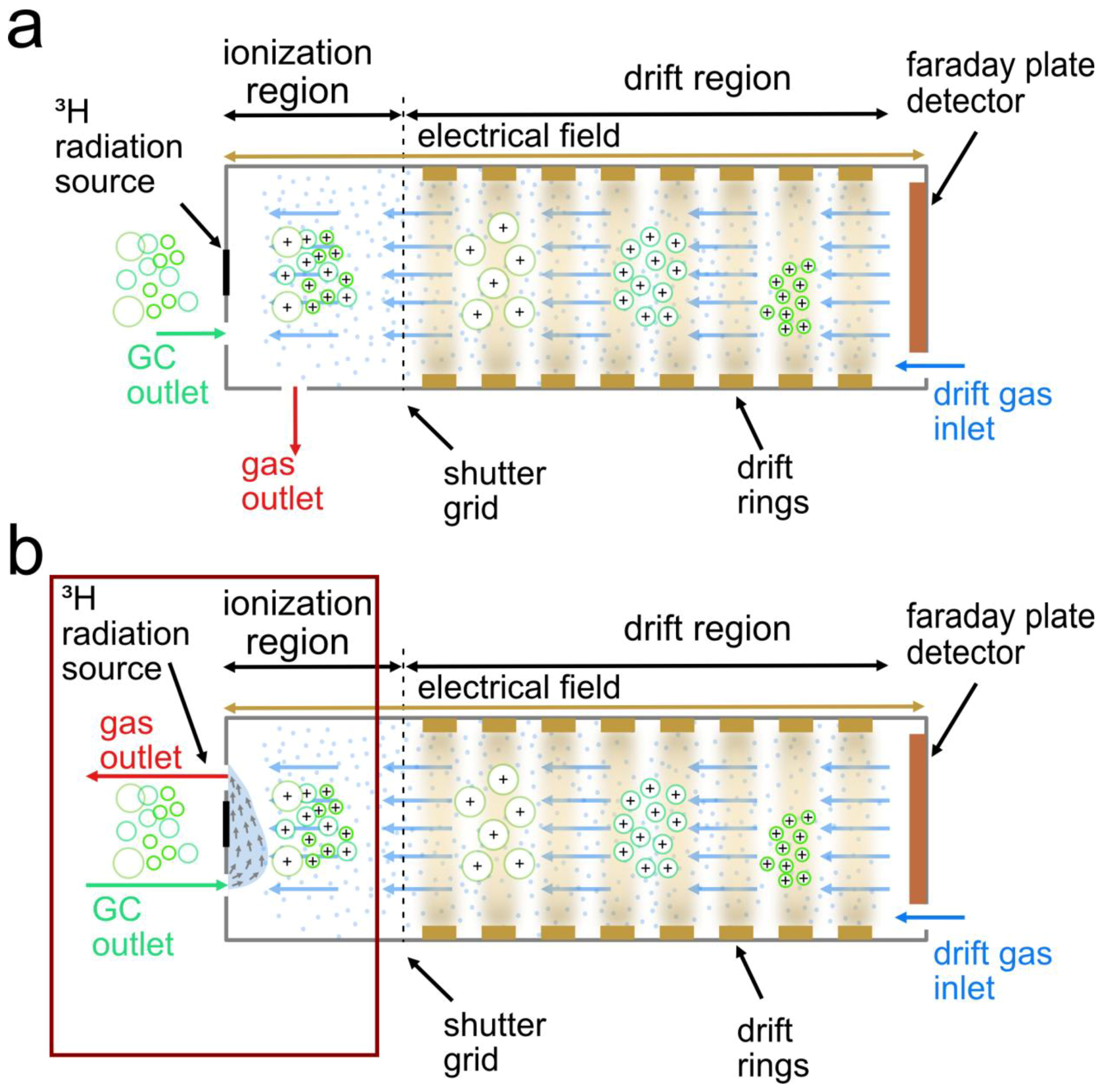
2.2. Instrumentation
2.3. Experimental Design for High-Temperature Focus IMS
2.4. Data Processing and Evaluation
2.4.1. Reactant Ion Peak (RIP) and Background Calculations
2.4.2. Data Processing of the Experiments for High-Temperature Focus IMS
3. Results and Discussion
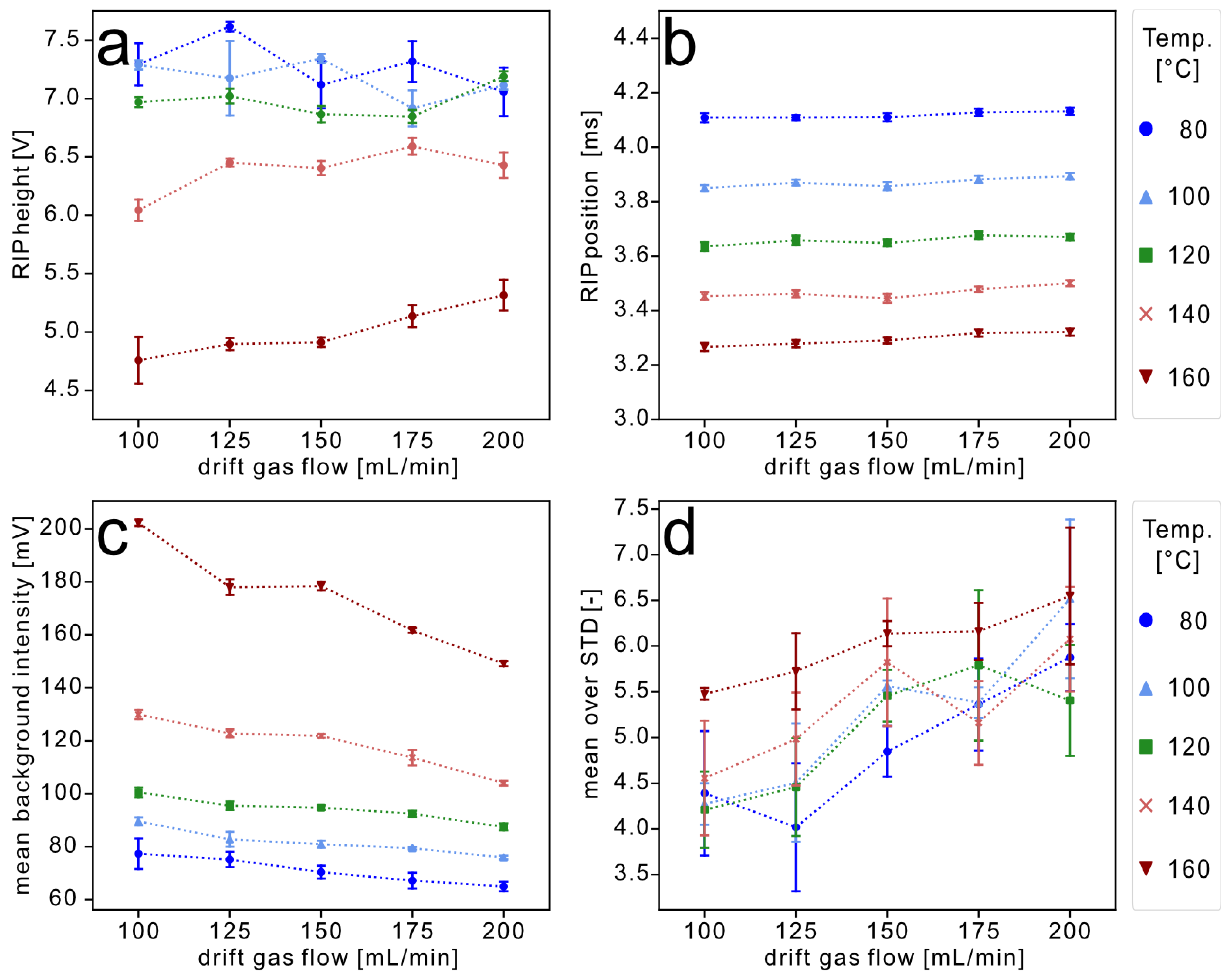
3.1. RIP and Background Calculations
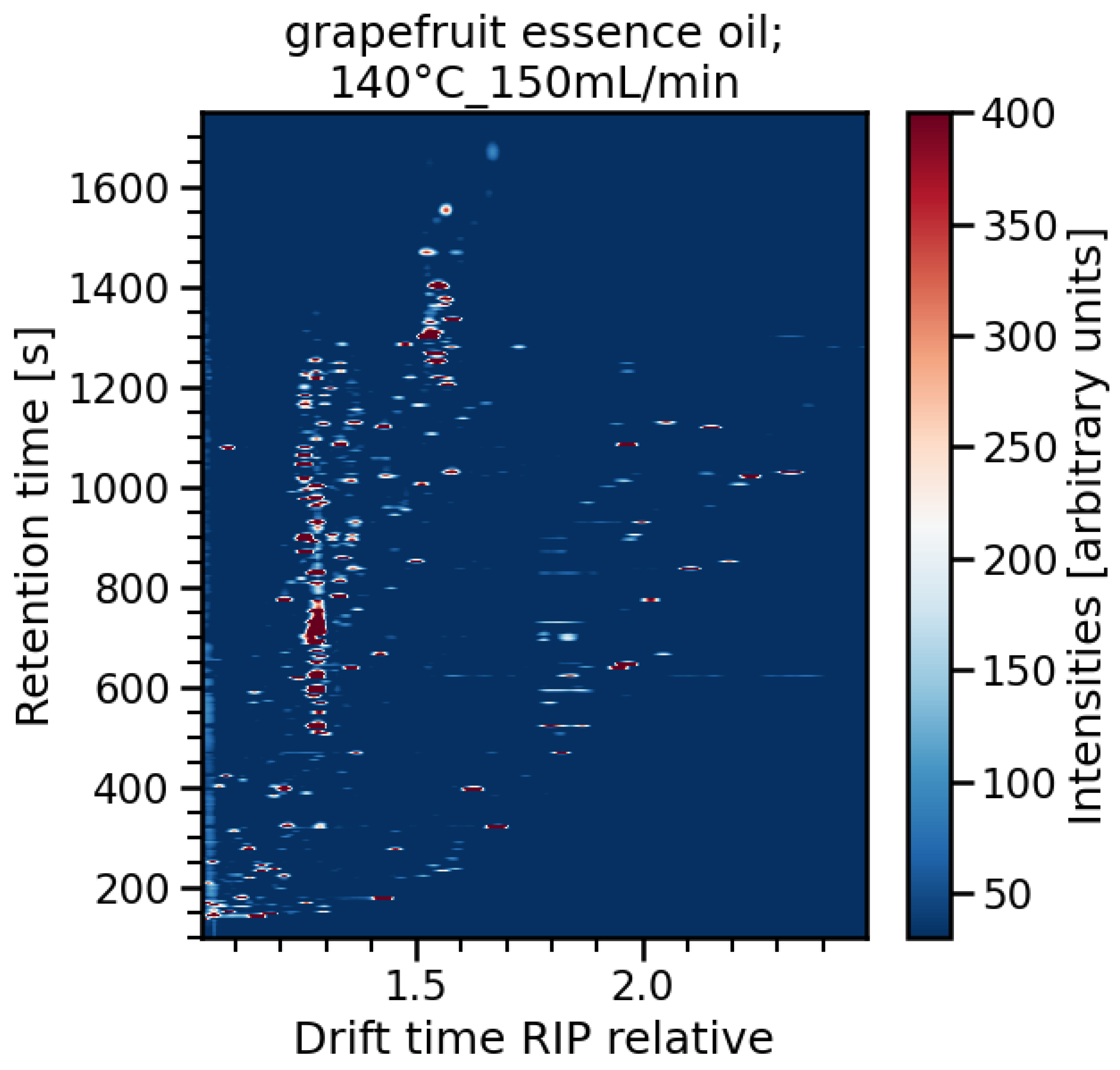
3.2. Data Evaluation of the Experiments for High-Temperature Focus IMS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hernández-Mesa, M.; Ropartz, D.; García-Campaña, A.M.; Rogniaux, H.; Dervilly-Pinel, G.; Le Bizec, B. Ion Mobility Spectrometry in Food Analysis: Principles, Current Applications and Future Trends. Molecules 2019, 24, 2706. [Google Scholar] [CrossRef]
- Vautz, W.; Zimmermann, D.; Hartmann, M.; Baumbach, J.I.; Nolte, J.; Jung, J. Ion mobility spectrometry for food quality and safety. Food Addit. Contam. 2006, 23, 1064–1073. [Google Scholar] [CrossRef] [PubMed]
- Brendel, R.; Schwolow, S.; Rohn, S.; Weller, P. Comparison of PLSR, MCR-ALS and Kernel-PLSR for the quantification of allergenic fragrance compounds in complex cosmetic products based on nonlinear 2D GC-IMS data. Chemom. Intell. Lab. Syst. 2020, 205, 104128. [Google Scholar] [CrossRef]
- Parastar, H.; Weller, P. Towards greener volatilomics: Is GC-IMS the new Swiss army knife of gas phase analysis? TrAC Trends Anal. Chem. 2024, 170, 117438. [Google Scholar] [CrossRef]
- Gerhardt, N.; Birkenmeier, M.; Schwolow, S.; Rohn, S.; Weller, P. Volatile-Compound Fingerprinting by Headspace-Gas-Chromatography Ion-Mobility Spectrometry (HS-GC-IMS) as a Benchtop Alternative to 1H NMR Profiling for Assessment of the Authenticity of Honey. Anal. Chem. 2018, 90, 1777–1785. [Google Scholar] [CrossRef]
- Gerhardt, N.; Schwolow, S.; Rohn, S.; Pérez-Cacho, P.R.; Galán-Soldevilla, H.; Arce, L.; Weller, P. Quality assessment of olive oils based on temperature-ramped HS-GC-IMS and sensory evaluation: Comparison of different processing approaches by LDA, kNN, and SVM. Food Chem. 2019, 278, 720–728. [Google Scholar] [CrossRef]
- Brendel, R.; Schwolow, S.; Rohn, S.; Weller, P. Volatilomic Profiling of Citrus Juices by Dual-Detection HS-GC-MS-IMS and Machine Learning-An Alternative Authentication Approach. J. Agric. Food Chem. 2021, 69, 1727–1738. [Google Scholar] [CrossRef]
- Garrido-Delgado, R.; Del Dobao-Prieto, M.M.; Arce, L.; Valcárcel, M. Determination of volatile compounds by GC-IMS to assign the quality of virgin olive oil. Food Chem. 2015, 187, 572–579. [Google Scholar] [CrossRef]
- Rodríguez-Maecker, R.; Vyhmeister, E.; Meisen, S.; Rosales Martinez, A.; Kuklya, A.; Telgheder, U. Identification of terpenes and essential oils by means of static headspace gas chromatography-ion mobility spectrometry. Anal. Bioanal. Chem. 2017, 409, 6595–6603. [Google Scholar] [CrossRef]
- Schanzmann, H.; Augustini, A.L.R.M.; Sanders, D.; Dahlheimer, M.; Wigger, M.; Zech, P.-M.; Sielemann, S. Differentiation of Monofloral Honey Using Volatile Organic Compounds by HS-GCxIMS. Molecules 2022, 27, 7554. [Google Scholar] [CrossRef]
- Lv, W.; Lin, T.; Ren, Z.; Jiang, Y.; Zhang, J.; Bi, F.; Gu, L.; Hou, H.; He, J. Rapid discrimination of Citrus reticulata ‘Chachi’ by headspace-gas chromatography-ion mobility spectrometry fingerprints combined with principal component analysis. Food Res. Int. 2020, 131, 108985. [Google Scholar] [CrossRef] [PubMed]
- Capitain, C.C.; Zischka, M.; Sirkeci, C.; Weller, P. Evaluation of IMS drift tube temperature on the peak shape of high boiling fragrance compounds towards allergen detection in complex cosmetic products and essential oils. Talanta 2023, 257, 124397. [Google Scholar] [PubMed]
- Breitmaier, E. Terpenes: Flavors, Fragrances, Pharmaca, Pheromones; 1. Reprint; Wiley-VCH: Weinheim, Germany, 2008; ISBN 9783527317868. [Google Scholar]
- Schrader, J.; Bohlmann, J. Biotechnology of Isoprenoids; Springer International Publishing: Cham, Switzerland, 2015; ISBN 978-3-319-20106-1. [Google Scholar]
- Borsdorf, H.; Rudolph, M. Gas-phase ion mobility studies of constitutional isomeric hydrocarbons using different ionization techniques. Int. J. Mass Spectrom. 2001, 208, 67–72. [Google Scholar] [CrossRef]
- Borsdorf, H.; Eiceman, G.A. Ion Mobility Spectrometry: Principles and Applications. Appl. Spectrosc. Rev. 2006, 41, 323–375. [Google Scholar] [CrossRef]
- European Parliament and the Council of the European Union. Council Directive 2013/59/Euratom of 5 December 2013 Laying Down Basic Safety Standards for Protection against the Dangers Arising from Exposure to Ionising Radiation. Off. J. Eur. Union 2013. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32013L0059 (accessed on 2 April 2025).
- Garrido-Delgado, R.; Dobao-Prieto, M.M.; Arce, L.; Aguilar, J.; Cumplido, J.L.; Valcárcel, M. Ion mobility spectrometry versus classical physico-chemical analysis for assessing the shelf life of extra virgin olive oil according to container type and storage conditions. J. Agric. Food Chem. 2015, 63, 2179–2188. [Google Scholar] [CrossRef]
- Eiceman, G.A.; Nazarov, E.G.; Rodriguez, J.E.; Berglof, J.F. Positive reactant ion chemistry for analytical, high temperature ion mobility spectrometry (IMS): Effects of electric field of the drift tube and moisture, temperature, and flow of the drift gas. Int. J. Ion Mobil. Spectrom. 1998, 1, 28–37. [Google Scholar]
- Ewing, R.G.; Eiceman, G.A.; Stone, J. Proton-bound cluster ions in ion mobility spectrometry. Int. J. Mass Spectrom. 1999, 193, 57–68. [Google Scholar] [CrossRef]
- Pomareda, V.; Guamán, A.V.; Mohammadnejad, M.; Calvo, D.; Pardo, A.; Marco, S. Multivariate curve resolution of nonlinear ion mobility spectra followed by multivariate nonlinear calibration for quantitative prediction. Chemom. Intell. Lab. Syst. 2012, 118, 219–229. [Google Scholar] [CrossRef]
- Mason, E.A.; Schamp, H.W. Mobility of gaseous lons in weak electric fields. Ann. Phys. 1958, 4, 233–270. [Google Scholar] [CrossRef]
- G.A.S. Gesellschaft für Analytische Sensorsysteme mbH. FOCUS-Ion Mobility Spectrometer®: Sensitivity meets Selectivity: Welcome the New Level of IMS Performance. 2021. Available online: https://www.gas-dortmund.de/data-live-gas/docs/pdf/Technical_Note_FOCUS_2108.pdf (accessed on 27 March 2025).
- Morton, D.W.; Young, C.L. Analysis of Peak Profiles Using Statistical Moments. J. Chromatogr. Sci. 1995, 33, 514–524. [Google Scholar] [CrossRef]
- Grushka, E. Characterization of exponentially modified Gaussian peaks in chromatography. Anal. Chem. 1972, 44, 1733–1738. [Google Scholar] [CrossRef] [PubMed]
- Kalambet, Y. Data acquisition and integration. In Gas Chromatography, 2nd ed.; Poole, C.F., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 505–524. ISBN 9780128206751. [Google Scholar]
- Kalambet, Y.; Kozmin, Y.; Mikhailova, K.; Nagaev, I.; Tikhonov, P. Reconstruction of chromatographic peaks using the exponentially modified Gaussian function. J. Chemom. 2011, 25, 352–356. [Google Scholar] [CrossRef]
- Pápai, Z.; Pap, T.L. Analysis of peak asymmetry in chromatography. J. Chromatogr. A 2002, 953, 31–38. [Google Scholar] [CrossRef] [PubMed]
- European Parliament and the council of the European Union. Commission Regulation (EU) 2023/1545 of 26 July 2023 amending Regulation (EC) No 1223/2009 of the European Parliament and of the Council as regards labelling of fragrance allergens in cosmetic products. Off. J. Eur. Union 2023. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32023R1545 (accessed on 2 April 2025).
- European Parliament and the council of the European Union. Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on Cosmetic Products. Off. J. Eur. Union 2009. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32009R1223 (accessed on 2 April 2025).
- Wang, Y.; Wang, S.; Fabroni, S.; Feng, S.; Rapisarda, P.; Rouseff, R. Chemistry of citrus flavor. In The Genus Citrus; Talon, M., Caruso, M., Gmitter, F.G., Eds.; Woodhead Publishing, an Imprint of Elsevier: Duxford, UK, 2020; pp. 447–470. ISBN 9780128121634. [Google Scholar]
- Rouseff, R.L.; Ruiz Perez-Cacho, P.; Jabalpurwala, F. Historical review of citrus flavor research during the past 100 years. J. Agric. Food Chem. 2009, 57, 8115–8124. [Google Scholar] [CrossRef]
- Berk, Z. Production of citrus juice concentrates. In Citrus Fruit Processing; Elsevier: Amsterdam, The Netherlands, 2016; pp. 187–217. ISBN 9780128031339. [Google Scholar]
- Wolford, R.W.; Kesterson, J.W.; Attaway, J.A. Physicochemical properties of citrus essential oils from Florida. J. Agric. Food Chem. 1971, 19, 1097–1105. [Google Scholar] [CrossRef]
- Nitschke, A.; Hitzemann, M.; Winkelholz, J.; Kirk, A.T.; Lippmann, M.; Thoben, C.; Wittwer, J.A.; Zimmermann, S. A hyper-fast gas chromatograph coupled to an ion mobility spectrometer with high repetition rate and flow-optimized ion source to resolve the short chromatographic peaks. J. Chromatogr. A 2024, 1736, 465376. [Google Scholar] [CrossRef]
- Harris, C.R.; Millman, K.J.; van der Walt, S.J.; Gommers, R.; Virtanen, P.; Cournapeau, D.; Wieser, E.; Taylor, J.; Berg, S.; Smith, N.J.; et al. Array programming with NumPy. Nature 2020, 585, 357–362. [Google Scholar] [CrossRef]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0: Fundamental algorithms for scientific computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef]
- Wang, H.; Wang, P.; Wang, F.; Chen, H.; Chen, L.; Hu, Y.; Liu, Y. Integrated HS-GC-IMS and UPLC-Q-Orbitrap HRMS-based metabolomics revealed the characteristics and differential volatile and nonvolatile metabolites of different citrus peels. Curr. Res. Food Sci. 2024, 8, 100755. [Google Scholar] [CrossRef]
- Lin, Y.; Yu, G.; Zhang, S.; Zhu, G.; Yi, F. Comparative analysis of the differences in volatile organic components of three lavender essential oils in Ili region using sensory evaluation, GC-IMS and GC-MS techniques. J. Chromatogr. A 2024, 1731, 465197. [Google Scholar] [CrossRef]


| Compound | Molar Mass [g/mol] | Boiling Point [°C] | Structure | Appearance |
|---|---|---|---|---|
| Ethyl butyrate (CAS: 105-54-4) | 116.16 | 120–121 |  | Sweet oranges (C. sinensis (L.) Osbeck), mandarins (C. reticulata) |
| Sabinene (CAS: 3387-41-5) | 136.23 | 163–164 | 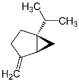 | All Citrus fruits |
| β—Pinene (CAS: 18172-67-3) | 136.23 | 165–167 |  | All Citrus fruits |
| (R)-(+)-Limonene (CAS: 5989-27-5) | 136.23 | 176–177 |  | All Citrus fruits |
| γ—Terpinene (CAS: 99-85-4) | 136.23 | 181–183 | 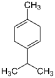 | All Citrus fruits |
| α—Terpineol (CAS: 98-55-5) | 154.25 | 218–221 | 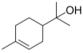 | All Citrus fruits |
| (S)-(+)-Carvone (CAS: 2244-16-8) | 150.22 | 230–231 |  | All Citrus fruits |
| Geraniol (CAS: 106-24-1) | 154.25 | 229–230 |  | All Citrus fruits |
| Geranyl acetate (CAS: 105-87-3) | 196.29 | 240–245 | 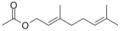 | All Citrus fruits |
| β-Citronellol (CAS: 106-22-9) | 156.26 | 224 | 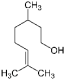 | All Citrus fruits |
| Citral (CAS: 5392-40-5) | 152.23 | 227–229 | 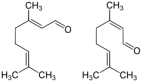 | All Citrus fruits |
| Methyl-N-Methylanthranilate (CAS: 85-91-6) | 165.19 | 255–256 |  | Mandarins (C. reticulata) |
| β-Caryophyllene (CAS: 87-44-5) | 204.35 | 256–259 | 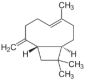 | Grapefruits (C. × paradisi) |
| Headspace Sampling and GC Settings | |
|---|---|
| Incubation time | 12 min |
| Incubation temperature | 85 °C |
| Injection volume | 1.0 mL |
| Syringe temperature | 100 °C |
| Inlet temperature | 200 °C |
| Split ratio | 1:20 |
| Inlet pressure | 6.7 psi, const. pressure |
| GC column | HP-5 (30 m × 0.32 mm × 0.25 μm) |
| Oven program | 40 °C → 120 °C with 5 °C/min 120 °C → 240 °C with 12 °C/min |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bodenbender, L.; Rohn, S.; Weller, P. Pushing Peak Shapes to Perfection by High-Temperature Focus GC-IMS. Chemosensors 2025, 13, 131. https://doi.org/10.3390/chemosensors13040131
Bodenbender L, Rohn S, Weller P. Pushing Peak Shapes to Perfection by High-Temperature Focus GC-IMS. Chemosensors. 2025; 13(4):131. https://doi.org/10.3390/chemosensors13040131
Chicago/Turabian StyleBodenbender, Lukas, Sascha Rohn, and Philipp Weller. 2025. "Pushing Peak Shapes to Perfection by High-Temperature Focus GC-IMS" Chemosensors 13, no. 4: 131. https://doi.org/10.3390/chemosensors13040131
APA StyleBodenbender, L., Rohn, S., & Weller, P. (2025). Pushing Peak Shapes to Perfection by High-Temperature Focus GC-IMS. Chemosensors, 13(4), 131. https://doi.org/10.3390/chemosensors13040131






