Volatile Organic Components and MS-e-nose Profiles of Indonesian and Malaysian Palm Sugars from Different Plant Origins
Abstract
:1. Introduction
2. Materials and Methods
2.1. Palm Sugar Samples
2.2. Volatile Composition Analysis
2.3. MS-e-nose Analysis
2.4. Statistical Analysis
3. Results
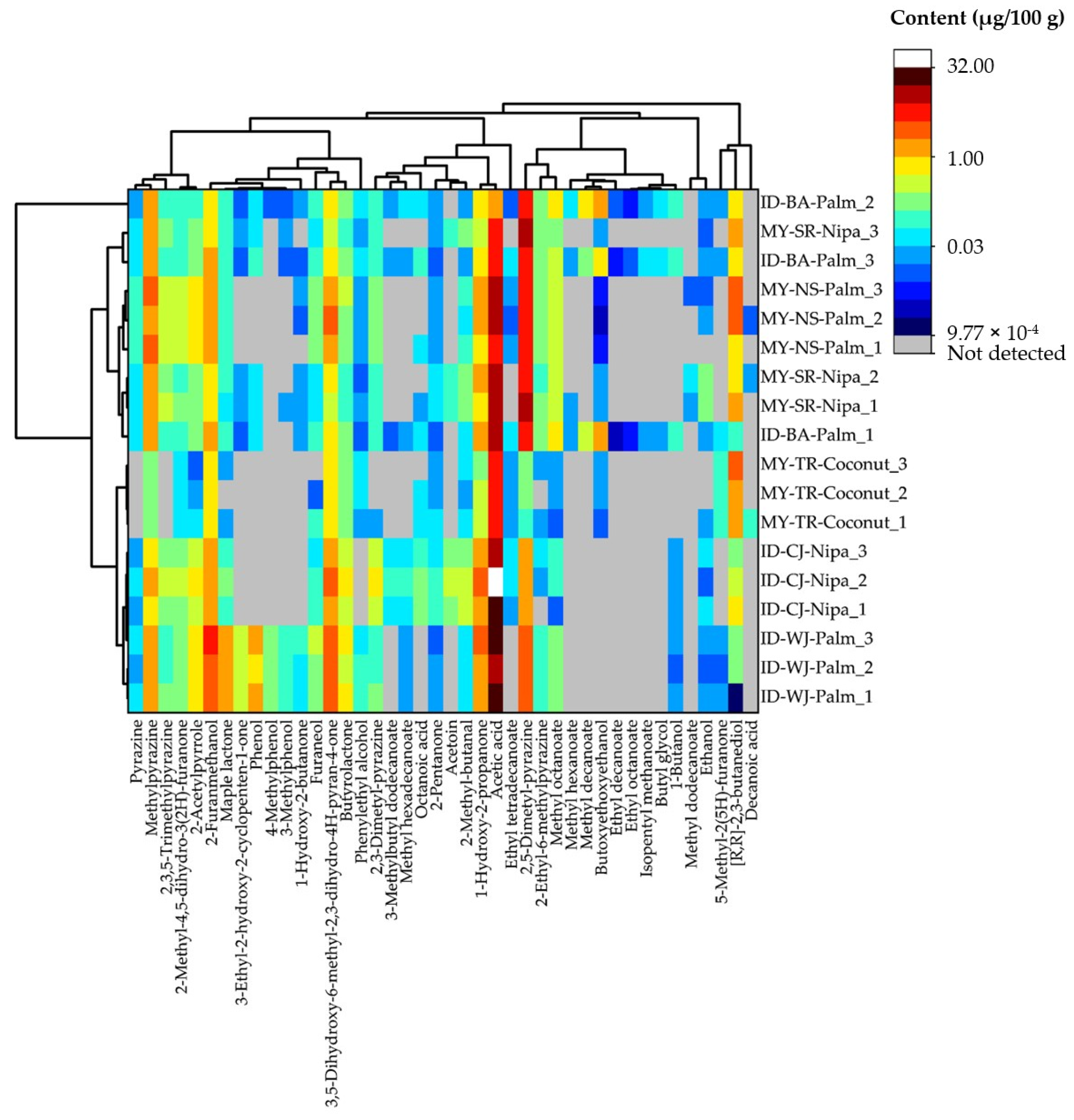
3.1. Volatile Compositions of Indonesian and Malaysian Palm Sugars
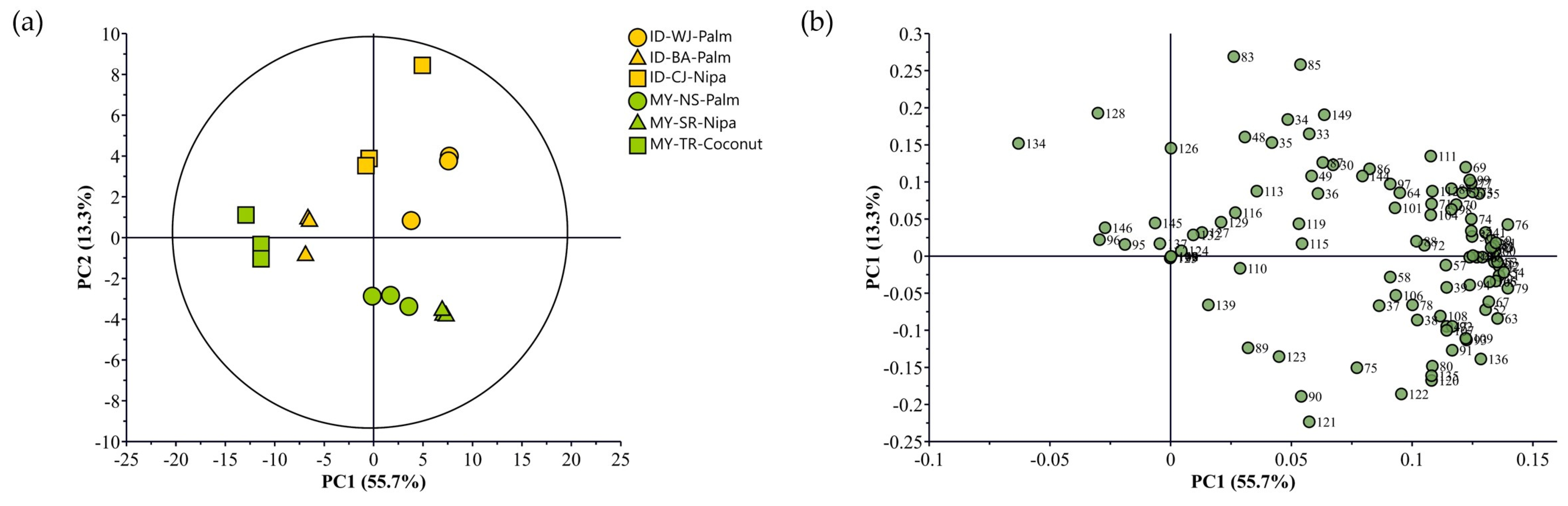
3.2. MS-e-nose Profiles of Indonesian and Malaysian Palm Sugars
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sarkar, T.; Mukherjee, M.; Roy, S.; Chakraborty, R. Palm sap sugar an unconventional source of sugar exploration for bioactive compounds and its role on functional food development. Heliyon 2023, 9, e14788. [Google Scholar] [CrossRef] [PubMed]
- Saputro, A.D.; Van de Walle, D.; Dewettinck, K. Palm sap sugar: A review. Sugar Tech 2019, 21, 862–867. [Google Scholar] [CrossRef]
- Asikin, M.N.; Mashrom, M.; Awal, A. Sugar palm (Arenga pinnata Wurmb Merr.): Its potential, limitation, and impact on socio-economic development of rural communities in Malaysia. J. Nat. Fibre Polym. Compos. 2023, 3, 5. [Google Scholar]
- Hebbar, K.B.; Pandiselvam, R.; Manikantan, M.R.; Arivalagan, M.; Beegum, S.; Chowdappa, P.J.S.T. Palm sap—Quality profiles, fermentation chemistry, and preservation methods. Sugar Tech 2018, 20, 621–634. [Google Scholar] [CrossRef]
- Asghar, M.T.; Yusof, Y.A.; Mokhtar, M.N.; Ya’acob, M.E.; Mohd Ghazali, H.; Chang, L.S.; Manaf, Y.N. Coconut (Cocos nucifera L.) sap as a potential source of sugar: Antioxidant and nutritional properties. Food Sci. Nutr. 2020, 8, 1777–1787. [Google Scholar] [CrossRef] [PubMed]
- Srikaeo, K.; Sangkhiaw, J.; Likittrakulwong, W. Productions and functional properties of palm sugars. Walailak J. Sci. Technol. 2019, 16, 897–907. [Google Scholar] [CrossRef]
- Verma, P.; Chavanpatil, V.; Shah, N.G.; Mahajani, S.M. Scientific understanding of the traditional practices followed in India for the production of non-centrifugal sugar. Sugar Tech 2021, 23, 1393–1412. [Google Scholar] [CrossRef]
- Zidan, D.; Azlan, A. Non-centrifugal sugar (NCS) and health: A review on functional components and health benefits. Appl. Sci. 2022, 12, 460. [Google Scholar] [CrossRef]
- Asikin, Y.; Nakaza, Y.; Oe, M.; Kaneda, H.; Maeda, G.; Takara, K.; Wada, K. Volatile component composition, retronasal aroma release profile, and sensory characteristics of non-centrifugal cane sugar obtained at different evaporation temperatures. Appl. Sci. 2024, 14, 11617. [Google Scholar] [CrossRef]
- Saputro, A.D.; Van de Walle, D.; Dewettinck, K. Physicochemical properties of coarse palm sap sugars as natural alternative sweetener. Food Biosci. 2020, 38, 100780. [Google Scholar] [CrossRef]
- Aeimsard, R.; Thumthanaruk, B.; Jumnongpon, R.; Lekhavat, S. Effect of drying on total phenolic compounds, antioxidant activities, and physical properties of palm sugar. J. Food Sci. Agric. Technol. 2015, 1, 126–130. [Google Scholar]
- Hou, X.; Jiang, J.; Luo, C.; Rehman, L.; Li, X.; Xie, X. Advances in detecting fruit aroma compounds by combining chromatography and spectrometry. J. Sci. Food Agric. 2023, 103, 4755–4766. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.L.; Arora, B.; Sethi, S.; Sharma, R.R. Electronic nose (E-nose) application in the food industry. In Sensor-Based Quality Assessment Systems for Fruits and Vegetables; Apple Academic Press: Point Pleasant, NJ, USA, 2020; pp. 143–167. [Google Scholar]
- Ayustaningwarno, F.; Asikin, Y.; Amano, R.; Vu, N.T.; Hajar-Azhari, S.; Anjani, G.; Wada, K. Composition of minerals and volatile organic components of non-centrifugal cane sugars from Japan and ASEAN Countries. Foods 2023, 12, 1406. [Google Scholar] [CrossRef]
- Worley, B.; Powers, R. Multivariate analysis in metabolomics. Curr. Metabolomics 2013, 1, 92–107. [Google Scholar] [PubMed]
- Zhang, X.H.; Liu, R.J.; Zheng, J.J.; Qing, X.D.; Yang, K.L.; Zhang, Y.Q.; Nie, J.F. Authentication of the production season of Xinyang Maojian green tea using two-dimensional fingerprints coupled with chemometric multivariate calibration and pattern recognition analysis. LWT 2023, 176, 114556. [Google Scholar] [CrossRef]
- Qu, D.; Xi, L.; Li, Y.; Yang, H.; Chen, X.; Jin, W.; Yan, F. Characterizing the composition of volatile compounds in different types of Chinese bacon using GC–MS, E-nose, and GC–IMS. J. Chromatogr. A 2024, 1730, 465056. [Google Scholar] [CrossRef]
- Liu, X.; Fang, Y.; Ma, H.; Zhang, N.; Li, C. Performance comparison of three scaling algorithms in NMR-based metabolomics analysis. Open Life Sci. 2023, 18, 20220556. [Google Scholar] [CrossRef]
- Asghar, M.T.; Yusof, Y.A.; Mokhtar, M.N.; Yaacob, M.E.; Ghazali, H.M.; Chang, L.S. A review of nutritional facts, production, availability, and future aspects of coconut palm sugar. J. Nutr. Food Sci. 2021, 11, 793. [Google Scholar]
- Phetrit, R.; Chaijan, M.; Sorapukdee, S.; Panpipat, W. Characterization of nipa palm (Nypa fruticans Wurmb.) sap and syrup as functional food ingredients. Sugar Tech 2020, 22, 191–201. [Google Scholar] [CrossRef]
- Ma, Q.L.; Hamid, N.; Bekhit, A.E.D.; Robertson, J.; Law, T.F. Optimization of headspace solid-phase microextraction (HS-SPME) for gas chromatography–mass spectrometry (GC–MS) analysis of aroma compounds in cooked beef using response surface methodology. Microchem. J. 2013, 111, 16–24. [Google Scholar] [CrossRef]
- Lu, J.; Jiang, Z.; Dang, J.; Li, D.; Yu, D.; Qu, C.; Wu, Q. GC–MS combined with fast GC E-Nose for the analysis of volatile components of chamomile (Matricaria chamomilla L.). Foods 2024, 13, 1865. [Google Scholar] [CrossRef] [PubMed]
- Loreto, F.; Dicke, M.; Schnitzler, J.P.; Turlings, T.C. Plant volatiles and the environment. Plant Cell Environ. 2014, 37, 1905–1908. [Google Scholar] [CrossRef]
- Ge, Y.; Li, K.; Xie, C.; Xu, Y.; Shi, C.; Hang, F.; Doherty, W.O. Formation of volatile and aroma compounds during the dehydration of membrane-clarified sugarcane juice to non-centrifugal sugar. Foods 2021, 10, 1561. [Google Scholar] [CrossRef] [PubMed]
- Phaeon, N.; Chapanya, P.; Mueangmontri, R.; Pattamasuwan, A.; Lipan, L.; Carbonell-Barrachina, Á.A.; Nitayapat, N. Acrylamide in non-centrifugal sugars and syrups. J. Sci. Food Agric. 2021, 101, 4561–4569. [Google Scholar] [CrossRef]
- Weerawatanakorn, M.; Asikin, Y.; Kamchonemenukool, S.; Tamaki, H.; Takara, K.; Wada, K. Physicochemical, antioxidant, volatile component, and mass spectrometry-based electronic nose analyses differentiated unrefined non-centrifugal cane, palm, and coconut sugars. J. Food Meas. Charact. 2021, 15, 1563–1577. [Google Scholar] [CrossRef]
- Wan Aida, W.M.; Ho, C.W.; Maskat, M.Y.; Osman, H. Relating descriptive sensory analysis to gas chromatography/mass spectrometry of palm sugars using partial least squares regression. ASEAN Food J. 2008, 15, 35–45. [Google Scholar]
- Wang, L.; Deng, W.; Wang, P.; Huang, W.; Wu, J.; Zheng, T.; Chen, J. Degradations of aroma characteristics and changes of aroma-related compounds, PPO activity, and antioxidant capacity in sugarcane juice during thermal process. J. Food Sci. 2020, 85, 1140–1150. [Google Scholar] [CrossRef] [PubMed]
- Wrage, J.; Burmester, S.; Kuballa, J.; Rohn, S. Coconut sugar (Cocos nucifera L.): Production process, chemical characterization, and sensory properties. LWT 2019, 112, 108227. [Google Scholar] [CrossRef]
- Jara’ee, J.; Awg-Adeni, D.S.; Bilung, L.M.; Azmin, P. Effects of selected preservation techniques on the shelf-life of nipa sap. Food Res. 2025, 9, 40–48. [Google Scholar] [CrossRef]
- Huynh Thi Le, D.; Lu, W.C.; Li, P.H. Sustainable processes and chemical characterization of natural food additives: Palmyra palm (Borassus flabellifer Linn.) granulated sugar. Sustainability 2020, 12, 2650. [Google Scholar] [CrossRef]
- Shetty, P.; D’Souza, A.; Poojari, S.; Narayana, J.; Rajeeva, P. Study of fermentation kinetics of palm sap from Cocos nucifera. Int. J. Appl. Sci. Biotechnol. 2017, 5, 375–381. [Google Scholar] [CrossRef]
- Arroyo-Manzanares, N.; Markiv, B.; Hernández, J.D.; López-García, I.; Guillén, I.; Vizcaíno, P.; Viñas, P. Headspace gas chromatography coupled to mass spectrometry for the assessment of the contamination of mayonnaise by yeasts. Food Chem. 2019, 289, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Okada, T.; Mochamad Afendi, F.; Altaf-Ul-Amin, M.; Takahashi, H.; Nakamura, K.; Kanaya, S. Metabolomics of medicinal plants: The importance of multivariate analysis of analytical chemistry data. Curr. Comput.-Aided Drug Des. 2010, 6, 179–196. [Google Scholar] [CrossRef] [PubMed]
- Diez-Simon, C.; Mumm, R.; Hall, R.D. Mass spectrometry-based metabolomics of volatiles as a new tool for understanding aroma and flavor chemistry in processed food products. Metabolomics 2019, 15, 41. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katherinatama, A.; Asikin, Y.; Amano, R.; Hajar-Azhari, S.; Yudianto, D.; Widyahapsari, D.A.N.; Widarta, I.W.R.; Takara, K.; Wada, K. Volatile Organic Components and MS-e-nose Profiles of Indonesian and Malaysian Palm Sugars from Different Plant Origins. Chemosensors 2025, 13, 155. https://doi.org/10.3390/chemosensors13050155
Katherinatama A, Asikin Y, Amano R, Hajar-Azhari S, Yudianto D, Widyahapsari DAN, Widarta IWR, Takara K, Wada K. Volatile Organic Components and MS-e-nose Profiles of Indonesian and Malaysian Palm Sugars from Different Plant Origins. Chemosensors. 2025; 13(5):155. https://doi.org/10.3390/chemosensors13050155
Chicago/Turabian StyleKatherinatama, Aldia, Yonathan Asikin, Ryo Amano, Siti Hajar-Azhari, David Yudianto, Dhina Aprilia Nurani Widyahapsari, I Wayan Rai Widarta, Kensaku Takara, and Koji Wada. 2025. "Volatile Organic Components and MS-e-nose Profiles of Indonesian and Malaysian Palm Sugars from Different Plant Origins" Chemosensors 13, no. 5: 155. https://doi.org/10.3390/chemosensors13050155
APA StyleKatherinatama, A., Asikin, Y., Amano, R., Hajar-Azhari, S., Yudianto, D., Widyahapsari, D. A. N., Widarta, I. W. R., Takara, K., & Wada, K. (2025). Volatile Organic Components and MS-e-nose Profiles of Indonesian and Malaysian Palm Sugars from Different Plant Origins. Chemosensors, 13(5), 155. https://doi.org/10.3390/chemosensors13050155





