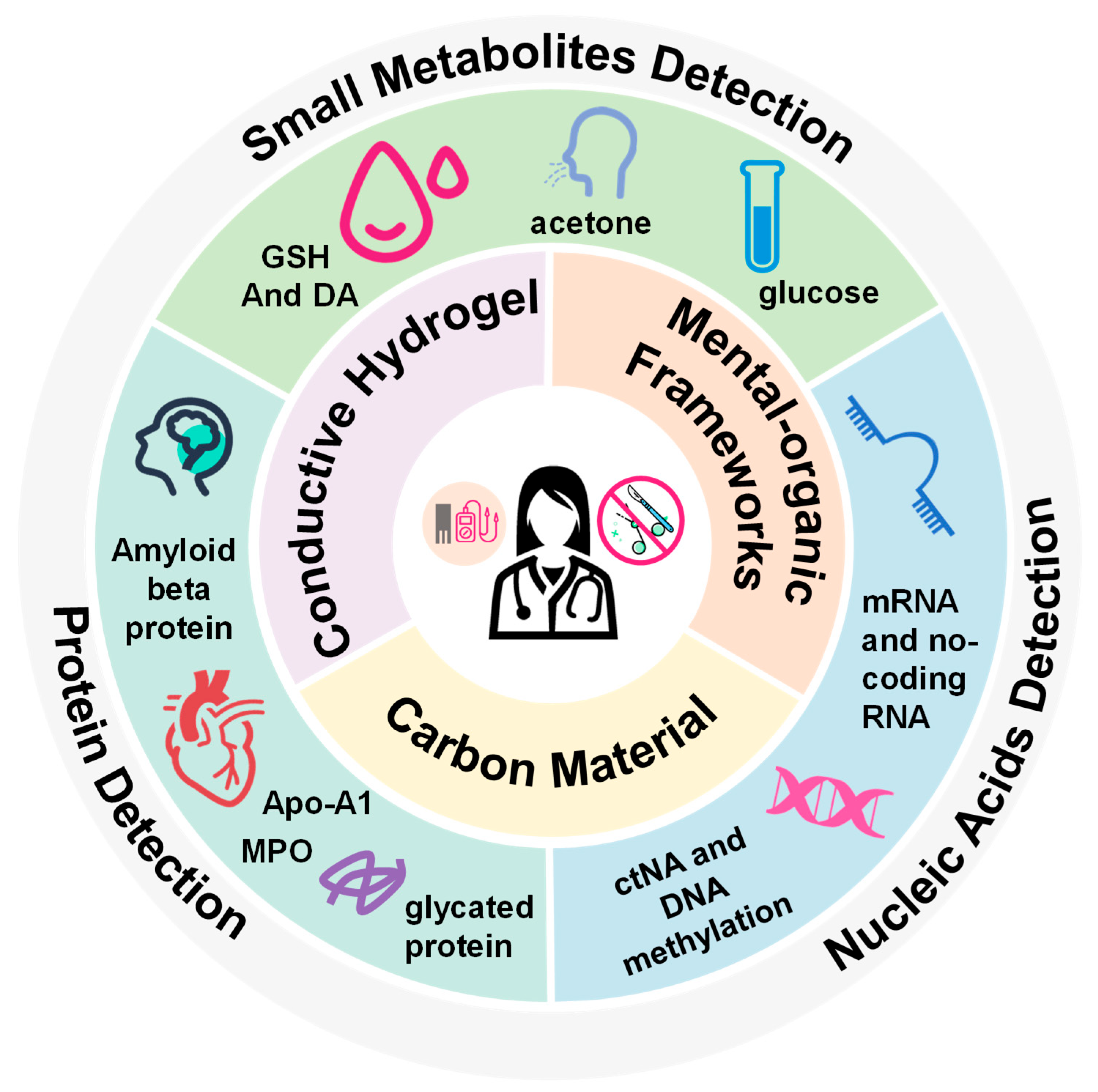
Abstract
The development of cost-effective, rapid-response, and user-friendly biosensing platforms has become paramount importance for achieving precise biomarker quantification in early disease detection. Implementing timely diagnostic interventions through accurate biomarker analysis not only significantly improves treatment outcomes but also enables effective disease management strategies, ultimately leading to substantial reductions in patient mortality rates. These clinical imperatives have consequently driven the innovation of portable point-of-care (POC) diagnostic systems. Electrochemical biosensors are attractive in the early diagnosis of diseases due to their low cost, simple operation, and high sensitivity. This review examines prevalent material innovations in electrode functionalization for electrochemical biosensing platforms, with specific emphasis on their translational applications in early-stage disease detection. The analysis included three important early diagnostic biomarker types: proteins, nucleic acids, and small molecule metabolites. Furthermore, the work proposes novel research trajectories for next-generation biosensor development, advocating the synergistic integration of artificial intelligence-driven analytics, Internet of Medical Things (IoMT)-enabled diagnostic networks, and advanced micro/nanofabrication techniques.
1. Introduction
One of the major challenges facing medicine today is the early diagnosis of diseases, which allows for early intervention, increasing the chances of curing patients and reducing healthcare costs. Inadequate infrastructure, limited access to healthcare, and expensive diagnostic costs in some developing regions contribute to delays in the early diagnosis of diseases [1]. Electrochemical sensors have many advantages such as simple equipment, fast analysis speed, high sensitivity, good selectivity, and can be monitored in complex systems, and have a broad application prospect in the field of instant detection medical diagnosis [2].
Electrochemical biosensors are an important subclass of electrochemical sensors. With the continuous development of medical research, electrochemical biosensors have gained great popularity in recent years as one of the most promising diagnostic technology tools due to the numerous advantages mentioned above [3,4]. Electrochemical biosensors differ from conventional sensors (Table 1). They consist of a biorecognition element that selectively binds the target analyte and provides analyte specificity, an electrode that acts as a transducer, and an output of current/impedance information related to the concentration of the analyte. Compared to laboratory analytical tools such as immunoassays and molecular diagnostics, which require expensive instruments as well as skilled technicians, electrochemical-based biosensors are miniaturized and portable [5,6]. In addition, electrochemical biosensors are capable of responding to very small sample volumes, and they can be unaffected by luminescent groups, fluorescent groups, etc., compared to optical detection [7]. The advantages of timely response, cost-effectiveness and in situ sensing can be used to develop real-time diagnostic point-of-care (POC) miniaturized devices [8]. This allows for real-time monitoring and diagnosis of patients regardless of time, place, or equipment. Thus, timely diagnosis of diseases is achieved, the cure rate of patients is increased, and the mortality rate is reduced.

Table 1.
Comparison of advantages and limitations of various types of biosensors.
In this paper, we will introduce the basic principles of electrochemical biosensors, the currently popular electrode modification materials (carbon materials, MOF, hydrogel), and their applications in the early detection of diseases (Scheme 1).
Scheme 1.
Materials and applications of electrochemical biosensors for point-of-care medical diagnostics.
2. Fundamentals of Electrochemical Biosensors
The basic principle of electrochemical biosensors is a redox reaction in which one chemical loses electrons and another gains them. The substance that loses electrons is oxidized and the substance that gains electrons is reduced. The two substances involved in electron transfer are called redox pairs. Electrochemical biosensors are able to bias the electron flow between redox pairs by applying an external voltage. The target analyte can participate in the electron transfer as one of the redox pairs. Electrochemical biosensors are used to infer the presence and concentration of analytes by applying different patterns of external voltages. There are three commonly used electrochemical techniques: amperometry, voltammetry, and impedance spectroscopy [14].
Amperometry measures current by applying a constant potential value. At any given point in time after the potential is applied, the current value is proportional to the concentration of the analyte in the body solution. Voltammetry measures current by applying a time-varying potential; cyclic voltammetry (CV) is the most common voltammetry. The potential applied by cyclic voltammetry varies repeatedly between two different potential levels [15].
Unlike amperometry, voltammetry usually treats current as a function of the applied voltage, rather than current as a function of time [16]. A plot of current versus potential is called a voltammogram. The shape of the voltammogram responds to the transition from an electrochemical current to a diffusion current. The key parameter that determines the shape of the voltammogram is the scan rate; the faster the scan rate, the higher the current. The shape of the voltammogram can be used to infer important information about the electrochemical cell, such as whether the reaction is a reversible electrochemical reaction and the magnitude of the cell’s capacitance and background resistance. For a defined surface area, diffusion coefficient and voltage scan rate, the peak current will be proportional to the volumetric concentration of the analyte.
Electrochemical impedance spectroscopy (EIS) is an impedance technique to analyze electrochemical systems in the frequency domain. A sinusoidal current is obtained by applying an alternating sinusoidal voltage over a series of frequencies. The complex impedance can be obtained by calculating these sinusoidal currents. The system’s characteristics are determined through visualization and equivalent circuit modeling of the complex impedance spectra [17].
For electrochemical biosensors, the sample analysis process consists of two main stages: recognition and signal conversion. Recognition is carried out by means of recognition elements, and they are prepared according to the properties of the analyte. Signal conversion refers to the conversion of the analyte into a measurable electrical signal, which is then output through the instrument as the basis for quantitative analysis. The biorecognition element is a key part of the electrochemical biosensor. The recognition element selectively captures the target analyte through specific molecular interactions, subsequently transducing the signal into quantifiable electrical responses via the transducer component.
Also noteworthy is the potentiometric method, which does not involve redox processes [18,19]. It mainly works through the following non-redox mechanisms: (1) interfacial charge accumulation: based on the Nernst equation, the interfacial potential difference is altered by ion exchange/complexation between the sensitive membrane and the target; (2) capacitance effect: changes in the capacitance of the bilayer induced by the binding of the target; and (3) modulation of the field effect: interactions between the target and the gate modification layer alter the semiconductor channel conductance.
The performance of electrochemical biosensors, such as sensitivity and selectivity, is related to the recognition element, and the sensors can be improved by appropriate modification of the electrodes. Three commonly used electrode modification materials are described below.
3. Engineered Intelligent Electrochemical Biosensors
3.1. Carbon Material
Graphene and its derivatives have been used in sensor fabrication due to their unique physicochemical properties. Graphene is a unique two-dimensional honeycomb carbon material with an exceptional basal structure, carrier mobility, thermal conductivity, broad electrochemical spectrum and a variety of unusual physicochemical properties [20]. The presence of carbon atoms in the sp2 hybridization state allows electrons to populate the π–π conjugate system, which allows them to move freely and gives the material excellent electron transfer properties. According to the number of layers of graphene, it is classified into single-layer graphene and multi-layer graphene. The number of layers of graphene has a significant effect on its application in electrochemistry. Single-layer graphene exhibits extremely high electrical conductivity, while multi-layer graphene is slightly lower than single-layer graphene. Multi-layer graphene has a larger surface area and is better able to adsorb and react with electrochemically active substances, enhancing their electrochemical properties. Moreover, compared with single-layer graphene, multi-layer graphene has a simpler production process and lower production costs, making it easier for large-scale factory production. In addition, graphene’s high specific surface area enables it to achieve high biomolecular loading and good detection sensitivity. These features make graphene more widely used in electrochemical biosensors.
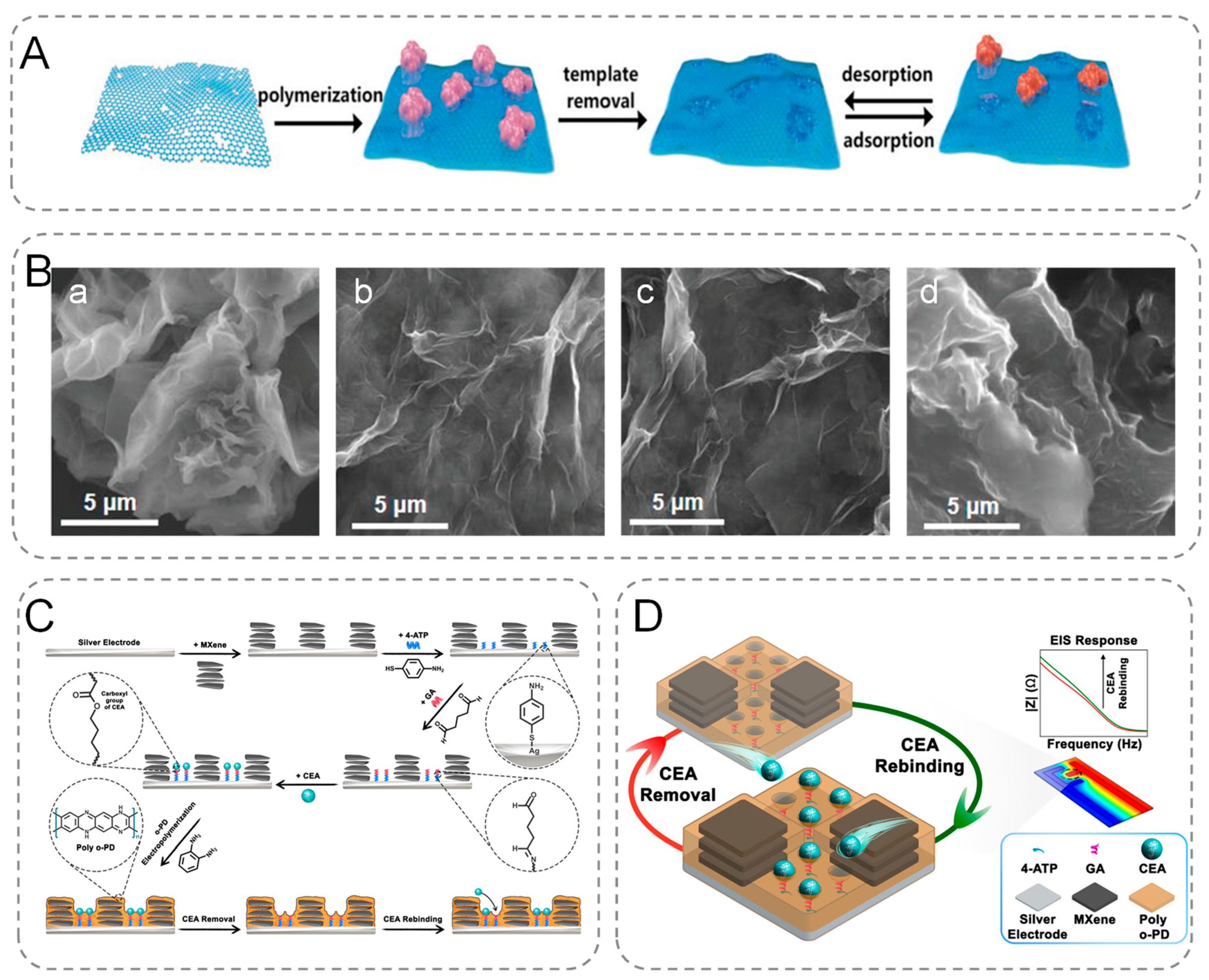
In most cases, the properties of graphene can be expanded by functionalization [21]. Graphene can be modified either non-covalently or covalently depending on the application. Graphene offers potential binding sites for many nanomaterials. Thus, in addition to using pristine-graphene [22] to modify the electrodes of electrochemical biosensors, the electrodes can be modified using graphene composites, such as metal-graphene [23], bimetallic-graphene [24], metal-oxide graphene [25], metal–organic framework-graphene [26], graphene-MXene [27], graphene-quantum dots [28], graphene-carbon nanotubes [29], and graphene-molecular imprinted polymers [30]. Li et al. reported a cost-effective reduced graphene oxide/polydopamine (rGO/PDA)-MIP sensing platform for the simultaneous detection of creatinine, urea, and HSA in serum and urine (Figure 1A,B). This system is capable of early screening and detection of chronic kidney disease (CKD) by differential pulse voltammetry (DPV) [31]. The platform shows very high sensitivity, selectivity, and robustness, and it records LoD at the femtomole level with a broad detection range and can be used as a POC platform by primary healthcare workers.
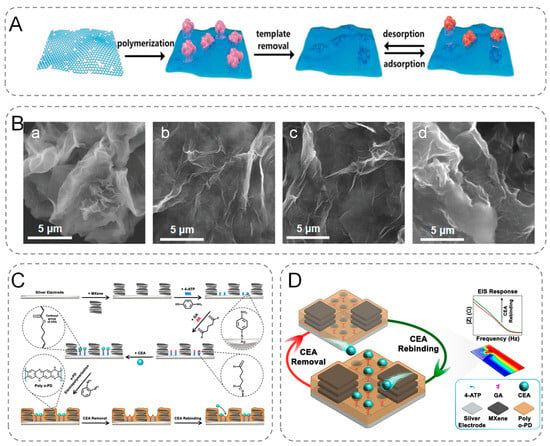
Figure 1.
(A) Overview of the surface MIP synthesis process based on the designed multiplexed POC sensing platform. (B) SEM images of (a) GO, (b) rGO/PDA-MIP (creatinine), (c) rGO/PDA-MIP (urea), (d) rGO/PDA-MIP (HSA). (C) Schematic diagram of the MIP biosensor manufacturing process steps. (D) CEA response process.
MXene was synthesized by extracting selected metal atoms from the MAX phase (etched with HF), which was identified as a layer of A atoms with the chemical formula Mn+1AXn (the A atoms represent any element between groups 13 and 16 of the periodic table) [32]. MXene has the unique physicochemical properties of typical 2D materials. MXene is highly conductive, biocompatible, ordered, and hydrophilic [33,34]. Additionally, MXene can be integrated with nanomaterials to enhance the surface-to-volume ratio [35], consequently optimizing active sites for enzymatic catalysis and molecular recognition.
Hadian et al. prepared a nano-constrained MXene-modified bionic sensor for the quantitative and label-free detection of Carcinoembryonic antigen (CEA). They modified disposable silver electrodes with Ti3C2Tx MXene and immobilized CEA on the surface using SAM (4-ATP) and glutaraldehyde (GA). MIP structures were prepared by electropolymerization of o-phenylenediamine (o-PD), and then proteins were extracted from the structures using an appropriate alkaline washing solution (Figure 1C,D). The nano-microcavities between the MXene nanoparticles create suitable sites for the attachment of the SAMs to the silver surface, providing a limited space for the trapping of the solution and analytes. Its integration with MIPs improves electrochemical biosensor performance [36]. Therefore, MXene can be used for electrochemical sensing of biomolecules and pathogens, making it one of the most promising 2D materials.
3.2. Mental-Organic Frameworks (MOFs)
MOFs are highly ordered porous crystal networks consisting of nodes of metal ions or metal clusters connected by poly-functional organic molecules called struts [37]. The porous architecture of MOFs offers an exceptionally high specific surface area, facilitating the adsorption and enrichment of target molecules, thereby significantly enhancing the sensor’s sensitivity and lowering its detection limit [38]. The pore structure of MOFs is highly tunable; by judiciously selecting various metal ions and organic ligands, it can be precisely engineered to accommodate target molecules of different sizes, thereby improving selectivity [39].
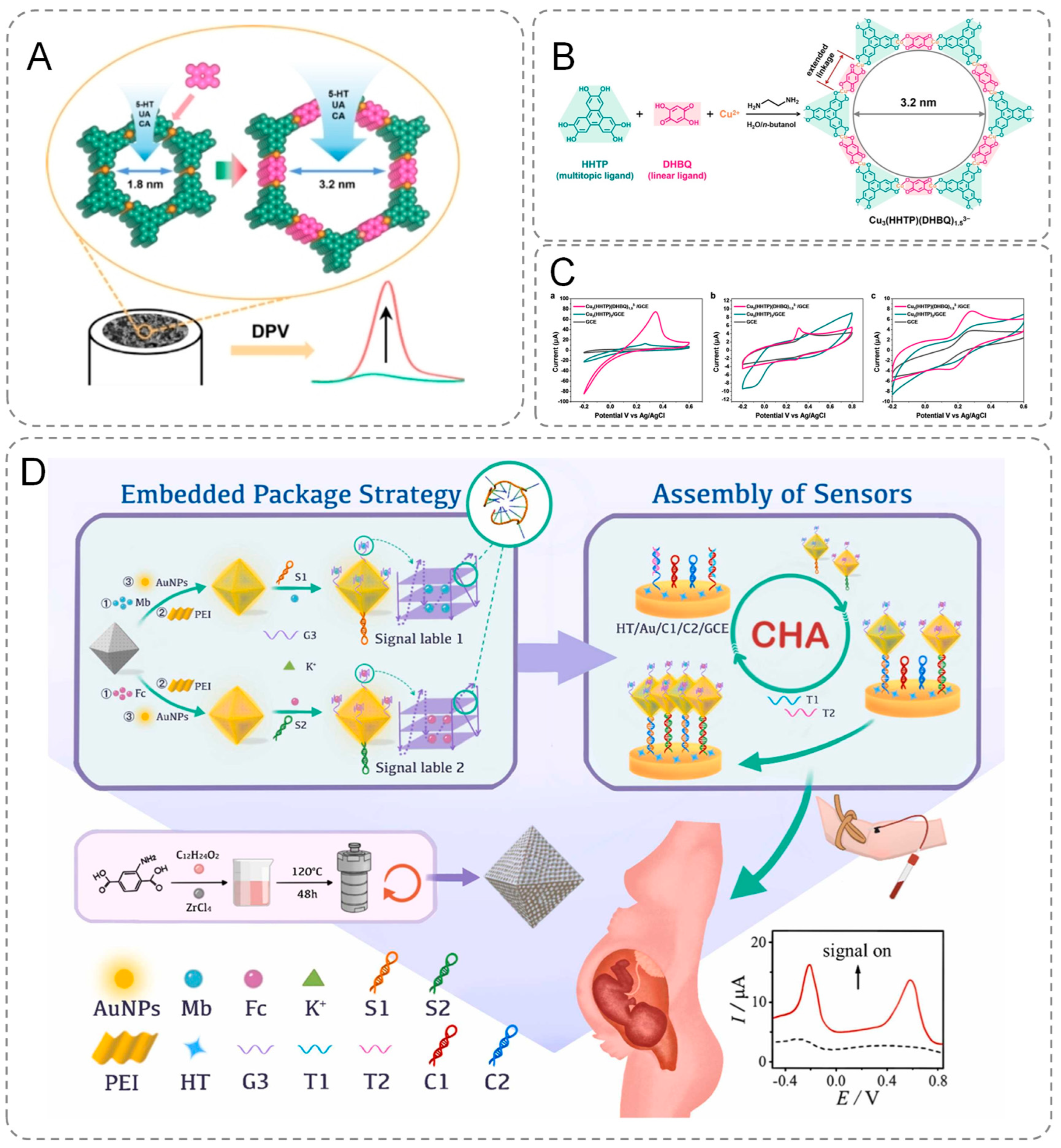
Two-dimensional conductive metal–organic frameworks (2D c-MOFs) with large pore sizes can be engineered through a ligand insertion strategy, wherein a linear ligand is incorporated into the framework, replacing the metal ion with a linear -M-ligand- unit. Leveraging this strategy, Wang et al. expanded upon the initially reported 2D conductive metal–organic framework (2D c-MOF), Cu3(HHTP)2, by incorporating the linear ligand 2,5-dihydroxybenzoquinone (DHBQ) to synthesize 2D c-MOFs featuring a mesopore size of 3.2 nm (Figure 2B,C). These materials were subsequently used to modify electrodes (Figure 2A), enabling the detection of several biologically significant molecules, including 5-hydroxytryptamine (5-HT), uric acid (UA), and caffeic acid (CA). The findings revealed that at a relatively high scan rate of 100 mV s⁻1, the current signals for all three analytes measured with the Cu3(HHTP)(DHBQ)1.53− modified electrode were substantially more pronounced compared to those obtained with bare glassy carbon electrodes (GCEs) and Cu3(HHTP)2-coated GCEs. This underscores the superior electrochemical activity of the Cu3(HHTP)(DHBQ)1.53− framework [40]. Furthermore, MOFs are inherently multifunctional and amenable to functionalization. They can concurrently perform catalytic, sensing, and adsorption roles, making them exceptionally suitable for diverse electrochemical detection applications. Additionally, their versatility allows for the introduction of specific functional groups, which further augments their capability to recognize and detect target molecules effectively [41]. These properties of MOFs make them widely applicable in the field of electrochemical biosensors. Ma et al. modified the MOF materials to prevent the leakage of MB/Fc in MOF and reduce the energy loss during electron transfer, thus improving the sensitivity and accuracy of detection [42]. The innovativeness of this study is reflected in the precise anchoring of gold nanoparticles (AuNPs) to the porous MOF surface through a polyethyleneimine (PEI)-mediated self-assembly strategy. Based on the directed modification technique of Au-S bonds, the research team successfully grafted guanine-enriched sequences to the AuNPs interface and achieved stable encapsulation of methylene blue (MB)/ferrocene (Fc) molecules through conformational restructuring of G-quadruplex (G-quadruplex). The material is capable of early identification of β-thalassemia-associated polygenic mutations (Figure 2D). Furthermore, by adapting the template, the technique can be extended to detect other disease-related mutations.
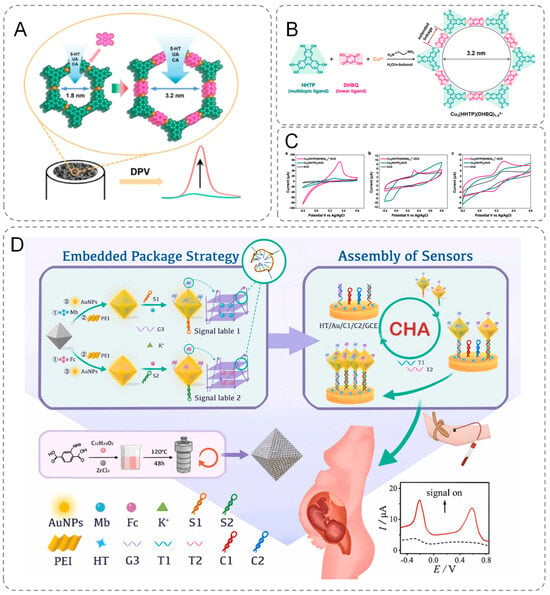
Figure 2.
(A) Modified electrodes. (B) Synthesis of Cu3(HHTP)(DHBQ)1.53− and electrode modification process. (C) CV curves (scan rate = 100 mVs−1) Cu3(HHTP)(DHBQ)1.53−-coated and Cu3(HHTP)2 -coated GCEs in the KCl solution (0.1 M). (D) Schematic representation of an electrochemical biosensor for simultaneous detection of multiple β-thalassemia genes.
3.3. Hydrogels
Hydrogels, a class of polymeric materials, have garnered significant attention in biomedical applications and flexible devices owing to their exceptional properties, including high water content, remarkable flexibility, and excellent biocompatibility [43]. The porous architecture of hydrogels facilitates efficient biomolecular transport and signal transduction, thereby enhancing the sensitivity and selectivity of sensors [44]. These porous structures also minimize interference from complex matrices, enabling more accurate sensing in real-world sample analyses. Additionally, the inherent flexibility and adhesive properties of hydrogels, combined with their ability to form intimate contact with diverse surfaces, provides distinct advantages in the design of compact, portable, and wearable electrochemical biosensors. Conductive Hydrogels 3D polymer networks include conductive components such as conductive polymers (CPs), carbon-based materials, or metal nanoparticles. The synthesis of conductive hydrogels can be broadly categorized into four methods: (1) introduction of CPs to form CP hydrogels; (2) introduction of conductive nanoparticles to form conductive composite hydrogels; (3) introduction of conductive ions to form conductive ionic hydrogels; and (4) incorporation of hybridized conductive fillers to form conductive hydrogels [45]. Through strategic modifications of the internal structure and functional groups of hydrogels, the selectivity and responsiveness of sensors can be significantly enhanced, thereby optimizing their performance for biomedical applications such as point-of-care diagnostics and real-time monitoring [46].
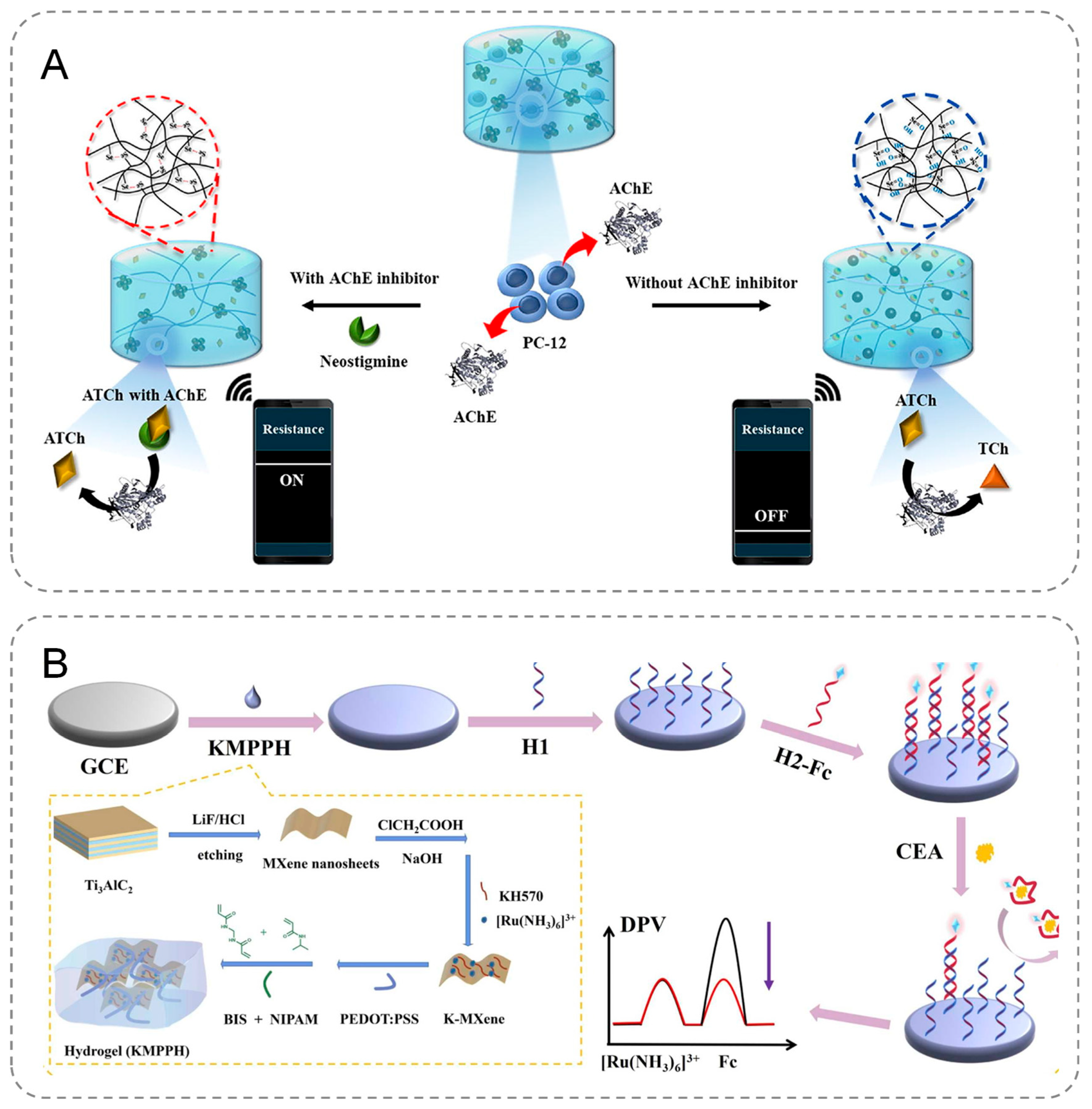
Seul Gi Kim et al. developed an innovative electrochemical biosensor designed for the precise detection of acetylcholinesterase (AChE) and its activity, leveraging the redox-responsive properties of carbodiselenide crosslinked polymer dot-loaded polydopamine (PDA@dsPD) embedded within a polyvinyl alcohol (PVA)/chitosan matrix. During the redox reaction, the diselenide bonds within the PDA@dsPD break down, releasing PDA, which simultaneously alters the electrical and optical properties of the hydrogel (Figure 3A). Furthermore, the biosensor can be integrated with a wireless device, enabling real-time monitoring of AChE levels and facilitating the rapid generation of accurate results [47]. Geng et al. fabricated MXene nanosheets with a large surface area to serve as the conductive framework for the anti-fouling hydrogel, incorporating a significant amount of [Ru(NH3)6]3+ onto the nanosheets (Figure 3B). To further enhance the stability and conductivity of the anti-fouling hydrogel and expand its applicability in electrochemical analysis, KH570 and PEDOT:PSS were introduced. Additionally, the encapsulated [Ru(NH3)6]3+ acted as a correction factor to improve the biosensor’s detection accuracy. The developed biosensor demonstrated excellent sensitivity and precision in detecting carcinoembryonic antigen (CEA) in human serum, enabling the trace-level detection of biological targets in serum samples [48].
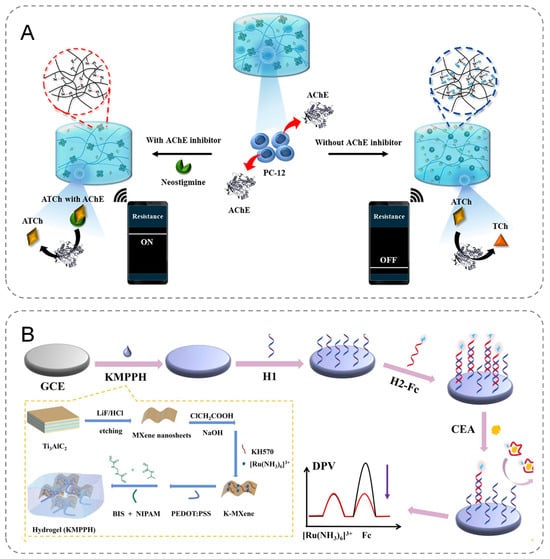
Figure 3.
(A) The course of PDA@dsPD-hydrogels in response to AChE. (B) Construction process of an efficient electrochemical biosensor based on a double-conductive antifouling hydrogel.
Overall, the inherent biocompatibility, flexibility, and high hydrophilicity of hydrogels endow them with great potential in the development of electrochemical biosensors and wearable devices.
4. Electrochemical Biosensors for Biomarker Detection in Early-Stage Disease Diagnostics
Fluctuations in biomarker concentrations (presence, absence, or quantitative variations) within biological specimens serve as critical indicators for tracking pathological progression. Biomarkers can be categorized as diagnostic, prognostic, and predictive [49]. Prognostic biomarkers provide information about the course of disease recurrence. Predictive biomarkers estimate the response to treatment. Diagnostic biomarkers are associated with the detection of diseases. This article focuses on diagnostic biomarkers. Biomarkers encompass a diverse array of molecules including proteins, nucleic acids, and small metabolites. The monitoring of these parameters requires a fully equipped laboratory or clinic. Countries and regions with relatively poor economic development have inadequate medical equipment, which makes early diagnosis inadequate. Therefore, the development of low-cost, convenient, and highly sensitive POC monitoring devices has become necessary for the early detection of disease and the effective management of the disease. Among the many methods, electrochemical biosensors are of interest because of their high sensitivity, fast analysis, and low cost. Recent advancements in analytical characterization and high-specificity electrochemical detection of biomarkers have significantly enhanced clinical capacity for early-stage disease diagnosis.
4.1. Protein
Alterations in the function of proteins involved in the regulation of the organism may have deleterious effects on normal physiological processes and lead to the development of disease. Proteins exhibiting significant alterations in expression levels or biological activity during disease onset may serve as candidate biomarkers. The detection and quantification of such biomarker proteins in biological specimens therefore holds clinical diagnostic value.
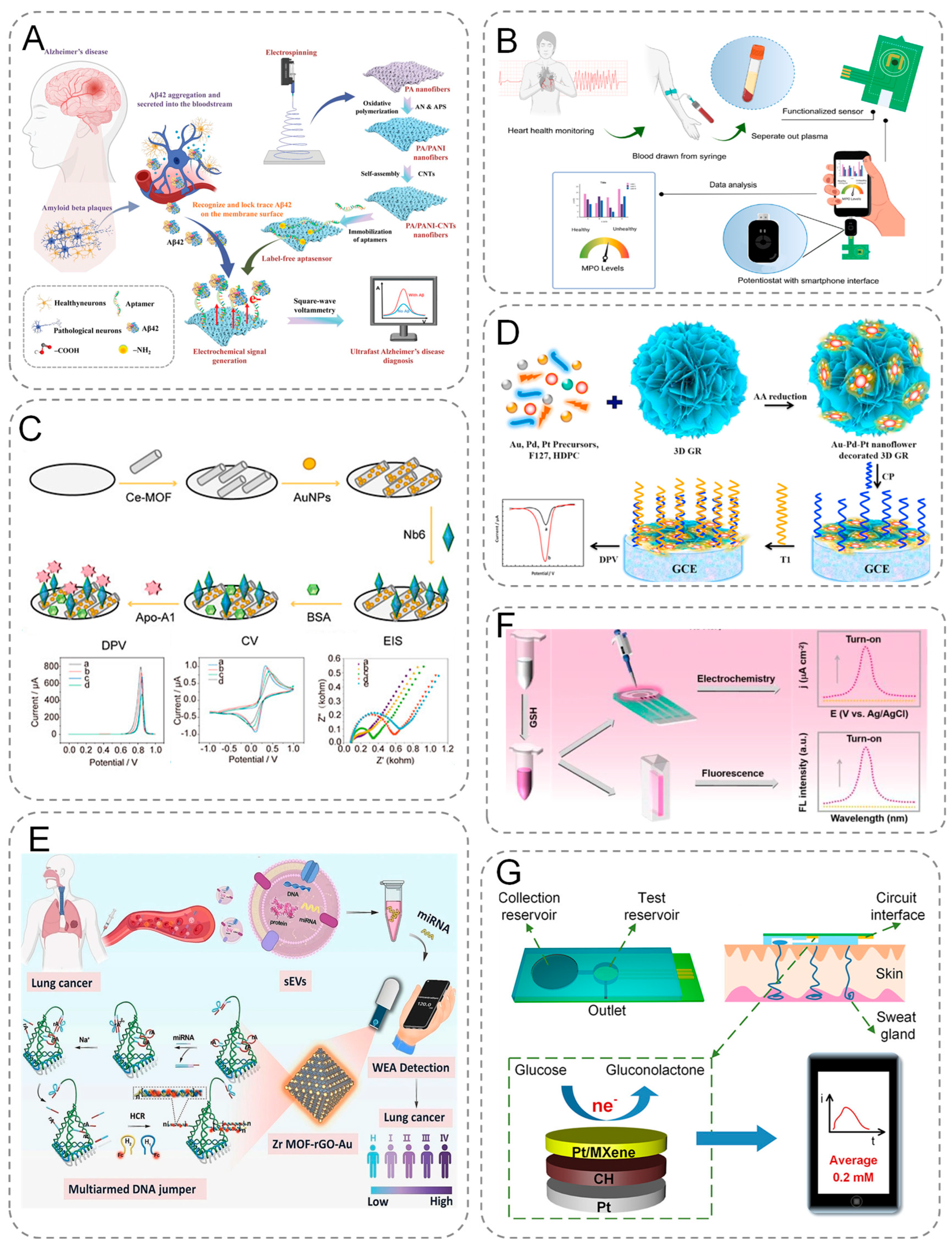
For example, amyloid beta protein (Aβ) causes gradual cognitive decline in Alzheimer’s patients [50,51,52]. Therefore, amyloid β-protein aggregation in the brain is a major hallmark of AD [53]. Conventional analytical techniques used to detect Aβ plaques have limitations such as high cost, invasive procedures, and high surgical risk, which make early diagnosis of AD difficult. Liu et al. constructed an electrochemical detection device that provides rapid, low-cost, and sensitive detection of Aβ42. They used an electrospinning technique to weave polyamide/polyaniline-carbon nanotubes (PA/PANI-CNTs) nanofibers into a freestanding backbone for DNA probe binding [54]. They successfully constructed an ultrafast label-free aptasensor capable of detecting A β42 in less than 4 min. The sensor also showed excellent properties in human serum samples (Figure 4A). It provides strong support for early diagnosis, research, and treatment of AD, and offers promising prospects for clinical applications and POC detection.
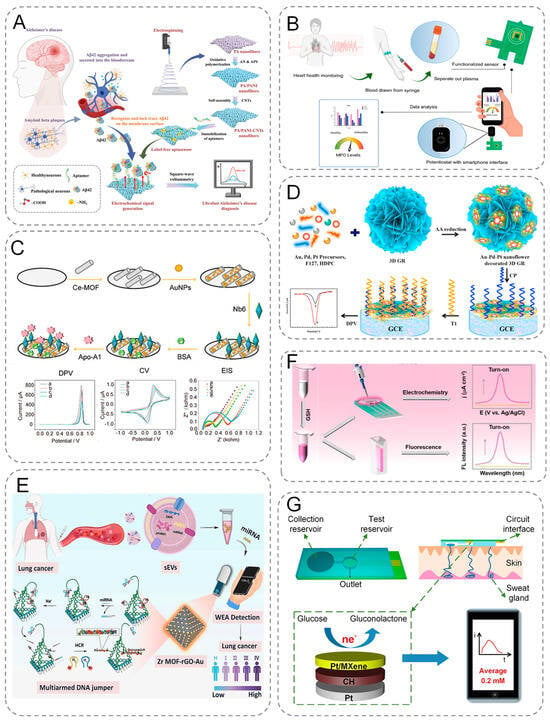
Figure 4.
(A) Schematic representation of PA/PANI-CNTs-based electrochemical biosensors for the early diagnosis of AD through simple and low-cost rapid detection of A β 42 in human blood. (B) Illustration of sample acquisition and testing using functionalized printed circuit board (PCB) sensors and schematic diagram of the PCB electrode functionalization and modification process. (C) Schematic diagram of the assembly process of Ce-MOF@Au-Based electrochemical sensor, and electrochemical behavior. (D) Schematic diagram of the principle of electrochemical signal generation by CRISPR/Cas9-triggered ESDR based on 3D GR/AuPtPd nanoflower biosensor. (E) Schematic diagram of electrochemical analysis of small extracellular vesicle-derived microRNAs (sEV-miRNAs) for lung cancer diagnosis. (F) Dual-mode, turn-on electrochemical and fluorescence sensing proto-schematic of GSH. (G) Schematic of sweat collection and glucose testing using wearable devices.
In addition, electrochemical biosensors enable specific protein detection in the clinical diagnosis of cardiovascular disease [55]. The gold standard for the diagnosis of CVD is through the detection of biomarkers in plasma, serum, and whole blood concentrations [9,56,57]. Ruchira Nandeshwar et al. electrodeposited methylene blue on the surface of printed electrodes (PCBs) to enhance electrode activity (Figure 4B). In situ synthesis of gold nanoparticles by acid-functionalized multiwalled carbon nanotubes as scaffolds and immobilization of antibodies to the target on gold nanoparticles for the detection of biomarkers of cardiovascular diseases (myeloperoxidase, MPO) [58]. They integrated capillary pump-driven microfluidic channels on PCB electrodes, where samples could be seamlessly introduced for testing. The results demonstrated that the sensor was able to discriminate between normal and abnormal concentrations, with a LOD of 15.79 ng/mL (the risk concentration of MPO in blood is 200 ng/mL). This indicates that the developed electrochemical biosensor is suitable for point-of-care biosensors. Sun et al. developed an electrochemical immunosensor for the detection of Apolipoprotein A1 (Apo-A1), taking advantage of the special strengths of electrochemical sensors and nanobodies (Figure 4C). They modified Ce-MOF@AuNPs nanocomplexes on a glassy carbon electrode to improve the adsorption and conductivity of the electrode. The nanocomplexes were combined with a highly specific and high affinity nanobody (Nb6) to target the novel biomarker Apo-A1 with a detection limit of 36 fg/mL. The sensor was used for serum sample analysis and also showed good performance. The platform shows the potential to develop durable, cost-effective and miniaturizable devices for the detection of cardiovascular disease biomarkers [59].
In addition, based on the specific recognition mechanism of cell surface protein receptors, electrochemical biosensors can achieve whole-cell detection function. As tumor-derived circulating cancer cells, the concentration of circulating tumor cells (CTCs) in peripheral blood is significantly and positively correlated with the degree of cancer progression [60]. Based on this, Sha’s team developed Folic Acid Functionalized Magnetic Beads (FA-MBs) to construct a highly sensitive CTC electrochemical detection platform through its specific binding ability with overexpressed folate receptor (FR) on the surface of cancer cells, in conjunction with CRISPR-Cas system signal amplification strategy [61].
In summary, electrochemical biosensors show great potential for protein-based biomarker detection for early disease detection due to their miniaturization, high sensitivity, and real-time monitoring capabilities.
4.2. Nucleic Acids
Nucleic acids are important biomarkers for disease (cancer, neurodegenerative diseases, infectious diseases) detection, monitoring, and treatment [62]. Nucleic acids, as fundamental carriers of genetic information, exhibit dynamic expression profiles that are intrinsically linked to molecular mechanisms underlying disease pathogenesis [63]. DNA encodes hereditary traits through its nucleotide sequences, while RNA not only regulates gene expression but also serves as a direct indicator of epigenetic dynamics. Disease-specific nucleic acid signatures—such as mutant DNA or dysregulated non-coding RNAs—can thus act as molecular fingerprints for early-stage pathophysiological processes. The high-specificity recognition of nucleic acid biomarkers facilitates both the tracing of genetic disorder origins and the molecular-level risk stratification for tumor predisposition and subclinical disease states. The development of ultrasensitive and highly selective biosensors for nucleic acids is of great significance for the early diagnosis and monitoring of the treatment process [64]. Electrochemical biosensors are considered to be an ideal tool for the early detection of cancer due to their simplicity of instrumentation, low sample volume requirement, fast response, high sensitivity, and portability [65].
Malignant tumors pose a great threat to human health. Early diagnosis of cancer is the key to reducing mortality and improving survival [66,67,68]. Liquid biopsy of peripheral blood from cancer patients using electrochemical biosensors enables real-time monitoring [69,70]. Peripheral blood ctDNA is a double-stranded circulating DNA fragment that carries tumor-specific sequence mutations and is present in the cell-free fraction of the blood. ctDNA plays an important role in the early diagnosis of many types of cancer [71]. The ctDNA contained in peripheral blood is a double-stranded circular DNA fragment that carries tumor-specific sequence mutations and is present in the cell-free portion of the blood. ctDNA plays an important role in the early diagnosis of many cancers, but ctDNA in the blood accounts for only 1% of cell-free DNA. Chen et al. constructed a 3D GR (three-dimensional structural of graphene)/AuPtPd nanoflower biosensor based on entropy-driven strand displacement reaction (ESDR) and CRISPR/CAS [72]. No significant change in peak current was observed when incubating the CP/3D GR/AuPtPd/GCE complex or the trigger strand with the substrate probe. However, upon addition of targets cleaved by the Cas9/sgRNA system, the current signal was significantly reduced. ESDR and subsequent nucleic acid hybridization with CP/3D GR/AuPtPd nanoflowers resulted in the electrode surfaces being modified, which induced signal changes (Figure 4D). The highly branched and flower-like structure of 3D GR/AuPtPd exhibits improved catalytic activity. The system successfully detected mutant epidermal growth factor receptor (EGFR) ctDNA for the first time with high specificity and sensitivity.
With advancements in electrochemical sensing technologies and clinical diagnostics, electrochemical biosensors are evolving toward miniaturized, user-friendly, and cost-effective configurations. Qiu et al. designed a portable wireless USB-type analyser biosensor for the detection of small extracellular vesicle-derived microRNAs (sEV-miRNAs) using paper-based electrodes [73]. The portable biosensor is used to detect miRNAs with a LOD as low as 34.6 aM. The signal generated by the sensor is able to increase proportionally to the NSCLC stage (Figure 4E). Their proposed electrochemical biosensor holds the promise of monitoring different tumor biomarkers in POC biosensing through a simple, accurate, low-cost and less time-demanding approach.
In summary, by combining advanced signal amplification technology and nanomaterials, electrochemical biosensors are able to achieve the detection of biomarkers at very low concentrations, providing a powerful tool for the detection of nucleic acid markers. In the future, with further technological development, electrochemical biosensors will play an even more important role in personalized medicine and large-scale diseases screening.
4.3. Small Metabolites
Disruptions in metabolic pathways often lead to abnormal levels of small molecule metabolites, which are recognized disease biomarkers [74]. Measuring these metabolites enables disease diagnosis, prognosis evaluation, and treatment monitoring. Among the many methods, electrochemical biosensors are one of the most popular due to their non-invasive diagnostic and monitoring capabilities with high specificity and selectivity [75].
For example, Parkinson’s disease (PD) is a neurodegenerative disease [76]. Oxidative stress is a key mechanism in the development of PD [77]. Glutathione (GSH), an important antioxidant, is significantly reduced in the brains of Parkinson’s patients, making cells more vulnerable to oxidative damage [78]. Compared to normal individuals, Parkinson’s patients have lower levels of GSH in brain tissue, lymphocytes, and also blood. Therefore, the quantification of serum GSH levels is valuable for early diagnosis, prevention, and treatment of PD. Dong et al. developed a highly selective molecular probe for the detection of GSH levels in a serum PD model using a dual-mode sensor concept (single probe with a dual-signal electrochemical ratio strategy and an ‘on’ fluorescence method) [79]. This dual-mode assay provides a sensitive and accurate tool for GSH detection, with potential applications in the early prevention of PD and related neurodegenerative diseases (Figure 4F).
Glucose levels can be used as a biomarker for early diagnosis of diabetes. Over the past few decades, electrochemical blood glucose sensors have seen significant improvements in performance in terms of sample volume, speed, miniaturization, accuracy and precision, mass production, and connectivity to smartphones [80]. However, accurate and stable non-invasive glucose monitoring devices remain a great challenge. Recent advancements in wearable sensor technology and non-invasive diagnostics have positioned sweat analysis as a viable approach for glucose monitoring [81]. Li et al. designed a direct electrochemical glucose sensor based on nitrogen-doped carbon nanocages (NCNCs) [82]. The sensor enables efficient communication between glucose oxidase (GOx) and the electrode without the need for an electron mediator via direct electron transfer (DET) technology, with a sensitivity of 13.7 μA(mmolL)−1 cm−2, a response time of only 5 s, and the ability to detect sweat as with as low as 0.05 mmol/L glucose concentration. While conventional blood glucose monitoring relies on fingertip blood collection, which is prone to infection and psychological burden, the sensor enables painless detection through sweat analysis and can be integrated into wearable devices to support continuous monitoring. It helps to promote the screening and management of pre-diabetes. Clinical studies have demonstrated that systematic implementation of these advanced biosensors enables not only early-stage diabetes diagnosis through dynamic glucose profiling but also facilitates preventive interventions that reduce diabetes-related mortality. Furthermore, real-time blood glucose tracking with effective supervision can significantly reduce the risk of life-threatening complications. However, for glucose sweat detection, sweat collection is a challenge for developing efficient wearable glucose biosensors. To solve this problem, Li et al. fabricated two microfluidic patches with reservoirs (Figure 4G). The collectors can collect sweat directly from the skin for glucose testing. They integrated Pt/MXene nanomaterials, conductive hydrogels, and microfluidic patches onto the flexible sensor to develop a wearable flexible glucose sensor [83].
Based on the demand for multidimensional information resolution for disease state assessment, the development of integrated biosensors with multi-target parallel detection capability is of great value. Zhang’s team developed a spiral core-sheath fully integrated electrochemical biosensing fiber, which integrates six groups of independent sensing units within a miniaturized chip area of 45 mm2 through the strategy of functionalized modification of microelectrode arrays [84]. The platform utilizes a 1 μL sweat sample to synchronize pH dynamic monitoring, Na⁺, K⁺, urea, lactate, and glucose metabolite detection. The integrated wireless transmission module can synchronize the multimodal data to the mobile terminal application interface in real time, forming a closed-loop system for continuous monitoring of sweat biomarkers.
Overall, electrochemical biosensors demonstrate the technological advantages of high sensitivity, real-time dynamic monitoring, and minimally/non-invasive integration in small metabolites detection, which significantly enhances the efficacy of early diagnosis of disease through the fusion of nano-material interface engineering and intelligent algorithms.
5. Trends and Challenges for the Future
In summary, as an emerging technology in the field of personalized medicine, the future development of electrochemical biosensors will focus on the development of point-of-care (PoC) systems, focusing on the construction of a new generation of detection platforms with mobile convenience, rapid detection, and reliable results. To achieve this goal, next-generation electrochemical biosensors need to meet the following core criteria: (1) ultra-high sensitivity detection; (2) micro-sample requirements; (3) excellent reproducibility; and (4) well-integrated PoC functionality. Moreover, self-powered electrochemical biosensors (SPES) are gaining interest. Compared to conventional electrochemical sensors, SPES do not require a power supply and modulation system, thus saving energy and cost. These devices also offer greater simplicity and are therefore more suitable for applications in wearable sensor devices as well as in vivo and in situ use [85]. In addition, electrochemical biosensor development should focus on translation from laboratory to clinical applications. There are challenges to achieving this goal: lack of sensitivity and specificity in complex media, poor reproducibility, biocompatibility issues, cumbersome sample handling requirements, and clear regulatory guidelines that are lacking.
At the level of technological innovation, it is first necessary to break through the existing detection limits through the development of new electrode materials. The application of advanced materials, such as nanomaterials, can significantly improve the sensitivity and selectivity of the sensor while enhancing its stability in complex biological matrices. Secondly, micro–nano manufacturing technology is a key breakthrough in the realization of PoC devices. Microfluidic chip technology, by integrating sample pre-treatment, and the separation and detection modules into micron-sized channels, not only significantly reduces the size of the device, but also realizes the intelligent operation of “Lab-on-a-chip”. It is noteworthy that the integration of modern information technology injects new momentum into the development of biosensors. Smartphones can be converted into portable electrochemical analyzers via integrated custom adapters and screen-printed electrodes, coupled with Bluetooth/WiFi modules for cloud-based data sharing and remote diagnostic capabilities.
The synergistic integration of transformative technological paradigms—particularly the Internet of Medical Things (IoMT) for real-time data telemetry and Artificial Intelligence (AI)-enabled predictive analytics—is revolutionizing early disease diagnostics by empowering electrochemical biosensors with intelligent decision-making capabilities and cloud-connected autonomous operation. By coupling these biosensors with IoMT, a network of connected medical devices and applications, real-time data transmission and monitoring becomes possible. Artificial intelligence algorithms can analyze large amounts of complex data from sensors and detect patterns, trends and correlations that may not be perceived by human observation through machine learning-trained neural networks. The ability of AI to process large datasets quickly and accurately allows for the detection of subtle and rapid changes in biomarker levels, which are critical in early detection and enable timely intervention in disease. Additionally, the combination of IoT and AI facilitates remote and point-of-care monitoring, allowing hospitals to access real-time data on patients wherever they are. In summary, the synergy of IoT and AI with electrochemical biosensors has the potential to transform cancer diagnosis by enabling real-time monitoring, early detection, personalized medicine, and data-driven decision support. This convergence represents a paradigm shift in cancer care, enhancing the diagnostic capabilities of healthcare professionals and ultimately contributing to improved patient outcomes and quality of life.
Overall, in the future, the development of electrochemical biosensors will trend toward portable, miniaturized, real-time monitoring, and other PoC applications.
Author Contributions
Conceptualization, J.L.; methodology, J.L.; software, J.L.; validation, Y.C.; formal analysis, Y.C.; investigation, J.L. and Y.C.; resources, J.L. and Y.C.; data curation, J.L. and Y.C.; writing—original draft preparation, J.L.; writing—review and editing, Y.C., H.J. and X.L.; visualization, Y.C.; supervision, X.W.; project administration, H.J. and X.L.; funding acquisition, X.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (82027806, 92461308, 82372220, 82061148012).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dashtian, K.; Hajat, S.; Karimi, R.; Keyhan, M. Near-Infrared-Responsive Photoelectrochemical Biosensors. Trends Anal. Chem. 2024, 179, 117890. [Google Scholar] [CrossRef]
- Kny, E.; Hasler, R.; Luczak, W.; Knoll, W.; Szunerits, S.; Kleber, C. State of the Art and Future Research Directions of Materials Science Applied to Electrochemical Biosensor Developments. Anal. Bioanal. Chem. 2024, 416, 2247–2259. [Google Scholar] [CrossRef] [PubMed]
- Heydari-Bafrooei, E.; Ensafi, A.A. Nanomaterials-Based Biosensing Strategies for Biomarkers Diagnosis, a Review. Biosens. Bioelectron. X 2023, 13, 100245. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, H.; Liu, X.; Wang, X. Engineered Electrochemiluminescence Biosensors for Monitoring Heavy Metal Ions: Current Status and Prospects. Biosensors 2024, 14, 9. [Google Scholar] [CrossRef]
- Mehrvar, M.; Abdi, M. Recent Developments, Characteristics, and Potential Applications of Electrochemical Biosensors. Anal. Sci. 2004, 20, 1113–1126. [Google Scholar] [CrossRef]
- Grieshaber, D.; MacKenzie, R.; Vörös, J.; Reimhult, E. Electrochemical Biosensors—Sensor Principles and Architectures. Sensors 2008, 8, 1400–1458. [Google Scholar] [CrossRef]
- Sankar, K.; Kuzmanović, U.; Schaus, S.E.; Galagan, J.E.; Grinstaff, M.W. Strategy, Design, and Fabrication of Electrochemical Biosensors: A Tutorial. ACS Sens. 2024, 9, 2254–2274. [Google Scholar] [CrossRef]
- Suhito, I.R.; Koo, K.M.; Kim, T.H. Recent Advances in Electrochemical Sensors for the Detection of Biomolecules and Whole Cells. Biomedicines 2021, 9, 15. [Google Scholar] [CrossRef]
- Ghormade, P.S.; Kumar, N.B.; Tingne, C.V.; Keoliya, A.N. Distribution & Diagnostic Efficacy of Cardiac Markers Ck-Mb & Ldh in Pericardial Fluid for Postmortem Diagnosis of Ischemic Heart Disease. J. Forensic Leg. Med. 2014, 28, 42–46. [Google Scholar]
- Sarkar, S.; Hazra, S.; Patra, S.; Gogoi, M. Biosensors for Cancer Detection: A Review. TrAC Trends Anal. Chem. 2024, 180, 117978. [Google Scholar] [CrossRef]
- Chen, G.-Y.; Chai, T.-Q.; Zhang, H.; Yang, F.-Q. Applications of Mild-Condition Synthesized Metal Complexes with Enzyme-Like Activity in the Colorimetric and Fluorescence Analysis. Coord. Chem. Rev. 2024, 508, 215761. [Google Scholar] [CrossRef]
- Wu, D.; Tang, D. Recent Advances on Portable Photoelectrochemical Biosensors for Diagnostics. Electroanalysis 2023, 12, 35. [Google Scholar] [CrossRef]
- Barhoum, A.; Altintas, Z.; Devi, K.S.S.; Forster, R.J. Electrochemiluminescence Biosensors for Detection of Cancer Biomarkers in Biofluids: Principles, Opportunities, and Challenges. Nanotoday 2023, 50, 101874. [Google Scholar] [CrossRef]
- Zou, A.; Zhu, X.; Fu, R.; Wang, Z.; Wang, Y.; Ruan, Z.; Xianyu, Y.; Zhang, J. Harnessing Nanomaterials for Next-Generation DNA Methylation Biosensors. Small 2025, 21, 2408246. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Neppolian, B.; Das, J. Electrochemical Fabrication of Aupt Nanoalloy Embedded Nitrogen-Doped Reduced Graphene Oxide (Aupt@N-Ergo) Based Combinatorial Dual-Analyte Sensor. J. Alloys Compd. 2024, 1004, 175712. [Google Scholar] [CrossRef]
- Boonkaew, S.; Szot-Karpińska, K.; Niedziółka-Jönsson, J.; de Marco, A.; Jönsson-Niedziółka, M. Nfc Smartphone-Based Electrochemical Microfluidic Device Integrated with Nanobody Recognition for C-Reactive Protein. ACS Sens. 2024, 9, 3066–3074. [Google Scholar] [CrossRef]
- Zhang, Z.; Ji, H.; Xu, Y.; Zhuang, X.; Zeng, C.; Liu, J.; Cui, F.; Ding, W.; Zhu, S. Multivalent Acetylated-Sialic Acid as Recognition Elements for the Electrochemical Sensing of Viral Antigens. Biosens. Bioelectron. 2025, 268, 116883. [Google Scholar] [CrossRef]
- Irina, S.; Muratova, L.A.; Kartsova; Konstantin, N.M. Voltammetric Vs. Potentiometric Sensing of Dopamine Advantages and Disadvantages, Novel Cell Designs, Fundamental Limitations and Promising Options. Sens. Actuators B Chem. 2015, 207, 900–906. [Google Scholar]
- Durka, M.; Durka, K.; Adamczyk-Woźniak, A.; Wróblewski, W. Dopamine/2-Phenylethylamine Sensitivity of Ion-Selective Electrodes Based on Bifunctional-Symmetrical Boron Receptors. Sensors 2019, 19, 283. [Google Scholar] [CrossRef]
- Reddy, Y.V.M.; Shin, J.H.; Palakollu, V.N.; Sravani, B.; Choi, C.-H.; Park, K.; Kim, S.-K.; Madhavi, G.; Park, J.P.; Shetti, N.P. Strategies, Advances, and Challenges Associated with the Use of Graphene-Based Nanocomposites for Electrochemical Biosensors. Adv. Colloid Interface Sci. 2022, 304, 102664. [Google Scholar] [CrossRef]
- Mohan, V.B.; Lau, K.-T.; Hui, D.; Bhattacharyya, D. Graphene-Based Materials and Their Composites: A Review on Production, Applications and Product Limitations. Compos. Part B Eng. 2018, 143, 200–220. [Google Scholar] [CrossRef]
- Ali, M.A.; Hu, C.; Yuan, B.; Jahan, S.; Saleh, S.M.; Guo, Z.; Gellman, A.J.; Panat, R. Breaking the Barrier to Biomolecule Limit-of-Detection Via 3d Printed Multi-Length-Scale Graphene-Coated Electrodes. Nat. Commun. 2021, 12, 7077. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.-W.; Park, J.-H.; Kang, M.-J.; Lee, J.-H.; Kim, Y.K.; Kim, T.-H.; Luo, Z.T. Electrochemical Detection of Dopamine Release from Living Neurons Using Graphene Oxide-Incorporated Polypyrrole/Gold Nanocluster Hybrid Nanopattern Arrays. Small 2023, 19, 2304271. [Google Scholar] [CrossRef] [PubMed]
- Lei, P.; Zhou, Y.; Li, B.; Liu, Y.; Dong, C.; Shuang, S. Gold/Palladium–Polypyrrole/Graphene Nanocomposites for Simultaneous Electrochemical Detection of DNA Bases. ACS Appl. Nano Mater. 2022, 5, 1635–1643. [Google Scholar] [CrossRef]
- Kumar, R.S.; Govindan, K.; Ramakrishnan, S.; Kim, J.S.; Kim, A.R.; Yoo, D.J. Fe3O4 Nanorods Decorated on Polypyrrole/Reduced Graphene Oxide for Electrochemical Detection of Dopamine and Photocatalytic Degradation of Acetaminophen. Appl. Surf. Sci. 2021, 556, 149765. [Google Scholar] [CrossRef]
- Junior, D.W.; Deroco, P.B.; Kubota, L.T. A Copper-Based Metal-Organic Framework/Reduced Graphene Oxide-Modified Electrode for Electrochemical Detection of Paraquat. Microchim. Acta 2022, 189, 278. [Google Scholar] [CrossRef]
- Qu, G.; Zhang, Y.; Zhou, J.; Tang, H.; Ji, W.; Yan, Z.; Pan, K.; Ning, P. Simultaneous Electrochemical Detection of Dimethyl Bisphenol a and Bisphenol a Using a Novel Pt@Swcnts-Mxene-Rgo Modified Screen-Printed Sensor. Chemosphere 2023, 337, 139315. [Google Scholar] [CrossRef]
- Gu, S.; Hsieh, C.-T.; Mallick, B.C.; Fu, C.-C.; Juang, R.-S.; Gandomi, Y.A.; Kihm, K.D. Non-Enzymatic Electrochemical Detection of Hydrogen Peroxide on Highly Amidized Graphene Quantum Dot Electrodes. Appl. Surf. Sci. 2020, 528, 146936. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, Y.; Shen, J.; Qi, W.; Wang, H. Design of Organic/Inorganic Nanocomposites for Ultrasensitive Electrochemical Detection of a Cancer Biomarker Protein. Talanta 2020, 212, 120794. [Google Scholar] [CrossRef]
- da Conceição, E.; Buffon, E.; Beluomini, M.A.; Falone, M.F.; de Andrade, F.B.; Contiero, J.; Stradiotto, N.R. Electrochemical Detection of Poly(3-Hydroxybutyrate) Production from Burkholderia Glumae Ma13 Using a Molecularly Imprinted Polymer-Reduced Graphene Oxide Modified Electrode. Microchim. Acta 2024, 191, 492. [Google Scholar] [CrossRef]
- Li, Y.; Luo, L.; Kong, Y.; George, S.; Li, Y.; Guo, X.; Li, X.; Yeatman, E.; Davenport, A.; Li, Y.; et al. A Point-of-Care Sensing Platform for Multiplexed Detection of Chronic Kidney Disease Biomarkers Using Molecularly Imprinted Polymers. Adv. Funct. Mater. 2024, 34, 2316865. [Google Scholar] [CrossRef]
- Wang, X.; Fan, X.; Zhu, W.; Li, M.; Xue, J.; Ye, F.; Cheng, L. Structure and Electromagnetic Properties of Ti3c2tx Mxene Derived from Ti3alc2 with Different Microstructures. Ceram. Int. 2021, 47, 13628–13634. [Google Scholar] [CrossRef]
- Bu, F.; Moustafa, M.Z.; Ibrahim, Y.; Elzatahry, A.; Ma, B.; Zhao, D. Porous Mxenes Synthesis, Structures, and Applications. Nanotoday 2020, 30, 100803. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, P.; Hu, Z.; Liang, Y.; Han, H.; Yang, M.; Luo, X.; Hou, C.; Huo, D. Amino-Functionalized Multilayer Ti3c2tx Enabled Electrochemical Sensor for Simultaneous Determination of Cd2+ and Pb2+ in Food Samples. Food Chem. 2023, 402, 134269. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, P.; Liang, Y.; Ma, Y.; Liu, Y.; Zhao, J.; Hou, J.; Hou, C.; Huo, D. A Sensitive Electrochemical Sensor Based on 3d Porous Melamine-Doped Rgo/Mxene Composite Aerogel for the Detection of Heavy Metal Ions in the Environment. Talanta 2023, 256, 124294. [Google Scholar] [CrossRef]
- Hadian, M.; Rabbani, M.; Shariati, L.; Ghasemi, F.; John, F.P.; Sanati, A. Mxene Nanoconfinement of Sam-Modified Molecularly Imprinted Electrochemical Biosensor for Point-of-Care Monitoring of Carcinoembryonic Antigen. ACS Sens. 2025, 10, 857–867. [Google Scholar] [CrossRef]
- Ayalew, H.; Assen, O.Y.; Shekhah, O.; Eddaoudi, M.; Khaled, N.S. Mofs for the Sensitive Detection of Ammonia: Deployment of Fcu-Mof Thin Films as Effective Chemical Capacitive Sensors. ACS Sens. 2017, 2, 1294–1301. [Google Scholar]
- Dong, J.; Wen, L.; Yang, H.; Zhao, J.; He, C.; Hu, Z.; Peng, L.; Hou, C.; Huo, D. Catalytic Hairpin Assembly-Driven Ratiometric Dual-Signal Electrochemical Biosensor for Ultrasensitive Detection of Microrna Based on the Ratios of Fe-Mofs and Mb-Ga-Uio-66-Nh2. Anal. Chem. 2022, 94, 5846–5855. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, H.; Shi, L.; Zheng, W.; Jing, X. Electroactive Cu2O Nanoparticles and Ag Nanoparticles Driven Ratiometric Electrochemical Aptasensor for Prostate Specific Antigen Detection. Sens. Actuators B Chem. 2020, 315, 128155. [Google Scholar] [CrossRef]
- Wang, X.-Z.; Chen, Y.; Cao, X.-M.; Li, R.-Y.; Chen, W.-Y.; Li, Y.; Guo, D.-S. Ligand-Insertion Strategy for Constructing 2d Conjugated Metal–Organic Framework with Large Pore Size for Electrochemical Analytics. Angew. Chem. Int. Ed. 2025, 64, e202413115. [Google Scholar] [CrossRef]
- Daniel, M.; Mathew, G.; Anpo, M.; Neppolian, B. Mof Based Electrochemical Sensors for the Detection of Physiologically Relevant Biomolecules: An Overview. Coord. Chem. Rev. 2022, 468, 214627. [Google Scholar] [CrossRef]
- Ma, Y.; Zhou, Y.; Guo, Z.; Wang, Z.; Peng, Q.; Huang, J.; Qing, T.; Li, N.; Ruan, J.; Su, H. A Mof-Embedded/G4-Packaged Electrochemical Labeling Strategy-Based Biosensor for the Simultaneous Detection of Β-Thalassemia Mutations. Sens. Actuators B Chem. 2025, 432, 137443. [Google Scholar] [CrossRef]
- Huang, L.; Zhou, Y.; Hu, X.; Yang, Z. Emerging Combination of Hydrogel and Electrochemical Biosensors. Small 2024, 21, 2409711. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Lu, W.; Zhu, Y.; Jin, H.; Yao, Y.; Zhang, H.; Zhao, Y. Porous Hydrogel-Encapsulated Photonic Barcodes for Multiplex Detection of Cardiovascular Biomarkers. ACS Sens. 2019, 4, 1384–1390. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.-Y.; Han, L.; Cai, H.-Q.; Zhang, K.; Sun, Z.-C.; Liu, R.-P.; Wang, T.-H.; Pan, F.-H.; Man, W.-T.; Wang, D.; et al. Conductive Hydrogel-Based Neural Interfaces: From Fabrication Methods, Properties, to Applications. Small Struct. 2025, 6, 2400696. [Google Scholar] [CrossRef]
- Hu, X.-B.; Qin, Y.; Fan, W.-T.; Liu, Y.-L.; Huang, W.-H. A Three-Dimensional Electrochemical Biosensor Integrated with Hydrogel Enables Real-Time Monitoring of Cells under Their in Vivo-Like Microenvironment. Anal. Chem. 2021, 93, 7917–7924. [Google Scholar] [CrossRef]
- Kim, S.G.; Ryplida, B.; Jo, H.J.; Lee, G.; Park, S.Y. Stimuli-Responsive Conductive Hydrogel Touch Sensor for Electrochemical and Fluorescence Monitoring of Acetylcholinesterase Activity and Inhibition. Chem. Eng. J. 2023, 452, 139028. [Google Scholar] [CrossRef]
- Geng, F.; Li, Y.; Wu, Q.; Ding, C. An Efficient Electrochemical Biosensor Based on Double-Conductive Hydrogel as Antifouling Interface for Ultrasensitive Analysis of Biomarkers in Complex Serum Medium. Sens. Actuators B Chem. 2025, 422, 136625. [Google Scholar] [CrossRef]
- Jayanthi, V.S.A.; Das, A.B.; Saxena, U. Recent Advances in Biosensor Development for the Detection of Cancer Biomarkers. Biosens. Bioelectron. 2017, 91, 15–23. [Google Scholar] [CrossRef]
- Thal, D.R.; Walter, J.; Takaomi, S.C.; Fändrich, M. Neuropathology and Biochemistry of Aβ and Its Aggregates in Alzheimer’s Disease. Acta Neuropathol. 2014, 129, 167–182. [Google Scholar] [CrossRef]
- Da, Y.; Luo, S.; Tian, Y. Real-Time Monitoring of Neurotransmitters in the Brain of Living Animals. ACS Appl. Mater. Interfaces 2022, 15, 138–157. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ding, X.; Zhang, D. Challenges and Strategies Faced in the Electrochemical Biosensing Analysis of Neurochemicals in Vivo: A Review. Talanta 2024, 266, 124933. [Google Scholar] [CrossRef] [PubMed]
- Mikuła, E. Recent Advancements in Electrochemical Biosensors for Alzheimer’s Disease Biomarkers Detection. Curr. Med. Chem. 2021, 28, 4049–4073. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yuan, X.; Liu, T.; Zhang, W.; Dong, H.; Chu, Z. Freestanding Nanofiber-Assembled Aptasensor for Precisely and Ultrafast Electrochemical Detection of Alzheimer’s Disease Biomarkers. Adv. Healthc. Mater. 2024, 13, 2304355. [Google Scholar] [CrossRef]
- Lakshmanakumar, M.; Nesakumar, N.; Sethuraman, S.; Krishnan, U.M.; Rajan, K.S.; Rayappan, J.B.B. Functionalized Graphene Quantum Dot Interfaced Electrochemical Detection of Cardiac Troponin I: An Antibody Free Approach. Sci. Rep. 2019, 9, 17348. [Google Scholar] [CrossRef]
- Li, P.-R.; Boilla, S.K.; Wang, C.-H.; Lin, P.-C.; Kuo, C.-N.; Tsai, T.-H.; Lee, G.-B. A Self-Driven, Microfluidic, Integrated-Circuit Biosensing Chip for Detecting Four Cardiovascular Disease Biomarkers. Biosens. Bioelectron. X 2024, 249, 115931. [Google Scholar] [CrossRef]
- Qin, X.; Li, D.; Qin, X.; Chen, F.; Guo, H.; Gui, Y.; Zhao, J.; Jiang, L.; Luo, D. Electrochemical Detection of the Cardiac Biomarker Cardiac Troponin, I. View 2024, 5, 20240025. [Google Scholar] [CrossRef]
- Nandeshwar, R.; Tallur, S. Electrochemical Detection of Myeloperoxidase (Mpo) in Blood Plasma with Surface-Modified Electroless Nickel Immersion Gold (Enig) Printed Circuit Board (Pcb) Electrodes. Biosens. Bioelectron. X 2024, 246, 115891. [Google Scholar] [CrossRef]
- Sun, B.; Li, G.; Wu, Y.; Gai, J.; Zhu, M.; Ji, W.; Wang, X.; Zhang, F.; Li, W.; Hu, J.; et al. Ce-Mof@Au-Based Electrochemical Immunosensor for Apolipoprotein A1 Detection Using Nanobody Technology. ACS Appl. Mater. Interfaces 2024, 16, 58405–58416. [Google Scholar] [CrossRef]
- Nolan, E.; Lindeman, G.J.; Visvader, J.E. Deciphering Breast Cancer: From Biology to the Clinic. Cell 2023, 186, 1708–1728. [Google Scholar] [CrossRef]
- Sha, L.; Cao, Y.; Wang, L.; Qin, Y.; Zhu, S.; Li, G.; Zhao, J. An Electrochemical Biosensor Based on Mild Reduction-Activated Crispr/Cas12a System for Sensitive Detection of Circulating Tumor Cells. Biosens. Bioelectron. 2024, 262, 116550. [Google Scholar] [CrossRef] [PubMed]
- Gilboa, T.; Garden, P.M.; Cohen, L. Single-Molecule Analysis of Nucleic Acid Biomarkers—A Review. Anal. Chim. Acta 2020, 1115, 61–85. [Google Scholar] [CrossRef] [PubMed]
- Michałowska, A.; Kudelski, A. Applications of Surface Enhanced Raman Scattering (Sers) Spectroscopy for Detection of Nucleic Acids. Nanophotonics 2024, 13, 4577–4603. [Google Scholar] [CrossRef]
- Ghorbani, F.; Abbaszadeh, H.; Dolatabadi, J.E.N.; Aghebati-Maleki, L.; Yousefi, M. Application of Various Optical and Electrochemical Aptasensors for Detection of Human Prostate Specific Antigen: A Review. Biosens. Bioelectron. X 2019, 142, 111484. [Google Scholar] [CrossRef]
- Liu, C.-S.; Li, J.; Pang, H. Metal-Organic Framework-Based Materials as an Emerging Platform for Advanced Electrochemical Sensing. Coord. Chem. Rev. 2020, 410, 213222. [Google Scholar] [CrossRef]
- Bagheri, A.K.; Aghaie, A.; Vahedi, E.; Qazvini, A.; Ghanei, M.; Afkhami, A.; Hasan, A.H. Electrochemical Biosensors for the Detection of Lung Cancer Biomarkers: A Review. Talanta 2020, 206, 120251. [Google Scholar]
- Zhang, S.; Rong, F.; Guo, C.; Duan, F.; He, L.; Wang, M.; Zhang, Z.; Kang, M.; Du, M. Metal–Organic Frameworks (Mofs) Based Electrochemical Biosensors for Early Cancer Diagnosis in Vitro. Coord. Chem. Rev. 2021, 439, 213948. [Google Scholar] [CrossRef]
- Gu, C.; Guo, C.; Li, W.Z.; Zhou, N.; He, L.; Zhang, Z.; Du, M. Bimetallic Zrhf-Based Metal-Organic Framework Embedded with Carbon Dots: Ultra-Sensitive Platform for Early Diagnosis of Her2 and Her2-Overexpressed Living Cancer Cells. Biosens. Bioelectron. X 2019, 134, 8–15. [Google Scholar] [CrossRef]
- Pan, D.; Lin, Y.; Liu, X.; Xin, Y.; Tian, Q.; Zhang, J. Ultrasensitive and Preprocessing-Free Electrochemical Biosensing Platform for the Detection of Cancer-Derived Exosomes Based on Spiky-Shaped Aptamer-Magnetic Beads. Biosens. Bioelectron. X 2022, 217, 114705. [Google Scholar] [CrossRef]
- Yan, R.; Lu, N.; Han, S.; Lu, Z.; Xiao, Y.; Zhao, Z.; Zhang, M. Simultaneous Detection of Dual Biomarkers Using Hierarchical Mos2 Nanostructuring and Nano-Signal Amplification-Based Electrochemical Aptasensor toward Accurate Diagnosis of Prostate Cancer. Biosens. Bioelectron. X 2022, 197, 113797. [Google Scholar] [CrossRef]
- Markou, A.; Tzanikou, E.; Lianidou, E. The Potential of Liquid Biopsy in the Management of Cancer Patients. Semin. Cancer Biol. 2022, 84, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wu, D.; Tu, S.; Yang, C.; Chen, D.; Xu, Y. Crispr/Cas9 Cleavage Triggered Esdr for Circulating Tumor DNA Detection Based on a 3d Graphene/Auptpd Nanoflower Biosensor. Biosens. Bioelectron. X 2021, 173, 112821. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Yang, H.; Shen, M.; Xu, H.; Wang, Y.; Liu, S.; Liu, Q.; Sun, M.; Ding, Z.; Zhang, L.; et al. Multiarmed DNA Jumper and Metal-Organic Frameworks–Functionalized Paper-Based Bioplatform for Small Extracellular Vesicle–Derived Mirnas Assay. J. Nanobiotechnol. 2024, 22, 274. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Cai, Y.; Yao, H.; Lin, C.; Xie, Y.; Tang, S.; Zhang, A. Small Molecule Metabolites Discovery of Biomarkers and Therapeutic. Signal Transduct. Target. Ther. 2023, 8, 132. [Google Scholar]
- Wang, D.; Zhao, H.-M.; Guo, L.; Zhang, L.; Zhao, H.-B.; Fang, X.; Li, S.; Wang, G. Facile Synthesis of Cuo–Co3o4 Prickly-Sphere-Like Composite for Non-Enzymatic Glucose Sensors. Rare Met. 2022, 41, 1911–1920. [Google Scholar] [CrossRef]
- Nicholas, H.S.; Johnson, A.J.; Ahmed, J.; Baysah, C.Z.; Ryan, A.D.; Tyler, D.B.; Alexandra, G.T.; Tessa, C.F.; Courtney, M.; Donnelly, S.K. Protein Mimetic 2d Fast Rescues Alpha Synuclein Aggregation Mediated Early and Post Disease Parkinson’s Phenotypes. Nat. Commun. 2024, 15, 3658. [Google Scholar]
- Zarkali, A.; George, E.C.; Thomas, H.Z.; Rimona, S.W. Neuroimaging and Fluid Biomarkers in Parkinson’s Disease in an Era of Targeted Interventions. Nat. Commun. 2024, 15, 5661. [Google Scholar] [CrossRef]
- Chen, X.D.; Yong, C.; Yang, X.; Chen, Y.S.; Peng, L.H. Signaling Pathways in Parkinson’s Disease: Molecular Mechanisms and Therapeutic Interventions. Signal Transduct. Target. Ther. 2023, 8, 73. [Google Scholar] [CrossRef]
- Dong, H.; Chen, W.; Xu, K.; Zheng, L.; Wei, B.; Liu, R.; Yang, J.; Wang, T.; Zhou, Y.; Zhang, Y.; et al. High Selectivity Fluorescence and Electrochemical Dual-Mode Detection of Glutathione in the Serum of Parkinson’s Disease Model Mice and Humans. Anal. Chem. 2025, 97, 1041–1466. [Google Scholar] [CrossRef]
- Yu, T.; Meng, X.; Hao, X.; Dong, Z.; Wang, Y.; Sun, S.; Cheng, P. Ysz-Based Mixed-Potential Acetone Sensor with Labaco2o5+Δ Sensitive Electrode for Diabetic Diagnosis. Sens. Actuators B Chem. 2024, 418, 136273. [Google Scholar] [CrossRef]
- Kudo, H.; Sawada, T.; Kazawa, E.; Yoshida, H.; Iwasaki, Y.; Mitsubayashi, K. A Flexible and Wearable Glucose Sensor Based on Functional Polymers with Soft-Mems Techniques. Biosens. Bioelectron. X 2006, 22, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhao, Y.; Pan, L.; Xu, J.; Shi, Y. A Direct Electrochemical Biosensor for Rapid Glucose Detection Based on Nitrogen-Doped Carbon Nanocages. Rare Met. 2024, 43, 2184–2192. [Google Scholar] [CrossRef]
- Li, Q.-F.; Chen, X.; Wang, H.; Liu, M.; Peng, H.-L. Pt/Mxene-Based Flexible Wearable Non-Enzymatic Electrochemical Sensor for Continuous Glucose Detection in Sweat. ACS Appl. Mater. Interfaces 2023, 15, 12611–13824. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Zhou, L.; Liang, Q.; Wang, X.; Hu, X.; Jia, K.; Chu, H.; Luo, Y.; Qiu, L.; Peng, H.; et al. All-in-One Multifunctional and Stretchable Electrochemical Fiber Enables Health-Monitoring Textile with Trace Sweat. Sci. China Mater. 2024, 67, 251–260. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Offenhäusser, A.; Mourzina, Y. Hydrogen Peroxide Fuel Cells and Self-Powered Electrochemical Sensors Based on the Principle of a Fuel Cell with Biomimetic and Nanozyme Catalysts. Biosensors 2025, 15, 124. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).