Construction of 2D TiO2@MoS2 Heterojunction Nanosheets for Efficient Toluene Gas Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Sensor Materials
2.2. Gas-Sensing Performance Measurement
3. Results and Discussion
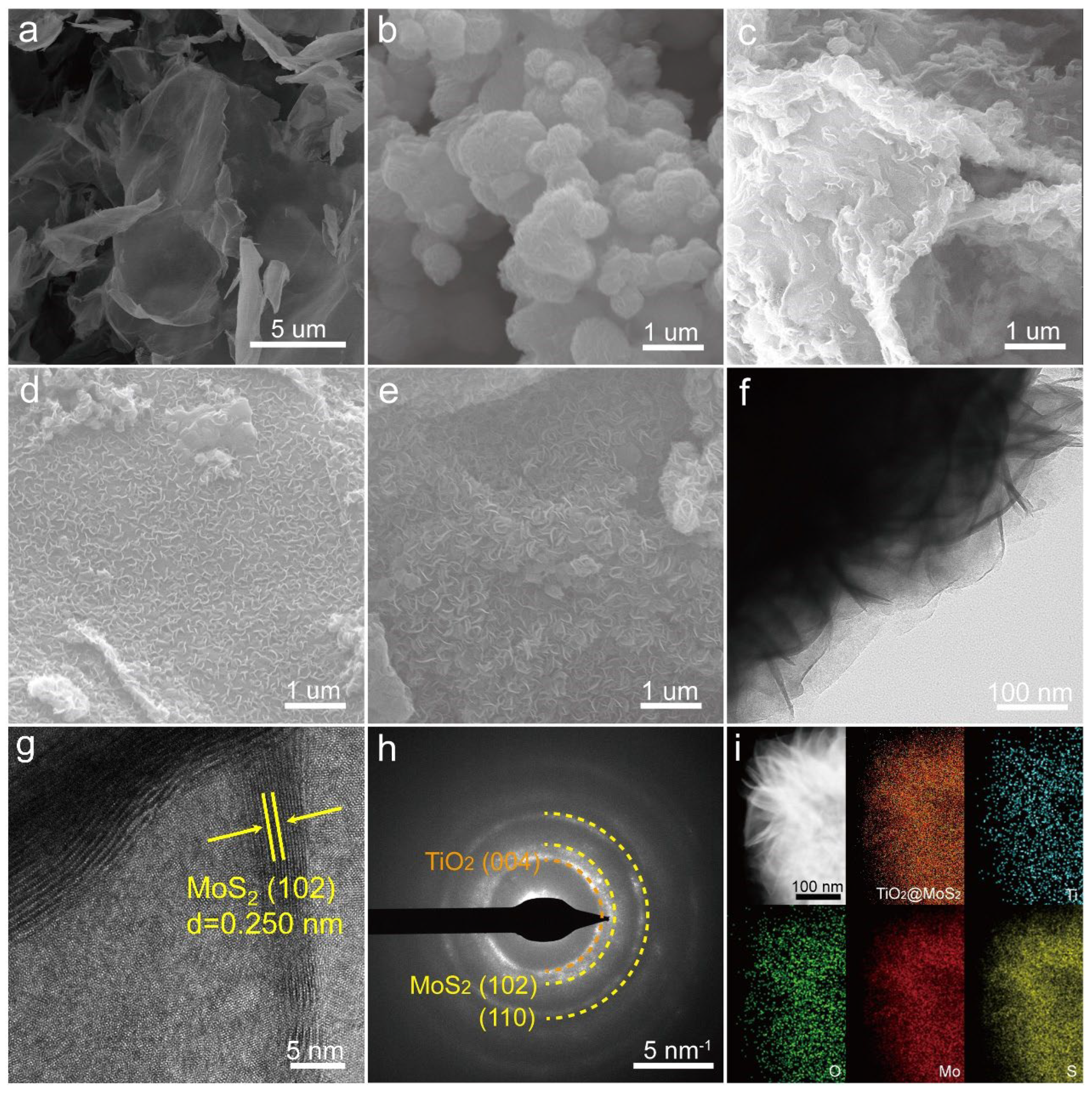
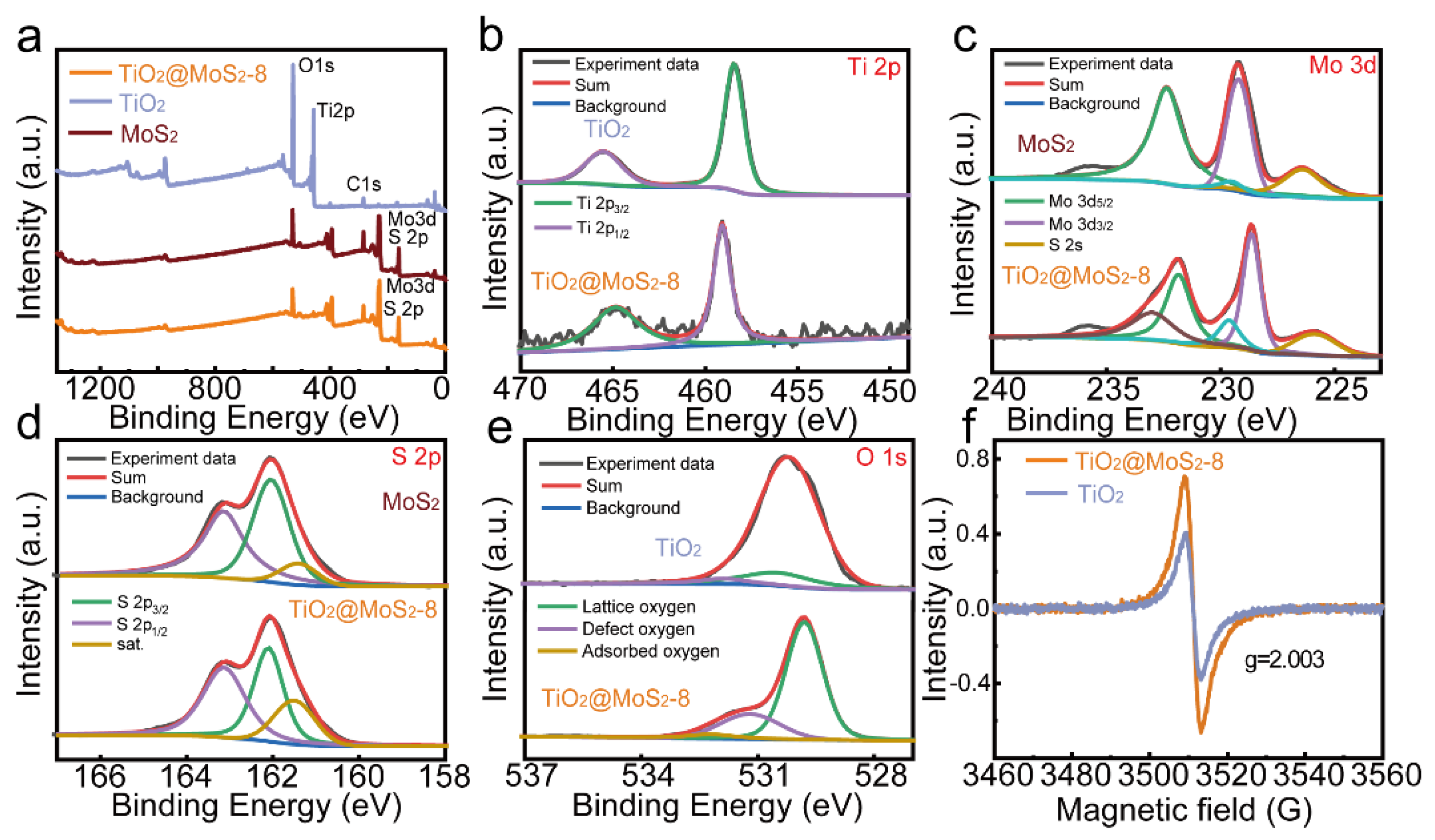
3.1. Morphology and Structure Characterization
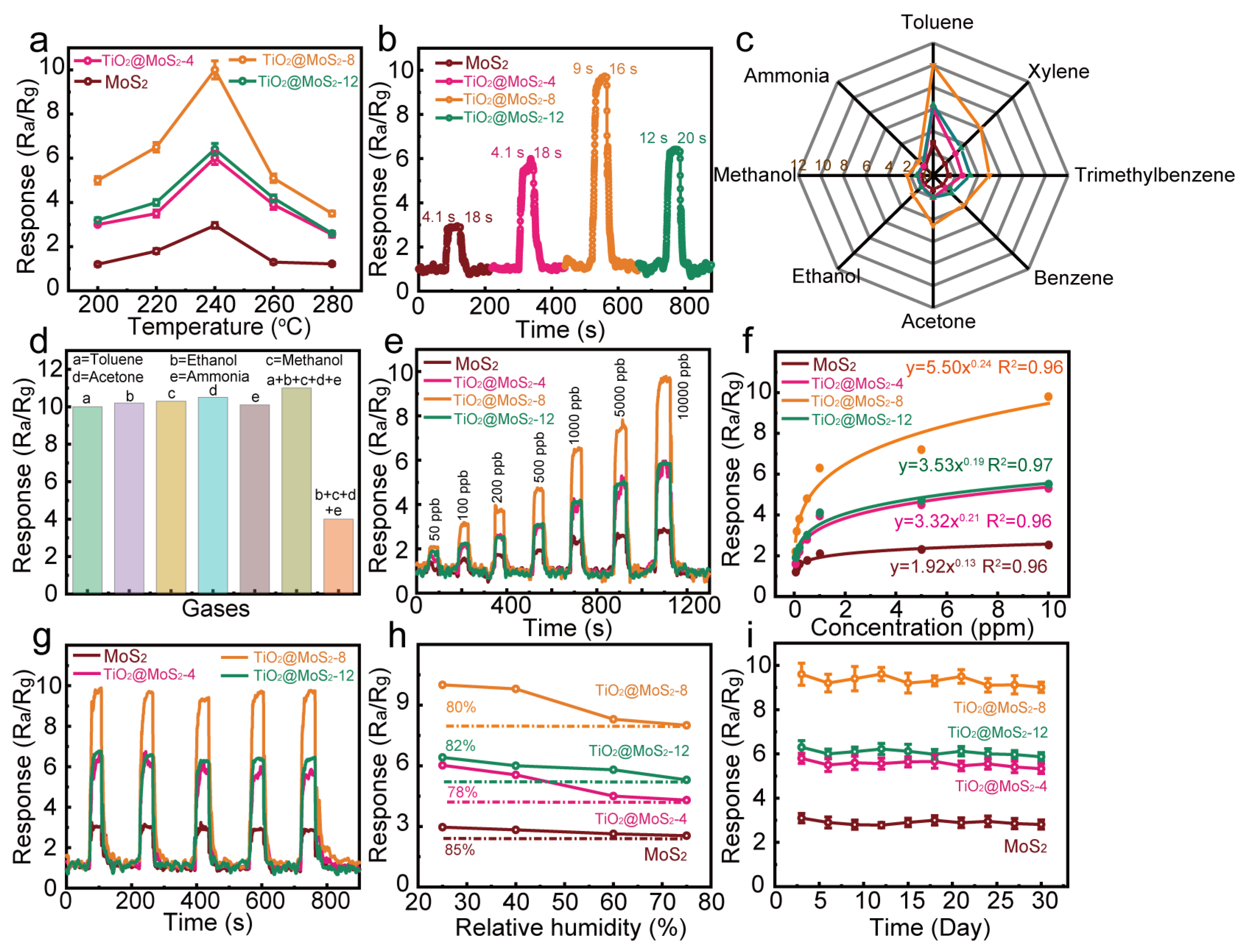
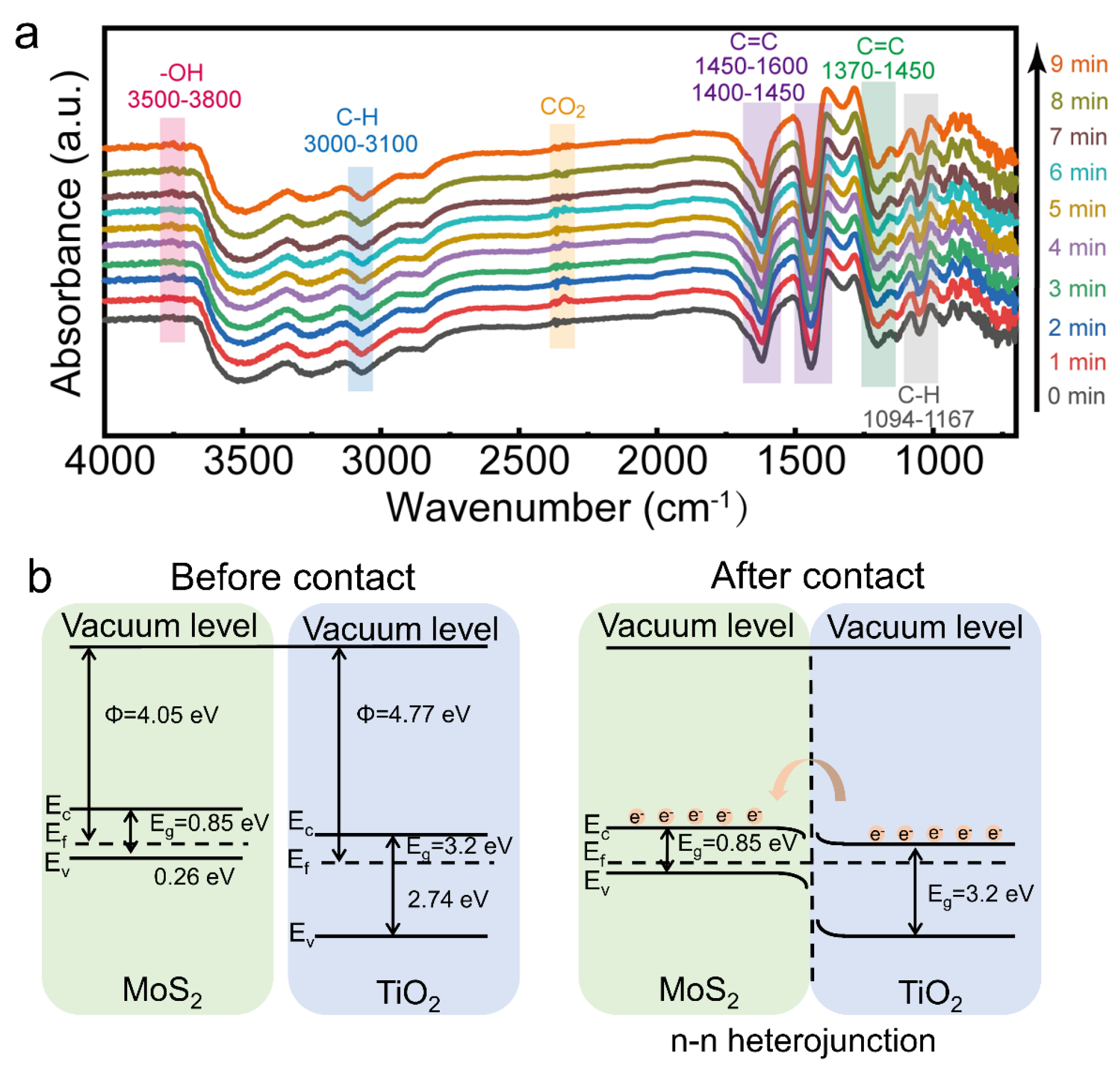
3.2. Gas-Sensitive Property
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ueda, T.; Hayashi, H.; Tsukahara, R.; Shimizu, Y.; Hyodo, T. Effects of Structure and Thickness of Ce0.9Pr0.1O2 Electrodes of YSZ-Based Gas Sensors on VOC-Sensing Properties. Sens. Actuators B Chem. 2025, 422, 136580. [Google Scholar] [CrossRef]
- Zhang, Y.-L.; Jia, C.-W.; Tian, R.-N.; Guan, H.-T.; Chen, G.; Dong, C.-J. Hierarchical Flower-like NiFe2O4 with Core–Shell Structure for Excellent Toluene Detection. Rare Met. 2021, 40, 1578–1587. [Google Scholar] [CrossRef]
- Wang, T.; Liu, S.; Sun, P.; Wang, Y.; Shimanoe, K.; Lu, G. Unexpected and Enhanced Electrostatic Adsorption Capacity of Oxygen Vacancy-Rich Cobalt-Doped In2O3 for High-Sensitive MEMS Toluene Sensor. Sens. Actuators B Chem. 2021, 342, 129949. [Google Scholar] [CrossRef]
- Liao, Z.; Chu, N.; Yuan, Z.; Shen, Y.; Meng, F. Highly Sensitive Toluene Sensor Based on Silver Ear-Like F-Doped Co3O4 Templated from Co(OH)F Guided by SiO2 Nanospheres. Chem. Eng. J. 2024, 498, 155688. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, J.; Li, M.; Li, Z.; Yuan, Y.; Yang, X.; Guo, L. Highly Efficient Toluene Gas Sensor Based on Spinel Structured Hollow Urchin-like Core-Shell ZnFe2O4 Spheres. Sens. Actuators B Chem. 2021, 349, 130734. [Google Scholar] [CrossRef]
- Siraj, S.; Bansal, G.; Hasita, B.; Srungaram, S.; S, S.K.; Rybicki, F.J.; Sonkusale, S.; Sahatiya, P. MXene/MoS2 Piezotronic Acetone Gas Sensor for Management of Diabetes. ACS Appl. Nano Mater. 2024, 7, 11350–11361. [Google Scholar] [CrossRef]
- Thayil, R.; Krishna, K.G.; Cherukulappurath, S.; Kathirvelu, V.; Parne, S.R. MoS2 and MoS2-Based Nanocomposites for Enhanced Toluene Sensing Response at Room Temperature. Surf. Interfaces 2024, 46, 104134. [Google Scholar] [CrossRef]
- Tang, T.; Li, Z.; Liu, Y.Y.; Chen, Y.L.; Cheng, Y.F.; Liang, Y.; Zhuang, J.H.; Hu, X.Y.; Jannat, A.; Ou, R.; et al. Ultrathin two-dimensional titanium oxysulfide for enhanced sensitivity and stability of room temperature NO2 sensing. Ceram. Int. 2025, 51, 3216–3223. [Google Scholar] [CrossRef]
- Guan, X.; Zhao, L.; Zhang, P.; Liu, J.; Song, X.; Gao, L. Electrode Material of Core-Shell Hybrid MoS2@C/CNTs with Carbon Intercalated Few-Layer MoS2 Nanosheets. Mater. Today Energy 2020, 16, 100379. [Google Scholar] [CrossRef]
- Gough, J.J.; McEvoy, N.; O’ Brien, M.; Bell, A.P.; McCloskey, D.; Boland, J.B.; Coleman, J.N.; Duesberg, G.S.; Bradley, A.L. Dependence of Photocurrent Enhancements in Quantum Dot (QD)-Sensitized MoS2 Devices on MoS2 Film Properties. Adv. Funct. Mater. 2018, 28, 1706149. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Pei, X.; Yuan, Z.; Zhang, Y.; Zhao, Z.; Hao, H.; Long, R.; Liu, N. Stretchable MoS2 Artificial Photoreceptors for E-Skin. Adv. Funct. Mater. 2022, 32, 2107524. [Google Scholar] [CrossRef]
- Chen, Y.L.; Li, Z.; Tang, T.; Cheng, Y.F.; Cheng, L.; Wang, X.X.; Haidry, A.A.; Jannat, A.; Ou, J.Z. Room-Temperature Optoelectronic NO2 Sensing Using Two-Dimensional Gallium Oxyselenides. ACS Appl. Nano Mater. 2024, 7, 3229–3238. [Google Scholar] [CrossRef]
- Wang, H.; Shao, Z.; Shi, X.; Tang, Z.; Sun, B. Rapidly Detecting the Carcinogen Acetaldehyde: Preparation and Application of a Flower-like MoS2 Cataluminescence Sensor at Low Working Temperature. Anal. Methods 2023, 15, 5620–5629. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Zeng, J.; Liu, X.; Li, J.; Wang, Q.; Li, H.; de Rooij, N.F.; Wang, Y.; Zhou, G. A Photovoltaic Self-Powered Gas Sensor Based on All-Dry Transferred MoS2/GaSe Heterojunction for ppb-Level NO2 Sensing at Room Temperature. Adv. Sci. 2021, 8, 2100472. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Yao, Y.T.; Zheng, S.L.; Wan, Y.; Wei, C.D.; Yang, G.C.; Yuan, Y.; Tsai, H.-S.; Wang, Y.; Hao, J.Y. Te@Se Core–Shell Heterostructures with Tunable Shell Thickness for Ultra-Stable NO2 Detection. ACS Sens. 2025, 10, 283–291. [Google Scholar] [CrossRef]
- Shi, J.; Xiong, J.; Qiao, L.; Liu, C.; Zeng, Y. Facile MOF-on-MOF Isomeric Strategy for ZnO@Co3O4 Single-Shelled Hollow Cubes with High Toluene Detection Capability. Appl. Surf. Sci. 2023, 609, 155271. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Z.; Cao, G.; Pan, G.; Yang, X.; Qiu, M.; Sun, C.; Shao, J.; Li, Z.; Zhang, H. Construction of Hollow NiO/ZnO p-n Heterostructure for Ultrahigh Performance Toluene Gas Sensor. Mater. Sci. Semicond. Process. 2022, 141, 106435. [Google Scholar] [CrossRef]
- Ma, S.; Xu, J. Nanostructured Metal Oxide Heterojunctions for Chemiresistive Gas Sensors. J. Mater. Chem. A 2023, 11, 23742–23771. [Google Scholar] [CrossRef]
- Wang, Y.; Tan, L.; Tan, M.H.; Zhang, P.P.; Yoshiharu, Y.; Yang, G.H.; Tsubaki, N. Rationally Designing Bifunctional Catalysts as an Efficient Strategy to Boost CO2 Hydrogenation Producing Value-Added Aromatics. ACS Catal. 2018, 9, 895–901. [Google Scholar] [CrossRef]
- Hermawan, A.; Asakura, Y.; Inada, M.; Yin, S. A Facile Method for Preparation of Uniformly Decorated-Spherical SnO2 by CuO Nanoparticles for Highly Responsive Toluene Detection at High Temperature. J. Mater. Sci. Technol. 2020, 51, 119–129. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Z.; Sun, C.; Shao, J.; Li, Z.; Zhang, H.; Qiu, M.; Pan, G.; Yang, X. Construction of Co3O4/Fe3O4 Heterojunctions from Metal Organic Framework Derivatives for High Performance Toluene Sensor. Sens. Actuators B Chem. 2023, 375, 132863. [Google Scholar] [CrossRef]
- Raghu, A.V.; Karuppanan, K.K.; Nampoothiri, J.; Pullithadathil, B. Wearable, Flexible Ethanol Gas Sensor Based on TiO2 Nanoparticles-Grafted 2D-Titanium Carbide Nanosheets. ACS Appl. Nano Mater. 2019, 2, 1152–1163. [Google Scholar] [CrossRef]
- Cao, S.; Sui, N.; Zhang, P.; Zhou, T.; Tu, J.; Zhang, T. TiO2 Nanostructures with Different Crystal Phases for Sensitive Acetone Gas Sensors. J. Colloid Interf. Sci. 2022, 607, 357–366. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, J.-G.; Ryu, G.H.; Ko, K.Y.; Woo, W.J.; Kim, Y.; Kim, D.; Lim, J.H.; Lee, S.; Lee, Z.; et al. Low-Temperature Synthesis of 2D MoS2 on a Plastic Substrate for a Flexible Gas Sensor. Nanoscale 2018, 10, 9338–9345. [Google Scholar] [CrossRef]
- Kumar, R.; Zheng, W.; Liu, X.; Zhang, J.; Kumar, M. MoS2-Based Nanomaterials for Room-Temperature Gas Sensors. Adv. Mater. Technol. 2020, 5, 1901062. [Google Scholar] [CrossRef]
- Verma, G.; Gokarna, A.; Kadiri, H.; Nomenyo, K.; Lerondel, G.; Gupta, A. Multiplexed Gas Sensor: Fabrication Strategies, Recent Progress, and Challenges. ACS Sens. 2023, 8, 3320–3337. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Cho, B.K. Metal Oxide Composites in Conductometric Gas Sensors: Achievements and Challenges. Sens. Actuators B Chem. 2017, 244, 182–210. [Google Scholar] [CrossRef]
- Gong, C.; Chen, M.; Song, F.; Yin, P.; Zhao, X.; You, X.; Fu, H.; Yu, S.; Liu, X.; Zhang, K.; et al. A Highly Sensitive Toluene Gas Sensor Based on Pd/PdO Decorated SnO2 Prepared by Electrospinning. ACS Appl. Electron. Mater. 2024, 6, 6036–6048. [Google Scholar] [CrossRef]
- Karmakar, S.; Deepak, M.S.; Nanda, O.P.; Sett, A.; Maity, P.C.; Karmakar, G.; Sha, R.; Badhulika, S.; Bhattacharyya, T.K. 2D V2 C MXene Based Flexible Gas Sensor for Highly Selective and Sensitive Toluene Detection at Room Temperature. ACS Appl. Electron. Mater. 2024, 6, 3717–3725. [Google Scholar] [CrossRef]
- Gao, H.; Zhao, L.; Wang, L.; Sun, P.; Lu, H.; Liu, F.; Chuai, X.; Lu, G. Ultrasensitive and Low Detection Limit of Toluene Gas Sensor Based on SnO2-Decorated NiO Nanostructure. Sens. Actuators B Chem. 2018, 255, 3505–3515. [Google Scholar] [CrossRef]
- Jung, G.; Shin, W.; Hong, S.; Jeong, Y.; Park, J.; Kim, D.; Bae, J.-H.; Park, B.-G.; Lee, J.-H. Comparison of the Characteristics of Semiconductor Gas Sensors with Different Transducers Fabricated on the Same Substrate. Sens. Actuators B Chem. 2021, 335, 129661. [Google Scholar] [CrossRef]
- Hu, J.W.; Wang, F.; Yu, J.J.; Hong, Z.J.; Zhang, W.H.; Li, H.-J.; Lai, Z.C.; Wang, D.; Deng, Y.H.; Li, G.S. MOF-derived porous Co3O4 nanosheets array assembled on SnO2 nanofibers for humidity-resistant high efficiency acetone detection. Chin. Chem. Lett. 2025, 110863. [Google Scholar] [CrossRef]
- Lashkov, A.V.; Fedorov, F.S.; Vasilkov, M.Y.; Kochetkov, A.V.; Belyaev, I.V.; Plugin, I.A.; Varezhnikov, A.S.; Filipenko, A.N.; Romanov, S.A.; Nasibulin, A.G.; et al. The Ti Wire Functionalized with Inherent TiO2 Nanotubes by Anodization as One-Electrode Gas Sensor: A Proof-of-Concept Study. Sens. Actuators B Chem. 2020, 306, 127615. [Google Scholar] [CrossRef]
- Shokri, A.; Salami, N. Gas Sensor Based on MoS2 Monolayer. Sens. Actuators B Chem. 2016, 236, 378–385. [Google Scholar] [CrossRef]
- Hou, J.-L.; Lin, Y.-T.; Hsueh, T.-J. Room-Temperature TiO2 Gas Sensor with Conical-TSV Using a Thermal Oxidation Process. ACS Appl. Electron. Mater. 2023, 5, 6542–6548. [Google Scholar] [CrossRef]
- Liu, H.; Shen, W.; Chen, X. A Room Temperature Operated Ammonia Gas Sensor Based on Ag-Decorated TiO2 Quantum Dot Clusters. RSC Adv. 2019, 9, 24519–24526. [Google Scholar] [CrossRef]
- Hu, J.W.; Zou, Y.D.; Deng, Y.; Li, H.-J.; Xu, H.; Wang, D.; Wu, L.M.; Deng, Y.H.; Li, G.S. Recent Advances in Non-Ionic Surfactant Templated Synthesis of Porous Metal Oxide Semiconductors for Gas Sensing Applications. Prog. Mater. Sci. 2025, 150, 101409. [Google Scholar] [CrossRef]
- Hou, L.; Duan, J.; Xiong, F.; Carraro, C.; Shi, T.; Maboudian, R.; Long, H. Low Power Gas Sensors: From Structure to Application. ACS Sens. 2024, 9, 6327–6357. [Google Scholar] [CrossRef]
- Doan, T.H.P.; Ta, Q.T.H.; Sreedhar, A.; Hang, N.T.; Yang, W.; Noh, J.-S. Highly Deformable Fabric Gas Sensors Integrating Multidimensional Functional Nanostructures. ACS Sens. 2020, 5, 2255–2262. [Google Scholar] [CrossRef]


| Materials | T (°C) | Concentration (ppm) | Detection Limit (ppm) | Tres/Trec (s) | Ref. |
|---|---|---|---|---|---|
| Pd/PdO-decorated SnO2 | 285 | 100 | 0.05 | 50/138 | [28] |
| V2C MXene | 25 | 200 | 0.047 | 14/34 | [29] |
| SnO2-decorated NiO | 100 | 250 | 0.01 | - | [30] |
| MoS2-Fe3O4 | 25 | 20 | 5 | 58/23 | [7] |
| ZnO@Co3O4 | 290 | 100 | 5 | 11.2/12.5 | [16] |
| NiO/ZnO | 100 | 300 | 0.1 | 2/33 | [17] |
| TiO2@MoS2 | 240 | 10 | 0.05 | 9/16 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Hu, J.; Xu, H.; Wang, D.; Li, G. Construction of 2D TiO2@MoS2 Heterojunction Nanosheets for Efficient Toluene Gas Detection. Chemosensors 2025, 13, 154. https://doi.org/10.3390/chemosensors13050154
Wang D, Hu J, Xu H, Wang D, Li G. Construction of 2D TiO2@MoS2 Heterojunction Nanosheets for Efficient Toluene Gas Detection. Chemosensors. 2025; 13(5):154. https://doi.org/10.3390/chemosensors13050154
Chicago/Turabian StyleWang, Dehui, Jinwu Hu, Hui Xu, Ding Wang, and Guisheng Li. 2025. "Construction of 2D TiO2@MoS2 Heterojunction Nanosheets for Efficient Toluene Gas Detection" Chemosensors 13, no. 5: 154. https://doi.org/10.3390/chemosensors13050154
APA StyleWang, D., Hu, J., Xu, H., Wang, D., & Li, G. (2025). Construction of 2D TiO2@MoS2 Heterojunction Nanosheets for Efficient Toluene Gas Detection. Chemosensors, 13(5), 154. https://doi.org/10.3390/chemosensors13050154





