Photodynamic Therapy (PDT) in Prosthodontics: Disinfection of Human Teeth Exposed to Streptococcus mutans and the Effect on the Adhesion of Full Ceramic Veneers, Crowns, and Inlays: An In Vitro Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Prosthodontic Preparation
2.2. Bacterial Contamination
2.3. Photodynamic Therapy Protocol
2.4. Fabrication and Cementation of Prosthodontic Restorations (Veneers, Inlays, and Crowns)
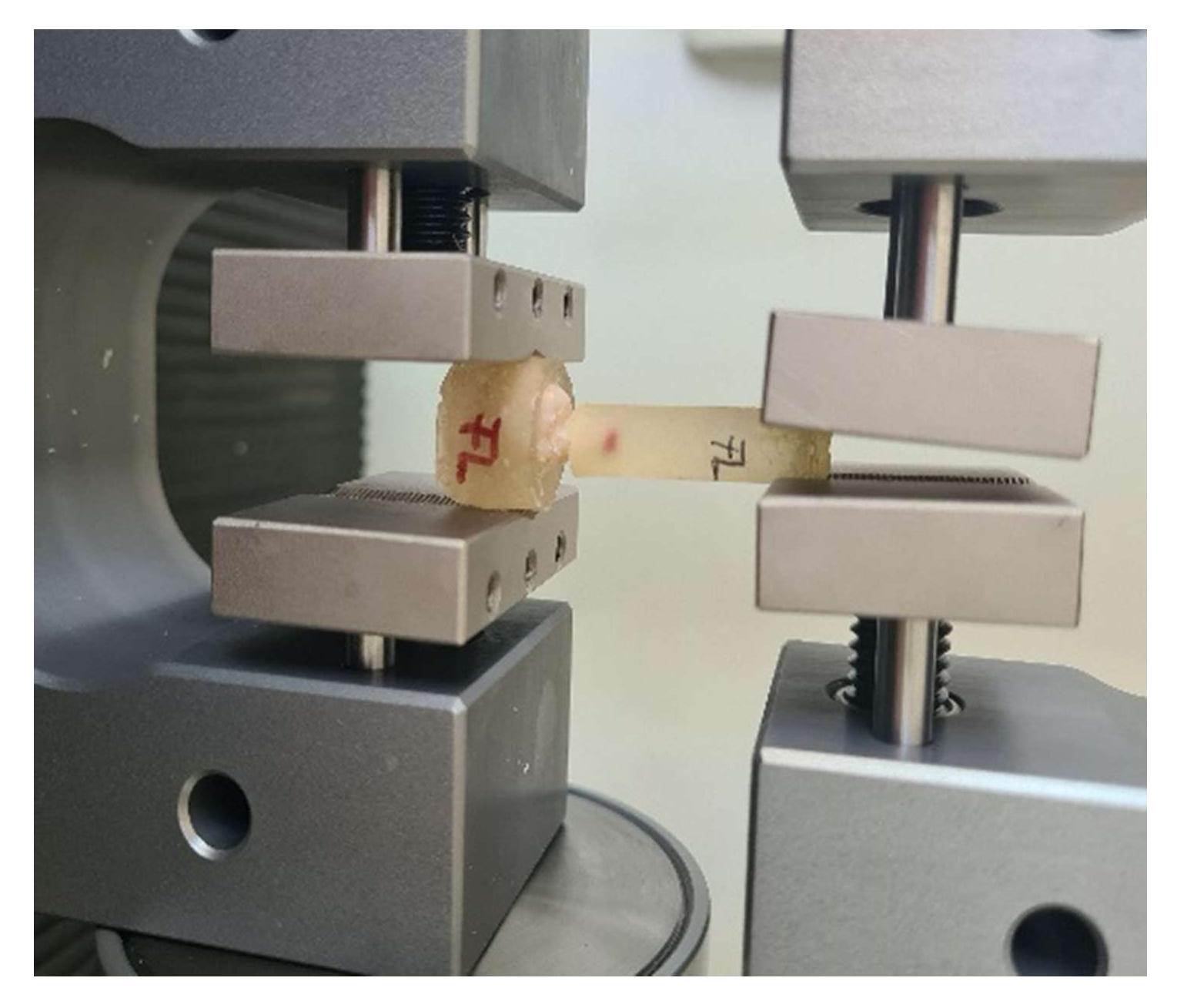
2.5. Pull-Out Test
3. Results
3.1. Effectiveness of Disinfection through Photodynamic Therapy
3.2. Appearance of Tooth–Prosthesis Interface after Adhesive Cementation
3.3. Adhesion Pull-Out Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stájer, A.; Kajári, S.; Gajdács, M.; Musah-Eroje, A.; Baráth, Z. Utility of photodynamic therapy in dentistry: Current concepts. Dent. J. 2020, 8, 43. [Google Scholar] [CrossRef] [PubMed]
- Merigo, E.; Conti, S.; Ciociola, T.; Manfredi, M.; Vescovi, P.; Fornaini, C. Antimicrobial Photodynamic Therapy Protocols on Streptococcus mutans with Different Combinations of Wavelengths and Photosensitizing Dyes. Bioengineering 2019, 6, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishiyama, K.; Nakamura, K.; Ikai, H.; Kanno, T.; Kohno, M.; Sasaki, K.; Niwano, Y. Bactericidal action of photogenerated singlet oxygen from photosensitizers used in plaque disclosing agents. PLoS ONE 2012, 7, e37871. [Google Scholar] [CrossRef]
- Nemezio, M.A.; de Souza Farias, S.S.; Borsatto, M.C.; Aires, C.P.; Corona, S.A.M. Effect of methylene blue-induced photodynamic therapy on a Streptococcus mutans biofilm model. Photodiagnosis Photodyn. Ther. 2017, 20, 234–237. [Google Scholar] [CrossRef]
- Misba, L.; Zaidi, S.; Khan, A.U. Efficacy of photodynamic therapy against Streptococcus mutans biofilm: Role of singlet oxygen. J. Photochem. Photobiol. B. 2018, 183, 16–21. [Google Scholar] [CrossRef]
- Garcia, M.T.; Pereira, A.H.C.; Figueiredo-Godoi, L.M.A.; Jorge, A.O.C.; Strixino, J.F.; Junqueira, J.C. Photodynamic therapy mediated by chlorin-type photosensitizers against Streptococcus mutans biofilms. Photodiagnosis Photodyn. Ther. 2018, 24, 256–261. [Google Scholar] [CrossRef]
- Weber, K.; Delben, J.; Bromage, T.G.; Duarte, S. Comparison of SEM and VPSEM imaging techniques with respect to Streptococcus mutans biofilm topography. FEMS Microbiol. Lett. 2014, 350, 175–179. [Google Scholar] [CrossRef] [Green Version]
- Shuwaish, M.S.B. Impact of photodynamic therapy on the push out bond strength of fiber post to root dentin: A systematic review. Photodiagnosis Photodyn. Ther. 2020, 32, 102010. [Google Scholar] [CrossRef]
- Ramos, A.T.P.R.; Belizário, L.G.; Venção, A.C.; Jordão-Basso, K.C.F.; de Souza Rastelli, A.N.; de Andrade, M.F.; Kuga, M.C. Effects of photodynamic therapy on the adhesive interface of fiber posts cementation protocols. JOE 2018, 44, 173–178. [Google Scholar] [CrossRef] [Green Version]
- Ramos, A.T.P.R.; Belizário, L.G.; Jordão-Basso, K.C.F.; Shinohara, A.L.; Kuga, M.C. Effects of photodynamic therapy on the adhesive interface using two fiber posts cementation systems. Photodiagnosis Photodyn. Ther. 2018, 24, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Sahyon, H.B.S.; da Silva, P.P.; de Oliveira, M.S.; Cintra, L.T.A.; Gomes-Filho, J.E.; dos Santos, P.H.; Sivieri-Araujo, G. Effect of photodynamic therapy on the mechanical properties and bond strength of glass-fiber posts to endodontically treated intraradicular dentin. J. Prosthet. Dent. 2018, 120, 317.E1–317.E7. [Google Scholar] [CrossRef] [Green Version]
- Mirhashemi, A.; Hormozi, S.; Noroozian, M.; Chiniforush, N. The effect of antimicrobial photodynamic therapy on shear bond strength of orthodontic bracket: An in vitro study. Photodiagnosis Photodyn. Ther. 2021, 34, 102244. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.H.D.; Lima, E.D.; Miranda, R.B.D.P.; Favero, S.S.; Lohbauer, U.; Cesar, P.F. Dental ceramics: A review of new materials and processing methods. Braz. Oral Res. 2017, 31 (Suppl. S1), 133–146. [Google Scholar] [CrossRef]
- Warreth, A.; Elkareimi, Y. All-ceramic restorations: A review of the literature. Saudi. Dent. J. 2020, 32, 365–372. [Google Scholar] [CrossRef]
- Kara, O.; Ozturk, A.N. The effect of surface treatments on the bonding strength of ceramic inlays to dentin. J. Adhes. Sci. Tech. 2017, 31, 2490–2502. [Google Scholar] [CrossRef]
- Nada, H.E.; Ahmed, S.E.; Fayza, H.A. Shear bond strength of ceramic laminate veneers to enamel and enamel–dentine complex bonded with different adhesive luting systems. ADJ 2016, 41, 131–137. [Google Scholar] [CrossRef] [Green Version]
- Stawarczyk, B.; Basler, T.; Ender, A.; Roos, M.; Özcan, M.; Hämmerle, C. Effect of surface conditioning with airborne-particle abrasion on the tensile strength of polymeric CAD/CAM crowns luted with self-adhesive and conventional resin cements. J. Prosthet. Dent. 2012, 107, 94–101. [Google Scholar] [CrossRef] [Green Version]
- Carrera, E.T.; Dias, H.B.; Corbi, S.C.T.; Marcantonio, R.A.C.; Bernardi, A.C.A.; Bagnato, V.S.; Rastelli, A. The application of antimicrobial photodynamic therapy (aPDT) in dentistry: A critical review. Laser Phys. 2016, 26, 123001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsui, R.; Cvitkovitch, D. Acid tolerance mechanisms utilized by Streptococcus mutans. Future Microbiol. 2010, 5, 403–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, X.; Zou, Z.; Zou, Z.; Li, C.; Dong, X.; Yin, H.; Yan, G. Effect of antibacterial photodynamic therapy on Streptococcus mutans plaque biofilm in vitro. J. Innov. Opt. Health Sci. 2020, 13, 2050022. [Google Scholar] [CrossRef]
- Azizi, A.; Shohrati, P.; Goudarzi, M.; Lawaf, S.; Rahimi, A. Comparison of the effect of photodynamic therapy with curcumin and methylene Blue on streptococcus mutans bacterial colonies. Photodiagnosis Photodyn. Ther. 2019, 27, 203–209. [Google Scholar] [CrossRef]
- Asahi, Y.; Miura, J.; Tsuda, T.; Kuwabata, S.; Tsunashima, K.; Noiri, Y.; Hayashi, M. Simple observation of Streptococcus mutans biofilm by scanning electron microscopy using ionic liquids. AMB Express 2015, 5, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borges, C.C.; Palma-Dibb, R.G.; Rodrigues, F.C.C.; Plotegher, F.; Rossi-Fedele, G.; de Sousa-Neto, M.D.; Souza-Gabriel, A.E. The effect of diode and Er, Cr: YSGG lasers on the bond strength of fiber posts. Photobiomodul. Photomed. Laser Surg. 2020, 38, 66–74. [Google Scholar] [CrossRef]
- Gounder, R.; Gounder, S. Laser science and its applications in prosthetic rehabilitation. J. Lasers Med. Sci. 2016, 7, 209–213. [Google Scholar] [CrossRef] [Green Version]
- Ladiz, M.A.R.; Mirzaei, A.; Hendi, S.S.; Najafi-Vosough, R.; Hooshyarfard, A.; Gholami, L. Effect of photobiomodulation with 810 and 940 nm diode lasers on human gingival fibroblasts. Dent. Med. Probl. 2020, 57, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Sterczała, B.; Grzech-Leśniak, K.; Michel, O.; Trzeciakowski, W.; Dominiak, M.; Jurczyszyn, K. Assessment of human gingival fibroblast proliferation after laser stimulation in vitro using different laser types and wavelengths (1064, 980, 635, 450, and 405 nm)—preliminary report. J. Pers. Med. 2021, 11, 98. [Google Scholar] [CrossRef] [PubMed]
- Kubasiewicz-Ross, P.; Hadzik, J.; Gedrange, T.; Dominiak, M.; Jurczyszyn, K.; Pitułaj, A.; Fleischer, M. Antimicrobial Efficacy of Different Decontamination Methods as Tested on Dental Implants with Various Types of Surfaces. Med. Sci. Monit. 2020, 26, e920513. [Google Scholar] [CrossRef]
- Kubasiewicz-Ross, P.; Fleischer, M.; Pitułaj, A.; Hadzik, J.; Nawrot-Hadzik, I.; Bortkiewicz, O.; Jurczyszyn, K. Evaluation of the three methods of bacterial decontamination on implants with three different surfaces. Adv. Clin. Exp. Med. 2020, 29, 177–182. [Google Scholar] [CrossRef]
- Dhaliwal, J.S.; Abd Rahman, N.A.; Ming, L.C.; Dhaliwal, S.K.S.; Knights, J.; Albuquerque Junior, R.F. Microbial Biofilm Decontamination on Dental Implant Surfaces: A Mini Review. Front. Cell. Infect. Microbiol. 2021, 11, 736186. [Google Scholar] [CrossRef]
- Schneider, M.; Kirfel, G.; Berthold, M.; Frentzen, M.; Krause, F.; Braun, A. The impact of antimicrobial photodynamic therapy in an artificial biofilm model. Lasers Med. Sci. 2012, 27, 615–620. [Google Scholar] [CrossRef]
- Rolim, J.P.; De-Melo, M.A.; Guedes, S.F.; Albuquerque-Filho, F.B.; De Souza, J.R.; Nogueira, N.A.; Rodrigues, L.K. The antimicrobial activity of photodynamic therapy against Streptococcus mutans using different photosensitizers. J. Photochem. Photobiol. B. 2012, 106, 40–46. [Google Scholar] [CrossRef]
- Dascalu, L.M.; Moldovan, M.; Prodan, D.; Ciotlaus, I.; Popescu, V.; Baldea, I.; Carpa, R.; Sava, S.; Chifor, R.; Badea, M.E. Assessment and Characterization of Some New Photosensitizers for Antimicrobial Photodynamic Therapy (aPDT). Materials 2020, 13, 3012. [Google Scholar] [CrossRef] [PubMed]
- Salem, S.K.; Shalaby, M.M. Fracture strength and marginal gap of re-pressed IPS E.max PRESS crowns with different concentrations. E. D. J. 2019, 65, 1939–1948. [Google Scholar] [CrossRef] [Green Version]
- Mobilio, N.; Fasiol, A.; Mollica, F.; Catapano, S. Effect of different luting agents on the retention of lithium disilicate ceramic crowns. Materials 2015, 8, 1604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mester, A.; Moldovan, M.; Cuc, S.; Tomuleasa, C.; Pasca, S.; Filip, M.; Piciu, A.; Onisor, F. Characteristics of Dental Resin-Based Composites in Leukemia Saliva: An In Vitro Analysis. Biomedicines 2021, 9, 1618. [Google Scholar] [CrossRef]
- Chirca, O.; Biclesanu, C.; Florescu, A.; Burcea, A.; Motelica, L.; Holban, A. Comparative study on adhesion to the dental structure of the total ceramic crown with different adhesive cements. Rom. J. Mater. 2021, 51, 309–318. [Google Scholar]
- Bastos, N.A.; Bitencourt, S.B.; Carneiro, R.F.; Ferrairo, B.M.; Strelhow, S.S.F.; Dos Santos, D.M.; Bombonatti, J.F.S. Marginal and internal adaptation of lithium disilicate partial restorations: A systematic review and meta-analysis. J. Indian Prosthodont. Soc. 2020, 20, 338. [Google Scholar] [CrossRef] [PubMed]
- Riccitiello, F.; Amato, M.; Leone, R.; Spagnuolo, G.; Sorrentino, R. In vitro evaluation of the marginal fit and internal adaptation of zirconia and lithium disilicate single crowns: Micro-CT comparison between different manufacturing procedures. Open Dent. J. 2018, 12, 160. [Google Scholar] [CrossRef] [PubMed]
- Heintze, S.D. Crown pull-off test (crown retention test) to evaluate the bonding effectiveness of luting agents. Dent. Mater. 2010, 26, 193–206. [Google Scholar] [CrossRef]
- Johnson, G.H.; Lepe, X.; Patterson, A.; Schäfer, O. Simplified cementation of lithium disilicate crowns: Retention with various adhesive resin cement combinations. J. Prosthet. Dent. 2018, 119, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Streiff, K.R.; Lepe, X.; Johnson, G.H. Long-term retention of lithium disilicate crowns with a current bioactive cement. J. Esthet. Restor. Dent. 2021, 33, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Upadhyaya, V.; Arora, A.; Singhal, J.; Kapur, S.; Sehgal, M. Comparative analysis of shear bond strength of lithium disilicate samples cemented using different resin cement systems: An in vitro study. J. Indian Prosthodont. Soc. 2019, 19, 240. [Google Scholar] [CrossRef] [PubMed]
- Levartovsky, S.; Bohbot, H.; Shem-Tov, K.; Brosh, T.; Pilo, R. Effect of Different Surface Treatments of Lithium Disilicate on the Adhesive Properties of Resin Cements. Materials 2021, 14, 3302. [Google Scholar] [CrossRef] [PubMed]
- Alsaif, A.; Tahmassebi, J.F.; Wood, S.R. Treatment of dental plaque biofilms using photodynamic therapy: A randomised controlled study. Eur. Arch. Paediatr. Dent. 2021, 22, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Tahmassebi, J.; Drogkari, E.; Wood, S.R. A study of the control of oral plaque biofilms via antibacterial photodynamic therapy. Eur. Arch. Paediatr. Dent. 2015, 16, 433–440. [Google Scholar] [CrossRef]






| Type of Prosthesis | Load at Maximum Load (N) | Tensile Strength (MPa) | Load at Upper Yield (N) | |
|---|---|---|---|---|
| Crown | With PDT | 265.82 ± 34.6781 a | 84.6 ± 8.52369 c | 209 ± 42.7055 d |
| Without PDT | 257.22 ± 51.8807 a | 64.7 ± 12.7614 c | 188 ± 55.1160 d | |
| Veneer | With PDT | 248.43 ± 20.9848 a | 79.079 ± 7.64638 c | 248.43 ± 35.0042 d |
| Without PDT | 239.55 ± 35.7009 a | 80.571 ± 10.3334 c | 228.88 ± 24.2239 d | |
| Inlay | With PDT | 305.98 ± 41.05021 b | 97.396 ± 11.03891 c | 290.83 ± 28.9973 e |
| Without PDT | 302.11 ± 84.5941 b | 90.557 ± 15.2840 c | 290.05 ± 20.9471 e | |
| p value | 0.00316 | 0.2135 | 0.02715 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tisler, C.E.; Chifor, R.; Badea, M.E.; Moldovan, M.; Prodan, D.; Carpa, R.; Cuc, S.; Chifor, I.; Badea, A.F. Photodynamic Therapy (PDT) in Prosthodontics: Disinfection of Human Teeth Exposed to Streptococcus mutans and the Effect on the Adhesion of Full Ceramic Veneers, Crowns, and Inlays: An In Vitro Study. Biomedicines 2022, 10, 144. https://doi.org/10.3390/biomedicines10010144
Tisler CE, Chifor R, Badea ME, Moldovan M, Prodan D, Carpa R, Cuc S, Chifor I, Badea AF. Photodynamic Therapy (PDT) in Prosthodontics: Disinfection of Human Teeth Exposed to Streptococcus mutans and the Effect on the Adhesion of Full Ceramic Veneers, Crowns, and Inlays: An In Vitro Study. Biomedicines. 2022; 10(1):144. https://doi.org/10.3390/biomedicines10010144
Chicago/Turabian StyleTisler, Corina Elena, Radu Chifor, Mindra Eugenia Badea, Marioara Moldovan, Doina Prodan, Rahela Carpa, Stanca Cuc, Ioana Chifor, and Alexandru Florin Badea. 2022. "Photodynamic Therapy (PDT) in Prosthodontics: Disinfection of Human Teeth Exposed to Streptococcus mutans and the Effect on the Adhesion of Full Ceramic Veneers, Crowns, and Inlays: An In Vitro Study" Biomedicines 10, no. 1: 144. https://doi.org/10.3390/biomedicines10010144
APA StyleTisler, C. E., Chifor, R., Badea, M. E., Moldovan, M., Prodan, D., Carpa, R., Cuc, S., Chifor, I., & Badea, A. F. (2022). Photodynamic Therapy (PDT) in Prosthodontics: Disinfection of Human Teeth Exposed to Streptococcus mutans and the Effect on the Adhesion of Full Ceramic Veneers, Crowns, and Inlays: An In Vitro Study. Biomedicines, 10(1), 144. https://doi.org/10.3390/biomedicines10010144








