Materials for Dentoalveolar Bioprinting: Current State of the Art
Abstract
:1. Introduction
2. Bioink Requirements for Dentoalveolar Tissue Engineering
2.1. General Requirements for a Bioink Material
2.2. Requirements of Bioinks for Dental Pulp Regeneration
2.3. Requirements of Bioinks for Dentin
2.4. Requirements of Bioinks for Periodontal Ligament Bioprinting
2.5. Requirements of Bioinks for Alveolar Bone Bioprinting
3. Bioinks
3.1. Natural Polymers
3.1.1. Collagen-Based Materials
3.1.2. Materials Based on Hyaluronic Acid
3.1.3. Fibrin-Based Materials
3.1.4. Alginate-Based Materials
3.2. Synthetic Polymers
4. Discussion and Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Petersen, P.E. World Health Organization global policy for improvement of oral health—World Health Assembly 2007. Int. Dent. J. 2008, 58, 115–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Political Declaration of the High-Level Meeting of the General Assembly on the Prevention and Control of Non-Communicable Diseases: Draft Resolution. 2011. 13p. Available online: http://digitallibrary.un.org/record/710899 (accessed on 20 August 2020).
- Khodakaram-Tafti, A.; Mehrabani, D.; Shaterzadeh-Yazdi, H.; Zamiri, B.; Omidi, M. Tissue Engineering in Maxillary Bone Defects. World J. Plast. Surg. 2018, 7, 3–11. [Google Scholar] [PubMed]
- Shaddox, L.M.; Walker, C.B. Treating chronic periodontitis: Current status, challenges, and future directions. Clin. Cosmet. Investig. Dent. 2010, 2, 79–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kassebaum, N.J.; Bernabé, E.; Dahiya, M.; Bhandari, B.; Murray, C.J.L.; Marcenes, W. Global burden of severe periodontitis in 1990–2010: A Systematic review and meta-regression. J. Dent. Res. 2014, 93, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Al-Ani, A.H.; Antoun, J.S.; Thomson, W.M.; Merriman, T.R.; Farella, M. Hypodontia: An Update on Its Etiology, Classification, and Clinical Management. BioMed Res. Int. 2017, 2017, 9378325. [Google Scholar] [CrossRef]
- WHO/Europe Disease Prevention—Oral Health. Available online: https://www.euro.who.int/en/health-topics/disease-prevention/oral-health (accessed on 28 July 2020).
- Hanif, A.; Qureshi, S.; Sheikh, Z.; Rashid, H. Complications in implant dentistry. Eur. J. Dent. 2017, 11, 135–140. [Google Scholar] [CrossRef] [Green Version]
- Martin, K.; Nathwani, S.; Bunyan, R. Autotransplantation of teeth: An evidence-based approach. Br. Dent. J. 2018, 224, 861–864. [Google Scholar] [CrossRef]
- Denys, D.; Shahbazian, M.; Jacobs, R.; Laenen, A.; Wyatt, J.; Vinckier, F.; Willems, G. Importance of root development in autotransplantations: A retrospective study of 137 teeth with a follow-up period varying from 1 week to 14 years. Eur. J. Orthod. 2013, 35, 680–688. [Google Scholar] [CrossRef]
- Gupta, S.; Goel, M.; Sachdeva, G.; Sharma, B.; Malhotra, D. Autotransplantation. J. Conserv. Dent. 2015, 18, 500–503. [Google Scholar]
- Shahbazian, M.; Wyatt, J.; Willems, G.; Jacobs, R. Clinical application of a stereolithographic tooth replica and surgical guide in tooth autotransplantation. Virtual Phys. Prototyp. 2012, 7, 211–218. [Google Scholar] [CrossRef]
- Ezeldeen, M.; Wyatt, J.; Al-Rimawi, A.; Coucke, W.; Shaheen, E.; Lambrichts, I.; Willems, G.; Politis, C.; Jacobs, R. Use of CBCT Guidance for Tooth Autotransplantation in Children. J. Dent. Res. 2019, 98, 406–413. [Google Scholar] [CrossRef]
- Langer, R.; Vacanti, J. Advances in tissue engineering. J. Pediatr. Surg. 2016, 51, 8–12. [Google Scholar] [CrossRef] [Green Version]
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [Green Version]
- Na, S.; Zhang, H.; Huang, F.; Wang, W.; Ding, Y.; Li, D.; Jin, Y. Regeneration of dental pulp/dentine complex with a three-dimensional and scaffold-free stem-cell sheet-derived pellet. J. Tissue Eng. Regen. Med. 2013, 10, 261–270. [Google Scholar] [CrossRef]
- Kim, N.R.; Lee, D.H.; Chung, P.-H.; Yang, H.-C. Distinct differentiation properties of human dental pulp cells on collagen, gelatin, and chitosan scaffolds. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2009, 108, e94–e100. [Google Scholar] [CrossRef]
- Cordeiro, M.M.; Dong, Z.; Kaneko, T.; Zhang, Z.; Miyazawa, M.; Shi, S.; Smith, A.J.; Nör, J.E. Dental Pulp Tissue Engineering with Stem Cells from Exfoliated Deciduous Teeth. J. Endod. 2008, 34, 962–969. [Google Scholar] [CrossRef]
- Galler, K.M.; Hartgerink, J.D.; Cavender, A.C.; Schmalz, G.; D’Souza, R.N. A Customized Self-Assembling Peptide Hydrogel for Dental Pulp Tissue Engineering. Tissue Eng. Part A 2012, 18, 176–184. [Google Scholar] [CrossRef]
- Chen, F.-M.; Zhang, J.; Zhang, M.; An, Y.; Chen, F.; Wu, Z.-F. A review on endogenous regenerative technology in periodontal regenerative medicine. Biomaterials 2010, 31, 7892–7927. [Google Scholar] [CrossRef]
- Smith, B.T.; Shum, J.; Wong, M.; Mikos, A.G.; Young, S. Bone Tissue Engineering Challenges in Oral & Maxillofacial Surgery. Adv. Exp. Med. Biol. 2015, 881, 57–78. [Google Scholar] [CrossRef]
- Kim, S.G.; Zheng, Y.; Zhou, J.; Chen, M.; Embree, M.C.; Song, K.; Jiang, N.; Mao, J.J. Dentin and dental pulp regeneration by the patient’s endogenous cells. Endod. Top. 2013, 28, 106–117. [Google Scholar] [CrossRef]
- Abou Neel, E.A.; Chrzanowski, W.; Salih, V.M.; Kim, H.-W.; Knowles, J.C. Tissue engineering in dentistry. J. Dent. 2014, 42, 915–928. [Google Scholar] [CrossRef] [Green Version]
- Galler, K.M.; Eidt, A.; Schmalz, G. Cell-free Approaches for Dental Pulp Tissue Engineering. J. Endod. 2014, 40, S41–S45. [Google Scholar] [CrossRef] [Green Version]
- Scantlebury, T.; Ambruster, J. The Development of Guided Regeneration: Making the Impossible Possible and the Unpredictable Predictable. J. Evid. Based Dent. Pract. 2012, 12, 101–117. [Google Scholar] [CrossRef]
- Obregon, F.; Vaquette, C.; Ivanovski, S.; Hutmacher, D.W.; Bertassoni, L. Three-Dimensional Bioprinting for Regenerative Dentistry and Craniofacial Tissue Engineering. J. Dent. Res. 2015, 94, 143S–152S. [Google Scholar] [CrossRef]
- Jammalamadaka, U.; Tappa, K. Recent Advances in Biomaterials for 3D Printing and Tissue Engineering. J. Funct. Biomater. 2018, 9, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolati, F.; Yu, Y.; Zhang, Y.; De Jesus, A.M.; Sander, E.A.; Ozbolat, I.T. In vitro evaluation of carbon-nanotube-reinforced bioprintable vascular conduits. Nanotechnology 2014, 25, 145101. [Google Scholar] [CrossRef] [Green Version]
- Kolesky, D.B.; Truby, R.L.; Gladman, A.S.; Busbee, T.A.; Homan, K.A.; Lewis, J.A. 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv Mater. 2014, 26, 3124–3130. [Google Scholar] [CrossRef] [PubMed]
- Pateman, C.J.; Harding, A.J.; Glen, A.; Taylor, C.S.; Christmas, C.R.; Robinson, P.P.; Rimmer, S.; Boissonade, F.M.; Claeyssens, F.; Haycock, J.W. Nerve guides manufactured from photocurable polymers to aid peripheral nerve repair. Biomaterials 2015, 49, 77–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, R.; Emami, K.; Wu, H.; Sun, W. Biofabrication of a three-dimensional liver micro-organ as an in vitro drug metabolism model. Biofabrication 2010, 2, 045004. [Google Scholar] [CrossRef] [PubMed]
- Amler, A.-K.; Dinkelborg, P.; Schlauch, D.; Spinnen, J.; Stich, S.; Lauster, R.; Sittinger, M.; Nahles, S.; Heiland, M.; Kloke, L.; et al. Comparison of the Translational Potential of Human Mesenchymal Progenitor Cells from Different Bone Entities for Autologous 3D Bioprinted Bone Grafts. Int. J. Mol. Sci. 2021, 22, 796. [Google Scholar] [CrossRef] [PubMed]
- Amler, A.-K.; Thomas, A.; Tüzüner, S.; Lam, T.; Geiger, M.-A.; Kreuder, A.-E.; Palmer, C.; Nahles, S.; Lauster, R.; Kloke, L. 3D bioprinting of tissue-specific osteoblasts and endothelial cells to model the human jawbone. Sci. Rep. 2021, 11, 4876. [Google Scholar] [CrossRef]
- Walladbegi, J.; Schaefer, C.; Pernevik, E.; Sämfors, S.; Kjeller, G.; Gatenholm, P.; Sándor, G.K.; Rasmusson, L. Three-dimensional bioprinting using a coaxial needle with viscous inks in bone tissue engineering—An In vitro study. Ann. Maxillofac. Surg. 2020, 10, 370–376. [Google Scholar] [CrossRef]
- Dubey, N.; Ferreira, J.A.; Malda, J.; Bhaduri, S.B.; Bottino, M.C. Extracellular Matrix/Amorphous Magnesium Phosphate Bioink for 3D Bioprinting of Craniomaxillofacial Bone Tissue. ACS Appl. Mater. Interfaces 2020, 12, 23752–23763. [Google Scholar] [CrossRef]
- Chimene, D.; Miller, L.; Cross, L.M.; Jaiswal, M.K.; Singh, I.; Gaharwar, A.K. Nanoengineered Osteoinductive Bioink for 3D Bioprinting Bone Tissue. ACS Appl. Mater. Interfaces 2020, 12, 15976–15988. [Google Scholar] [CrossRef]
- Ma, Y.; Ji, Y.; Zhong, T.; Wan, W.; Yang, Q.; Li, A.; Zhang, X.; Lin, M. Bioprinting-Based PDLSC-ECM Screening for in Vivo Repair of Alveolar Bone Defect Using Cell-Laden, Injectable and Photocrosslinkable Hydrogels. ACS Biomater. Sci. Eng. 2017, 3, 3534–3545. [Google Scholar] [CrossRef]
- Kuss, M.A.; Harms, R.; Wu, S.; Wang, Y.; Untrauer, J.B.; Carlson, M.A.; Duan, B. Short-term hypoxic preconditioning promotes prevascularization in 3D bioprinted bone constructs with stromal vascular fraction derived cells. RSC Adv. 2017, 7, 29312–29320. [Google Scholar] [CrossRef] [Green Version]
- Kang, H.-W.; Lee, S.J.; Ko, I.K.; Kengla, C.; Yoo, J.J.; Atala, A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol. 2016, 34, 312–319. [Google Scholar] [CrossRef]
- Duarte Campos, D.F.; Blaeser, A.; Buellesbach, K.; Sen, K.S.; Xun, W.; Tillmann, W.; Fischer, H. Bioprinting Organotypic Hydrogels with Improved Mesenchymal Stem Cell Remodeling and Mineralization Properties for Bone Tissue Engineering. Adv. Healthc. Mater. 2016, 5, 1336–1345. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, M.; Liu, Y.; Shi, C.; Wang, Y.; Liu, T.; Huang, Y.; Zhong, P.; Dai, J.; Liu, X. The performance of 3D bioscaffolding based on a human periodontal ligament stem cell printing technique. J. Biomed. Mater. Res. Part A 2020, 109, 1209–1219. [Google Scholar] [CrossRef]
- Ono, T.; Tomokiyo, A.; Ipposhi, K.; Yamashita, K.; Alhasan, M.A.; Miyazaki, Y.; Kunitomi, Y.; Tsuchiya, A.; Ishikawa, K.; Maeda, H. Generation of biohybrid implants using a multipotent human periodontal ligament cell line and bioactive core materials. J. Cell. Physiol. 2021, 236, 6742–6753. [Google Scholar] [CrossRef]
- Lee, U.-L.; Yun, S.; Cao, H.-L.; Ahn, G.; Shim, J.-H.; Woo, S.-H.; Choung, P.-H. Bioprinting on 3D Printed Titanium Scaffolds for Periodontal Ligament Regeneration. Cells 2021, 10, 1337. [Google Scholar] [CrossRef]
- Thattaruparambil Raveendran, N.; Vaquette, C.; Meinert, C.; Samuel Ipe, D.; Ivanovski, S. Optimization of 3D bioprinting of periodontal ligament cells. Dent. Mater. 2019, 35, 1683–1694. [Google Scholar] [CrossRef]
- Ma, Y.; Ji, Y.; Huang, G.; Ling, K.; Zhang, X.; Xu, F. Bioprinting 3D cell-laden hydrogel microarray for screening human periodontal ligament stem cell response to extracellular matrix. Biofabrication 2015, 7, 044105. [Google Scholar] [CrossRef]
- Lin, Y.-T.; Hsu, T.-T.; Liu, Y.-W.; Kao, C.-T.; Huang, T.-H. Bidirectional Differentiation of Human-Derived Stem Cells Induced by Biomimetic Calcium Silicate-Reinforced Gelatin Methacrylate Bioink for Odontogenic Regeneration. Biomedicines 2021, 9, 929. [Google Scholar] [CrossRef]
- Han, J.; Jeong, W.; Kim, M.-K.; Nam, S.-H.; Park, E.-K.; Kang, H.-W. Demineralized Dentin Matrix Particle-Based Bio-Ink for Patient-Specific Shaped 3D Dental Tissue Regeneration. Polymers 2021, 13, 1294. [Google Scholar] [CrossRef]
- Han, J.; Kim, D.S.; Jang, H.; Kim, H.-R.; Kang, H.-W.; Shaddox, L.M. Bioprinting of three-dimensional dentin-pulp complex with local differentiation of human dental pulp stem cells. J. Tissue Eng. 2019, 10. [Google Scholar] [CrossRef]
- Athirasala, A.; Tahayeri, A.; Thrivikraman, G.; Franca, C.; Monteiro, N.; Tran, V.; Ferracane, J.; Bertassoni, L.E. A dentin-derived hydrogel bioink for 3D bioprinting of cell laden scaffolds for regenerative dentistry. Biofabrication 2017, 10, 024101. [Google Scholar] [CrossRef]
- Park, J.H.; Gillispie, G.J.; Copus, J.S.; Zhang, W.; Atala, A.; Yoo, J.J.; Yelick, P.C.; Lee, S.J. The effect of BMP-mimetic peptide tethering bioinks on the differentiation of dental pulp stem cells (DPSCs) in 3D bioprinted dental constructs. Biofabrication 2020, 12, 035029. [Google Scholar] [CrossRef]
- Duarte Campos, D.F.; Zhang, S.; Kreimendahl, F.; Köpf, M.; Fischer, H.; Vogt, M.; Blaeser, A.; Apel, C.; Esteves-Oliveira, M. Hand-held bioprinting for de novo vascular formation applicable to dental pulp regeneration. Connect. Tissue Res. 2020, 61, 205–215. [Google Scholar] [CrossRef]
- Betsch, M.; Cristian, C.; Lin, Y.-Y.; Blaeser, A.; Schöneberg, J.; Vogt, M.; Buhl, E.M.; Fischer, H.; Campos, D.F.D. Incorporating 4D into Bioprinting: Real-Time Magnetically Directed Collagen Fiber Alignment for Generating Complex Multilayered Tissues. Adv. Health Mater. 2018, 7, e1800894. [Google Scholar] [CrossRef]
- Hospodiuk, M.; Dey, M.; Sosnoski, D.; Ozbolat, I.T. The bioink: A comprehensive review on bioprintable materials. Biotechnol. Adv. 2017, 35, 217–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Freeman, F.; Kelly, D.J. Tuning Alginate Bioink Stiffness and Composition for Controlled Growth Factor Delivery and to Spatially Direct MSC Fate within Bioprinted Tissues. Sci. Rep. 2017, 7, 17042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Gaihre, B.; George, M.N.; Miller, A.L., 2nd; Xu, H.; Waletzki, B.E.; Lu, L. 3D bioprinting of oligo(poly[ethylene glycol] fumarate) for bone and nerve tissue engineering. J. Biomed. Mater. Res. A 2020, 109, 6–17. [Google Scholar] [CrossRef]
- Amorim, P.; D’Ávila, M.; Anand, R.; Moldenaers, P.; Van Puyvelde, P.; Bloemen, V. Insights on shear rheology of inks for extrusion-based 3D bioprinting. Bioprinting 2021, 22, e00129. [Google Scholar] [CrossRef]
- Mandrycky, C.; Wang, Z.; Kim, K.; Kim, D.-H. 3D bioprinting for engineering complex tissues. Biotechnol. Adv. 2015, 34, 422–434. [Google Scholar] [CrossRef] [Green Version]
- Sloan, A.J. Chapter 29—Biology of the Dentin-Pulp Complex. In Ramalingam MBT-SCB and TE in DS; Vishwakarma, A., Sharpe, P., Shi, S., Eds.; Academic Press: Boston, MA, USA, 2015; pp. 371–378. [Google Scholar]
- Abbass, M.M.S.; El-Rashidy, A.A.; Sadek, K.M.; El Moshy, S.; Radwan, I.A.; Rady, D.; Dörfer, C.E.; El-Sayed, K.M.F. Hydrogels and Dentin–Pulp Complex Regeneration: From the Benchtop to Clinical Translation. Polymers 2020, 12, 2935. [Google Scholar] [CrossRef]
- Erisken, C.; Kalyon, D.; Zhou, J.; Kim, S.G.; Mao, J.J. Viscoelastic Properties of Dental Pulp Tissue and Ramifications on Biomaterial Development for Pulp Regeneration. J. Endod. 2015, 41, 1711–1717. [Google Scholar] [CrossRef]
- Liu, N.; Zhou, M.; Zhang, Q.; Zhang, T.; Tian, T.; Ma, Q.; Xue, C.; Lin, S.; Cai, X. Stiffness regulates the proliferation and osteogenic/odontogenic differentiation of human dental pulp stem cells via the WNT signalling pathway. Cell Prolif. 2018, 51, e12435. [Google Scholar] [CrossRef] [Green Version]
- Bouillaguet, S. Biological risks of resin-based materials to the dentin-pulp complex. Crit. Rev. Oral Biol. Med. Off. Publ. Am. Assoc. Oral Biol. 2004, 15, 47–60. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.-R.; Du, W.; Zhou, X.-D.; Yu, H.-Y. Review of research on the mechanical properties of the human tooth. Int. J. Oral Sci. 2014, 6, 61–69. [Google Scholar] [CrossRef] [Green Version]
- Panseri, S.; Montesi, M.; Dozio, S.M.; Savini, E.; Tampieri, A.; Sandri, M. Biomimetic Scaffold with Aligned Microporosity Designed for Dentin Regeneration. Front. Bioeng. Biotechnol. 2016, 4, 48. [Google Scholar] [CrossRef] [Green Version]
- Abdulqader, S.T.; Rahman, I.A.; Thirumulu, K.P.; Ismail, H.; Mahmood, Z. Effect of biphasic calcium phosphate scaffold porosities on odontogenic differentiation of human dental pulp cells. J. Biomater. Appl. 2016, 30, 1300–1311. [Google Scholar] [CrossRef]
- Dean, R. The Periodontal Ligament: Development, Anatomy and Function. Oral Health Dent. Manag. 2017, 2017, 1–7. [Google Scholar]
- Fill, T.S.; Toogood, R.W.; Major, P.W.; Carey, J.P. Analytically determined mechanical properties of, and models for the periodontal ligament: Critical review of literature. J. Biomech. 2012, 45, 9–16. [Google Scholar] [CrossRef]
- Fill, T.S.; Carey, J.; Toogood, R.W.; Major, P.W. Experimentally Determined Mechanical Properties of, and Models for, the Periodontal Ligament: Critical Review of Current Literature. J. Dent. Biomech. 2011, 2, 312980. [Google Scholar] [CrossRef] [Green Version]
- Dorow, C.; Krstin, N.; Sander, F.-G. Experiments to Determine the Material Properties of the Periodontal Ligament. J. Orofac. Orthop. 2002, 63, 94–104. [Google Scholar] [CrossRef]
- Qian, L.; Todo, M.; Morita, Y.; Matsushita, Y.; Koyano, K. Deformation analysis of the periodontium considering the viscoelasticity of the periodontal ligament. Dent. Mater. 2009, 25, 1285–1292. [Google Scholar] [CrossRef]
- Natali, A.N.; Pavan, P.G.; Carniel, E.L.; Dorow, C. Viscoelastic Response of the Periodontal Ligament: An Experimental–Numerical Analysis. Connect. Tissue Res. 2004, 45, 222–230. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Su, M.-Z.; Chang, H.-H.; Chiang, Y.-C.; Tao, S.-H.; Cheng, J.-H.; Fuh, L.-J.; Lin, C.-P. Tension-compression viscoelastic behaviors of the periodontal ligament. J. Formos. Med. Assoc. 2012, 111, 471–481. [Google Scholar] [CrossRef] [Green Version]
- Mestrović, S.; Slaj, M.; Rajić, P. Finite element method analysis of the tooth movement induced by orthodontic forces. Coll. Antropol. 2003, 27, 17–21. [Google Scholar] [PubMed]
- Jin, S.; He, D.; Wang, Y.; Zhang, T.; Yu, H.; Li, Z.; Zhu, L.; Zhou, Y.; Liu, Y. Mechanical force modulates periodontal ligament stem cell characteristics during bone remodelling via TRPV4. Cell Prolif. 2020, 53, e12912. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Zhou, M.; Zhang, Q.; Yong, L.; Zhang, T.; Tian, T.; Ma, Q.; Lin, S.; Zhu, B.; Cai, X. Effect of substrate stiffness on proliferation and differentiation of periodontal ligament stem cells. Cell Prolif. 2018, 51, e12478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorrel, C.; Andersson, S.; Verhaert, L. Chapter 4—Anatomy of the teeth and periodontium. In Verhaert LBT-VD for the GP, 2nd ed.; Gorrel, C., Andersson, S., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2013; pp. 37–41. [Google Scholar]
- Son, C.; Choi, M.S.; Park, J. Different Responsiveness of Alveolar Bone and Long Bone to Epithelial-Mesenchymal Interaction-Related Factor. JBMR Plus 2020, 4, e10382. [Google Scholar] [CrossRef] [PubMed]
- Goudouri, O.-M.; Kontonasaki, E.; Boccaccini, A.R. Layered scaffolds for periodontal regeneration. In Biomaterials for Oral and Dental Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2017; pp. 279–295. [Google Scholar] [CrossRef]
- Almasoud, N.N.; Tanneru, N.; Marei, H. Alveolar bone density and its clinical implication in the placement of dental implants and orthodontic mini-implants. Saudi Med. J. 2016, 37, 684–689. [Google Scholar] [CrossRef]
- Park, H.-S.; Lee, Y.-J.; Jeong, S.-H.; Kwon, T.-G. Density of the alveolar and basal bones of the maxilla and the mandible. Am. J. Orthod. Dentofac. Orthop. 2008, 133, 30–37. [Google Scholar] [CrossRef]
- Scaglione, S.; Giannoni, P.; Bianchini, P.; Sandri, M.; Marotta, R.; Firpo, G.; Valbusa, U.; Tampieri, A.; Diaspro, A.; Bianco, P.; et al. Order versus Disorder: In vivo bone formation within osteoconductive scaffolds. Sci. Rep. 2012, 2, 274. [Google Scholar] [CrossRef] [Green Version]
- Wan, Z.; Zhang, P.; Liu, Y.; Lv, L.; Zhou, Y. Four-dimensional bioprinting: Current developments and applications in bone tissue engineering. Acta Biomater. 2019, 101, 26–42. [Google Scholar] [CrossRef]
- Gungor-Ozkerim, P.S.; Inci, I.; Zhang, Y.S.; Khademhosseini, A.; Dokmeci, M.R. Bioinks for 3D bioprinting: An overview. Biomater. Sci. 2018, 6, 915–946. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.X.B. (Ed.) Biomaterials for Bioprinting BT—Extrusion Bioprinting of Scaffolds for Tissue Engineering Applications; Springer: Cham, Switherland, 2019; pp. 33–48. [Google Scholar]
- Yao, L.; Flynn, N. Dental pulp stem cell-derived chondrogenic cells demonstrate differential cell motility in type I and type II collagen hydrogels. Spine J. 2018, 18, 1070–1080. [Google Scholar] [CrossRef]
- Osidak, E.; Kozhukhov, I.; Osidak, M.; Domogatsky, S. Collagen as Bioink for Bioprinting: A Comprehensive Review. Int. J. Bioprint. 2020, 22, 6. [Google Scholar]
- Marques, C.; Diogo, G.S.; Pina, S.; Oliveira, J.M.; Silva, T.H.; Reis, R.L. Collagen-based bioinks for hard tissue engineering applications: A comprehensive review. J. Mater. Sci. Mater. Med. 2019, 30, 32. [Google Scholar] [CrossRef]
- Koch, L.; Deiwick, A.; Schlie, S.; Michael, S.; Gruene, M.; Coger, V.; Zychlinski, D.; Schambach, A.; Reimers, K.; Vogt, P.M.; et al. Skin tissue generation by laser cell printing. Biotechnol. Bioeng. 2012, 109, 1855–1863. [Google Scholar] [CrossRef]
- Shi, Y.; Xing, T.L.; Zhang, H.B.; Yin, R.X.; Yang, S.M.; Wei, J.; Zhang, W.J. Tyrosinase-doped bioink for 3D bioprinting of living skin constructs. Biomed. Mater. 2018, 13, 035008. [Google Scholar] [CrossRef]
- Kim, W.J.; Yun, H.-S.; Kim, G.H. An innovative cell-laden α-TCP/collagen scaffold fabricated using a two-step printing process for potential application in regenerating hard tissues. Sci. Rep. 2017, 7, 3181. [Google Scholar] [CrossRef] [Green Version]
- Lee, A.; Hudson, A.R.; Shiwarski, D.J.; Tashman, J.W.; Hinton, T.J.; Yerneni, S.; Bliley, J.M.; Campbell, P.G.; Feinberg, A.W. 3D bioprinting of collagen to rebuild components of the human heart. Science 2019, 365, 482–487. [Google Scholar] [CrossRef]
- Mazzocchi, A.; Devarasetty, M.; Huntwork, R.; Soker, S.; Skardal, A. Optimization of collagen type I-hyaluronan hybrid bioink for 3D bioprinted liver microenvironments. Biofabrication 2018, 11, 015003. [Google Scholar] [CrossRef]
- Lee, Y.-B.; Polio, S.; Lee, W.; Dai, G.; Menon, L.; Carroll, R.S.; Yoo, S.-S. Bio-printing of collagen and VEGF-releasing fibrin gel scaffolds for neural stem cell culture. Exp. Neurol. 2010, 223, 645–652. [Google Scholar] [CrossRef]
- Prasadh, S.; Wong, R.C.W. Unraveling the mechanical strength of biomaterials used as a bone scaffold in oral and maxillofacial defects. Oral Sci. Int. 2018, 15, 48–55. [Google Scholar] [CrossRef]
- Yang, G.H.; Kim, M.; Kim, G. Additive-manufactured polycaprolactone scaffold consisting of innovatively designed microsized spiral struts for hard tissue regeneration. Biofabrication 2016, 9, 15005. [Google Scholar] [CrossRef]
- Jaipan, P.; Nguyen, A.; Narayan, R.J. Gelatin-based hydrogels for biomedical applications. MRS Commun. 2017, 7, 416–426. [Google Scholar] [CrossRef]
- Sun, M.; Sun, X.; Wang, Z.; Guo, S.; Yu, G.; Yang, H. Synthesis and Properties of Gelatin Methacryloyl (GelMA) Hydrogels and Their Recent Applications in Load-Bearing Tissue. Polymers 2018, 10, 1290. [Google Scholar] [CrossRef] [Green Version]
- Monteiro, N.; Thrivikraman, G.; Athirasala, A.; Tahayeri, A.; Franca, C.; Ferracane, J.L.; Bertassoni, L.E. Photopolymerization of cell-laden gelatin methacryloyl hydrogels using a dental curing light for regenerative dentistry. Dent. Mater. 2018, 34, 389–399. [Google Scholar] [CrossRef]
- Athirasala, A.; Lins, F.; Tahayeri, A.; Hinds, M.; Smith, A.J.; Sedgley, C.; Ferracane, J.; Bertassoni, L.E. A Novel Strategy to Engineer Pre-Vascularized Full-Length Dental Pulp-like Tissue Constructs. Sci. Rep. 2017, 7, 3323. [Google Scholar] [CrossRef] [Green Version]
- Khayat, A.; Monteiro, N.; Smith, E.E.; Pagni, S.; Zhang, W.; Khademhosseini, A.; Yelick, P.C. GelMA-Encapsulated hDPSCs and HUVECs for Dental Pulp Regeneration. J. Dent. Res. 2017, 96, 192–199. [Google Scholar] [CrossRef] [Green Version]
- Zhan, H.; Löwik, D.W.P.M. A Hybrid Peptide Amphiphile Fiber PEG Hydrogel Matrix for 3D Cell Culture. Adv. Funct. Mater. 2019, 29, 1808505. [Google Scholar] [CrossRef]
- Highley, C.B.; Prestwich, G.D.; Burdick, J.A. Recent advances in hyaluronic acid hydrogels for biomedical applications. Curr. Opin. Biotechnol. 2016, 40, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Pescosolido, L.; Schuurman, W.; Malda, J.; Matricardi, P.; Alhaique, F.; Coviello, T.; van Weeren, P.R.; Dhert, W.J.A.; Hennink, W.E.; Vermonden, T. Hyaluronic Acid and Dextran-Based Semi-IPN Hydrogels as Biomaterials for Bioprinting. Biomacromolecules 2011, 12, 1831–1838. [Google Scholar] [CrossRef]
- Skardal, A.; Zhang, J.; Prestwich, G.D. Bioprinting vessel-like constructs using hyaluronan hydrogels crosslinked with tetrahedral polyethylene glycol tetracrylates. Biomaterials 2010, 31, 6173–6181. [Google Scholar] [CrossRef]
- Hamlet, S.M.; Vaquette, C.; Shah, A.; Hutmacher, D.W.; Ivanovski, S. 3-Dimensional functionalized polycaprolactone-hyaluronic acid hydrogel constructs for bone tissue engineering. J. Clin. Periodontol. 2017, 44, 428–437. [Google Scholar] [CrossRef]
- Silva, C.R.; Babo, P.S.; Gulino, M.; Costa, L.; Oliveira, J.M.; Silva-Correia, J.; Domingues, R.M.; Reis, R.L.; Gomes, M.E. Injectable and tunable hyaluronic acid hydrogels releasing chemotactic and angiogenic growth factors for endodontic regeneration. Acta Biomater. 2018, 77, 155–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, Q.; Zünd, G.; Benedikt, P.; Jockenhoevel, S.; Hoerstrup, S.P.; Sakyama, S.; Hubbell, J.; Turina, M. Fibrin gel as a three dimensional matrix in cardiovascular tissue engineering. Eur. J. Cardio-Thorac. Surg. 2000, 17, 587–591. [Google Scholar] [CrossRef]
- Ahmed, T.A.E.; Dare, E.V.; Hincke, M. Fibrin: A versatile scaffold for tissue engineering applications. Tissue Eng. Part B Rev. 2008, 14, 199–215. [Google Scholar] [CrossRef]
- England, S.; Rajaram, A.; Schreyer, D.J.; Chen, X. Bioprinted fibrin-factor XIII-hyaluronate hydrogel scaffolds with encapsulated Schwann cells and their in vitro characterization for use in nerve regeneration. Bioprinting 2017, 5, 1–9. [Google Scholar] [CrossRef]
- Hakam, M.S.; Imani, R.; Abolfathi, N.; Fakhrzadeh, H.; Sharifi, A.M. Evaluation of fibrin-gelatin hydrogel as biopaper for application in skin bioprinting: An in-vitro study. Bio-Med. Mater. Eng. 2017, 27, 669–682. [Google Scholar] [CrossRef]
- Chiesa, I.; De Maria, C.; Lapomarda, A.; Fortunato, G.M.; Montemurro, F.; Di Gesù, R.; Tuan, R.S.; Vozzi, G.; Gottardi, R. Endothelial cells support osteogenesis in an in vitro vascularized bone model developed by 3D bioprinting. Biofabrication 2020, 12, 025013. [Google Scholar] [CrossRef]
- Cui, X.; Boland, T. Human microvasculature fabrication using thermal inkjet printing technology. Biomaterials 2009, 30, 6221–6227. [Google Scholar] [CrossRef] [Green Version]
- Lu, Q.; Pandya, M.; Rufaihah, A.J.; Rosa, V.; Tong, H.J.; Seliktar, D.; Toh, W.S. Modulation of Dental Pulp Stem Cell Odontogenesis in a Tunable PEG-Fibrinogen Hydrogel System. Stem Cells Int. 2015, 2015, 525367. [Google Scholar] [CrossRef]
- Mastrullo, V.; Cathery, W.; Velliou, E.; Madeddu, P.; Campagnolo, P. Angiogenesis in Tissue Engineering: As Nature Intended? Front. Bioeng. Biotechnol. 2020, 8, 188. [Google Scholar] [CrossRef] [Green Version]
- Szekalska, M.; Puciłowska, A.; Szymańska, E.; Ciosek, P.; Winnicka, K. Alginate: Current Use and Future Perspectives in Pharmaceutical and Biomedical Applications. Int. J. Polym. Sci. 2016, 2016, 7697031. [Google Scholar] [CrossRef] [Green Version]
- Sandvig, I.; Karstensen, K.; Rokstad, A.M.; Aachmann, F.L.; Formo, K.; Sandvig, A.; Skjåk-Braek, G.; Strand, B.L. RGD-peptide modified alginate by a chemoenzymatic strategy for tissue engineering applications. J. Biomed. Mater. Res. Part A 2014, 103, 896–906. [Google Scholar] [CrossRef]
- Bhoj, M.; Zhang, C.; Green, D.W. A First Step in De Novo Synthesis of a Living Pulp Tissue Replacement Using Dental Pulp MSCs and Tissue Growth Factors, Encapsulated within a Bioinspired Alginate Hydrogel. J. Endod. 2015, 41, 1100–1107. [Google Scholar] [CrossRef]
- Sevari, S.P.; Shahnazi, F.; Chen, C.; Mitchell, J.C.; Ansari, S.; Moshaverinia, A. Bioactive glass-containing hydrogel delivery system for osteogenic differentiation of human dental pulp stem cells. J. Biomed. Mater. Res. Part A 2019, 108, 557–564. [Google Scholar] [CrossRef]
- Wüst, S.; Godla, M.E.; Müller, R.; Hofmann, S. Tunable hydrogel composite with two-step processing in combination with innovative hardware upgrade for cell-based three-dimensional bioprinting. Acta Biomater. 2014, 10, 630–640. [Google Scholar] [CrossRef]
- Jia, W.; Gungor-Ozkerim, P.S.; Zhang, Y.S.; Yue, K.; Zhu, K.; Liu, W.; Pi, Q.; Byambaa, B.; Dokmeci, M.R.; Shin, S.R.; et al. Direct 3D bioprinting of perfusable vascular constructs using a blend bioink. Biomaterials 2016, 106, 58–68. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Zhang, Y.; Martin, J.A.; Ozbolat, I.T. Evaluation of Cell Viability and Functionality in Vessel-like Bioprintable Cell-Laden Tubular Channels. J. Biomech. Eng. 2013, 135, 91011. [Google Scholar] [CrossRef] [Green Version]
- Sell, S.A.; Wolfe, P.S.; Garg, K.; McCool, J.M.; Rodriguez, I.A.; Bowlin, G.L. The Use of Natural Polymers in Tissue Engineering: A Focus on Electrospun Extracellular Matrix Analogues. Polymers 2010, 2, 522–553. [Google Scholar] [CrossRef]
- Hersel, U.; Dahmen, C.; Kessler, H. RGD modified polymers: Biomaterials for stimulated cell adhesion and beyond. Biomaterials 2003, 24, 4385–4415. [Google Scholar] [CrossRef]
- Wang, X. Advanced Polymers for Three-Dimensional (3D) Organ Bioprinting. Micromachines 2019, 10, 814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amiryaghoubi, N.; Pesyan, N.N.; Fathi, M.; Omidi, Y. Injectable thermosensitive hybrid hydrogel containing graphene oxide and chitosan as dental pulp stem cells scaffold for bone tissue engineering. Int. J. Biol. Macromol. 2020, 162, 1338–1357. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, P.; Kandasubramanian, B. Review of alginate-based hydrogel bioprinting for application in tissue engineering. Biofabrication 2019, 11, 042001. [Google Scholar] [CrossRef]
- Lee, J.M.; Suen, S.K.Q.; Ng, W.L.; Ma, W.C.; Yeong, W.Y. Bioprinting of Collagen: Considerations, Potentials, and Applications. Macromol. Biosci. 2020, 21, e2000280. [Google Scholar] [CrossRef] [PubMed]
- De Melo, B.A.G.; Jodat, Y.A.; Cruz, E.M.; Benincasa, J.C.; Shin, S.R.; Porcionatto, M.A. Strategies to use fibrinogen as bioink for 3D bioprinting fibrin-based soft and hard tissues. Acta Biomater. 2020, 117, 60–76. [Google Scholar] [CrossRef]
- Hauptstein, J.; Böck, T.; Bartolf-Kopp, M.; Forster, L.; Stahlhut, P.; Nadernezhad, A.; Blahetek, G.; Zernecke-Madsen, A.; Detsch, R.; Jüngst, T.; et al. Hyaluronic Acid-Based Bioink Composition Enabling 3D Bioprinting and Improving Quality of Deposited Cartilaginous Extracellular Matrix. Adv. Health Mater. 2020, 9, 2000737. [Google Scholar] [CrossRef]
- Qu, T.; Liu, X. Nano-structured gelatin/bioactive glass hybrid scaffolds for the enhancement of odontogenic differentiation of human dental pulp stem cells. J. Mater. Chem. B 2013, 1, 4764–4772. [Google Scholar] [CrossRef] [Green Version]
- Casagrande, L.; Cordeiro, M.M.; Nör, S.A.; Nör, J.E. Dental pulp stem cells in regenerative dentistry. Odontology 2011, 99, 1–7. [Google Scholar] [CrossRef]
- Kang, J.; Fan, W.; Deng, Q.; He, H.; Huang, F. Stem Cells from the Apical Papilla: A Promising Source for Stem Cell-Based Therapy. BioMed Res. Int. 2019, 2019, 6104738. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Gong, Y.; Liu, L.; Zhou, Y.; Fang, X.; Zhang, C.; Li, Y.; Li, J. The use of human umbilical vein endothelial cells (HUVECs) as an in vitro model to assess the toxicity of nanoparticles to endothelium: A review. J. Appl. Toxicol. 2017, 37, 1359–1369. [Google Scholar] [CrossRef]
- Yu, C.; Miller, K.L.; Schimelman, J.; Wang, P.; Zhu, W.; Ma, X.; Tang, M.; You, S.; Lakshmipathy, D.; He, F.; et al. A sequential 3D bioprinting and orthogonal bioconjugation approach for precision tissue engineering. Biomaterials 2020, 258, 120294. [Google Scholar] [CrossRef]
- Xu, L.; Varkey, M.; Jorgensen, A.; Ju, J.H.; Jin, Q.; Park, J.H.; Fu, Y.; Zhang, G.; Ke, D.; Zhao, W.; et al. Bioprinting small diameter blood vessel constructs with an endothelial and smooth muscle cell bilayer in a single step. Biofabrication 2020, 12, 045012. [Google Scholar] [CrossRef]
- Yao, R.; Alkhawtani, A.Y.F.; Chen, R.; Luan, J.; Xu, M. Rapid and efficient in vivo angiogenesis directed by electro-assisted bioprinting of alginate/collagen microspheres with human umbilical vein endothelial cell coating layer. Int. J. Bioprint. 2019, 5, 194. [Google Scholar] [CrossRef]
- Duarte Campos, D.F.; Lindsay, C.D.; Roth, J.G.; LeSavage, B.L.; Seymour, A.J.; Krajina, B.A.; Ribeiro, R.; Costa, P.F.; Blaeser, A.; Heilshorn, S.C. Bioprinting Cell- and Spheroid-Laden Protein-Engineered Hydrogels as Tissue-on-Chip Platforms. Front. Bioeng. Biotechnol. 2020, 8, 374. [Google Scholar] [CrossRef]
- Afghah, F.; Altunbek, M.; Dikyol, C.; Koc, B. Preparation and characterization of nanoclay-hydrogel composite support-bath for bioprinting of complex structures. Sci. Rep. 2020, 10, 5257. [Google Scholar] [CrossRef]
- Li, L.; Qin, S.; Peng, J.; Chen, A.; Nie, Y.; Liu, T.; Song, K. Engineering gelatin-based alginate/carbon nanotubes blend bioink for direct 3D printing of vessel constructs. Int. J. Biol. Macromol. 2019, 145, 262–271. [Google Scholar] [CrossRef]
- Gonzalez-Fernandez, T.; Rathan, S.; Hobbs, C.; Pitacco, P.; Freeman, F.E.; Cunniffe, G.M.; Dunne, N.J.; McCarthy, H.O.; Nicolosi, V.; O’Brien, F.J.; et al. Pore-forming bioinks to enable spatio-temporally defined gene delivery in bioprinted tissues. J. Control. Release 2019, 301, 13–27. [Google Scholar] [CrossRef]
- Kérourédan, O.; Bourget, J.-M.; Rémy, M.; Crauste-Manciet, S.; Kalisky, J.; Catros, S.; Thébaud, N.B.; Devillard, R. Micropatterning of endothelial cells to create a capillary-like network with defined architecture by laser-assisted bioprinting. J. Mater. Sci. Mater. Med. 2019, 30, 28. [Google Scholar] [CrossRef]
- Moshaverinia, A.; Chen, C.; Akiyama, K.; Xu, X.; Chee, W.W.L.; Schricker, S.R.; Shi, S. Encapsulated dental-derived mesenchymal stem cells in an injectable and biodegradable scaffold for applications in bone tissue engineering. J. Biomed. Mater. Res. Part A 2013, 101, 3285–3294. [Google Scholar] [CrossRef]
- Yang, Z.; Jin, F.; Zhang, X.; Ma, D.; Han, C.; Huo, N.; Wang, Y.; Zhang, Y.; Lin, Z.; Jin, Y. Tissue Engineering of Cementum/Periodontal-Ligament Complex Using a Novel Three-Dimensional Pellet Cultivation System for Human Periodontal Ligament Stem Cells. Tissue Eng. Part C Methods 2009, 15, 571–581. [Google Scholar] [CrossRef]
- Sun, B.; Lian, M.; Han, Y.; Mo, X.; Jiang, W.; Qiao, Z.; Dai, K. A 3D-Bioprinted dual growth factor-releasing intervertebral disc scaffold induces nucleus pulposus and annulus fibrosus reconstruction. Bioact. Mater. 2020, 6, 179–190. [Google Scholar] [CrossRef]
- Gori, M.; Giannitelli, S.M.; Torre, M.; Mozetic, P.; Abbruzzese, F.; Trombetta, M.; Traversa, E.; Moroni, L.; Rainer, A. Biofabrication of Hepatic Constructs by 3D Bioprinting of a Cell-Laden Thermogel: An Effective Tool to Assess Drug-Induced Hepatotoxic Response. Adv. Health Mater. 2020, 9, 2001163. [Google Scholar] [CrossRef]
- Sun, Y.; You, Y.; Jiang, W.; Wang, B.; Wu, Q.; Dai, K. 3D bioprinting dual-factor releasing and gradient-structured constructs ready to implant for anisotropic cartilage regeneration. Sci. Adv. 2020, 6, eaay1422. [Google Scholar] [CrossRef] [PubMed]
- Toprakhisar, B.; Nadernezhad, A.; Bakirci, E.; Khani, N.; Skvortsov, G.A.; Koc, B. Development of Bioink from Decellularized Tendon Extracellular Matrix for 3D Bioprinting. Macromol. Biosci. 2018, 18, e1800024. [Google Scholar] [CrossRef] [PubMed]
- Laternser, S.; Keller, H.; Leupin, O.; Rausch, M.; Graf-Hausner, U.; Rimann, M. A Novel Microplate 3D Bioprinting Platform for the Engineering of Muscle and Tendon Tissues. SLAS Technol. Transl. Life Sci. Innov. 2018, 23, 599–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merceron, T.K.; Burt, M.; Seol, Y.-J.; Kang, H.-W.; Lee, S.J.; Yoo, J.J.; Atala, A. A 3D bioprinted complex structure for engineering the muscle–tendon unit. Biofabrication 2015, 7, 035003. [Google Scholar] [CrossRef]
- Kupfer, M.E.; Lin, W.-H.; Ravikumar, V.; Qiu, K.; Wang, L.; Gao, L.; Bhuiyan, D.B.; Lenz, M.; Ai, J.; Mahutga, R.R.; et al. In Situ Expansion, Differentiation, and Electromechanical Coupling of Human Cardiac Muscle in a 3D Bioprinted, Chambered Organoid. Circ. Res. 2020, 127, 207–224. [Google Scholar] [CrossRef]
- Attalla, R.; Puersten, E.; Jain, N.; Selvaganapathy, P.R. 3D bioprinting of heterogeneous bi- and tri-layered hollow channels within gel scaffolds using scalable multi-axial microfluidic extrusion nozzle. Biofabrication 2018, 11, 015012. [Google Scholar] [CrossRef]
- Demirtaş, T.T.; Irmak, G.; Gümüşderelioğlu, M. A bioprintable form of chitosan hydrogel for bone tissue engineering. Biofabrication 2017, 9, 035003. [Google Scholar] [CrossRef]
- Wongsupa, N.; Nuntanaranont, T.; Kamolmattayakul, S.; Thuaksuban, N. Assessment of bone regeneration of a tissue-engineered bone complex using human dental pulp stem cells/poly(ε-caprolactone)-biphasic calcium phosphate scaffold constructs in rabbit calvarial defects. J. Mater. Sci. Mater. Med. 2017, 28, 77. [Google Scholar] [CrossRef]
- Pandula, P.K.C.P.; Samaranayake, L.P.; Jin, L.J.; Zhang, C.F. Human umbilical vein endothelial cells synergize osteo/odontogenic differentiation of periodontal ligament stem cells in 3D cell sheets. J. Periodontal Res. 2013, 49, 299–306. [Google Scholar] [CrossRef]
- Walmsley, G.G.; Ransom, R.C.; Zielins, E.R.; Leavitt, T.; Flacco, J.S.; Hu, M.S.; Lee, A.S.; Longaker, M.T.; Wan, M.C. Stem Cells in Bone Regeneration. Stem Cell Rev. Rep. 2016, 12, 524–529. [Google Scholar] [CrossRef] [Green Version]
- Ding, X.; Li, X.; Li, C.; Qi, M.; Zhang, Z.; Sun, X.; Wang, L.; Zhou, Y. Chitosan/Dextran Hydrogel Constructs Containing Strontium-Doped Hydroxyapatite with Enhanced Osteogenic Potential in Rat Cranium. ACS Biomater. Sci. Eng. 2019, 5, 4574–4586. [Google Scholar] [CrossRef]
- Yang, W.S.; Kim, W.J.; Ahn, J.Y.; Lee, J.; Ko, D.W.; Park, S.; Kim, J.Y.; Jang, C.H.; Lim, J.M.; Kim, G.H. New Bioink Derived from Neonatal Chicken Bone Marrow Cells and Its 3D-Bioprinted Niche for Osteogenic Stimulators. ACS Appl. Mater. Interfaces 2020, 12. [Google Scholar] [CrossRef]
- Rukavina, P.; Koch, F.; Wehrle, M.; Tröndle, K.; Stark, G.B.; Koltay, P.; Zimmermann, S.; Zengerle, R.; Lampert, F.; Strassburg, S.; et al. In vivo evaluation of bioprinted prevascularized bone tissue. Biotechnol. Bioeng. 2020, 117, 3902–3911. [Google Scholar] [CrossRef]
- Zhang, J.; Wehrle, E.; Adamek, P.; Paul, G.R.; Qin, X.-H.; Rubert, M.; Müller, R. Optimization of mechanical stiffness and cell density of 3D bioprinted cell-laden scaffolds improves extracellular matrix mineralization and cellular organization for bone tissue engineering. Acta Biomater. 2020, 114, 307–322. [Google Scholar] [CrossRef]
- He, H.; Li, D.; Lin, Z.; Peng, L.; Yang, J.; Wu, M.; Cheng, D.; Pan, H.; Ruan, C. Temperature-programmable and enzymatically solidifiable gelatin-based bioinks enable facile extrusion bioprinting. Biofabrication 2020, 12, 045003. [Google Scholar] [CrossRef]
- Ahlfeld, T.; Cubo, N.; Cometta, S.; Guduric, V.; Vater, C.; Bernhardt, A.; Akkineni, A.R.; Lode, A.; Gelinsky, M. A Novel Plasma-Based Bioink Stimulates Cell Proliferation and Differentiation in Bioprinted, Mineralized Constructs. ACS Appl. Mater. Interfaces 2020, 12, 12557–12572. [Google Scholar] [CrossRef]
- Kim, W.; Kim, G. Collagen/bioceramic-based composite bioink to fabricate a porous 3D hASCs-laden structure for bone tissue regeneration. Biofabrication 2019, 12, 15007. [Google Scholar] [CrossRef]
- Gao, G.; Yonezawa, T.; Hubbell, K.; Dai, G.; Cui, X. Inkjet-bioprinted acrylated peptides and PEG hydrogel with human mesenchymal stem cells promote robust bone and cartilage formation with minimal printhead clogging. Biotechnol. J. 2015, 10, 1568–1577. [Google Scholar] [CrossRef]
- Ahlfeld, T.; Cidonio, G.; Kilian, D.; Duin, S.; Akkineni, A.R.; Dawson, J.I.; Yang, S.; Lode, A.; Oreffo, R.O.C.; Gelinsky, M. Development of a clay based bioink for 3D cell printing for skeletal application. Biofabrication 2017, 9, 034103. [Google Scholar] [CrossRef]
- Zhao., C.; Qazvini, N.T.; Sadati, M.; Zeng, Z.; Huang, S.; De La Lastra, A.L.; Zhang, L.; Feng, Y.; Liu, W.; Huang, B.; et al. A pH-Triggered, Self-Assembled, and Bioprintable Hybrid Hydrogel Scaffold for Mesenchymal Stem Cell Based Bone Tissue Engineering. ACS Appl Mater. Interfaces 2019, 11, 8749–8762. [Google Scholar] [CrossRef]
- Brinkman, W.T.; Nagapudi, K.; Thomas, A.B.S.; Chaikof, E.L. Photo-Cross-Linking of Type I Collagen Gels in the Presence of Smooth Muscle Cells: Mechanical Properties, Cell Viability, and Function. Biomacromolecules 2003, 4, 890–895. [Google Scholar] [CrossRef]
- Lawson, A.C.; Czernuszka, J.T. Collagen-calcium phosphate composites. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 1998, 212, 413–425. [Google Scholar] [CrossRef]
- Davidenko, N.; Schuster, C.F.; Bax, D.V.; Raynal, N.; Farndale, R.W.; Best, S.M.; Cameron, R.E. Control of crosslinking for tailoring collagen-based scaffolds stability and mechanics. Acta Biomater. 2015, 25, 131–142. [Google Scholar] [CrossRef] [Green Version]
- VijayaVenkataRaman, S.; Vialli, N.; Fuh, J.Y.H.; Lu, W.F. Conductive Collagen/PPy-b-PCL hydrogel for bioprinting of neural tissue constructs. Int. J. Bioprint. 2019, 5, 229. [Google Scholar] [CrossRef]
- Melchels, F.P.W.; Dhert, W.J.A.; Hutmacher, D.W.; Malda, J. Development and characterisation of a new bioink for additive tissue manufacturing. J. Mater. Chem. B 2014, 2, 2282–2289. [Google Scholar] [CrossRef] [Green Version]
- Billiet, T.; Gevaert, E.; De Schryver, T.; Cornelissen, M.; Dubruel, P. The 3D printing of gelatin methacrylamide cell-laden tissue-engineered constructs with high cell viability. Biomaterials 2013, 35, 49–62. [Google Scholar] [CrossRef]
- Zuo, Y.; Liu, X.; Wei, D.; Sun, J.; Xiao, W.; Zhao, H.; Guo, L.; Wei, Q.; Fan, H.; Zhang, X. Photo-Cross-Linkable Methacrylated Gelatin and Hydroxyapatite Hybrid Hydrogel for Modularly Engineering Biomimetic Osteon. ACS Appl. Mater. Interfaces 2015, 7, 10386–10394. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, L.; Liao, J.; Tan, Y.; Ouyang, K.; Ning, C.; Ni, G.; Tan, G. Cell-laden photocrosslinked GelMA-DexMA copolymer hydrogels with tunable mechanical properties for tissue engineering. J. Mater. Sci. Mater. Med. 2014, 25, 2173–2183. [Google Scholar] [CrossRef]
- Billiet, T.; Van Gasse, B.; Gevaert, E.; Cornelissen, M.; Martins, J.C.; Dubruel, P. Quantitative Contrasts in the Photopolymerization of Acrylamide and Methacrylamide-Functionalized Gelatin Hydrogel Building Blocks. Macromol. Biosci. 2013, 13, 1531–1545. [Google Scholar] [CrossRef] [PubMed]
- Hoshikawa, A.; Nakayama, Y.; Matsuda, T.; Oda, H.; Nakamura, K.; Mabuchi, K. Encapsulation of Chondrocytes in Photopolymerizable Styrenated Gelatin for Cartilage Tissue Engineering. Tissue Eng. 2006, 12, 2333–2341. [Google Scholar] [CrossRef] [PubMed]
- Mũnoz, Z.; Shih, H.; Lin, C.-C. Gelatin hydrogels formed by orthogonal thiol–norbornene photochemistry for cell encapsulation. Biomater. Sci. 2014, 2, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Dikovsky, D.; Bianco-Peled, H.; Seliktar, D. The effect of structural alterations of PEG-fibrinogen hydrogel scaffolds on 3-D cellular morphology and cellular migration. Biomaterials 2006, 27, 1496–1506. [Google Scholar] [CrossRef] [PubMed]
- Kjaergard, H.K.; Weis-Fogh, U.S. Important factors influencing the strength of autologous fibrin glue; the fibrin concentration and reaction time—Comparison of strength with commercial fibrin glue. Eur. Surg. Res. 1994, 26, 273–276. [Google Scholar] [CrossRef] [PubMed]
- Law, N.; Doney, B.; Glover, H.; Qin, Y.; Aman, Z.; Sercombe, T.; Liew, L.; Dilley, R.J.; Doyle, B. Characterisation of hyaluronic acid methylcellulose hydrogels for 3D bioprinting. J. Mech. Behav. Biomed. Mater. 2018, 77, 389–399. [Google Scholar] [CrossRef]
- Petta, D.; D’Amora, U.; Ambrosio, L.; Grijpma, D.; Eglin, D.; D’Este, M. Hyaluronic acid as a bioink for extrusion-based 3D printing. Biofabrication 2020, 12, 032001. [Google Scholar] [CrossRef]
- Radhakrishnan, J.; Subramanian, A.; Krishnan, U.M.; Sethuraman, S. Injectable and 3D Bioprinted Polysaccharide Hydrogels: From Cartilage to Osteochondral Tissue Engineering. Biomacromolecules 2016, 18, 1–26. [Google Scholar] [CrossRef]
- Khunmanee, S.; Jeong, Y.; Park, H. Crosslinking method of hyaluronic-based hydrogel for biomedical applications. J. Tissue Eng. 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.-Y.; Peng, H.-H.; Chen, M.-H.; Sun, J.-S.; Liu, T.-Y.; Chen, M.-H. In situ forming hydrogel composed of hyaluronate and polygalacturonic acid for prevention of peridural fibrosis. J. Mater. Sci. Mater. Electron. 2015, 26, 168. [Google Scholar] [CrossRef]
- Sanjuan-Alberte, P.; Vaithilingam, J.; Moore, J.; Wildman, R.; Tuck, C.; Alexander, M.; Hague, R.; Rawson, F. Development of Conductive Gelatine-Methacrylate Inks for Two-Photon Polymerisation. Polymers 2021, 13, 1038. [Google Scholar] [CrossRef]
- Leach, J.K.; Whitehead, J. Materials-Directed Differentiation of Mesenchymal Stem Cells for Tissue Engineering and Regeneration. ACS Biomater. Sci. Eng. 2017, 4, 1115–1127. [Google Scholar] [CrossRef]
- Sharma, R.I.; Snedeker, J.G. Biochemical and biomechanical gradients for directed bone marrow stromal cell differentiation toward tendon and bone. Biomaterials 2010, 31, 7695–7704. [Google Scholar] [CrossRef]
- Rahman, C.V.; Kuhn, G.; White, L.J.; Kirby, G.T.S.; Varghese, O.P.; McLaren, J.S.; Cox, H.C.; Rose, F.R.A.J.; Müller, R.; Hilborn, J.; et al. PLGA/PEG-hydrogel composite scaffolds with controllable mechanical properties. J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 101B, 648–655. [Google Scholar] [CrossRef]
- Gao, Q.; He, Y.; Fu, J.-Z.; Liu, A.; Ma, L. Coaxial nozzle-assisted 3D bioprinting with built-in microchannels for nutrients delivery. Biomaterials 2015, 61, 203–215. [Google Scholar] [CrossRef]
- Ayan, B.; Celik, N.; Zhang, Z.; Zhou, K.; Kim, M.H.; Banerjee, D.; Wu, Y.; Costanzo, F.; Ozbolat, I.T. Aspiration-assisted freeform bioprinting of pre-fabricated tissue spheroids in a yield-stress gel. Commun. Phys. 2020, 3, 183. [Google Scholar] [CrossRef]
- Bernal, P.N.; Delrot, P.; Loterie, D.; Li, Y.; Malda, J.; Moser, C.; Levato, R. Volumetric Bioprinting of Complex Living-Tissue Constructs within Seconds. Adv. Mater. 2019, 31, e1904209. [Google Scholar] [CrossRef] [Green Version]
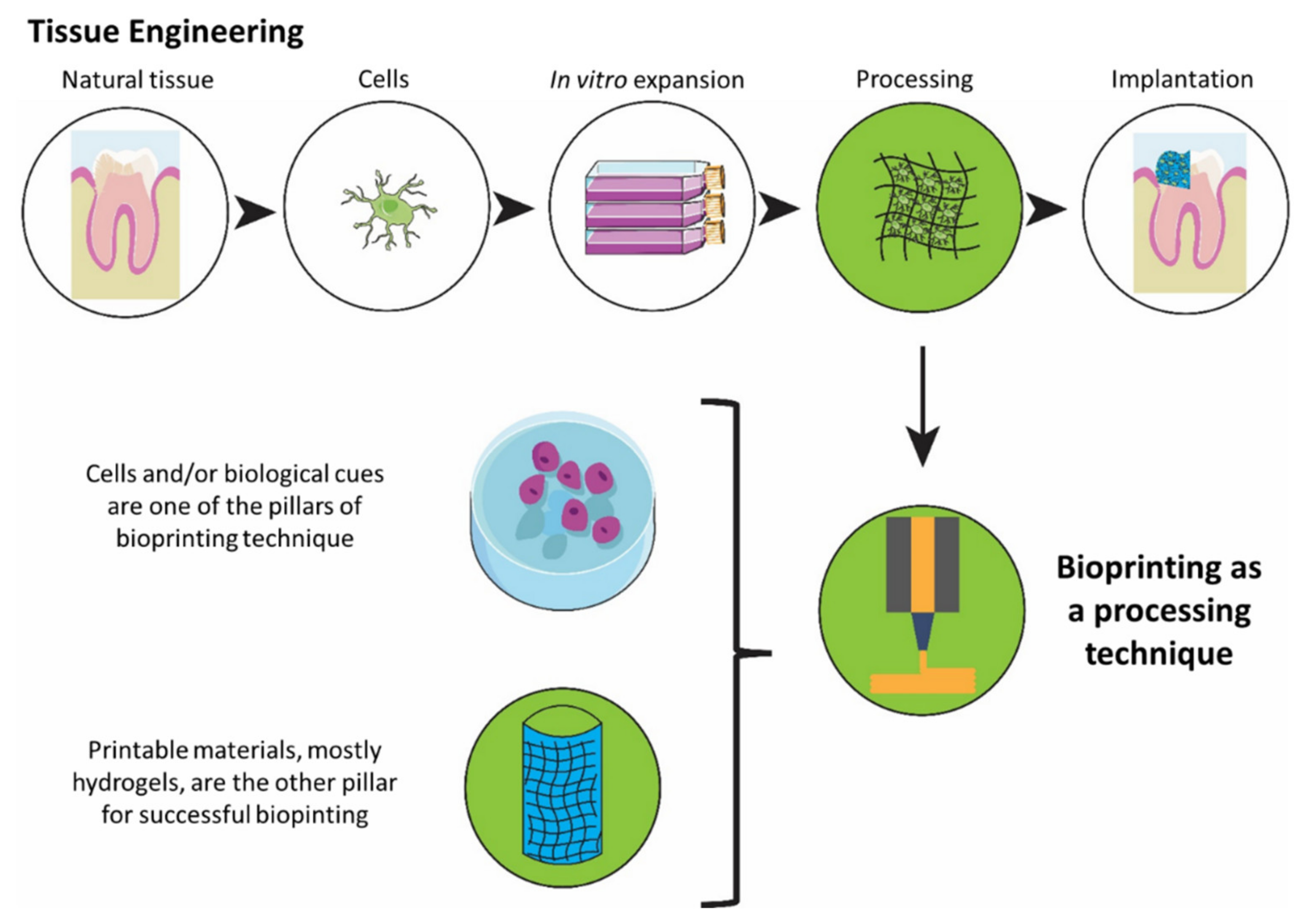
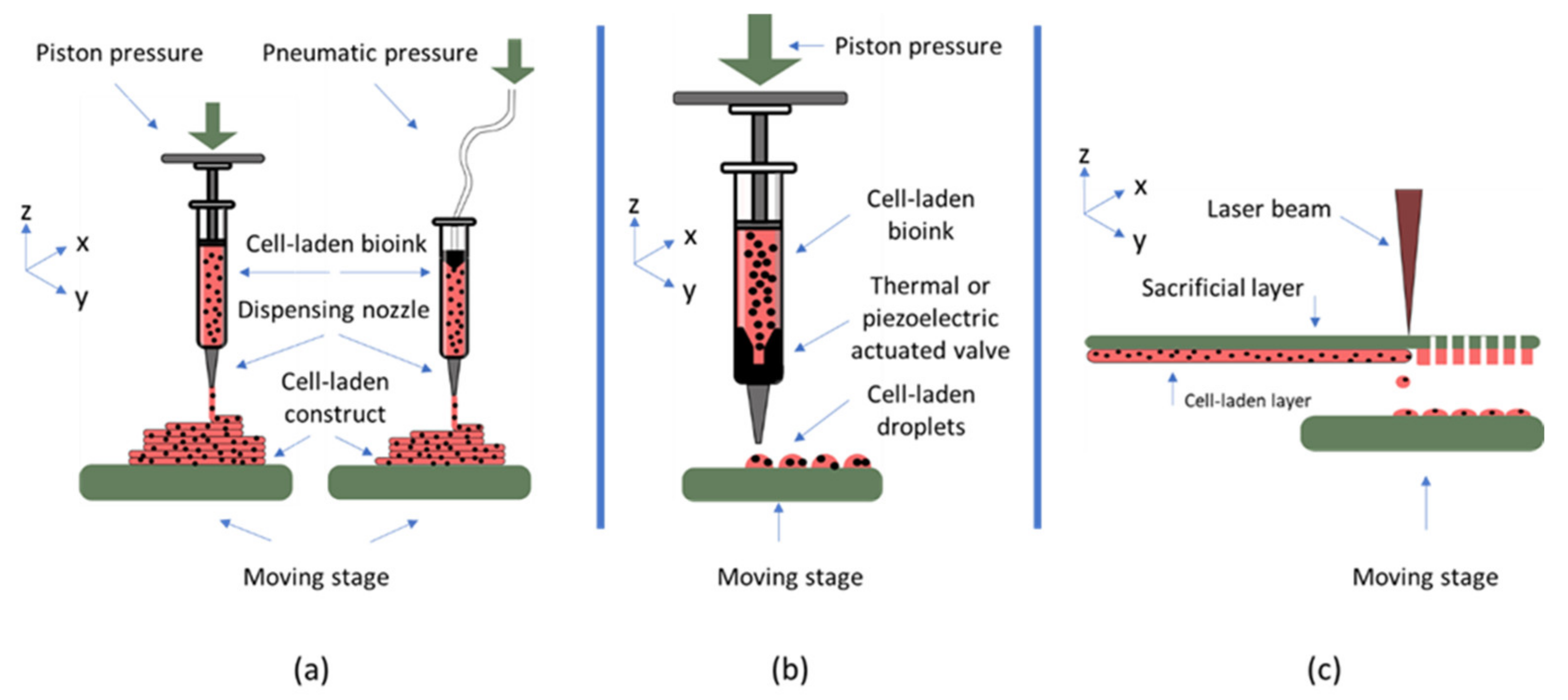
| Tissue | Bioprinting Strategy | Material Used | Nozzle Size | Study Type | Ref. |
|---|---|---|---|---|---|
| alveolar bone | Stereolitography | gelatin methacrylate | - | In vitro | Amler et al. 2021 [32] |
| alveolar bone | Stereolitography | gelatin methacrylate + methacrylated hyaluronic acid | - | In vitro | Amler et al. 2021 [33] |
| alveolar bone | Extrusion | β-TCP + nanofibrillated cellulose/alginate | Coaxial: ≈406–535 µm (22–18 G) ≈406–885 µm (22–16 G) ≈406–1295 µm (22–14 G) | In vitro | Walladbegi et al. 2020 [34] |
| alveolar bone | Inkjet | ECM-based hydrogel + self-assembling FEFEFKFK octapeptide + amorphous magnesium phosphates | - | In vitro + In vivo | Dubey et al. 2020 [35] |
| alveolar bone | Extrusion | gelMA + kappa-carrageenan + nanosilicates | 400 µm (≈22 G) | In vitro | Chimene et al. 2020 [36] |
| alveolar bone | Inkjet | gelatin methacrylate + poly (ethylene glycol) | 150 µm (≈30 G) | In vitro + In vivo | Ma et al. 2017 [37] |
| alveolar bone | Extrusion | methacrylated hyaluronic acid + gelatin methacrylate | 400 µm (≈22 G) | In vitro | Kuss et al. 2017 [38] |
| alveolar bone | Extrusion | gelatin + hyaluronic acid + fibrinogen + glycerol | 300 µm (≈23 G) | In vivo | Kang et al. 2016 [39] |
| alveolar bone | Extrusion | collagen type I + agarose | 600 µm (≈20 G) | In vitro | Campos et al. 2016 [40] |
| bone/alveolar bone | Extrusion | sodium alginate + gelatin + nano-hydroxyapatite | 400 µm (≈22 G) | In vitro | Tian et al. 2020 [41] |
| periodontal ligament | In-house—single-cell printing | - | ≈240 µm (26 G) | In vitro | Tomokiyo et al. 2021 [42] |
| periodontal ligament | Extrusion | collagen + FGF-2 | 400 µm (≈22 G) | In vitro + In vivo | Lee et al. 2021 [43] |
| periodontal ligament | Extrusion | gelatin methacrylate | ≈220 µm (25 or 27 G) | In vitro | Raveendran et al. 2019 [44] |
| periodontal ligament | Inkjet | gelatin methacrylate + poly (ethylene glycol) dimethacrylate | 150 µm (≈30 G) | In vitro | Ma et al. 2015 [45] |
| dentin pulp | Extrusion | Calcium silicate reinforced gelatin methacrylate | 30 G (≈150 µm) | In vitro | Lin et al. 2021 [46] |
| dentin pulp | Extrusion | Fibrinogen—gelatin—demineralized dentin matrix particles | 300 µm (≈23 G) | In vitro | Han et al. 2021 [47] |
| dentin pulp | Extrusion | fibrinogen + gelatin + hyaluronic acid + glycerol | 300 µm (≈23 G) | In vitro | Han et al. 2019 [48] |
| dentin pulp | Extrusion | alginate + dentin matrix | ≈450 µm (22 G) | In vitro | Athirasala et al. 2018 [49] |
| dental pulp | Extrusion | BMP-mimetic peptide modified GelMA + gelatin + hyaluronic acid + glycerol | 330 µm (≈23 G) | In vitro | Park et al. 2020 [50] |
| dental pulp | Inkjet | agarose + collagen type I + fibrinogen | 300 µm (≈23 G) | In vitro | Campos et al. 2019 [51] |
| dental pulp/cornea/articular cartilage | Inkjet | agarose + collagen type I | 300 µm (≈23 G) | In vitro | Betsch et al. 2018 [52] |
| Ref. | Material | Targeted Tissue | Cell Types Used | Bioprinted? (Tech) | Max Cell Viability (%) | Study Duration (Days) | In Vivo? | Suggestive Tissue | Suggestive Cell Types | Suggestive Biological Cues |
|---|---|---|---|---|---|---|---|---|---|---|
| [119] | alginate + matrigel + bioactive glass microparticles | pulp-dentin | dental pulp stem cells | No | 80 | 21 | ✕ | pulp-dentin | dental pulp stem cells (DPSCs) [131,132]/stem cells from apical papilla (SCAPs) [133]/human umbilical vein endothelial cells (HUVECs) [134]/odontoblast-like cells/stem cells from human exfoliated deciduous teeth | vascular endothelial growth factor (VEGF)/nerve growth factor (NGF)/bone morphogenetic protein 7 (BMP-7)/platelet-derived growth factor (PDGF) |
| [118] | RGD modified alginate | pulp-dentin | human umbilical vascular endothelial cells + human dental pulp stem cells | No | N/A | 14 | ✕ | |||
| [114] | fibrin + polyethylene glycol | pulp-dentin | dental pulp stem cells | No | 85 | 7 | ✕ | |||
| [107] | hyaluronic acid + cellulose nanocrystals + platelet lysate | pulp-dentin | dental pulp stem cell | No | N/A | 14 | ✕ | |||
| [135] | gelatin norbornene + thyiolated gelatin | vascularized cardiac tissue | human umbilical vein endothelial cells + iPSC-derived cardiomyocytes | L | 94 | 7 | ✕ | |||
| [136] | gelMA + gelatin + glycerol + hyaluronic acid | small blood vessels | human umbilical vein endothelial cells + smooth muscle cells | Ex | 89.8 | 7 | ✕ | |||
| [137] | alginate + type I collagen | microvasculature | human umbilical vein endothelial cells | IJ | N/A | 3 | ✓ | |||
| [138] | RGD modified elastin-like protein hydrogel | neural tissue model | neural progenitor cells + human induced pluripotent stem cells + human umbilical vein endothelial cells + human premalignant breast epithelial cells | IJ | 88.3 | 14 | ✕ | |||
| [139] | alginate in nanoclay support bath | complex vascular structures | NIH/3T3 fibroblasts | Ex | 94.3 | 7 | ✕ | |||
| [140] | alginate + gelatin + carbon nanotubes | vessel constructs | fibroblasts | Ex | 86.6 | 7 | ✕ | |||
| [141] | alginate-methylcellulose | bioinks for gene delivery | bone marrow-derived mesenchymal stem cells | Ex | N/A | N/A | ✓ | |||
| [142] | collagen type I | capillary network | stem cells from the apical papilla | L | N/A | N/A | ✕ |
| Ref. | Material | Targeted Tissue | Cell Types Used | Bioprinted? (Tech) | Max Cell Viability (%) | Study Duration (Days) | In Vivo? | Suggestive Tissue | Suggestive Cell Types | Suggestive Biological Cues |
|---|---|---|---|---|---|---|---|---|---|---|
| [143] | alginate + sodium periodate | periodontal ligament | periodontal ligament stem cells + gingival mesenchymal stem cells | No | 95 | 28 | ✓ | periodontal ligament | periodontal ligament stem cells (PDLSCs) [144]/gingival mesenchymal stem cells [145] | connective tissuee growth factor (CTGF) + transforming growth factor-β3 (TGF-β3) [146]/transforming growth factor B1 (TGFB1) |
| [147] | gelatin + fibrinogen + hyaluronic acid + glycerol + PCL support | anisotropic cartilage | bone marrow stromal cell | Ex | 75 | 21 | ✓ | |||
| [146] | pluronic + alginate | liver model | hepG2/C3A cell line | Ex | N/A | 7 | ✕ | |||
| [148] | decellularized tendon extracellular matrix | tendon tissue | NIH 3T3 cells | Ex | ≈ 85 | 3 | ✕ | |||
| [149] | methacryloyl-polyethylenglycol dimethacrylate | muscle and tendon tissues | primary human skeletal-muscle-derived cells + Primary rat tail tenocytes | IJ | 95 | <1 | ✕ | |||
| [150] | hyaluronic acid + gelatin + fibrinogen + polyurethane support | muscle tendon unit | C2C12 cell line + NIH/3T3 cell line | Ex | 80 | 7 | ✕ | |||
| [151] | gelMA, collagen methacrylate, fibronectin, laminin | cardiac muscle | human-induced pluripotent stem cells | Ex | N/A | 13 | ✕ | |||
| [152] | collagen + fibrinogen + alginate | multilayered vascular tissue constructs | human umbilical vein endothelial cells | Ex | N/A | 5 | ✕ | |||
| [153] | chitosan + chitosan-hyaluronic acid | bone tissue | MC3T3-E1 pre-osteoblast cell line | Ex | 95 | 9 | ✕ | Alveolar bone | dental pulp stem cells (DPSCs) [154]/human umbilical vein endothelial cells (HUVECs) [155]/bone marrow mesenchymal stem cells (BMSCs) [156]/osteoblast cell precursor MC3T3-E1 [157] | periostin + TGF-β [158] transforming growth factor–β3 (TGFβ3), bone + morphogenetic protein 4 (BMP4) [147]/basic fibroblast growth factor (bFGF)/vascular endothelial growth factor (VEGF) |
| [106] | hyaluronic acid + polycaprolactone | alveolar bone | osteoblasts | No | 75 | 7 | ✓ | |||
| [159] | gelatin + hyaluronic acid + fibrinogen + glycerol + hydroxyapatite + aprotinin | prevascularized bone tissue | human adipose-derived mesenchymal stem cells + human umbilical vein endothelial cells | IJ | 90 | <1 | ✓ | |||
| [160] | alginate + gelatin + glycerol | bone tissue | human mesenchymal stem cells | Ex | ≈85 | 7 | ✕ | |||
| [161] | Gelatin—ureido-pyrimidinone—tyramine | complex structures | human bone marrow mesenchymal stem cell + endothelial cells + | Ex | 90 | 1 | ✕ | |||
| [56] | oligo(poly[ethylene glycol] fumarate) + gelatin | bone and nerve | MC3T3-E1 pre-osteoblast cells | Ex | N/A | 7 | ✕ | |||
| [162] | blood plasma + alginate + methylcellulose + calcium phosphate cement support | bone tissue | mesenchymal stem cells | Ex | 75 | <1 | ✕ | |||
| [163] | collagen + β-Tricalcium phosphate | bone tissue | MC3T3-E1 pre-osteoblast cells + human adipose stem cells | Ex | 92 | <1 | ✕ | |||
| [164] | poly(ethylene glycol) dimethacrylate + acrylated GRGDS and MMP-sensitive peptides | bone and cartilage | human mesenchymal stem cells | IJ | ≈88 | 1 | ✕ | |||
| [165] | alginate + methylcellulose + laponite | bone tissue | immortalised human mesenchymal stem cells | Ex | 75 | 21 | ✕ | |||
| [166] | carboxymethyl chitosan + amorphous calcium phosphate | bone tissue | mesenchymal stem cell | Ex | N/A | 15 | ✓ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salar Amoli, M.; EzEldeen, M.; Jacobs, R.; Bloemen, V. Materials for Dentoalveolar Bioprinting: Current State of the Art. Biomedicines 2022, 10, 71. https://doi.org/10.3390/biomedicines10010071
Salar Amoli M, EzEldeen M, Jacobs R, Bloemen V. Materials for Dentoalveolar Bioprinting: Current State of the Art. Biomedicines. 2022; 10(1):71. https://doi.org/10.3390/biomedicines10010071
Chicago/Turabian StyleSalar Amoli, Mehdi, Mostafa EzEldeen, Reinhilde Jacobs, and Veerle Bloemen. 2022. "Materials for Dentoalveolar Bioprinting: Current State of the Art" Biomedicines 10, no. 1: 71. https://doi.org/10.3390/biomedicines10010071
APA StyleSalar Amoli, M., EzEldeen, M., Jacobs, R., & Bloemen, V. (2022). Materials for Dentoalveolar Bioprinting: Current State of the Art. Biomedicines, 10(1), 71. https://doi.org/10.3390/biomedicines10010071








