Oxidative Stress and Antioxidant Nanotherapeutic Approaches for Inflammatory Bowel Disease
Abstract
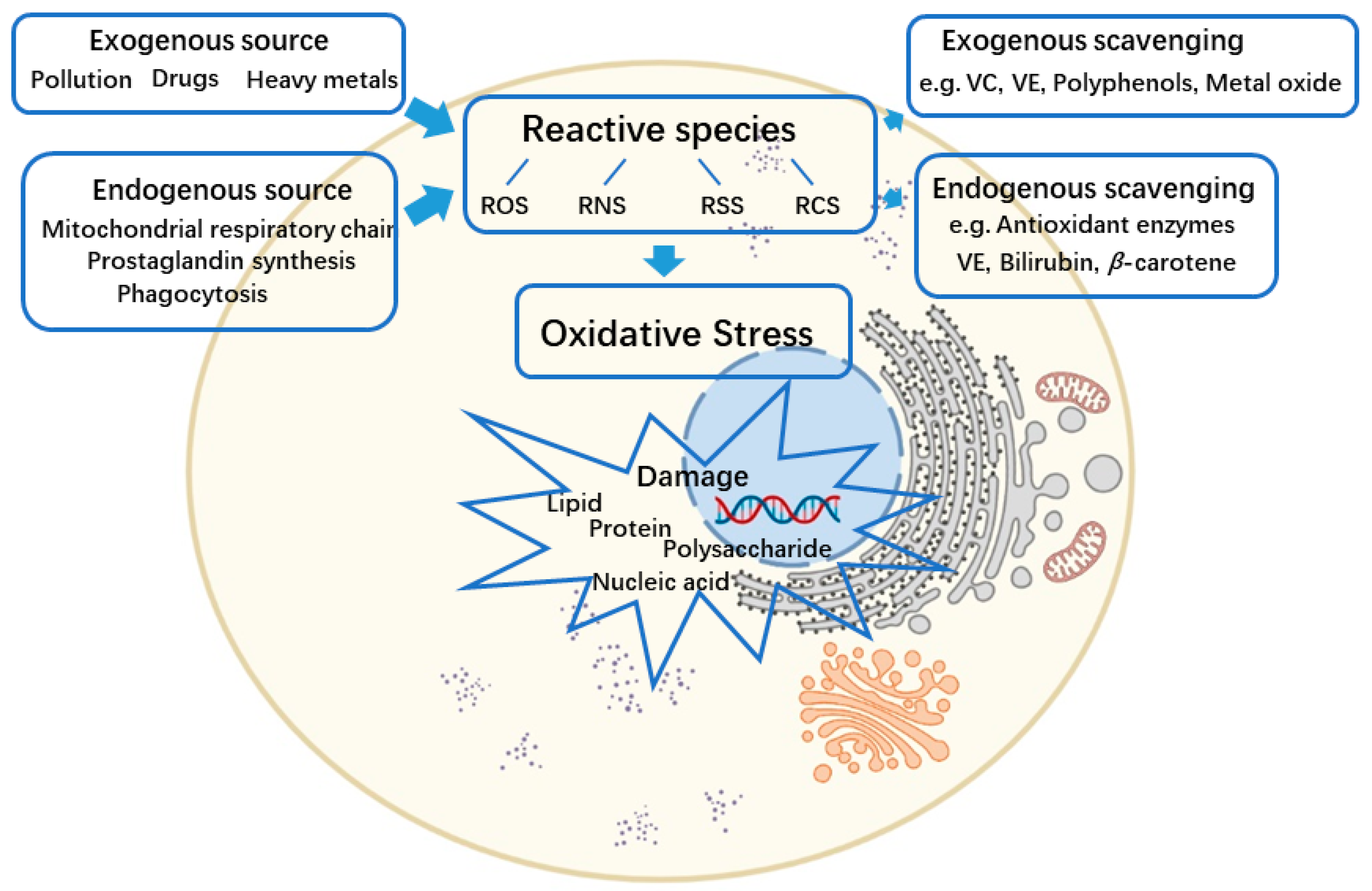
:1. Introduction
2. The Reactive Species and Oxidative Stress
3. Oxidative Stress and IBD
4. Antioxidative Nanotherapeutic Approaches for IBD
4.1. Nanosystem Delivery of Protein and Peptide Drugs to Impact Oxidative Stress
4.2. Nanosystem Delivery of Nucleic Acid Drugs to Interfere with Antioxidant Pathways
4.3. Nanosystem Delivery of Small-Molecule Antioxidants to Act as Reactive Species Scavengers
4.4. Nanozymes to Catalyze Oxidative Defense
5. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bourgonje, A.R.; Feelisch, M.; Faber, K.N.; Pasch, A.; Dijkstra, G.; van Goor, H. Oxidative stress and redox-modulating therapeutics in inflammatory bowel disease. Trends Mol. Med. 2020, 26, 1034–1046. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, S.M.U.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta 2017, 1863, 585–597. [Google Scholar] [CrossRef]
- Khor, B.; Gardet, A.; Xavier, R.J. Genetics and pathogenesis of inflammatory bowel disease. Nature 2011, 474, 307–317. [Google Scholar] [CrossRef] [Green Version]
- Abraham, C.; Cho, J.H. Inflammatory bowel disease. N. Eng. J. Med. 2009, 361, 2066–2078. [Google Scholar] [CrossRef]
- Lingappan, K. NF-κB in oxidative stress. Curr. Opin. Toxicol. 2018, 7, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Pérez, S.; Taléns-Visconti, R.; Rius-Pérez, S.; Finamor, I.; Sastre, J. Redox signaling in the gastrointestinal tract. Free Radic. Biol. Med. 2017, 104, 75–103. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, M.; Swierczynski, M.; Fichna, J.; Piechota-Polanczyk, A. The Nrf2 in the pathophysiology of the intestine: Molecular mechanisms and therapeutic implications for inflammatory bowel diseases. Pharmacol. Res. 2021, 163, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wardyn, J.D.; Ponsford, A.H.; Sanderson, C.M. Dissecting molecular cross-talk between Nrf2 and NF-κB response pathways. Biochem. Soc. Trans. 2015, 43, 621–626. [Google Scholar]
- Chung, C.H.; Jung, W.; Keum, H.; Kim, T.W.; Jon, S. Nanoparticles derived from the natural antioxidant rosmarinic acid ameliorate acute inflammatory bowel disease. ACS Nano 2020, 14, 6887–6896. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, Y.; Wang, W.; Tian, Y.; Gan, Z.; Wang, Y.; He, H.; Chen, W.; Zhang, X.; Wu, Y.; et al. In situ growth of nano-antioxidants on cellular vesicles for efficient reactive oxygen species elimination in acute inflammatory diseases. Nano Today 2021, 40, 1–6. [Google Scholar] [CrossRef]
- Huang, Z.; Gan, J.; Jia, L.; Guo, G.; Wang, C.; Zang, Y.; Ding, Z.; Chen, J.; Zhang, J.; Dong, L. An orally administrated nucleotide-delivery vehicle targeting colonic macrophages for the treatment of inflammatory bowel disease. Biomaterials 2015, 48, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and benefits for human health. Oxid. Med. Cell. Longev. 2017, 2017, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Li, C.W.; Li, L.L.; Chen, S.; Zhang, J.X.; Lu, W.L. Antioxidant nanotherapies for the treatment of inflammatory diseases. Front. Bioeng. Biotechnol. 2020, 8, 1–20. [Google Scholar] [CrossRef]
- Piechota-Polanczyk, A.; Fichna, J. Review article: The role of oxidative stress in pathogenesis and treatment of inflammatory bowel diseases. Naunyn. Schmiedebergs. Arch. Pharmacol. 2014, 387, 605–620. [Google Scholar] [CrossRef] [Green Version]
- Kumar, H.; Bhardwaj, K.; Nepovimova, E.; Kuča, K.; Dhanjal, D.S.; Bhardwaj, S.; Bhatia, S.K.; Verma, R.; Kumar, D. Antioxidant functionalized nanoparticles: A combat against oxidative stress. Nanomaterials 2020, 10, 1334. [Google Scholar] [CrossRef]
- Zhao, J.; Gao, W.; Cai, X.; Xu, J.; Zou, D.; Li, Z.; Hu, B.; Zheng, Y. Nanozyme-mediated catalytic nanotherapy for inflammatory bowel disease. Theranostics 2019, 9, 2843–2855. [Google Scholar] [CrossRef]
- Zhao, S.; Li, Y.; Liu, Q.; Li, S.; Cheng, Y.; Cheng, C.; Sun, Z.; Du, Y.; Butch, C.J.; Wei, H. An orally administered CeO2@montmorillonite nanozyme targets inflammation for inflammatory bowel disease therapy. Adv. Funct. Mater. 2020, 30, 1–14. [Google Scholar] [CrossRef]
- Zhao, J.; Cai, X.; Gao, W.; Zhang, L.; Zou, D.; Zheng, Y.; Li, Z.; Chen, H. Prussian blue nanozyme with multienzyme activity reduces colitis in mice. ACS Appl. Mater. Interfaces 2018, 10, 26108–26117. [Google Scholar] [CrossRef]
- Li, Y.; Li, W.; Bao, W.; Liu, B.; Li, D.; Jiang, Y.; Wei, W.; Ren, F. Bioinspired peptosomes with programmed stimuli-responses for sequential drug release and high-performance anticancer therapy. Nanoscale 2017, 9, 9317–9324. [Google Scholar] [CrossRef]
- Xiao, B.; Chen, Q.; Zhang, Z.; Wang, L.; Kang, Y.; Denning, T.; Merlin, D. TNFα gene silencing mediated by orally targeted nanoparticles combined with interleukin-22 for synergistic combination therapy of ulcerative colitis. J. Control Release 2018, 287, 235–246. [Google Scholar] [CrossRef]
- Bao, W.; Liu, X.; Lv, Y.; Lu, G.H.; Li, F.; Zhang, F.; Liu, B.; Li, D.; Wei, W.; Li, Y. Nanolongan with multiple on-demand conversions for ferroptosis-apoptosis combined anticancer therapy. ACS Nano 2019, 13, 260–273. [Google Scholar] [CrossRef] [PubMed]
- Picardo, M.; Dell’Anna, M.L. Oxidative Stress. In Vitiligo; Picardo, M., Taïeb, A., Eds.; Springer: Berlin, Germany, 2010; pp. 231–237. [Google Scholar]
- Zuo, L.; Huang, Z.; Dong, L.; Xu, L.; Zhu, Y.; Zeng, K.; Zhang, C.; Chen, J.; Zhang, J. Targeting delivery of anti-TNFα oligonucleotide into activated colonic macrophages protects against experimental colitis. Gut 2010, 59, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Manichanh, C.; Borruel, N.; Casellas, F.; Guarner, F. The gut microbiota in IBD. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 599–608. [Google Scholar] [CrossRef]
- Hartwig, O.; Shetab Boushehri, M.A.; Shalaby, K.S.; Loretz, B.; Lamprecht, A.; Lehr, C.M. Drug delivery to the inflamed intestinal mucosa—Targeting technologies and human cell culture models for better therapies of IBD. Adv. Drug Deliv. Rev. 2021, 175, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Li, Y.R. Oxidative stress and redox signaling mechanisms of inflammatory bowel disease: Updated experimental and clinical evidence. Exp. Biol. Med. 2012, 237, 474–480. [Google Scholar] [CrossRef]
- Kruidenier, L.; Van Meeteren, M.E.; Kuiper, I.; Jaarsma, D.; Lamers, C.B.H.W.; Zijlstra, F.J.; Verspaget, H.W. Attenuated mild colonic inflammation and improved survival from severe DSS-colitis of transgenic Cu/Zn-SOD mice. Free Radic. Biol. Med. 2003, 34, 753–765. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Nature 2017, 2, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Schreck, R.; Rieber, P.; Baeuerle, P.A. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-κB transcription factor and HIV-1. EMBO J. 1991, 10, 2247–2258. [Google Scholar] [CrossRef]
- Khor, T.O.; Huang, M.T.; Kwon, K.H.; Chan, J.Y.; Reddy, B.S.; Kong, A.N. Nrf2-deficient mice have an increased susceptibility to dextran sulfate sodium-induced colitis. Cancer Res. 2006, 66, 11580–11584. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, E.H.; Suzuki, T.; Funayama, R.; Nagashima, T.; Hayashi, M.; Sekine, H.; Tanaka, N.; Moriguchi, T.; Motohashi, H.; Nakayama, K.; et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Commun. 2016, 7, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Awuh, J.A.; Haug, M.; Mildenberger, J.; Marstad, A.; Ngoc Do, C.P.; Louet, C.; Stenvik, J.; Steigedal, M.; Damås, J.K.; Halaas, Ø.; et al. Keap1 regulates inflammatory signaling in Mycobacterium avium-infected human macrophages. Proc. Natl. Acad. Sci. USA 2015, 112, E4272–E4280. [Google Scholar] [CrossRef] [Green Version]
- Pan, H.; Wang, H.; Wang, X.; Zhu, L.; Mao, L. The absence of Nrf2 enhances NF-κB-dependent inflammation following scratch injury in mouse primary cultured astrocytes. Mediat. Inflamm. 2012, 2012, 1–9. [Google Scholar] [CrossRef]
- Nielsen, O.H.; Munck, L.K. Drug Insight: Aminosalicylates for the treatment of IBD. Nat. Clin. Pract. Gastroenterol. Hepatol. 2007, 4, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Ben-Horin, S.; Chowers, Y. Tailoring anti-TNF therapy in IBD: Drug levels and disease activity. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Hawthorne, A.B.; Hawkey, C.J. Immunosuppressive drugs in inflammatory bowel disease: A review of their mechanisms of efficacy and place in therapy. Drugs 1989, 38, 267–288. [Google Scholar] [CrossRef] [PubMed]
- Farokhzad, O.C.; Langer, R. Impact of nanotechnology on drug delivery. ACS Nano 2009, 3, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Langer, R.; Traverso, G. Nanoparticulate drug delivery systems targeting inflammation for treatment of inflammatory bowel disease. Nano Today 2017, 16, 82–96. [Google Scholar] [CrossRef]
- Iqbal, S.; Du, X.; Wang, J.; Li, H.; Yuan, Y.; Wang, J. Surface charge tunable nanoparticles for TNF-α siRNA oral delivery for treating ulcerative colitis. Nano Res. 2018, 11, 2872–2884. [Google Scholar] [CrossRef]
- Aviello, G.; Knaus, U.G. ROS in gastrointestinal inflammation: Rescue or Sabotage? Br. J. Pharmacol. 2017, 174, 1704–1718. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Z.; He, X.; Li, C.; Lin, S.; Chen, H.; Liu, L.; Feng, X. Oral delivery of antioxidant enzymes for effective treatment of inflammatory disease. Biomaterials 2021, 271, 1–10. [Google Scholar] [CrossRef]
- Wang, X.; Yan, J.; Wang, L.; Pan, D.; Xu, Y.; Wang, F.; Sheng, J.; Li, X.; Yang, M. Oral delivery of anti-TNF antibody shielded by natural polyphenol-mediated supramolecular assembly for inflammatory bowel disease therapy. Theranostics 2020, 10, 10808–10822. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhao, Y.; Cheng, J.; Guo, J.; Zhang, Q.; Zhang, X.; Ren, J.; Wang, F.; Huang, J.; Hu, H.; et al. A proresolving peptide nanotherapy for site-specific treatment of inflammatory bowel disease by regulating proinflammatory microenvironment and gut Microbiota. Adv. Sci. 2019, 6, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.C.; Huang, Y.C. Soluble eggshell membrane protein-loaded chitosan/fucoidan nanoparticles for treatment of defective intestinal epithelial cells. Int. J. Biol. Macromol. 2019, 131, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Xu, Z.; Viennois, E.; Zhang, Y.; Zhang, Z.; Zhang, M.; Han, M.K.; Kang, Y.; Merlin, D. Orally targeted delivery of tripeptide KPV via hyaluronic acid-functionalized nanoparticles efficiently alleviates ulcerative colitis. Mol. Ther. 2017, 25, 1628–1640. [Google Scholar] [CrossRef] [Green Version]
- Veiga, N.; Goldsmith, M.; Granot, Y.; Rosenblum, D.; Dammes, N.; Kedmi, R.; Ramishetti, S.; Peer, D. Cell specific delivery of modified mRNA expressing therapeutic proteins to leukocytes. Nat. Commun. 2018, 9, 1–9. [Google Scholar] [CrossRef]
- Tian, Y.; Xu, J.; Li, Y.; Zhao, R.; Du, S.; Lv, C.; Wu, W.; Liu, R.; Sheng, X.; Song, Y.; et al. MicroRNA-31 reduces inflammatory signaling and promotes regeneration in colon epithelium, and delivery of mimics in microspheres reduces colitis in mice. Gastroenterology 2019, 156, 2281–2296. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yang, W.; Liang, Q.; Shi, Y.; Zhang, W.; Wang, X.; Meng, F.; Zhong, Z.; Yin, L. Efficient and targeted drug/siRNA co-delivery mediated by reversibly crosslinked polymersomes toward anti-inflammatory treatment of ulcerative colitis (UC). Nano Res. 2019, 12, 659–667. [Google Scholar] [CrossRef]
- Huang, Y.; Guo, J.; Gui, S. Orally targeted galactosylated chitosan poly(lactic-co-glycolic acid) nanoparticles loaded with TNF-α; siRNA provide a novel strategy for the experimental treatment of ulcerative colitis. Eur. J. Pharm. Sci. 2018, 125, 232–243. [Google Scholar] [CrossRef]
- Jiang, J.; Xiao, J.; Zhao, Z.; Yuan, M.S.; Wang, J. One-step prepared nano-in-micro microcapsule delivery vehicle with sequential burst-sustained drug release for the targeted treatment of inflammatory bowel disease. Mater. Chem. Front. 2021, 5, 6027–6040. [Google Scholar] [CrossRef]
- Huguet-Casquero, A.; Xu, Y.; Gainza, E.; Pedraz, J.L.; Beloqui, A. Oral delivery of oleuropein-loaded lipid nanocarriers alleviates inflammation and oxidative stress in acute colitis. Int. J. Pharm. 2020, 586, 1–24. [Google Scholar] [CrossRef]
- Hu, B.; Yu, S.; Shi, C.; Gu, J.; Shao, Y.; Chen, Q.; Li, Y.; Mezzenga, R. Amyloid-polyphenol hybrid nanofilaments mitigate colitis and regulate gut microbial dysbiosis. ACS Nano 2020, 14, 2760–2776. [Google Scholar] [CrossRef]
- Xu, Y.; Zhu, B.-W.; Li, X.; Li, Y.-F.; Ye, X.-M.; Hu, J.-N. Glycogen-based pH and redox sensitive nanoparticles with ginsenoside Rh2 for effective treatment of ulcerative colitis. Biomaterials 2022, 280, 121077. [Google Scholar] [CrossRef]
- Oshi, M.A.; Lee, J.; Naeem, M.; Hasan, N.; Kim, J.; Kim, H.J.; Lee, E.H.; Jung, Y.; Yoo, J.W. Curcumin nanocrystal/pH-responsive polyelectrolyte multilayer core-shell nanoparticles for inflammation-targeted alleviation of ulcerative colitis. Biomacromolecules 2020, 21, 3571–3581. [Google Scholar] [CrossRef]
- Luo, R.; Lin, M.; Zhang, C.; Shi, J.; Zhang, S.; Chen, Q.; Hu, Y.; Zhang, M.; Zhang, J.; Gao, F. Genipin-crosslinked human serum albumin coating using a tannic acid layer for enhanced oral administration of curcumin in the treatment of ulcerative colitis. Food Chem. 2020, 330, 1–10. [Google Scholar] [CrossRef]
- Gou, S.; Huang, Y.; Wan, Y.; Ma, Y.; Zhou, X.; Tong, X.; Huang, J.; Kang, Y.; Pan, G.; Dai, F.; et al. Multi-bioresponsive silk fibroin-based nanoparticles with on-demand cytoplasmic drug release capacity for CD44-targeted alleviation of ulcerative colitis. Biomaterials 2019, 212, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, S.S.; Ahmad, R.; Bakkari, M.A.; Rajput, M.K.S.; Dachineni, R.; Valiveti, C.K.; Kapur, S.; Jayarama Bhat, G.; Singh, A.B.; Tummala, H. Site-directed non-covalent polymer-drug complexes for inflammatory bowel disease (IBD): Formulation development, characterization and pharmacological evaluation. J. Control Release 2018, 290, 165–179. [Google Scholar] [CrossRef]
- Pujara, N.; Wong, K.Y.; Qu, Z.; Wang, R.; Moniruzzaman, M.; Rewatkar, P.; Kumeria, T.; Ross, B.P.; McGuckin, M.; Popat, A. Oral Delivery of β-lactoglobulin-nanosphere-encapsulated resveratrol alleviates inflammation in Winnie Mice with spontaneous ulcerative colitis. Mol. Pharm. 2021, 18, 627–640. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Li, S.; Wu, Y.; Li, D.; Han, Y. Construction of chitosan/alginate nano-drug delivery system for improving dextran sodium sulfate-induced colitis in mice. Nanomaterials 2021, 11, 1884. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, N.; Galbis, E.; Díaz-Blanco, M.J.; Lucas, R.; Benito, E.; De-Paz, M.V. Nanostructured chitosan-based biomaterials for sustained and colon-specific resveratrol release. Int. J. Mol. Sci. 2019, 20, 398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Yan, J.J.; Wang, L.; Pan, D.; Yang, R.; Xu, Y.P.; Sheng, J.; Huang, Q.; Zhao, H.; Yang, M. Rational design of polyphenol-poloxamer nanovesicles for targeting inflammatory bowel disease therapy. Chem. Mater. 2018, 30, 4073–4080. [Google Scholar] [CrossRef]
- Diez-Echave, P.; Ruiz-Malagón, A.J.; Molina-Tijeras, J.A.; Hidalgo-García, L.; Vezza, T.; Cenis-Cifuentes, L.; Rodríguez-Sojo, M.J.; Cenis, J.L.; Rodríguez-Cabezas, M.E.; Rodríguez-Nogales, A.; et al. Silk fibroin nanoparticles enhance quercetin immunomodulatory properties in DSS-induced mouse colitis. Int. J. Pharm. 2021, 606, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Zhang, S.; Wu, Y.; Lu, T.; Liu, J.; Cao, X.; Liu, S.; Yan, L.; Shi, X.; Liu, G.; et al. Genistein-derived ROS-responsive nanoparticles relieve colitis by regulating mucosal homeostasis. ACS Appl. Mater. Interfaces 2021, 13, 40249–40266. [Google Scholar] [CrossRef]
- Nguyen, T.H.T.; Trinh, N.T.; Tran, H.N.; Tran, H.T.; Le, P.Q.; Ngo, D.N.; Tran-Van, H.; Van Vo, T.; Vong, L.B.; Nagasaki, Y. Improving silymarin oral bioavailability using silica-installed redox nanoparticle to suppress inflammatory bowel disease. J. Control. Release 2021, 331, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Marinho, S.; Illanes, M.; Ávila-Román, J.; Motilva, V.; Talero, E. Anti-inflammatory effects of rosmarinic acid-loaded nanovesicles in acute colitis through modulation of NLRP3 inflammasome. Biomolecules 2021, 11, 162. [Google Scholar] [CrossRef]
- Wang, T.; Fan, Q.; Hong, J.; Chen, Z.; Zhou, X.; Zhang, J.; Dai, Y.; Jiang, H.; Gu, Z.; Cheng, Y.; et al. Therapeutic nanoparticles from grape seed for modulating oxidative stress. Small 2021, 17, 2102485. [Google Scholar] [CrossRef]
- Zu, M.; Song, H.; Zhang, J.; Chen, Q.; Deng, S.; Canup, B.S.B.; Yuan, Y.; Xiao, B. Lycium barbarum lipid-based edible nanoparticles protect against experimental colitis. Colloids Surf. B Biointerfaces 2020, 187, 1–11. [Google Scholar] [CrossRef]
- Varthya, S.B.; Sarma, P.; Bhatia, A.; Shekhar, N.; Prajapat, M.; Kaur, H.; Thangaraju, P.; Kumar, S.; Singh, R.; Siingh, A.; et al. Efficacy of green tea, its polyphenols and nanoformulation in experimental colitis and the role of non-canonical and canonical nuclear factor kappa beta (NF-kB) pathway: A preclinical in-vivo and in-silico exploratory study. J. Biomol. Struct. Dyn. 2020, 39, 1–13. [Google Scholar] [CrossRef]
- Lee, Y.; Sugihara, K.; Gillilland, M.G.; Jon, S.; Kamada, N.; Moon, J.J. Hyaluronic acid–bilirubin nanomedicine for targeted modulation of dysregulated intestinal barrier, microbiome and immune responses in colitis. Nat. Mater. 2020, 19, 118–126. [Google Scholar] [CrossRef]
- Xu, C.; Qiao, L.; Ma, L.; Guo, Y.; Dou, X.; Yan, S.; Zhang, B.; Roman, A. Biogenic selenium nanoparticles synthesized by lactobacillus casei ATCC 393 alleviate intestinal epithelial barrier dysfunction caused by oxidative stress via Nrf2 signaling-mediated mitochondrial pathway. Int. J. Nanomed. 2019, 14, 4491–4502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, D.; Cheng, Y.; Li, X.; Wang, F.; Lu, Z.; Xiao, X.; Wang, Y. Biogenic nanoselenium particles effectively attenuate oxidative stress-induced intestinal epithelial barrier injury by activating the Nrf2 antioxidant pathway. ACS Appl. Mater. Interfaces 2017, 9, 14724–14740. [Google Scholar] [CrossRef]
- Zhu, C.; Zhang, S.; Song, C.; Zhang, Y.; Ling, Q.; Hoffmann, P.R.; Li, J.; Chen, T.; Zheng, W.; Huang, Z. Selenium nanoparticles decorated with Ulva lactuca polysaccharide potentially attenuate colitis by inhibiting NF-ΚB mediated hyper inflammation. J. Nanobiotechnol. 2017, 15, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.; Jiang, X.; Boudreau, M.D.; Feng, G.; Miao, Y.; Dong, S.; Wu, H.; Zeng, M.; Yin, J.J. Orally administered gold nanoparticles protect against colitis by attenuating Toll-like receptor 4- and reactive oxygen/nitrogen species-mediated inflammatory responses but could induce gut dysbiosis in mice. J. Nanobiotechnol. 2018, 16, 1–18. [Google Scholar] [CrossRef]
- Li, J.; Chen, H.; Wang, B.; Cai, C.; Yang, X.; Chai, Z.; Feng, W. ZnO nanoparticles act as supportive therapy in DSS-induced ulcerative colitis in mice by maintaining gut homeostasis and activating Nrf2 signaling. Sci. Rep. 2017, 7, 1–11. [Google Scholar]
- Zhang, C.; Shu, W.; Zhou, G.; Lin, J.; Chu, F.; Wu, H.; Liu, Z. Anti-TNF-α therapy suppresses proinflammatory activities of mucosal neutrophils in inflammatory bowel disease. Mediat. Inflamm. 2018, 2018, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Shi, J. Antioxidative nanomaterials and biomedical applications. Nano Today 2019, 27, 146–177. [Google Scholar] [CrossRef]
- Durán-lobato, M.; Niu, Z.; Alonso, M.J. Oral delivery of biologics for precision medicine. Adv. Mater. 2019, 1901935, 1–27. [Google Scholar] [CrossRef]
- Chacko, R.T.; Ventura, J.; Zhuang, J.; Thayumanavan, S. Polymer nanogels: A versatile nanoscopic drug delivery platform. J. Am. Chem. Soc. 2012, 64, 836–851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Wang, Z.; Gemeinhart, R.A. Progress in microRNA delivery. J. Control Release 2013, 172, 962–974. [Google Scholar] [CrossRef] [Green Version]
- Gebert, L.F.R.; MacRae, I.J. Regulation of microRNA function in animals. Nat. Rev. Mol. Cell Biol. 2019, 20, 21–37. [Google Scholar] [CrossRef]
- Espinosa-Diez, C.; Miguel, V.; Mennerich, D.; Kietzmann, T.; Sánchez-Pérez, P.; Cadenas, S.; Lamas, S. Antioxidant responses and cellular adjustments to oxidative stress. Redox Biol. 2015, 6, 183–197. [Google Scholar] [CrossRef] [Green Version]
- Cheng, T.Y.; Chen, M.H.; Chang, W.H.; Huang, M.Y.; Wang, T.W. Neural stem cells encapsulated in a functionalized self-assembling peptide hydrogel for brain tissue engineering. Biomaterials 2013, 34, 2005–2016. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Siegwart, D.J.; Anderson, D.G. Strategies, design, and chemistry in siRNA delivery systems. Adv. Drug Deliv. Rev. 2019, 144, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Lamprecht, A. Nanomedicines in gastroenterology and hepatology. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 195–204. [Google Scholar] [CrossRef]
- Zhao, R.; Sujuan, D.; Liu, Y.; Cong, L.; Song, Y.; Chen, X.; Zhang, B.; Li, D.; Gao, S.; Cui, W.; et al. Mucoadhesive-to-penetrating controllable peptosomes-in-microspheres co-loaded with anti-miR-31 oligonucleotide and Curcumin for targeted colorectal cancer therapy. Theranostics 2020, 10, 3594–3611. [Google Scholar] [CrossRef]
- Wilson, D.S.; Dalmasso, G.; Wang, L.; Sitaraman, S.V.; Merlin, D.; Murthy, N. Orally delivered thioketal nanoparticles loaded with TNF-α-siRNA target inflammation and inhibit gene expression in the intestines. Nat. Mater. 2010, 9, 923–928. [Google Scholar] [CrossRef]
- Xiao, B.; Laroui, H.; Ayyadurai, S.; Viennois, E.; Charania, M.A.; Zhang, Y.; Merlin, D. Mannosylated bioreducible nanoparticle-mediated macrophage-specific TNF-α RNA interference for IBD therapy. Biomaterials 2013, 34, 7471–7482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frede, A.; Neuhaus, B.; Klopfleisch, R.; Walker, C.; Buer, J.; Müller, W.; Epple, M.; Westendorf, A.M. Colonic gene silencing using siRNA-loaded calcium phosphate/PLGA nanoparticles ameliorates intestinal inflammation in vivo. J. Control Release 2016, 222, 86–96. [Google Scholar] [CrossRef]
- Aouadi, M.; Tesz, G.J.; Nicoloro, S.M.; Wang, M.; Chouinard, M.; Soto, E.; Ostroff, G.R.; Czech, M.P. Orally delivered siRNA targeting macrophage Map4k4 suppresses systemic inflammation. Nature 2009, 458, 1180–1184. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Tang, C.; Yin, C. Galactosylated trimethyl chitosan-cysteine nanoparticles loaded with Map4k4 siRNA for targeting activated macrophages. Biomaterials 2013, 34, 3667–3677. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, S.; Jon, S. Biotinylated bilirubin nanoparticles as a tumor microenvironment-responsive drug delivery system for targeted cancer therapy. Adv. Sci. 2018, 5, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.; Lee, S.; Lee, D.Y.; Yu, B.; Miao, W.; Jon, S. Multistimuli-responsive bilirubin nanoparticles for anticancer therapy. Angew. Chem. Int. Ed. 2016, 55, 10676–10680. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, H.; Kang, S.; Lee, J.; Park, J.; Jon, S. Bilirubin nanoparticles as a nanomedicine for anti-inflammation therapy. Angew. Chem. Int. Ed. 2016, 55, 7460–7463. [Google Scholar] [CrossRef]
- Salehi, B.; Stojanović-Radić, Z.; Matejić, J.; Sharifi-Rad, M.; Anil Kumar, N.V.; Martins, N.; Sharifi-Rad, J. The therapeutic potential of curcumin: A review of clinical trials. Eur. J. Med. Chem. 2019, 163, 527–545. [Google Scholar] [CrossRef]
- Xiao, B.; Zhang, Z.; Viennois, E.; Kang, Y.; Zhang, M.; Han, M.K.; Chen, J.; Merlin, D. Combination therapy for ulcerative colitis: Orally targeted nanoparticles prevent mucosal damage and relieve inflammation. Theranostics 2016, 6, 2250–2266. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wu, J.; Tang, Q.; Xu, C.; Huang, Y.; Huang, D.; Luo, F.; Wu, Y.; Yan, F.; Weng, Z.; et al. Nano-micelles based on hydroxyethyl starch-curcumin conjugates for improved stability, antioxidant and anticancer activity of curcumin. Carbohydr. Polym. 2020, 228, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.J.; Spencer, J.P.E.; Rice-Evans, C. Flavonoids: Antioxidants or signalling molecules? Free Radic. Biol. Med. 2004, 36, 838–849. [Google Scholar] [CrossRef]
- Vong, L.B.; Mo, J.; Abrahamsson, B.; Nagasaki, Y. Specific accumulation of orally administered redox nanotherapeutics in the inflamed colon reducing inflammation with dose-response efficacy. J. Control Release 2015, 210, 19–25. [Google Scholar] [CrossRef]
- Li, S.; Xie, A.; Li, H.; Zou, X.; Zhang, Q. A self-assembled, ROS-responsive Janus-prodrug for targeted therapy of inflammatory bowel disease. J. Control Release 2019, 316, 66–78. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Ni, D.; Rosenkrans, Z.T.; Huang, P.; Yan, X.; Cai, W. Nanozyme: New horizons for responsive biomedical applications. Chem. Soc. Rev. 2019, 48, 3683–3704. [Google Scholar] [CrossRef]
- Wang, H.; Wan, K.; Shi, X. Recent advances in nanozyme research. Adv. Mater. 2019, 31, 1–10. [Google Scholar] [CrossRef]
- Khurana, A.; Tekula, S.; Saifi, M.A.; Venkatesh, P.; Godugu, C. Therapeutic applications of selenium nanoparticles. Biomed. Pharmacother. 2019, 111, 802–812. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Qu, X. Cerium oxide nanoparticle: A remarkably versatile rare earth nanomaterial for biological applications. NPG Asia Mater. 2014, 6, 1–16. [Google Scholar] [CrossRef]
- Kaplan, G.G.; Windsor, J.W. The four epidemiological stages in the global evolution of inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 56–66. [Google Scholar] [CrossRef]
- Lee, B.C.; Lee, J.Y.; Kim, J.; Yoo, J.M.; Kang, I.; Kim, J.J.; Shin, N.; Kim, D.J.; Choi, S.W.; Kim, D.; et al. Graphene quantum dots as anti-inflammatory therapy for colitis. Sci. Adv. 2020, 6, 1–14. [Google Scholar] [CrossRef] [PubMed]


| Antioxidants | Type of Compounds | Nanosystem Components | Size and Surface Charge | Colitis Model | References |
|---|---|---|---|---|---|
| Protein/peptide | |||||
| SOD/CAT | Antioxidante enzyme | WCC | ~156 nm | DSS-induced mice | [43] |
| TNF-α antibody/tannic acid/ EGCG | Protein/polyphenol | DSPE-PEG | ~100 nm | DSS-induced mice | [44] |
| Ac2-26 | Peptide | PBAP conjugatedβ-CD | 202 ± 4 nm, −37.4 ± 0.6 mV | DSS-induced mice | [45] |
| SEP | Protein | Chitosan/Fucoidan | tunable | LPS-induced macrophage | [46] |
| Anti-TNF-α antibody | Protein | Galactose/PLGA | ~261 nm, ~−6 mV | DSS-induced mice | [22] |
| KPV | Peptide | PLGA/PVA/HA/chitosan | ~270 nm, −5.3 mV | DSS-induced mice | [47] |
| Nucleic acid | |||||
| IL-10 mRNA | Modified mRNA | Lipid | 63.7 ± 1.59 nm | DSS-induced mice | [48] |
| Anti-miRNA-31/Curcumin | MiRNA inhibitor/polyphenol | α-lactalbumin/OKGM (nano-in-micro) | ~25 μm, ~−7 mV | AOM-DSS-induced | [49] |
| TNF-α siRNA/Dexamethasone | SiRNA/small molecule | TKPR-PEG-P(TMC-DTC), PEG-P(TMC-DTC)-PEI | ~500 nm, ~0.6 mV | DSS-induced mice | [50] |
| TNF-α siRNA | SiRNA | PEG-b-PLGA | ~120 nm, −17 mV~31 mV | DSS-induced mice | [41] |
| TNF-α siRNA | SiRNA | PVA/PLGA | ~300 nm, ~20 mV | DSS-induced mice | [51] |
| Small molecule | |||||
| Curcumin/Dex | Polyphenol/ glucocorticoid | PLGA/HPMCAS-HF (nano-in-micro) | ~176 nm | HT29-MTX/T84 cell line | [52] |
| Curcumin | Polyphenol | Chitosan/alginate/cellulose | 421 ± 14 nm, −47 ± 3 mV | DSS-induced mice | [53] |
| Curcumin/tannic acid | Polyphenol | Genipin-crosslinked HBA | ~220 nm, −28.8 mV | TNBS-induced mice | [54] |
| Curcumin | Polyphenol | Silk fibroin/Chondroitin sulfate | ~175.4 nm, −35.5 mV | DSS-induced mice | [55] |
| Curcumin | Polyphenol | Eudragit® S100 | DSS-induced mice | [56] | |
| Resveratrol | Polyphenol | β-Lactoglobulin | 165 ± 2 nm, −34 ± 0.6 mV | Winnie mice | [57] |
| Resveratrol | Polyphenol | PLGA/chitosan/alginate | 255.9 ± 12.0 nm, 13.5 ± 3.9 mV | DSS-induced mice | [58] |
| Resveratrol | Polyphenol | Chitosan/pHEMA/in pDMAEMA (nano-in-gel) | 121 ± 1 nm, −170 ± 90 mV | DSS-induced mice | [59] |
| Rosmarinic acid | Polyphenol | Chitosan/nutriose | 63.5 ± 4.0 nm, −33.70 mV | DSS-induced mice | [60] |
| Rosmarinic acid | Polyphenol | PEG | 141.2 ± 12.3 nm, −25.30 ± 2.7 mV | DSS-induced mice | [10] |
| Oleuropein | Polyphenol | Lipid | ~ 150 nm, −25 mV | DSS-induced mice | [61] |
| EGCG | Polyphenol | Amyloid | - | DSS-induced mice | [62] |
| Tannicacid/EGCG/catechin | Polyphenol/glucocorticoid | Block PEG | ~130 nm, −27 mV | DSS-induced mice | [63] |
| Quercetin | Flavonoids | Silk fibroin | 175.8 ± 0.9 nm, −24.5 ± 4.1 mV | DSS-induced mice | [64] |
| Genistein/Tempol/VE | Flavonoids/ | β-CD/HMPBA/TPGS | 636 ± 94 nm/304 ± 60 nm | DSS-induced mice | [65] |
| Silymarin | Synthetic antioxidant compound | Silica-derived | −21.08 ± 1.51/6.63 ± 1.91 mV | DSS-induced mice | [66] |
| Ginsenoside | Flavonoids | Glycogen-derived | ~110 nm | DSS-induced mice | [67] |
| Grape seed extract/ | Steroid glycosides | Grape seed extract/ | 128.9 ± 0.3 nm, 1.3 ± 0.08 mV | DSS-induced mice | [68] |
| Horseradish peroxidase | Plant extract/antioxidant enzyme | Horseradish peroxidase | |||
| Lycium barbarum | Plant extract | Lipid | ~189.2 nm, ~−34.9 mV | DSS-induced mice | [69] |
| Green tea extract | Plant extract | PLA-PEG | ~163.1 nm, ~−7.92 mV | TNBS-induced rat | [70] |
| Bilirubin | Small molecule | HA | 86 ± 5 nm to 416 ± 9 nm | DSS-induced mice | [71] |
| −35.6 ± 1.6 mV to −46.2 ± 5.2 mV | |||||
| Nanozyme | |||||
| CeO2 | Nanozyme | Red blood vesicle/exosome | ~3 nm | DSS-induced mice | [11] |
| CeO2 | Nanozyme | MMT/CeO2 | 1.6 ± 0.2 nm, −30.3 ± 0.3 mV | DSS-induced mice | [19] |
| Prussian blue/Mn | Nanozyme | PVP | 60 nm~120 nm, −27.0 mV | DSS-induced mice | [18] |
| Prussian blue | Nanozyme | PVP | ~60 nm | DSS-induced mice | [20] |
| Se | Nonozyme | Lactobacillus casei produced | 50~80 nm | NCM460 cells | [72] |
| Se | Nanozyme | Enterobacter cloacae Z0206 produced | 139.43 ± 7.44 nm | DSS-induced mice | [73] |
| Se | Nanozyme | Ulva lactuca polysaccharide | 30 to 150 nm | DSS-induced mice | [74] |
| Gold | Nanozyme | PVP/Citrate | ~5 nm | DSS-induced mice | [75] |
| ZnO | Nanozyme | ZnO | 29.7 ± 4.0 nm, −59.4 ± 3.8 mV | DSS-induced mice | [76] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, P.; Li, Y.; Wang, R.; Ren, F.; Wang, X. Oxidative Stress and Antioxidant Nanotherapeutic Approaches for Inflammatory Bowel Disease. Biomedicines 2022, 10, 85. https://doi.org/10.3390/biomedicines10010085
Liu P, Li Y, Wang R, Ren F, Wang X. Oxidative Stress and Antioxidant Nanotherapeutic Approaches for Inflammatory Bowel Disease. Biomedicines. 2022; 10(1):85. https://doi.org/10.3390/biomedicines10010085
Chicago/Turabian StyleLiu, Ping, Yixuan Li, Ran Wang, Fazheng Ren, and Xiaoyu Wang. 2022. "Oxidative Stress and Antioxidant Nanotherapeutic Approaches for Inflammatory Bowel Disease" Biomedicines 10, no. 1: 85. https://doi.org/10.3390/biomedicines10010085
APA StyleLiu, P., Li, Y., Wang, R., Ren, F., & Wang, X. (2022). Oxidative Stress and Antioxidant Nanotherapeutic Approaches for Inflammatory Bowel Disease. Biomedicines, 10(1), 85. https://doi.org/10.3390/biomedicines10010085






