Interleukin-8 in Colorectal Cancer: A Systematic Review and Meta-Analysis of Its Potential Role as a Prognostic Biomarker
Abstract
:1. Introduction
2. Materials and Methods
2.1. Analysis of IL-8 Expression Levels in CRC and Normal Samples
2.2. Case Selection
2.3. Tissue MicroArray (TMA) Construction and ImmunoHistoChemistry (IHC)
2.4. DNA Extraction
2.5. Search Strategy
- PubMed: ((((“Interleukin-8”[Mesh]) AND (“Colorectal Neoplasms”[Mesh] AND “Prognosis”[Mesh]) AND (“1994/01/01”[PDat] : “2021/06/30”[PDat]))) OR (((((Prognosis) AND ((Colorectal Neoplas* OR CRC OR Colorectal Cancer OR Colorectal tumor)))) AND (((Interleukin-8 OR IL-8 OR interleukin8 OR IL8)))) AND (“1994/01/01”[PDat] : “2021/06/30”[PDat])))
- (‘colorectal neoplasm’/exp OR ‘colorectal neoplasm’ OR (colorectal AND (‘neoplasm’/exp OR neoplasm)) OR crc OR ‘colorectal cancer’/exp OR ‘colorectal cancer’ OR (colorectal AND (‘cancer’/exp OR cancer)) OR ‘colorectal tumor’/exp OR ‘colorectal tumor’ OR (colorectal AND (‘tumor’/exp OR tumor))) AND (‘interleukin 8’/exp OR ‘interleukin 8’ OR ((‘interleukin’/exp OR interleukin) AND 8) OR ‘il 8’/exp OR ‘il 8’ OR (il AND 8) OR interleukin8 OR il8) AND (1994:py OR 1995:py OR 1996:py OR 1997:py OR 1998:py OR 1999:py OR 2000:py OR 2001:py OR 2002:py OR 2003:py OR 2004:py OR 2005:py OR 2006:py OR 2007:py OR 2008:py OR 2009:py OR 2010:py OR 2011:py OR 2012:py OR 2013:py OR 2014:py OR 2015:py OR 2016:py OR 2017:py OR 2018:py OR 2019:py OR 2020:py OR 2021:py) AND (‘bevacizumab’/dd OR ‘monoclonal antibody’/dd) AND ‘colorectal cancer’/dm AND (‘clinical study’/de OR ‘clinical trial’/de OR ‘cohort analysis’/de OR ‘controlled clinical trial’/de OR ‘controlled study’/de OR ‘meta analysis’/de OR ‘multicenter study’/de OR ‘phase 2 clinical trial’/de OR ‘phase 2 clinical trial topic’/de OR ‘phase 3 clinical trial’/de OR ‘phase 3 clinical trial topic’/de OR ‘prospective study’/de OR ‘randomized controlled trial’/de OR ‘randomized controlled trial topic’/de OR ‘retrospective study’/de) AND ‘avastin’/tn
2.6. Inclusion and Exclusion Criteria
- Original papers;
- All IL-8 expression evaluations, including serum, plasma, and tissue;
- Studies with hazard ratio (HR) and 95% confidence intervals (95% CI) for OS or PFS.
- Papers reporting data which cannot be extracted;
- Non-English papers;
- Non-full-length papers;
- Reviews, systematic reviews, meta-analysis, book chapters, congress abstracts;
- Animal studies or basic research papers;
- Studies on gene polymorphisms;
- Studies also containing other histotypes different from CRC;
- Papers not relevant to the topic;
- Note publications, retracted articles.
3. Results
3.1. IL-8 in TSI
3.2. IL-8 Expression in CRC Patients
3.2.1. IL-8 Expression Analysis in RNA-Sequencing Data Resources
3.2.2. IL-8 Analysis in Tumor Specimens from CRC Patients
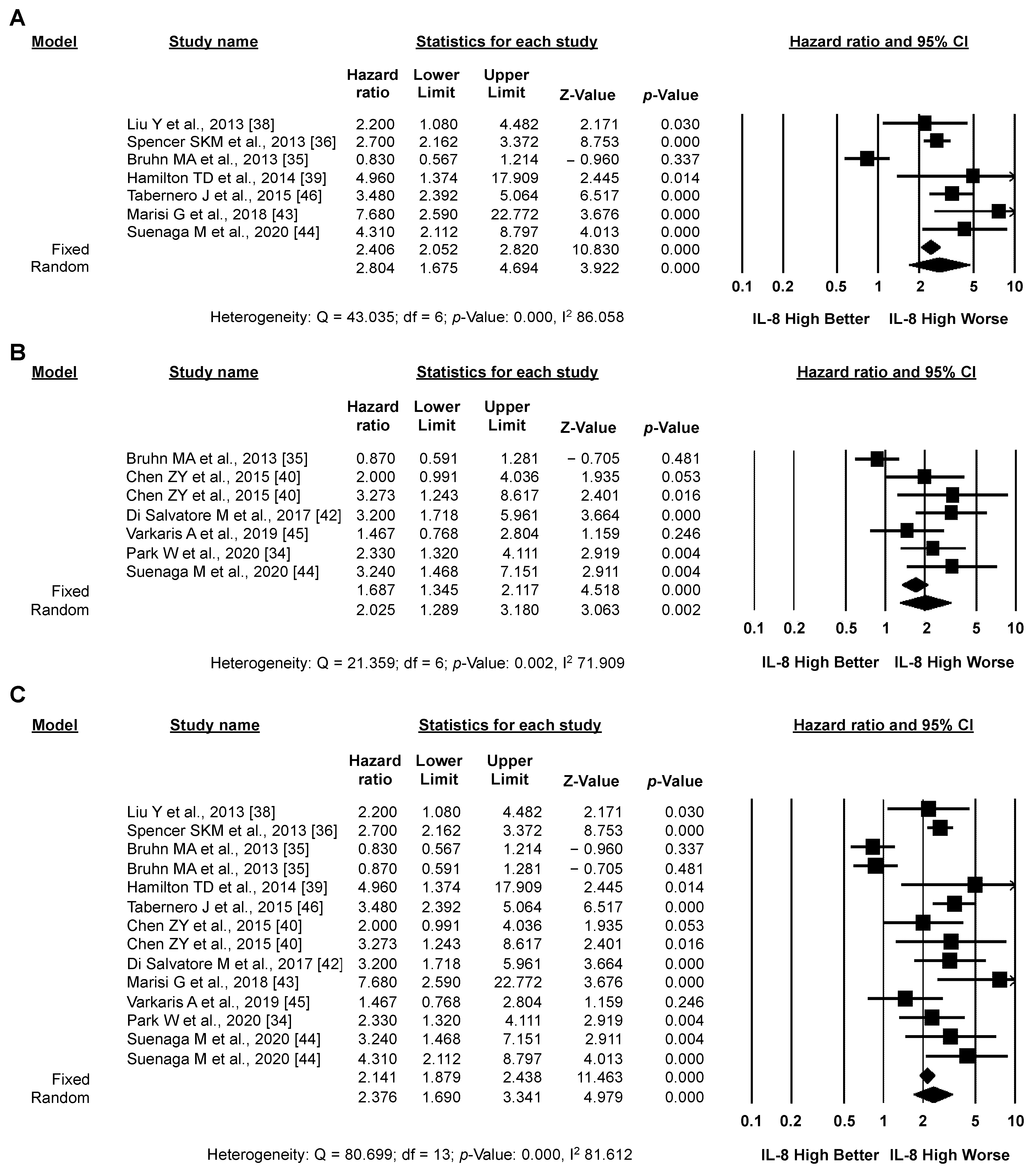
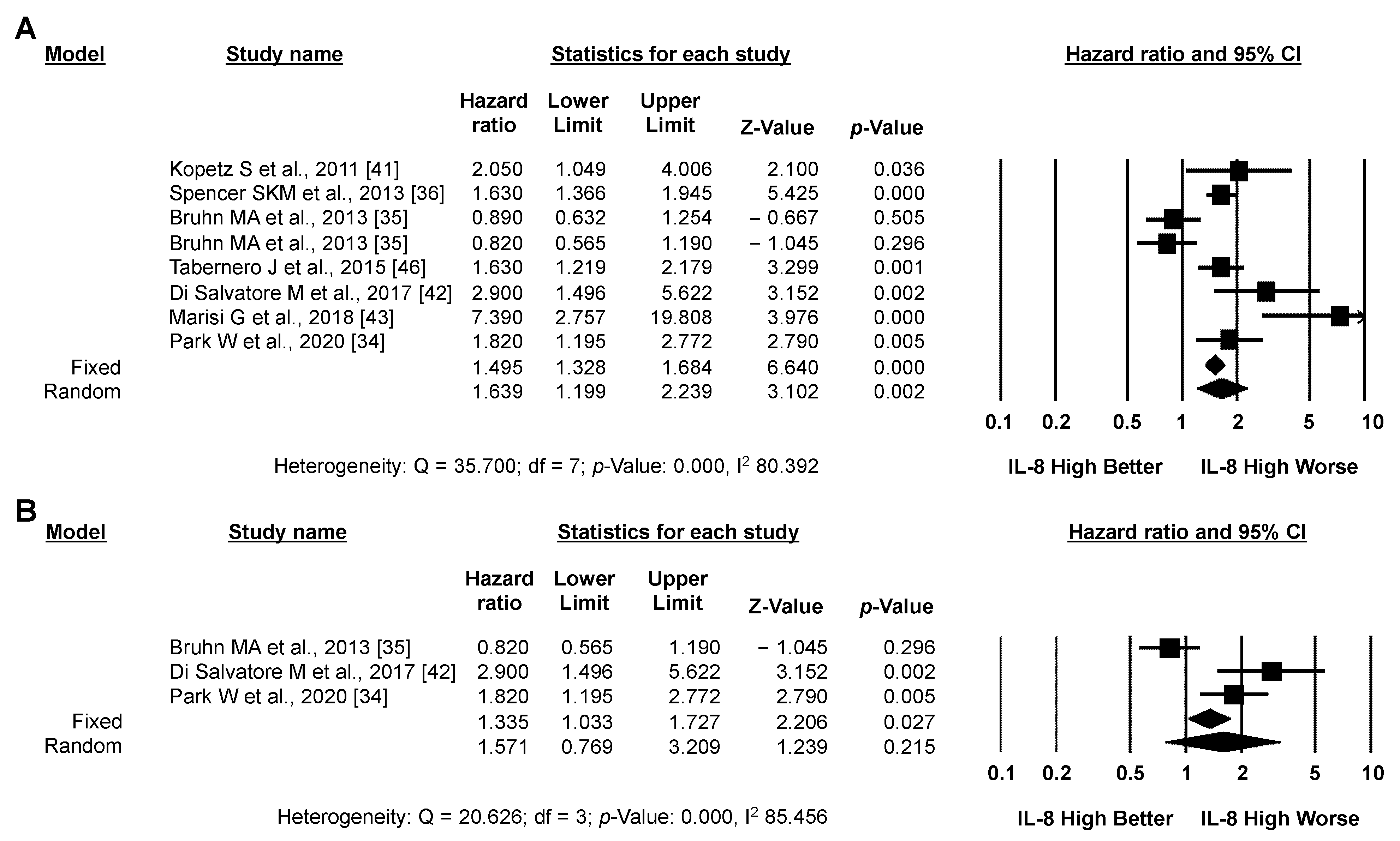
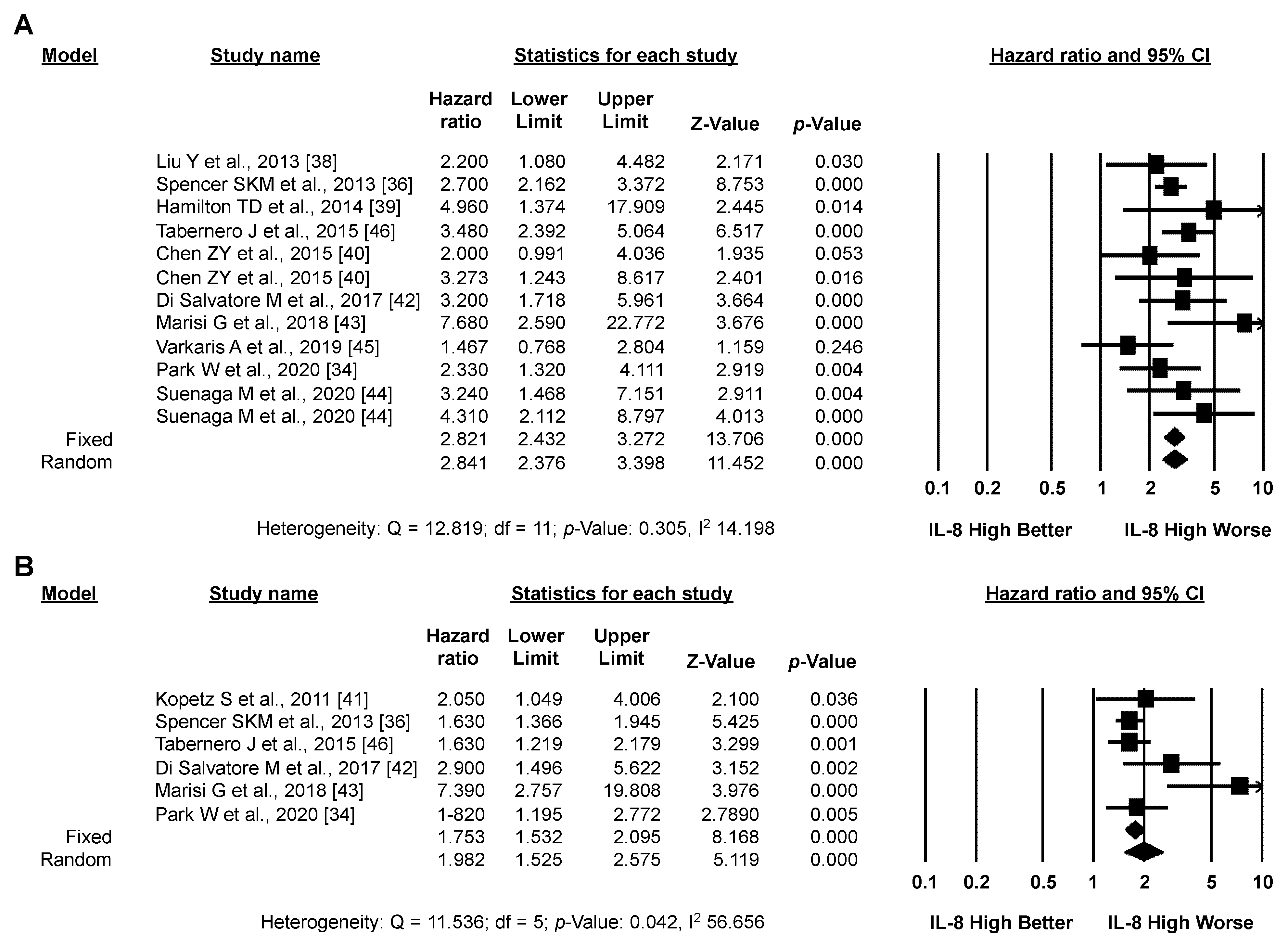
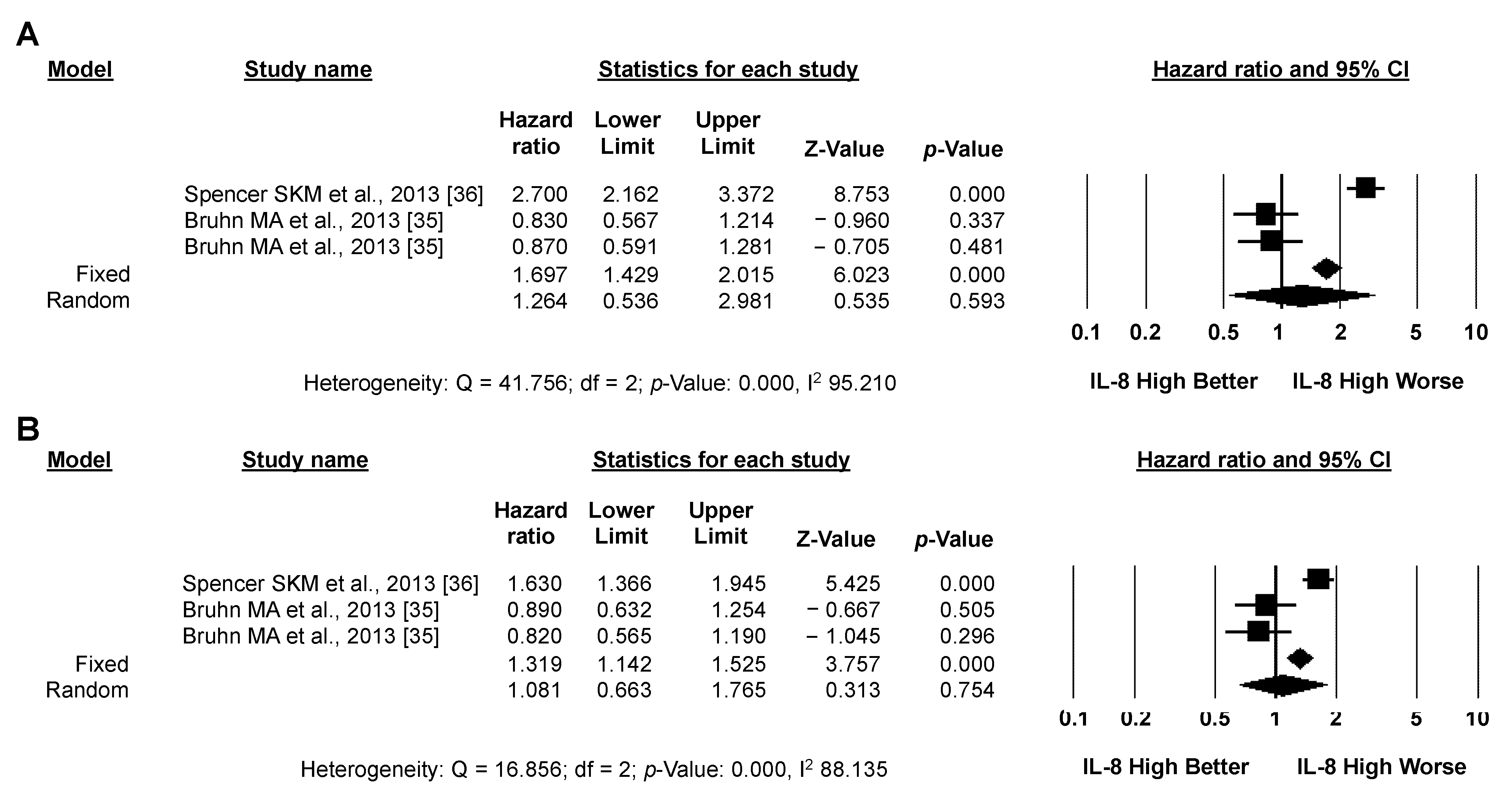
3.3. Meta-Analysis: IL-8 and CRC Prognosis
3.3.1. Primary Analysis: OS
3.3.2. Secondary Analysis: PFS
3.3.3. Exploratory Analysis: Sensitivity Analysis According to IL-8 Source and the Type of Systemic Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Q.-H.; Wang, Y.-Z.; Tu, J.; Liu, C.-W.; Yuan, Y.-J.; Lin, R.; He, W.-L.; Cai, S.-R.; He, Y.-L.; Ye, J.-N. Anti-EGFR therapy in metastatic colorectal cancer: Mechanisms and potential regimens of drug resistance. Gastroenterol. Rep. 2020, 8, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Malki, A.; Abu ElRuz, R.; Gupta, I.; Allouch, A.; Vranic, S.; Al Moustafa, A.-E. Molecular Mechanisms of Colon Cancer Progression and Metastasis: Recent Insights and Advancements. Int. J. Mol. Sci. 2020, 22, 130. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.-H.; Chen, Y.-X.; Fang, J.-Y. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct. Target. Ther. 2020, 5, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Wu, T.; Cai, X.; Dong, J.; Xia, C.; Zhou, Y.; Ding, R.; Yang, R.; Tan, J.; Zhang, L.; et al. Neoadjuvant Immunotherapy for MSI-H/dMMR Locally Advanced Colorectal Cancer: New Strategies and Unveiled Opportunities. Front. Immunol. 2022, 13, 795972. [Google Scholar] [CrossRef]
- Bazzichetto, C.; Conciatori, F.; Falcone, I.; Cognetti, F.; Milella, M.; Ciuffreda, L. Advances in Tumor-Stroma Interactions: Emerging Role of Cytokine Network in Colorectal and Pancreatic Cancer. J. Oncol. 2019, 2019, 5373580. [Google Scholar] [CrossRef]
- Itatani, Y.; Kawada, K.; Inamoto, S.; Yamamoto, T.; Ogawa, R.; Taketo, M.M.; Sakai, Y. The Role of Chemokines in Promoting Colorectal Cancer Invasion/Metastasis. Int. J. Mol. Sci. 2016, 17, 643. [Google Scholar] [CrossRef] [Green Version]
- Ha, H.; Debnath, B.; Neamati, N. Role of the CXCL8-CXCR1/2 Axis in Cancer and Inflammatory Diseases. Theranostics 2017, 7, 1543–1588. [Google Scholar] [CrossRef]
- Najdaghi, S.; Razi, S.; Rezaei, N. An overview of the role of interleukin-8 in colorectal cancer. Cytokine 2020, 135, 155205. [Google Scholar] [CrossRef]
- Li, A.; Dubey, S.; Varney, M.L.; Dave, B.J.; Singh, R.K. IL-8 Directly Enhanced Endothelial Cell Survival, Proliferation, and Matrix Metalloproteinases Production and Regulated Angiogenesis. J. Immunol. 2003, 170, 3369–3376. [Google Scholar] [CrossRef] [Green Version]
- Conciatori, F.; Bazzichetto, C.; Falcone, I.; Ferretti, G.; Cognetti, F.; Milella, M.; Ciuffreda, L. Colorectal cancer stem cells properties and features: Evidence of interleukin-8 involvement. Cancer Drug Resist 2019, 2, 968–979. [Google Scholar] [CrossRef]
- Xu, H.; Lai, W.; Zhang, Y.; Liu, L.; Luo, X.; Zeng, Y.; Wu, H.; Lan, Q.; Chu, Z. Tumor-associated macrophage-derived IL-6 and IL-8 enhance invasive activity of LoVo cells induced by PRL-3 in a KCNN4 channel-dependent manner. BMC Cancer 2014, 14, 330. [Google Scholar] [CrossRef] [Green Version]
- Xia, W.; Chen, W.; Zhang, Z.; Wu, D.; Wu, P.; Chen, Z.; Li, C.; Huang, J. Prognostic Value, Clinicopathologic Features and Diagnostic Accuracy of Interleukin-8 in Colorectal Cancer: A Meta-Analysis. PLoS ONE 2015, 10, e0123484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The ICGC/TCGA Pan-Cancer Analysis of Whole Genomes Consortium; Campbell, P.J.; Getz, G. Pan-cancer analysis of whole genomes. Nature 2020, 578, 82–93. [Google Scholar] [CrossRef] [Green Version]
- GTEx Consortium. The Genotype-Tissue Expression (GTEx) pilot analysis: Multitissue gene regulation in humans. Science 2015, 348, 648–660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cunningham, F.; Allen, J.E.; Allen, J.; Alvarez-Jarreta, J.; Amode, M.R.; Armean, I.M.; Austine-Orimoloye, O.; Azov, A.G.; Barnes, I.; Bennett, R.; et al. Ensembl 2022. Nucleic Acids Res. 2022, 50, D988–D995. [Google Scholar] [CrossRef]
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. TNM Classification of Malignant Tumours, 7th ed.; Blackwell Publishing Ltd.: Singapore, 2009. [Google Scholar]
- Conciatori, F.; Bazzichetto, C.; Amoreo, C.A.; Sperduti, I.; Donzelli, S.; Diodoro, M.G.; Buglioni, S.; Falcone, I.; Shirasawa, S.; Blandino, G.; et al. BRAF status modulates Interelukin-8 expression through a CHOP-dependent mechanism in colorectal cancer. Commun. Biol. 2020, 3, 546. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, E.; Dittrich-Breiholz, O.; Holtmann, H.; Kracht, M. Multiple control of interleukin-8 gene expression. J. Leukoc. Biol. 2002, 72, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Ciuffreda, L.; Del Bufalo, D.; Desideri, M.; Di Sanza, C.; Stoppacciaro, A.; Ricciardi, M.R.; Chiaretti, S.; Tavolaro, S.; Benassi, B.; Bellacosa, A.; et al. Growth-Inhibitory and Antiangiogenic Activity of the MEK Inhibitor PD0325901 in Malignant Melanoma with or without BRAF Mutations. Neoplasia 2009, 11, 720–731. [Google Scholar] [CrossRef] [Green Version]
- Ackermann, A.; Lafferton, B.; Plotz, G.; Zeuzem, S.; Brieger, A. Expression and secretion of the pro-inflammatory cytokine IL-8 is increased in colorectal cancer cells following the knockdown of non-erythroid spectrin αII. Int. J. Oncol. 2020, 56, 1551–1564. [Google Scholar] [CrossRef]
- Wu, H.-X.; Cheng, X.; Jing, X.-Q.; Ji, X.-P.; Chen, X.-Z.; Zhang, Y.-Q.; He, Y.-G.; Liu, K.; Ye, F.; Sun, H.-X.; et al. LIFR promotes tumor angiogenesis by up-regulating IL-8 levels in colorectal cancer. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2018, 1864, 2769–2784. [Google Scholar] [CrossRef]
- Itatani, Y.; Kawada, K.; Sakai, Y. Transforming Growth Factor-β Signaling Pathway in Colorectal Cancer and Its Tumor Microenvironment. Int. J. Mol. Sci. 2019, 20, 5822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogawa, R.; Yamamoto, T.; Hirai, H.; Hanada, K.; Kiyasu, Y.; Nishikawa, G.; Mizuno, R.; Inamoto, S.; Itatani, Y.; Sakai, Y.; et al. Loss of SMAD4 Promotes Colorectal Cancer Progression by Recruiting Tumor-Associated Neutrophils via the CXCL1/8–CXCR2 Axis. Clin. Cancer Res. 2019, 25, 2887–2899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, C.-J.; Chien, Y.; Lu, K.-H.; Chang, S.-C.; Chou, Y.-C.; Huang, C.-S.; Chang, C.-H.; Chen, K.-H.; Chang, Y.-L.; Tseng, L.-M.; et al. Oct4-related cytokine effects regulate tumorigenic properties of colorectal cancer cells. Biochem. Biophys. Res. Commun. 2011, 415, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; He, Y.; Li, P.; Wu, W.; Chen, Y.; Ruan, H. IL-8 regulates the doxorubicin resistance of colorectal cancer cells via modulation of multidrug resistance 1 (MDR1). Cancer Chemother. Pharmacol. 2018, 81, 1111–1119. [Google Scholar] [CrossRef] [PubMed]
- Ning, Y.; Manegold, P.C.; Hong, Y.K.; Zhang, W.; Pohl, A.; Lurje, G.; Winder, T.; Yang, D.; LaBonte, M.J.; Wilson, M.L.; et al. Interleukin-8 is associated with proliferation, migration, angiogenesis and chemosensitivity in vitro and in vivo in colon cancer cell line models. Int. J. Cancer 2011, 128, 2038–2049. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, K.; Shiga, K.; Maeda, A.; Harata, S.; Yanagita, T.; Suzuki, T.; Ushigome, H.; Maeda, Y.; Hirokawa, T.; Ogawa, R.; et al. Chitinase 3-like 1 secreted from cancer-associated fibroblasts promotes tumor angiogenesis via interleukin-8 secretion in colorectal cancer. Int. J. Oncol. 2022, 60, 3. [Google Scholar] [CrossRef]
- Yuen, K.C.; Liu, L.-F.; Gupta, V.; Madireddi, S.; Keerthivasan, S.; Li, C.; Rishipathak, D.; Williams, P.; Kadel, E.E., 3rd; Koeppen, H.; et al. High systemic and tumor-associated IL-8 correlates with reduced clinical benefit of PD-L1 blockade. Nat. Med. 2020, 26, 693–698. [Google Scholar] [CrossRef]
- Amorim, C.; Docasar, C.L.; Guimarães-Bastos, D.; Frony, A.C.; Barja-Fidalgo, C.; Renovato-Martins, M.; Moraes, J.A. Extracellular Vesicles Derived from MDA-MB-231 Cells Trigger Neutrophils to a Pro-Tumor Profile. Cells 2022, 11, 1875. [Google Scholar] [CrossRef]
- Shang, A.; Gu, C.; Zhou, C.; Yang, Y.; Chen, C.; Zeng, B.; Wu, J.; Lu, W.; Wang, W.; Sun, Z.; et al. Exosomal KRAS mutation promotes the formation of tumor-associated neutrophil extracellular traps and causes deterioration of colorectal cancer by inducing IL-8 expression. Cell Commun. Signal. 2020, 18, 52. [Google Scholar] [CrossRef] [Green Version]
- Burz, C.; Bojan, A.; Balacescu, L.; Pop, V.-V.; Silaghi, C.; Lupan, I.; Aldea, C.; Sur, D.; Samasca, G.; Cainap, C.; et al. Interleukin 8 as predictive factor for response to chemotherapy in colorectal cancer patients. Acta Clin. Belg. 2021, 76, 113–118. [Google Scholar] [CrossRef]
- Komura, T.; Yano, M.; Miyake, A.; Takabatake, H.; Miyazawa, M.; Ogawa, N.; Seki, A.; Honda, M.; Wada, T.; Matsui, S.; et al. Immune Condition of Colorectal Cancer Patients Featured by Serum Chemokines and Gene Expressions of CD4+ Cells in Blood. Can. J. Gastroenterol. Hepatol. 2018, 2018, 7436205. [Google Scholar] [CrossRef] [PubMed]
- Oladipo, O.; Conlon, S.; O’Grady, A.; Purcell, C.; Wilson, C.; Maxwell, P.J.; Johnston, P.G.; Stevenson, M.; Kay, E.W.; Wilson, R.H.; et al. The expression and prognostic impact of CXC-chemokines in stage II and III colorectal cancer epithelial and stromal tissue. Br. J. Cancer 2011, 104, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Chang, H.J.; Yeo, H.Y.; Han, N.; Kim, B.C.; Kong, S.-Y.; Kim, J.; Oh, J.H. The relationships between systemic cytokine profiles and inflammatory markers in colorectal cancer and the prognostic significance of these parameters. Br. J. Cancer 2020, 123, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Bruhn, M.A.; Townsend, A.R.; Lee, C.K.; Shivasami, A.; Price, T.J.; Wrin, J.; Arentz, G.; Tebbutt, N.C.; Hocking, C.; Cunningham, D.; et al. Proangiogenic tumor proteins as potential predictive or prognostic biomarkers for bevacizumab therapy in metastatic colorectal cancer. Int. J. Cancer 2013, 135, 731–741. [Google Scholar] [CrossRef]
- Spencer, S.K.M.; Pommier, A.J.C.; Morgan, S.R.; Barry, S.T.; Robertson, J.D.; Hoff, P.M.; Jürgensmeier, J.M. Prognostic/predictive value of 207 serum factors in colorectal cancer treated with cediranib and/or chemotherapy. Br. J. Cancer 2013, 109, 2765–2773. [Google Scholar] [CrossRef] [Green Version]
- Bilusic, M.; Heery, C.R.; Collins, J.M.; Donahue, R.N.; Palena, C.; Madan, R.A.; Karzai, F.; Marté, J.L.; Strauss, J.; Gatti-Mays, M.E.; et al. Phase I trial of HuMax-IL8 (BMS-986253), an anti-IL-8 monoclonal antibody, in patients with metastatic or unresectable solid tumors. J. Immunother. Cancer 2019, 7, 240. [Google Scholar] [CrossRef]
- Liu, Y.; Starr, M.D.; Bulusu, A.; Pang, H.; Wong, N.S.; Honeycutt, W.; Amara, A.; Hurwitz, H.I.; Nixon, A.B. Correlation of angiogenic biomarker signatures with clinical outcomes in metastatic colorectal cancer patients receiving capecitabine, oxaliplatin, and bevacizumab. Cancer Med. 2013, 2, 234–242. [Google Scholar] [CrossRef]
- Hamilton, T.D.; Leugner, D.; Kopciuk, K.; Dixon, E.; Sutherland, F.R.; Bathe, O.F. Identification of prognostic inflammatory factors in colorectal liver metastases. BMC Cancer 2014, 14, 542. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.-Y.; He, W.-Z.; Peng, L.-X.; Jia, W.-H.; Guo, R.-P.; Xia, L.-P.; Qian, C.-N. A prognostic classifier consisting of 17 circulating cytokines is a novel predictor of overall survival for metastatic colorectal cancer patients. Int. J. Cancer 2015, 136, 584–592. [Google Scholar] [CrossRef]
- Kopetz, S.; Hoff, P.M.; Morris, J.S.; Wolff, R.A.; Eng, C.; Glover, K.Y.; Adinin, R.; Overman, M.J.; Valero, V.; Wen, S.; et al. Phase II Trial of Infusional Fluorouracil, Irinotecan, and Bevacizumab for Metastatic Colorectal Cancer: Efficacy and Circulating Angiogenic Biomarkers Associated With Therapeutic Resistance. J. Clin. Oncol. 2011, 28, 453–459. [Google Scholar] [CrossRef]
- Di Salvatore, M.; Pietrantonio, F.; Orlandi, A.; Del Re, M.; Berenato, R.; Rossi, E.; Caporale, M.; Guarino, D.; Martinetti, A.; Basso, M.; et al. IL-8 and eNOS polymorphisms predict bevacizumab-based first line treatment outcomes in RAS mutant metastatic colorectal cancer patients. Oncotarget 2017, 8, 16887–16898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marisi, G.; Scarpi, E.; Passardi, A.; Nanni, O.; Pagan, F.; Valgiusti, M.; Gardini, A.C.; Neri, L.M.; Frassineti, G.L.; Amadori, D.; et al. IL-8 and thrombospondin-1 as prognostic markers in patients with metastatic colorectal cancer receiving bevacizumab. Cancer Manag. Res. 2018, 10, 5659–5666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suenaga, M.; Mashima, T.; Kawata, N.; Wakatsuki, T.; Dan, S.; Seimiya, H.; Yamaguchi, K. Serum IL-8 level as a candidate prognostic marker of response to anti-angiogenic therapy for metastatic colorectal cancer. Int. J. Color. Dis. 2020, 36, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Varkaris, A.; Katsiampoura, A.; Davis, J.S.; Shah, N.; Lam, M.; Frias, R.L.; Ivan, C.; Shimizu, M.; Morris, J.; Menter, D.; et al. Circulating inflammation signature predicts overall survival and relapse-free survival in metastatic colorectal cancer. Br. J. Cancer 2019, 120, 340–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabernero, J.; Lenz, H.-J.; Siena, S.; Sobrero, A.; Falcone, A.; Ychou, M.; Humblet, Y.; Bouché, O.; Mineur, L.; Barone, C.; et al. Analysis of circulating DNA and protein biomarkers to predict the clinical activity of regorafenib and assess prognosis in patients with metastatic colorectal cancer: A retrospective, exploratory analysis of the CORRECT trial. Lancet Oncol. 2015, 16, 937–948. [Google Scholar] [CrossRef]
- Xiao, Y.-C.; Yang, Z.-B.; Cheng, X.-S.; Fang, X.-B.; Shen, T.; Xia, C.-F.; Liu, P.; Qian, H.-H.; Sun, B.; Yin, Z.-F.; et al. CXCL8, overexpressed in colorectal cancer, enhances the resistance of colorectal cancer cells to anoikis. Cancer Lett. 2015, 361, 22–32. [Google Scholar] [CrossRef]
- Merz, V.; Zecchetto, C.; Santoro, R.; Simionato, F.; Sabbadini, F.; Mangiameli, D.; Piro, G.; Cavaliere, A.; Deiana, M.; Valenti, M.T.; et al. Plasma IL8 Is a Biomarker for TAK1 Activation and Predicts Resistance to Nanoliposomal Irinotecan in Patients with Gemcitabine-Refractory Pancreatic Cancer. Clin. Cancer Res. 2020, 26, 4661–4669. [Google Scholar] [CrossRef]
- Schalper, K.A.; Carleton, M.; Zhou, M.; Chen, T.; Feng, Y.; Huang, S.-P.; Walsh, A.M.; Baxi, V.; Pandya, D.; Baradet, T.; et al. Elevated serum interleukin-8 is associated with enhanced intratumor neutrophils and reduced clinical benefit of immune-checkpoint inhibitors. Nat. Med. 2020, 26, 688–692. [Google Scholar] [CrossRef]

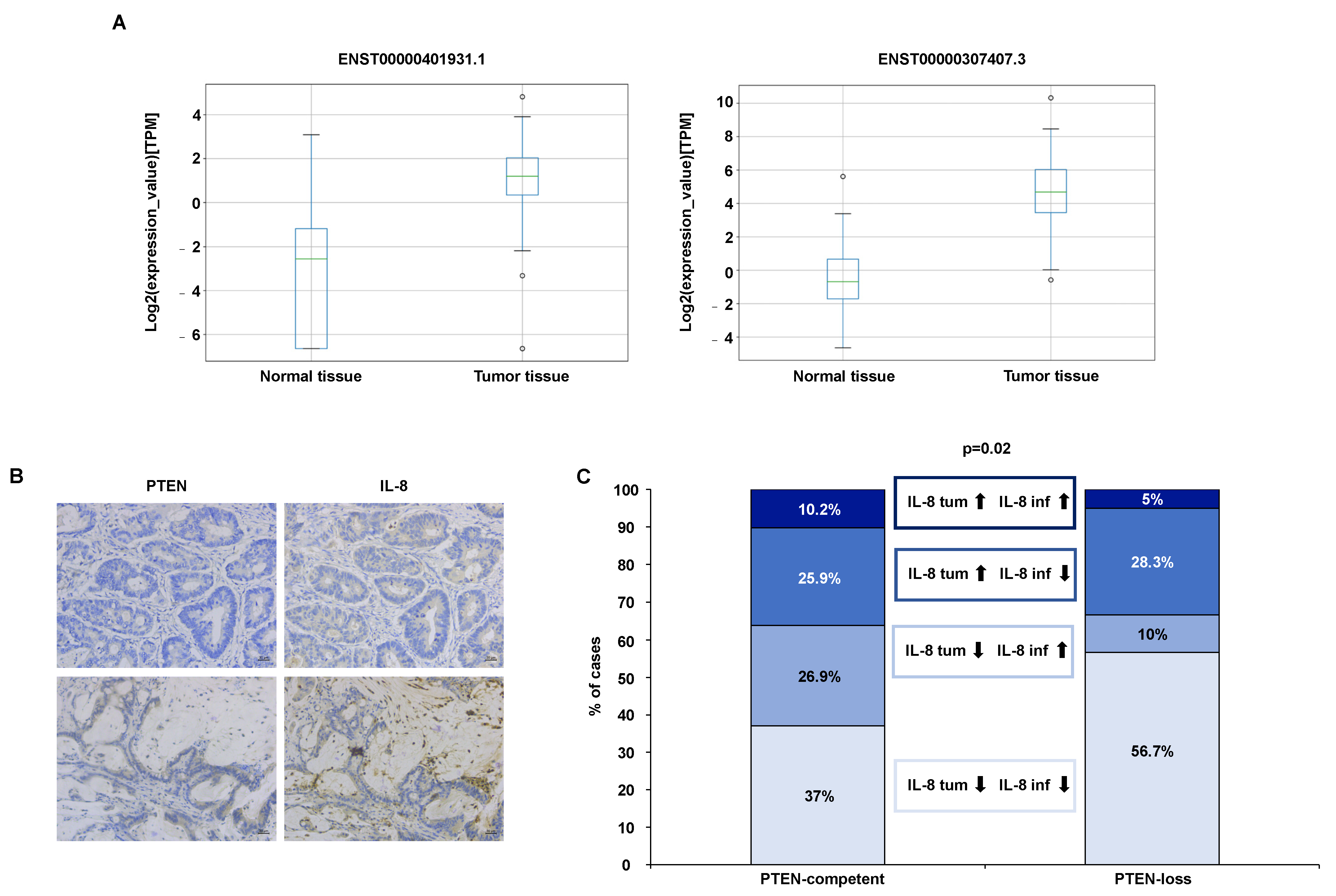

| Gene | Transcript (Ensembl Identifier) | Mean_Normal (TPM) | Mean_Tumor (TPM) |
|---|---|---|---|
| IL-8 (ENSG00000169429) | ENST00000307407.3 | 1.9 | 80.6 |
| IL-8 (ENSG00000169429) | ENST00000401931.1 | 0.4 | 3.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bazzichetto, C.; Milella, M.; Zampiva, I.; Simionato, F.; Amoreo, C.A.; Buglioni, S.; Pacelli, C.; Le Pera, L.; Colombo, T.; Bria, E.; et al. Interleukin-8 in Colorectal Cancer: A Systematic Review and Meta-Analysis of Its Potential Role as a Prognostic Biomarker. Biomedicines 2022, 10, 2631. https://doi.org/10.3390/biomedicines10102631
Bazzichetto C, Milella M, Zampiva I, Simionato F, Amoreo CA, Buglioni S, Pacelli C, Le Pera L, Colombo T, Bria E, et al. Interleukin-8 in Colorectal Cancer: A Systematic Review and Meta-Analysis of Its Potential Role as a Prognostic Biomarker. Biomedicines. 2022; 10(10):2631. https://doi.org/10.3390/biomedicines10102631
Chicago/Turabian StyleBazzichetto, Chiara, Michele Milella, Ilaria Zampiva, Francesca Simionato, Carla Azzurra Amoreo, Simonetta Buglioni, Chiara Pacelli, Loredana Le Pera, Teresa Colombo, Emilio Bria, and et al. 2022. "Interleukin-8 in Colorectal Cancer: A Systematic Review and Meta-Analysis of Its Potential Role as a Prognostic Biomarker" Biomedicines 10, no. 10: 2631. https://doi.org/10.3390/biomedicines10102631
APA StyleBazzichetto, C., Milella, M., Zampiva, I., Simionato, F., Amoreo, C. A., Buglioni, S., Pacelli, C., Le Pera, L., Colombo, T., Bria, E., Zeuli, M., Del Bufalo, D., Sperduti, I., & Conciatori, F. (2022). Interleukin-8 in Colorectal Cancer: A Systematic Review and Meta-Analysis of Its Potential Role as a Prognostic Biomarker. Biomedicines, 10(10), 2631. https://doi.org/10.3390/biomedicines10102631







