Implication of Neutrophils Extracellular Traps in the Pathogenesis of SARS-CoV-2 pneumonia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Study Design
2.3. Data Collection
2.4. Laboratory Determinations
2.5. Statistical Analysis
3. Results
3.1. PMNs-Related Parameters and Endothelial- and Platelets-Derived Variables in Patients and Controls
3.2. Outcomes of Patients during Hospitalization
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barnes, B.J.; Adrover, J.M.; Baxter-Stoltzfus, A.; Borczuk, A.; Cools-Lartigue, J.; Crawford, J.M.; Daßler-Plenker, J.; Guerci, P.; Huynh, C.; Knight, J.S.; et al. Targeting potential drivers of COVID-19: Neutrophil extracellular traps. J. Exp. Med. 2020, 217, e20200652. [Google Scholar] [CrossRef]
- Radermecker, C.; Detrembleur, N.; Guiot, J.; Cavalier, E.; Henket, M.; D’Emal, C.; Vanwinge, C.; Cataldo, D.; Oury, C.; Delvenne, P.; et al. Neutrophil extracellular traps infiltrate the lung airway, interstitial, and vascular compartments in severe COVID-19. J. Exp. Med. 2020, 217, e20201012. [Google Scholar] [CrossRef]
- Fox, S.E.; Akmatbekov, A.; Harbert, J.L.; Li, G.; Brown, J.Q.; Heide, R.S.V. Pulmonary and cardiac pathology in African American patients with COVID-19: An autopsy series from New Orleans. Lancet Respir. Med. 2020, 8, 681–686. [Google Scholar] [CrossRef]
- Zhou, Z.; Ren, L.; Zhang, L.; Zhong, J.; Xiao, Y.; Jia, Z.; Guo, L.; Yang, J.; Wang, C.; Jiang, S.; et al. Heightened Innate Immune Responses in the Respiratory Tract of COVID-19 Patients. Cell Host Microbe 2020, 27, 883–890.e2. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Q.; Yin, Y.; Zhang, Y.; Cao, Y.; Lin, X.; Huang, L.; Hoffmann, D.; Lu, M.; Qiu, Y. Excessive Neutrophils and Neutrophil Extracellular Traps in COVID-19. Front. Immunol. 2020, 11, 2063. [Google Scholar] [CrossRef]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus—Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, R.; He, G. Hematological findings in coronavirus disease 2019: Indications of progression of disease. Ann. Hematol. 2020, 99, 1421–1428. [Google Scholar] [CrossRef] [PubMed]
- Papayannopoulos, V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2017, 18, 134–147. [Google Scholar] [CrossRef]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Soehnlein, O.; Steffens, S.; Hidalgo, A.; Weber, C. Neutrophils as protagonists and targets in chronic inflammation. Nat. Rev. Immunol. 2017, 17, 248–261. [Google Scholar] [CrossRef]
- Ward, P.A.; Fattahi, F. New strategies for treatment of infectious sepsis. J. Leukoc. Biol. 2019, 106, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Gidari, A.; De Socio, G.V.; Sabbatini, S.; Francisci, D. Predictive value of National Early Warning Score 2 (NEWS2) for intensive care unit admission in patients with SARS-CoV-2 infection. Infect. Dis. 2020, 52, 698–704. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Du, X.; Chen, J.; Jin, Y.; Peng, L.; Wang, H.H.; Luo, M.; Chen, L.; Zhao, Y. Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J. Infect. 2020, 81, e6–e12. [Google Scholar] [CrossRef] [PubMed]
- Ji, P.; Zhu, J.; Zhong, Z.; Li, H.; Pang, J.; Li, B.; Zhang, J.F. Association of elevated inflammatory markers and severe COVID-19: A me-ta-analysis. Medicine 2020, 99, e23315. [Google Scholar] [CrossRef] [PubMed]
- Shakaroun, D.A.; Lazar, M.H.; Horowitz, J.C.; Jennings, J.H. Serum Ferritin as a Predictor of Outcomes in Hospitalized Patients with COVID-19 Pneumonia. J. Intensiv. Care Med. 2022. Online ahead of print. [Google Scholar] [CrossRef]
- Zhang, L.; Yan, X.; Fan, Q.; Liu, H.; Liu, X.; Liu, Z.; Zhang, Z. D-dimer levels on admission to predict in-hospital mortality in patients with COVID-19. J. Thromb. Haemost. 2020, 18, 1324–1329. [Google Scholar] [CrossRef] [Green Version]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Int. J. Surg. 2014, 12, 1495–1499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myrstad, M.; Ihle-Hansen, H.; Tveita, A.A.; Andersen, E.L.; Nygård, S.; Tveit, A.; Berge, T. National Early Warning Score 2 (NEWS2) on admission predicts severe disease and in-hospital mortality from COVID-19—A prospective cohort study. Scand. J. Trauma Resusc. Emerg. Med. 2020, 28, 66. [Google Scholar] [CrossRef]
- Brown, S.M.; Duggal, A.; Hou, P.C.; Tidswell, M.; Khan, A.; Exline, M.; Park, P.K.; Schoenfeld, D.A.; Liu, M.; Grissom, C.K.; et al. Nonlinear Imputation of PaO2/FIO2 From SpO2/FIO2 Among Mechanically Ventilated Patients in the ICU. Crit. Care Med. 2017, 45, 1317–1324. [Google Scholar] [CrossRef] [PubMed]
- Rice, T.W.; Wheeler, A.P.; Bernard, G.R.; Hayden, D.L.; Schoenfeld, D.A.; Ware, L.B. Comparison of the Sp o 2 /F io 2 Ratio and the Pa o 2 /F io 2 Ratio in Patients With Acute Lung Injury or ARDS. Chest 2007, 132, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Sánchez, C.; Ruiz-Limón, P.; Aguirre, M.; Jiménez-Gómez, Y.; la Rosa, I.A.-D.; Aguilera, M.C.; Rodriguez-Ariza, A.; Villegas, M.D.C.C.; Ortega-Castro, R.; Segui, P.; et al. Diagnostic potential of NETosis-derived products for disease activity, atherosclerosis and therapeutic effectiveness in Rheumatoid Arthritis patients. J. Autoimmun. 2017, 82, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Fan, Y.; Lai, Y.; Han, T.; Li, Z.; Zhou, P.; Pan, P.; Wang, W.; Hu, D.; Liu, X.; et al. Coronavirus infections and immune responses. J. Med. Virol. 2020, 92, 424–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ragab, D.; Eldin, H.S.; Taeimah, M.; Khattab, R.; Salem, R. The COVID-19 Cytokine Storm; What We Know So Far. Front. Immunol. 2020, 11, 1446. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Yalavarthi, S.; Shi, H.; Gockman, K.; Zuo, M.; Madison, J.A.; Blair, C.N.; Weber, A.; Barnes, B.J.; Egeblad, M.; et al. Neutrophil extracellular traps in COVID-19. JCI Insight 2020, 5, e138999. [Google Scholar] [CrossRef] [Green Version]
- Middleton, E.A.; He, X.-Y.; Denorme, F.; Campbell, R.A.; Ng, D.; Salvatore, S.P.; Mostyka, M.; Baxter-Stoltzfus, A.; Borczuk, A.C.; Loda, M.; et al. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood 2020, 136, 1169–1179. [Google Scholar] [CrossRef]
- Saithong, S.; Worasilchai, N.; Saisorn, W.; Udompornpitak, K.; Bhunyakarnjanarat, T.; Chindamporn, A.; Tovichayathamrong, P.; Torvorapanit, P.; Chiewchengchol, D.; Chancharoenthana, W.; et al. Neutrophil Extracellular Traps in Severe SARS-CoV-2 Infection: A Possible Impact of LPS and (1→3)-β-D-glucan in Blood from Gut Translocation. Cells 2022, 11, 1103. [Google Scholar] [CrossRef] [PubMed]
- Prével, R.; Dupont, A.; Labrouche-Colomer, S.; Garcia, G.; Dewitte, A.; Rauch, A.; Goutay, J.; Caplan, M.; Jozefowicz, E.; Lanoix, J.-P.; et al. Plasma Markers of Neutrophil Extracellular Trap Are Linked to Survival but Not to Pulmonary Embolism in COVID-19-Related ARDS Patients. Front. Immunol. 2022, 13, 851497. [Google Scholar] [CrossRef] [PubMed]
- Henry, B.M.; de Oliveira, M.H.S.; Cheruiyot, I.; Benoit, J.; Rose, J.; Favaloro, E.J.; Lippi, G.; Benoit, S.; Shakked, N.P. Cell-Free DNA, Neutrophil extracellular traps (NETs), and Endothelial Injury in Coronavirus Disease 2019– (COVID-19–) Associated Acute Kidney Injury. Mediat. Inflamm. 2022, 2022, 9339411. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, S.; Chelluboina, S.; Jedge, P.; Doke, P.; Palkar, S.; Mishra, A.C.; Arankalle, V.A. Elevated Levels of Neutrophil Activated Proteins, Alpha-Defensins (DEFA1), Calprotectin (S100A8/A9) and Myeloperoxidase (MPO) Are Associated With Disease Severity in COVID-19 Patients. Front. Cell. Infect. Microbiol. 2021, 11, 751232. [Google Scholar] [CrossRef]
- Veras, F.P.; Pontelli, M.C.; Silva, C.M.; Toller-Kawahisa, J.E.; de Lima, M.; Nascimento, D.C.; Schneider, A.H.; Caetité, D.; Tavares, L.A.; Paiva, I.M.; et al. SARS-CoV-2–triggered neutrophil extracellular traps mediate COVID-19 pathology. J. Exp. Med. 2020, 217, e20201129. [Google Scholar] [CrossRef] [PubMed]
- Narasaraju, T.; Yang, E.; Samy, R.P.; Ng, H.H.; Poh, W.P.; Liew, A.-A.; Phoon, M.C.; van Rooijen, N.; Chow, V.T. Excessive Neutrophils and Neutrophil Extracellular Traps Contribute to Acute Lung Injury of Influenza Pneumonitis. Am. J. Pathol. 2011, 179, 199–210. [Google Scholar] [CrossRef]
- Cicco, S.; Cicco, G.; Racanelli, V.; Vacca, A. Neutrophil Extracellular Traps (NETs) and Damage-Associated Molecular Patterns (DAMPs): Two Potential Targets for COVID-19 Treatment. Mediat. Inflamm. 2020, 2020, 7527953. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Joshi, M.; Filippova, M.; Erne, P.; Hasler, P.; Hahn, S.; Resink, T.J. Activated endothelial cells induce neutrophil extracellular traps and are susceptible to NETosis-mediated cell death. FEBS Lett. 2010, 584, 3193–3197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, A.K.; Hasler, P.; Holzgreve, W.; Gebhardt, S.; Hahn, S. Induction of Neutrophil Extracellular DNA Lattices by Placental Microparticles and IL-8 and Their Presence in Preeclampsia. Hum. Immunol. 2005, 66, 1146–1154. [Google Scholar] [CrossRef]
- Sil, P.; Wicklum, H.; Surell, C.; Rada, B. Macrophage-derived IL-1β enhances monosodium urate crystal-triggered NET formation. Agents Actions 2016, 66, 227–237. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Tecson, K.M.; McCullough, P.A. Endothelial dysfunction contributes to COVID-19-associated vascular inflammation and coagulopathy. Rev. Cardiovasc. Med. 2020, 21, 315–319. [Google Scholar] [CrossRef]
- Lampsas, S.; Tsaplaris, P.; Pantelidis, P.; Oikonomou, E.; Marinos, G.; Charalambous, G.; Souvaliotis, N.; Mystakidi, V.-C.; Goliopoulou, A.; Katsianos, E.; et al. The Role of Endothelial Related Circulating Biomarkers in COVID-19. A Systematic Review and Meta-analysis. Curr. Med. Chem. 2021, 29, 3790–3805. [Google Scholar] [CrossRef]
- Motloch, L.J.; Jirak, P.; Gareeva, D.; Davtyan, P.; Gumerov, R.; Lakman, I.; Tataurov, A.; Zulkarneev, R.; Kabirov, I.; Cai, B.; et al. Cardiovascular Biomarkers for Prediction of in-hospital and 1-Year Post-discharge Mortality in Patients With COVID-19 Pneumonia. Front. Med. 2022, 9, 906665. [Google Scholar] [CrossRef]
- Ledford, H. Coronavirus breakthrough: Dexamethasone is first drug shown to save lives. Nature 2020, 582, 469. [Google Scholar] [CrossRef] [PubMed]
- Wan, T.; Zhao, Y.; Fan, F.; Hu, R.; Jin, X. Dexamethasone Inhibits, S. aureus-Induced Neutrophil Extracellular Pathogen-Killing Mechanism, Possibly through Toll-Like Receptor Regulation. Front. Immunol. 2017, 8, 60. [Google Scholar] [CrossRef] [Green Version]
- Tamayo-Velasco, Á.; Bombín-Canal, C.; Cebeira, M.J.; Prada, L.S.-D.; Miramontes-González, J.P.; Martín-Fernández, M.; Peñarrubia-Ponce, M.J. Full Characterization of Thrombotic Events in All Hospitalized COVID-19 Patients in a Spanish Tertiary Hospital during the First 18 Months of the Pandemic. J. Clin. Med. 2022, 11, 3443. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, J.; Laurence, J. Long COVID endotheliopathy: Hypothesized mechanisms and potential therapeutic approaches. J. Clin. Investig. 2022, 132, e161167. [Google Scholar] [CrossRef] [PubMed]
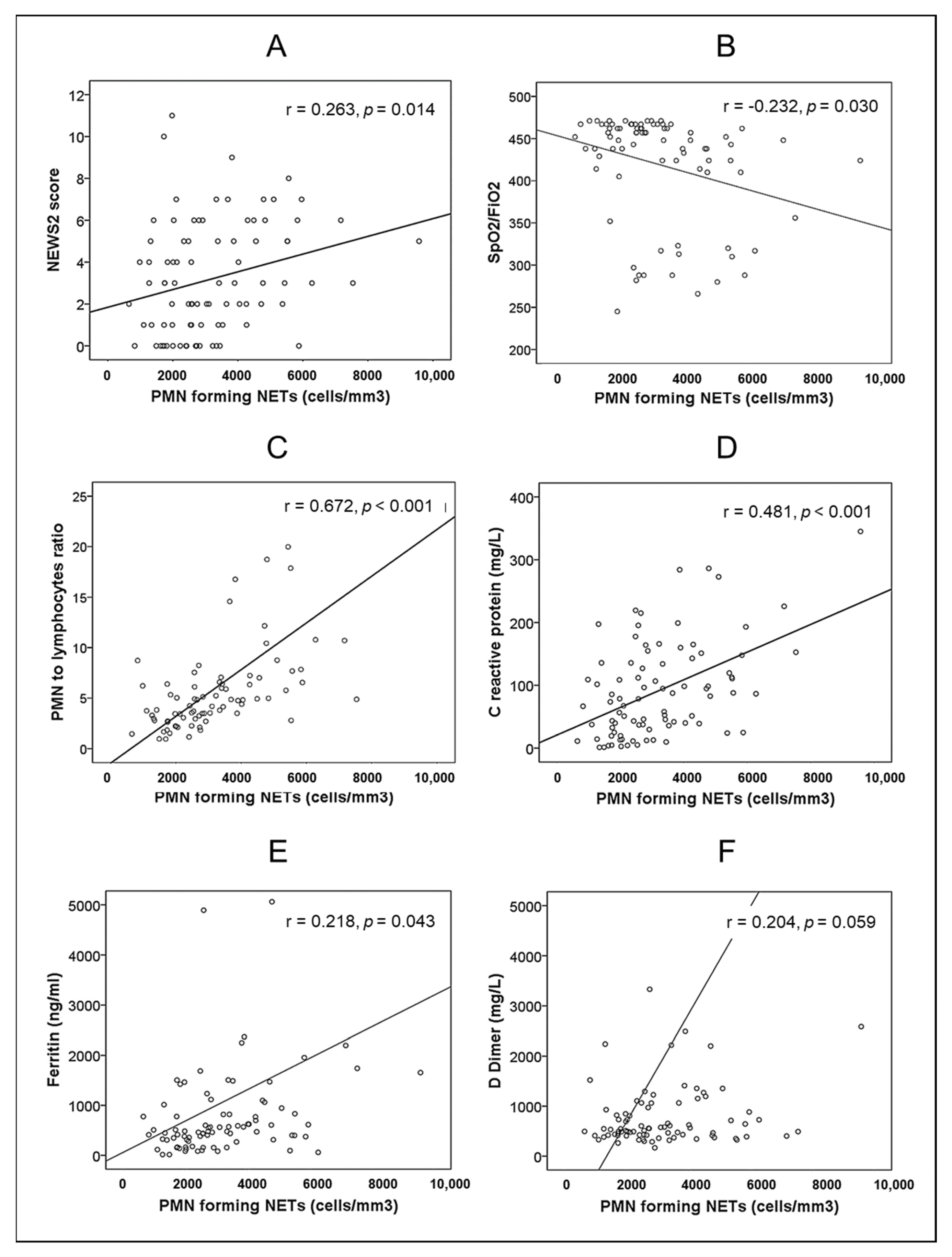
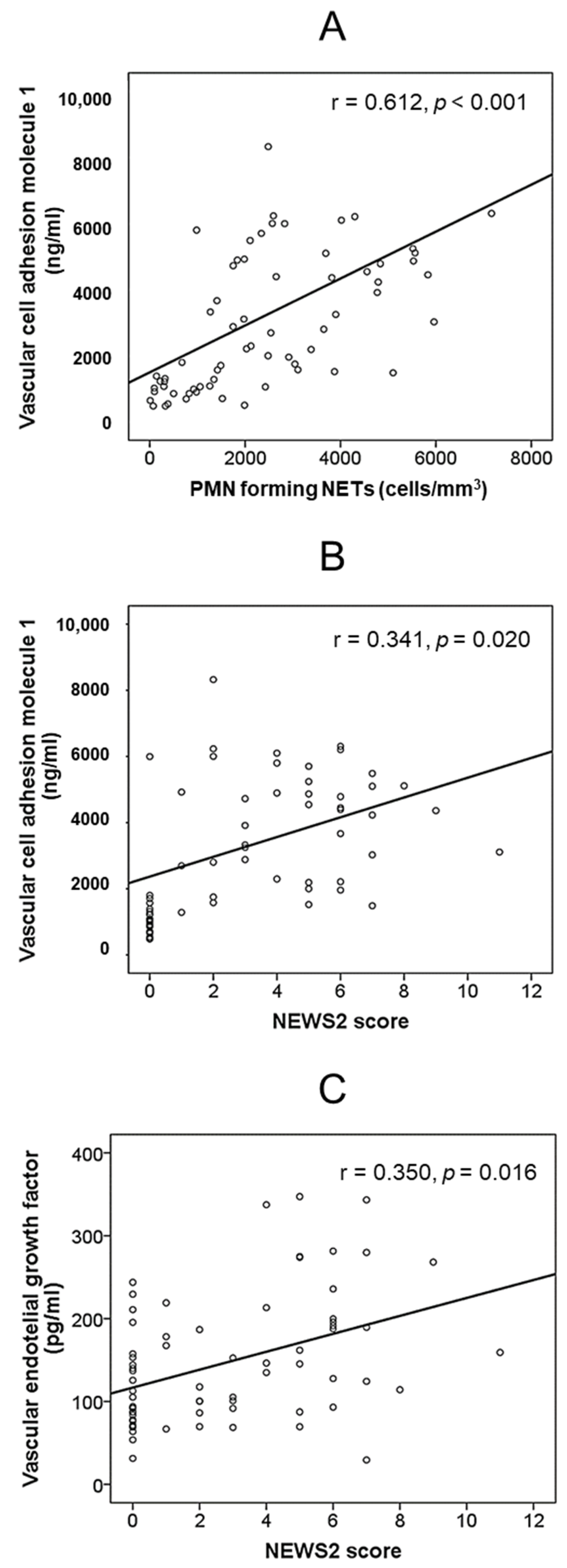

| Variable | Healthy Controls (n = 30) | Patients with SARS-CoV-2 Pneumonia (n = 87) | p | |
|---|---|---|---|---|
| Age (years) | 56 (48–70) | 56 (44–68) | 0.568 | |
| Sex male (n,%) | 18 (60) | 54 (62) | 1.000 | |
| Comorbidity | ||||
| Arterial hypertension (n,%) | 0 (0) | 33 (38) | ||
| Diabetes mellitus (n,%) | 0 (0) | 18 (21) | ||
| Obesity (body mass index > 30 kg/m2) (n,%) | 0 (0) | 22 (25) | ||
| Time from first symptom to admission (days) | 9 (7–11) | |||
| NEWS2 score | ||||
| NEWS2 (points) | 3 (1–5) | |||
| NEWS2 > 6 (n,%) | 10 (12) | |||
| SpO2/FiO2 | 448 (405–462) | |||
| Polymorphonuclear neutrophils (cells/mm3) | 3575 (2860–4250) | 3990 (2810–5650) | 0.168 | |
| Lymphocytes (cells/mm3) | 1990 (1608–2418) | 850 (660–1205) | <0.001 | |
| Neutrophil to lymphocyte ratio | 1.9 (1.5–2.4) | 4.8 (3.0–6.6) | <0.001 | |
| D-Dimer (mg/L) | 382 (273–466) | 547 (420–996) | <0.001 | |
| C-reactive protein (mg/L) | 2 (1–4) | 79 (33–136) | <0.001 | |
| Ferritin (ng/mL) | 78 (50–116) | 484 (313–1014) | <0.001 | |
| Variable | Healthy Controls (n = 30) | Patients with Pneumonia by SARS-CoV-2 (n = 87) | p | |
|---|---|---|---|---|
| PMN-derived parameters | ||||
| PMNs forming NETs (cells/mm3) | 324 (134–803) | 2751 (1983–4261) | <0.001 | |
| PMNs forming NETs (% of PMNs) | 10 (4–25) | 75 (65–89) | <0.001 | |
| Myeloperoxidase (ng/mL) | 680 (499–1233) | 875 (490–1498) | 0.373 | |
| DNAse I (ng/mL) | 8.8 (5.6–12.9) | 6.1 (2.4–12.4) | 0.079 | |
| Endothelial-derived molecules | ||||
| E-selectin (ng/mL) | 31 (21–45) | 33 (20–49) | 0.765 | |
| Vascular cell adhesion molecule 1 (ng/mL) | 989 (673–1224) | 4363 (2212–5487) | <0.001 | |
| Vascular endothelial growth factor (pg/mL) | 105 (74–149) | 146 (93–213) | 0.036 | |
| Platelet-derived molecules | ||||
| Platelet factor 4 (ng/mL) | 9589 (7910–12,223) | 7990 (6190–9697) | 0.006 | |
| P-selectin (ng/mL) | 54 (43–58) | 46 (32–58) | 0.043 | |
| Variable | Surviving Patients (n = 80) | Non-Surviving Patients (n = 7) | p | |
|---|---|---|---|---|
| Age (years) | 56 (44–68) | 62 (49–68) | 0.353 | |
| Sex male (n,%) | 50 (63) | 4 (57) | 1.000 | |
| Comorbidity | ||||
| Arterial hypertension (n,%) | 29 (36) | 4 (57) | 0.419 | |
| Diabetes mellitus (n,%) | 14 (17) | 4 (57) | 0.031 | |
| Obesity (body mass index > 30 kg/m2) (n,%) | 19 (24) | 3 (43) | 0.362 | |
| Time from first symptom to admission (days) | 9 (7–11) | 8 (7–11) | 0.437 | |
| NEWS2 score | ||||
| NEWS2 (points) | 2 (1–5) | 7 (6–7) | 0.001 | |
| NEWS2 > 6 (n,%) | 6 (8) | 4 (57) | 0.003 | |
| SpO2/FiO2 | 448 (417–466) | 310 (282–410) | 0.002 | |
| C-reactive protein (mg/L) | 79 (30–136) | 88 (42–165) | 0.657 | |
| Ferritin (ng/mL) | 481 (313–997) | 591 (282–5062) | 0.492 | |
| D-Dimer (mg/L) | 533 (415–973) | 734 (465–1066) | 0.533 | |
| Polymorphonuclear neutrophils (cells/mm3) | 3845 (2810–5585) | 5350 (4360–6340) | 0.180 | |
| Lymphocytes (cells/mm3) | 855 (638–1208) | 820 (750–1100) | 0.919 | |
| Neutrophil to lymphocyte ratio | 4.2 (2.8–6.5) | 5.0 (4.4–7.7) | 0.261 | |
| PMN forming NETs (cells/mm3) | 2725 (1974–4013) | 4018 (2105–4791) | 0.165 | |
| PMN forming NETs (% of PMNs) | 76 (65–89) | 69 (34–89) | 0.731 | |
| Myeloperoxidase (ng/mL) | 869 (500–1500) | 870 (490–1452) | 0.985 | |
| DNAse I (ng/mL) | 6.1 (2.5–12.4) | 6.1 (2.4–9.8) | 1.000 | |
| E-selectin (ng/mL) | 32 (19–47) | 45 (30–61) | 0.086 | |
| Vascular cell adhesion molecule 1 (ng/mL) | 3791 (2049–5162) | 5112 (4230–6101) | 0.118 | |
| Vascular endothelial growth factor (pg/mL) | 146 (88–195) | 213 (114–281) | 0.134 | |
| Platelet factor 4 (ng/mL) | 7993 (6198–9697) | 7885 (6120–12,200) | 0.450 | |
| P-selectin (ng/mL) | 45 (31–59) | 47 (34–48) | 0.826 | |
| Variable | At Admission (T0) | At Discharge (Tdi) | 14 Days after Discharge (T+14di) | p T0 vs. Tdi | p T0 vs. 14Tdi | p Tdi vs. T14di |
|---|---|---|---|---|---|---|
| SpO2FiO2 | 448 (417–466) | 465 (457–467) | 462 (457–467) | <0.001 | <0.001 | 0.251 |
| C-reactive protein (mg/L) | 79 (30–136) | 5 (2–8) | 1 (0–3) | <0.001 | <0.001 | <0.001 |
| Ferritin (ng/mL) | 481 (313–997) | 446 (277–930) | 201 (84–348) | 0.374 | <0.001 | <0.001 |
| D-Dimer (mg/L) | 533 (415–973) | 457 (299–736) | 334 (250–791) | <0.001 | <0.001 | 0.085 |
| Neutrophil to lymphocyte ratio | 4.2 (2.8–6.5) | 2.3 (1.5–3.5) | 2.1 (1.4–2.8) | <0.001 | <0.001 | 0.351 |
| PMNs forming NETs (cells/mm3) | 2725 (1974–4013) | 1270 (590–2305) | 702 (270–1305) | <0.001 | <0.001 | 0.003 |
| Myeloperoxidase (ng/mL) | 869 (500–1500) | 605 (456–1475) | 340 (258–500) | 0.278 | <0.001 | <0.001 |
| DNAse I (ng/mL) | 6.1 (2.5–12.4) | 23.9 (11.9–35.9) | 13.1 (7.1–23.8) | <0.001 | <0.001 | <0.001 |
| E-selectin (ng/mL) | 32 (19–47) | 30 (21–42) | 35 (22–46) | 0.350 | 0.677 | 0.128 |
| Vascular cell adhesion molecule 1 (ng/mL) | 3791 (2049–5162) | 2023 (1224–2582) | 1390 (1070–2045) | <0.001 | <0.001 | <0.001 |
| Vascular endotelial growth factor (pg/mL) | 146 (88–195) | 132 (92–285) | 97 (64–125) | 0.009 | <0.001 | <0.001 |
| Platelet factor 4 (ng/mL) | 7993 (6198–9697) | 10,793 (7128–14,524) | 7423 (4731–9611) | <0.001 | <0.001 | 0.053 |
| P-selectin (ng/mL) | 45 (31–59) | 58 (49–59) | 49 (42–58) | <0.001 | 0.066 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Guerrero, P.; Illanes-Álvarez, F.; Márquez-Ruiz, D.; Campaña-Gómez, I.; Cuesta-Sancho, S.; Márquez-Coello, M.; Girón-González, J.-A. Implication of Neutrophils Extracellular Traps in the Pathogenesis of SARS-CoV-2 pneumonia. Biomedicines 2022, 10, 2638. https://doi.org/10.3390/biomedicines10102638
Pérez-Guerrero P, Illanes-Álvarez F, Márquez-Ruiz D, Campaña-Gómez I, Cuesta-Sancho S, Márquez-Coello M, Girón-González J-A. Implication of Neutrophils Extracellular Traps in the Pathogenesis of SARS-CoV-2 pneumonia. Biomedicines. 2022; 10(10):2638. https://doi.org/10.3390/biomedicines10102638
Chicago/Turabian StylePérez-Guerrero, Patricia, Francisco Illanes-Álvarez, Denisse Márquez-Ruiz, Irene Campaña-Gómez, Sara Cuesta-Sancho, Mercedes Márquez-Coello, and José-Antonio Girón-González. 2022. "Implication of Neutrophils Extracellular Traps in the Pathogenesis of SARS-CoV-2 pneumonia" Biomedicines 10, no. 10: 2638. https://doi.org/10.3390/biomedicines10102638
APA StylePérez-Guerrero, P., Illanes-Álvarez, F., Márquez-Ruiz, D., Campaña-Gómez, I., Cuesta-Sancho, S., Márquez-Coello, M., & Girón-González, J.-A. (2022). Implication of Neutrophils Extracellular Traps in the Pathogenesis of SARS-CoV-2 pneumonia. Biomedicines, 10(10), 2638. https://doi.org/10.3390/biomedicines10102638







