Open Source Repository and Online Calculator of Prediction Models for Diagnosis and Prognosis in Oncology
Abstract
1. Introduction
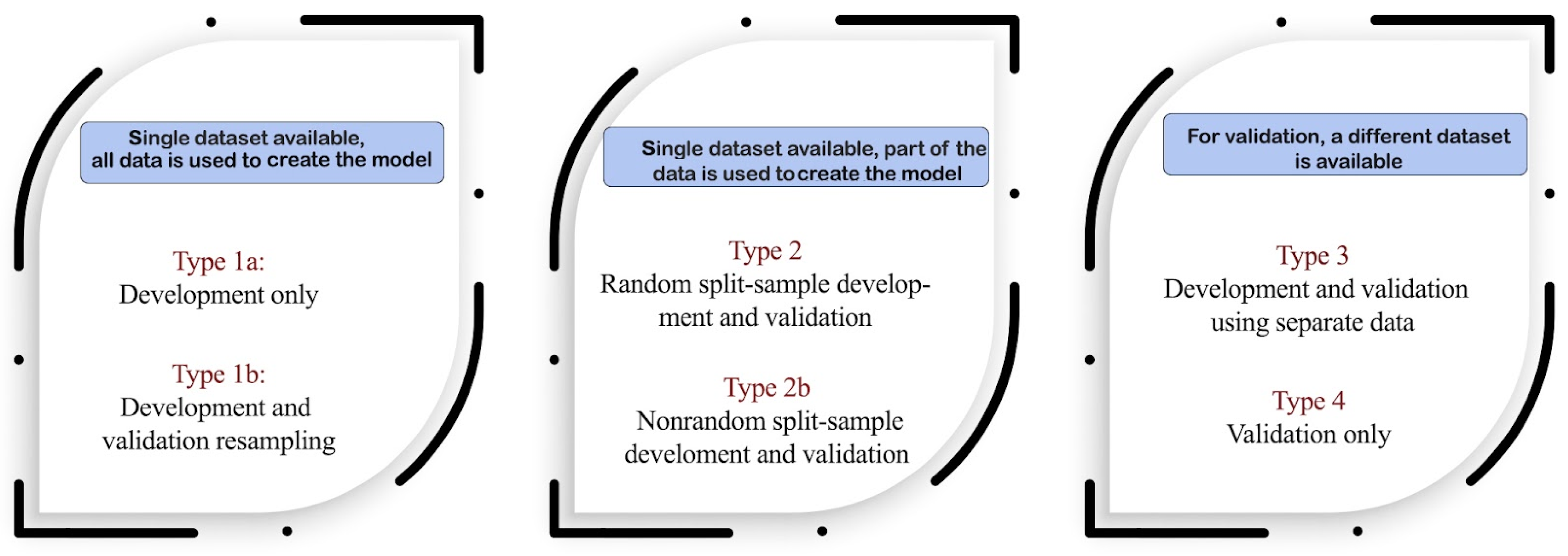
2. Materials and Methods
2.1. Obtaining Model Coefficients from a Nomogram
2.2. Application Development Process
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Craddock, M.; Crockett, C.; McWilliam, A.; Price, G.; Sperrin, M.; van der Veer, S.N.; Faivre-Finn, C. Evaluation of Prognostic and Predictive Models in the Oncology Clinic. Clin. Oncol. 2022, 34, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Chua, I.S.; Gaziel-Yablowitz, M.; Korach, Z.T.; Kehl, K.L.; Levitan, N.A.; Arriaga, Y.E.; Jackson, G.P.; Bates, D.W.; Hassett, M. Artificial intelligence in oncology: Path to implementation. Cancer Med. 2021, 10, 4138–4149. [Google Scholar] [CrossRef] [PubMed]
- Hogarth, R.M.; Karelaia, N. Heuristic and linear models of judgment: Matching rules and environments. Psychol. Rev. 2007, 114, 733–758. [Google Scholar] [CrossRef] [PubMed]
- Steyerberg, E. Clinical Prediction Models: A Practical Approach to Development, Validation, and Updating; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Prosperi, M.; Guo, Y.; Sperrin, M.; Koopman, J.S.; Min, J.S.; He, X.; Rich, S.; Wang, M.; Buchan, I.E.; Bian, J. Causal inference and counterfactual prediction in machine learning for actionable healthcare. Nat. Mach. Intell. 2020, 2, 369–375. [Google Scholar] [CrossRef]
- Wiens, J.; Saria, S.; Sendak, M.; Ghassemi, M.; Liu, V.X.; Doshi-Velez, F.; Jung, K.; Heller, K.; Kale, D.; Saeed, M.; et al. Do no harm: A roadmap for responsible machine learning for health care. Nat. Med. 2019, 25, 1337–1340. [Google Scholar] [CrossRef]
- Dash, S.; Acharya, B.R.; Mittal, M.; Abraham, A.; Kelemen, A. Deep Learning Techniques for Biomedical and Health Informatics; Springer Nature: London, UK, 2019. [Google Scholar]
- Faruqui, N.; Yousuf, M.A.; Whaiduzzaman, M.; Azad, A.K.M.; Barros, A.; Moni, M.A. LungNet: A hybrid deep-CNN model for lung cancer diagnosis using CT and wearable sensor-based medical IoT data. Comput. Biol. Med. 2021, 139, 104961. [Google Scholar] [CrossRef]
- Aurna, N.F.; Yousuf, M.A.; Taher, K.A.; Azad, A.K.M.; Moni, M.A. A classification of MRI brain tumor based on two stage feature level ensemble of deep CNN models. Comput. Biol. Med. 2022, 146, 105539. [Google Scholar] [CrossRef]
- He, B.; Chen, W.; Liu, L.; Hou, Z.; Zhu, H.; Cheng, H.; Zhang, Y.; Zhan, S.; Wang, S. Prediction Models for Prognosis of Cervical Cancer: Systematic Review and Critical Appraisal. Front. Public Health 2021, 9, 654454. [Google Scholar] [CrossRef]
- Dhiman, P.; Ma, J.; Navarro, C.A.; Speich, B.; Bullock, G.; Damen, J.A.; Kirtley, S.; Hooft, L.; Riley, R.D.; Van Calster, B.; et al. Reporting of prognostic clinical prediction models based on machine learning methods in oncology needs to be improved. J. Clin. Epidemiol. 2021, 138, 60–72. [Google Scholar] [CrossRef]
- van Wijk, Y.; Ramaekers, B.; Vanneste, B.G.L.; Halilaj, I.; Oberije, C.; Chatterjee, A.; Marcelissen, T.; Jochems, A.; Woodruff, H.C.; Lambin, P. Modeling-Based Decision Support System for Radical Prostatectomy Versus External Beam Radiotherapy for Prostate Cancer Incorporating an Clinical Trial and a Cost-Utility Study. Cancers 2021, 13, 2687. [Google Scholar] [CrossRef]
- Collins, G.S.; Reitsma, J.B.; Altman, D.G.; Moons, K.G.M.; members of the TRIPOD group. Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD): The TRIPOD Statement. Eur. Urol. 2015, 67, 1142–1151. [Google Scholar] [CrossRef]
- Toxopeus, E.L.A.; Nieboer, D.; Shapiro, J.; Biermann, K.; van der Gaast, A.; van Rij, C.M.; Steyerberg, E.W.; van Lanschot, J.J.B.; Wijnhoven, B.P.L. Nomogram for predicting pathologically complete response after neoadjuvant chemoradiotherapy for oesophageal cancer. Radiother. Oncol. 2015, 115, 392–398. [Google Scholar] [CrossRef]
- Cox, D.R.; Cox, D. Selected Statistical Papers of Sir David Cox: Volume 1, Design of Investigations, Statistical Methods and Applications; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Bonnin, R. Machine Learning for Developers: Uplift Your Regular Applications with the Power of Statistics, Analytics, and Machine Learning; Packt Publishing Ltd.: Birmingham, UK, 2017. [Google Scholar]
- Hendriks, L.; Keek, S.A.; Chatterjee, A.; Belderbos, J.; Bootsma, G.; van den Borne, B.; Dingemans, A.-M.C.; Gietema, H.; Groen, H.J.M.; Herder, G.; et al. 127P Does radiomics have added value in predicting the development of brain metastases in patients with radically treated stage III non-small cell lung cancer (NSCLC)? Ann. Oncol. 2022, 33, S91. [Google Scholar] [CrossRef]
- Rodrigues, G.; Warner, A.; Zindler, J.; Slotman, B.; Lagerwaard, F. A clinical nomogram and recursive partitioning analysis to determine the risk of regional failure after radiosurgery alone for brain metastases. Radiother. Oncol. 2014, 111, 52–58. [Google Scholar] [CrossRef]
- Molenaar, R.; Wiggenraad, R.; Verbeek-de Kanter, A.; Walchenbach, R.; Vecht, C. Relationship between volume, dose and local control in stereotactic radiosurgery of brain metastasis. Br. J. Neurosurg. 2009, 23, 170–178. [Google Scholar] [CrossRef]
- Chang, E.L.; Hassenbusch, S.J., 3rd; Shiu, A.S.; Lang, F.F.; Allen, P.K.; Sawaya, R.; Maor, M.H. The role of tumor size in the radiosurgical management of patients with ambiguous brain metastases. Neurosurgery 2003, 53, 272–280, discussion 280–281. [Google Scholar] [CrossRef]
- Chang, E.L.; Selek, U.; Hassenbusch SJ 3rd Maor, M.H.; Allen, P.K.; Mahajan, A.; Sawaya, R.; Woo, S.Y. Outcome variation among “radioresistant” brain metastases treated with stereotactic radiosurgery. Neurosurgery 2005, 56, 936–945, discussion 936–945. [Google Scholar]
- Chao, S.T.; Barnett, G.H.; Vogelbaum, M.A.; Angelov, L.; Weil, R.J.; Neyman, G.; Reuther, A.M.; Suh, J.H. Salvage stereotactic radiosurgery effectively treats recurrences from whole-brain radiation therapy. Cancer 2008, 113, 2198–2204. [Google Scholar] [CrossRef]
- Ernst-Stecken, A.; Ganslandt, O.; Lambrecht, U.; Sauer, R.; Grabenbauer, G. Phase II trial of hypofractionated stereotactic radiotherapy for brain metastases: Results and toxicity. Radiother. Oncol. 2006, 81, 18–24. [Google Scholar] [CrossRef]
- Higuchi, Y.; Serizawa, T.; Nagano, O.; Matsuda, S.; Ono, J.; Sato, M.; Iwadate, Y.; Saeki, N. Three-staged stereotactic radiotherapy without whole brain irradiation for large metastatic brain tumors. Int. J. Radiat. Oncol. Biol. Phys. 2009, 74, 1543–1548. [Google Scholar] [CrossRef]
- Lutterbach, J.; Cyron, D.; Henne, K.; Ostertag, C.B. Radiosurgery followed by planned observation in patients with one to three brain metastases. Neurosurgery 2008, 62 (Suppl. 2), 776–784. [Google Scholar] [CrossRef]
- Matsuo, T.; Shibata, S.; Yasunaga, A.; Iwanaga, M.; Mori, K.; Shimizu, T.; Hayashi, N.; Ochi, M.; Hayashi, K. Dose optimization and indication of Linac radiosurgery for brain metastases. Int. J. Radiat. Oncol. Biol. Phys. 1999, 45, 931–939. [Google Scholar] [CrossRef]
- Narayana, A.; Chang, J.; Yenice, K.; Chan, K.; Lymberis, S.; Brennan, C.; Gutin, P.H. Hypofractionated stereotactic radiotherapy using intensity-modulated radiotherapy in patients with one or two brain metastases. Stereotact. Funct. Neurosurg. 2007, 85, 82–87. [Google Scholar] [CrossRef]
- Saitoh, J.-I.; Saito, Y.; Kazumoto, T.; Kudo, S.; Ichikawa, A.; Hayase, N.; Kazumoto, K.; Sakai, H.; Shibuya, K. Therapeutic effect of linac-based stereotactic radiotherapy with a micro-multileaf collimator for the treatment of patients with brain metastases from lung cancer. Jpn. J. Clin. Oncol. 2010, 40, 119–124. [Google Scholar] [CrossRef][Green Version]
- Vogelbaum, M.A.; Angelov, L.; Lee, S.-Y.; Li, L.; Barnett, G.H.; Suh, J.H. Local control of brain metastases by stereotactic radiosurgery in relation to dose to the tumor margin. J. Neurosurg. 2006, 104, 907–912. [Google Scholar] [CrossRef]
- Wiggenraad, R.; Verbeek-de Kanter, A.; Kal, H.B.; Taphoorn, M.; Vissers, T.; Struikmans, H. Dose-effect relation in stereotactic radiotherapy for brain metastases. A systematic review. Radiother. Oncol. 2011, 98, 292–297. [Google Scholar] [CrossRef]
- Zindler, J.D.; Jochems, A.; Lagerwaard, F.J.; Beumer, R.; Troost, E.G.C.; Eekers, D.B.P.; Compter, I.; van der Toorn, P.-P.; Essers, M.; Oei, B.; et al. Individualized early death and long-term survival prediction after stereotactic radiosurgery for brain metastases of non-small cell lung cancer: Two externally validated nomograms. Radiother. Oncol. 2017, 123, 189–194. [Google Scholar] [CrossRef]
- Thor, M.; Owosho, A.A.; Clark, H.D.; Oh, J.H.; Riaz, N.; Hovan, A.; Tsai, J.; Thomas, S.D.; Yom, S.H.K.; Wu, J.S.; et al. Internal and external generalizability of temporal dose-response relationships for xerostomia following IMRT for head and neck cancer. Radiother. Oncol. 2017, 122, 200–206. [Google Scholar] [CrossRef]
- Ramaekers, B.L.T.; Grutters, J.P.C.; Pijls-Johannesma, M.; Lambin, P.; Joore, M.A.; Langendijk, J.A. Protons in head-and-neck cancer: Bridging the gap of evidence. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 1282–1288. [Google Scholar] [CrossRef] [PubMed]
- Ramaekers, B.L.T.; Joore, M.A.; Grutters, J.P.C.; van den Ende, P.; de Jong, J.; Houben, R.; Lambin, P.; Christianen, M.; Beetz, I.; Pijls-Johannesma, M.; et al. The impact of late treatment-toxicity on generic health-related quality of life in head and neck cancer patients after radiotherapy. Oral Oncol. 2011, 47, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Ramaekers, B.L.T.; Pijls-Johannesma, M.; Joore, M.A.; van den Ende, P.; Langendijk, J.A.; Lambin, P.; Kessels, A.G.H.; Grutters, J.P.C. Systematic review and meta-analysis of radiotherapy in various head and neck cancers: Comparing photons, carbon-ions and protons. Cancer Treat. Rev. 2011, 37, 185–201. [Google Scholar] [CrossRef] [PubMed]
- Beetz, I.; Schilstra, C.; van der Schaaf, A.; van den Heuvel, E.R.; Doornaert, P.; van Luijk, P.; Vissink, A.; van der Laan, B.F.A.M.; Leemans, C.R.; Bijl, H.P.; et al. NTCP models for patient-rated xerostomia and sticky saliva after treatment with intensity modulated radiotherapy for head and neck cancer: The role of dosimetric and clinical factors. Radiother. Oncol. 2012, 105, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Wopken, K.; Bijl, H.P.; van der Schaaf, A.; van der Laan, H.P.; Chouvalova, O.; Steenbakkers, R.J.H.M.; Doornaert, P.; Slotman, B.J.; Oosting, S.F.; Christianen, M.E.M.C.; et al. Development of a multivariable normal tissue complication probability (NTCP) model for tube feeding dependence after curative radiotherapy/chemo-radiotherapy in head and neck cancer. Radiother. Oncol. 2014, 113, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Dehing-Oberije, C.; De Ruysscher, D.; van der Weide, H.; Hochstenbag, M.; Bootsma, G.; Geraedts, W.; Pitz, C.; Simons, J.; Teule, J.; Rahmy, A.; et al. Tumor volume combined with number of positive lymph node stations is a more important prognostic factor than TNM stage for survival of non-small-cell lung cancer patients treated with (chemo)radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 1039–1044. [Google Scholar] [CrossRef]
- Wallington, M.; Saxon, E.B.; Bomb, M.; Smittenaar, R.; Wickenden, M.; McPhail, S.; Rashbass, J.; Chao, D.; Dewar, J.; Talbot, D.; et al. 30-day mortality after systemic anticancer treatment for breast and lung cancer in England: A population-based, observational study. Lancet Oncol. 2016, 17, 1203–1216. [Google Scholar] [CrossRef]
- Dehing-Oberije, C.; De Ruysscher, D.; van Baardwijk, A.; Yu, S.; Rao, B.; Lambin, P. The importance of patient characteristics for the prediction of radiation-induced lung toxicity. Radiother. Oncol. 2009, 91, 421–426. [Google Scholar] [CrossRef]
- Oberije, C.; Liao, Z.; De Ruysscher, D.; Tucker, S.; Lambin, P. Development and External Validation of a Model for Prediction of Radiation-Induced Dyspnea: An Approach combining Clinical Data with Information from Literature. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, S528. [Google Scholar] [CrossRef]
- Dehing-Oberije, C.; De Ruysscher, D.; Petit, S.; Van Meerbeeck, J.; Vandecasteele, K.; De Neve, W.; Dingemans, A.M.C.; El Naqa, I.; Deasy, J.; Bradley, J.; et al. Development, external validation and clinical usefulness of a practical prediction model for radiation-induced dysphagia in lung cancer patients. Radiother. Oncol. 2010, 97, 455–461. [Google Scholar] [CrossRef]
- Nalbantov, G.; Kietselaer, B.; Vandecasteele, K.; Oberije, C.; Berbee, M.; Troost, E.; Dingemans, A.-E.; van Baardwijk, A.; Smits, K.; Dekker, A.; et al. Cardiac comorbidity is an independent risk factor for radiation-induced lung toxicity in lung cancer patients. Radiother. Oncol. 2013, 109, 100–106. [Google Scholar] [CrossRef]
- Walsh, S.; van der Putten, W. A TCP model for external beam treatment of intermediate-risk prostate cancer. Med. Phys. 2013, 40, 031709. [Google Scholar] [CrossRef]
- Walsh, S.; Roelofs, E.; Kuess, P.; Lambin, P.; Jones, B.; Georg, D.; Verhaegen, F. A validated tumor control probability model based on a meta-analysis of low, intermediate, and high-risk prostate cancer patients treated by photon, proton, or carbon-ion radiotherapy. Med. Phys. 2016, 43, 734–747, Erratum in Med. Phys. 2016, 43, 5263. [Google Scholar] [CrossRef]
- Creutzberg, C.L.; van Stiphout, R.G.P.M.; Nout, R.A.; Lutgens, L.C.H.; Jürgenliemk-Schulz, I.M.; Jobsen, J.J.; Smit, V.T.H.B.; Lambin, P. Nomograms for Prediction of Outcome With or Without Adjuvant Radiation Therapy for Patients With Endometrial Cancer: A Pooled Analysis of PORTEC-1 and PORTEC-2 Trials. Int. J. Radiat. Oncol. Biol. Phys. 2015, 91, 530–539. [Google Scholar] [CrossRef]
- van Stiphout, R.G.P.M.; Lammering, G.; Buijsen, J.; Janssen, M.H.M.; Gambacorta, M.A.; Slagmolen, P.; Lambrecht, M.; Rubello, D.; Gava, M.; Giordano, A.; et al. Development and external validation of a predictive model for pathological complete response of rectal cancer patients including sequential PET-CT imaging. Radiother. Oncol. 2011, 98, 126–133. [Google Scholar] [CrossRef]
- Halilaj, I.; Chatterjee, A.; van Wijk, Y.; Wu, G.; van Eeckhout, B.; Oberije, C.; Lambin, P. Covid19Risk.ai: An open source repository and online calculator of prediction models for early diagnosis and prognosis of Covid-19. BioMed 2021, 1, 41–49. [Google Scholar] [CrossRef]






| Parameter | Equation | Nomogram Value | Equation Value | Points | Coefficient |
|---|---|---|---|---|---|
| Intercept | - | - | - | - | −1.47 |
| Tumor type | Adenocarcinoma | 0 | 0 | 0 | |
| Squamous cell carcinoma | 1 | 100 | 0.72 | ||
| Differentiation grade | 1 or 2 | 0 | 0 | 0 | |
| 3 | 1 | 47 | 0.34 | ||
| Gender | Male | 0 | 0 | 0 | |
| Female | 1 | 41 | 0.30 | ||
| T-stage | 3 or 4 | 0 | 0 | 0 | |
| 1 or 2 | 1 | 85 | 0.61 |
| Model Title | Cancer Type | Input Features | Output | Cohort Type | Tripod Type | Model Type |
|---|---|---|---|---|---|---|
| Brain | Tumor histology Age | Predicts the development of brain metastasis. | Primary stage III NSCLC brain cancer patients. | 2b | Linear regression |
| Brain | WHO performance status; Age; Volume of the largest brain metastasis; Number of treated brain metastases | Predicts the probability of occurrence of new distant brain recurrences. | Patients with 1 up to 3 BM treated with SRS. | 2b | Univariate logistic regression and Cox regression |
| Brain | Prescribed fraction SRS dose; Number of SRS fractions | Predicts local control probability at 1 year after stereotactic radiosurgery (SRS) for brain metastases (BM). | Patients treated with SRS for BM. | 2b | Linear-quadratic BED (LQ-BED), Linear regression, Cox regression |
| Brain | Age; Presence of extracranial metastases; WHO performance status; GTV largest metastasis; Volume; Sphere; diameter | Predicts the probability of early death (<3 months) and the probability of long-term survival (>12 months) with prognostic factors for survival. | Patients treated with SRS for 1, 2, or 3 BMs of NSCLC. | 3 | Multivariate Cox regression |
| Head and Neck | D-mean-contra; D-mean-ipsi | Predicts Xerostomia three months after intensity-modulated radiotherapy (IMRT). | Patients with locally advanced head and neck cancer, eligible for potentially curative loco-regional treatment, and have not been treated for another malignancy. | 3 | Logistic regression |
| Head and Neck | Mean dose of ipsilateral parotis; contralateral parotis; pharyngeal constrictor muscle superior; supraglottic area; Willingness to pay per QALY gained | Predicts the most cost-effective treatment. | 2b | Multivariate logistic regression | |
| Head and Neck | Mean dose contralater submandibular gland; Mean dose sublingual glands; Mean dose soft palate | Predicts the probability of sticky saliva 6 months after treatment. | Head and neck cancer (HNC) originating in the oral cavity, oropharynx, larynx, hypopharynx, or nasopharynx; 2. treated with curative intensity-modulated radiotherapy (IMRT) either alone or in combination with chemotherapy or cetuximab; 3. no previous surgery, radiotherapy and/or chemotherapy; 4. no previous malignancies; 5. no distant metastases; 6. planning CT and 3D-dose distributions available in DICOM format; 7. HRQoL assessments available prior to and 6 months after completion of (CH)RT. | 2b | Multivariate logistic regression |
| Head and Neck | T-classification; Baseline weight loss; Type of treatment; Mean dose PCM superior; Mean dose PCM inferior; Mean dose contralateral parotid; Mean dose cricopharyngeal muscle | Predicts the probability of tube feeding dependence 6 months after treatment. | Curative radiotherapy/chemoradiotherapy for head and neck cancer (HNC) may result in severe acute and late side effects, including tube-feeding dependence. | 2b | Multivariable logistic regression |
| Esophagus | Tumor histology; differentiation grade; Gender; T-stage | This tool predicts pathological complete response. | Patients with histologically proven carcinoma of the esophagus or gastro-esophageal junction, treated with neo-adjuvant chemoradiotherapy (CROSS) followed by surgery. | 2b | Logistic regression |
| Lung | Gender; WHO-PS; FEV1; Gross tumor volume; Number of nodal stations | Predicts the probability that a patient with non-small cell lung cancer (NSCLC) will be alive at 2 years post-radiotherapy treatment. | NSCLC patients, stage I-IIIB. | 3 | Kaplan–Meier and Cox regression |
| Lung | Age; Gender; Performance status; Status; Income deprivation; Previous treatment given; BMI | Predicts the probability of 30-day mortality. | NSCLC patients receiving systemic anti-cancer therapies (SACT). | 3 | Logistic regression |
| Lung | Age; Nicotine use; WHO-PS; FEV1; MLD | Predicts the probability of developing acute severe (≥grade 2) dyspnea: %. | NSCLC patients, stage I-IIIB and SCLC patients. | 3 | Multivariate logistic regression |
| Lung | Gender; Age; OTT; Mean esophagus dose; Max esophagus dose; Chemotherapy; WHO-PS | Predicts the probability of developing dysphagia ≥ grade 3: %. | NSCLC stage I-IIIB as well as SCLC patients with limited disease. | 3 | Multivariable logistic regression |
| Lung | Baseline dyspnea score* at the start of R(CH)T; Cardiac comorbidity; Tumor location; Forced expiratory volume; Sequential chemotherapy | Predicts the probability of dyspnea ≥ 2 within 6 months after the start of R(CH)T: %. | NSCLC patients, stage I-IIIB. Patients have to be treated with high-dose conformal radiotherapy alone (≤3 Gy per fraction) or high-dose conformal radiotherapy combined with chemotherapy (sequential or concurrent). | 3 | Univariate and multivariate logistic regression |
| Prostate | Age; BMI; Diabetes; Hemorrhoids; Uretra; Pre-treatment erectile function; PSA level; T-Stage; Primary Gleason score; No. of positive biopsy cores; No. of negative biopsy cores; ASA score; Anticoagulants; Nerve-sparing surgery; Androgen deprivation therapy; ADT length; Prior abdominal surgery; Irradiation of pelvic nodes; Mean trigone dose; Mean rectum dose; Rectum volume; | Compares the probability of biochemical failure, and the probability of developing chronic erectile dysfunction, urinary incontinence, or rectal bleeding for either prostatectomy or external beam radiotherapy for the treatment of prostate cancer. | Low- to intermediate-risk prostate cancer patients eligible for primary treatment with either external beam radiotherapy or prostatectomy. | 3 | Markov model |
| Prostate | Total dose D (45.0–82.8 Gy); Fractional dose d (1.2–10 Gy); Modality type; Risk group | Predicts the chance of 5-year biological no evidence of disease (5-year bNED). | External beam radiotherapy prostate cancer patients. | 3 | TCP, linear-quadratic (LQ) |
| Endometrium | Age; FIGO histological grade; Myometrial invasion depth; Vascular invasion; Radiotherapy | Predicts the probability that an endometrial cancer patient will have one of the following events within 5 years of follow-up: loco-regional recurrence (LRR), distant recurrence (DR), relapse or death (disease-free survival, DFS), and death (overall survival, OS). | Endometrium cancer patients. | 3 | Cox proportional hazards, Cox regression |
| Rectum | Tumor length (2.0–15.0 cm); SUVmax-pre (1.0–25.0); SUVmax-post (1.0–25.0) | Predicts the probability that a patient with locally advanced rectal cancer (LARC) will have a pathologic complete response after long course chemoradiotherapy (CRT) and surgery. | Patients with locally advanced rectal cancer (LARC). | 3 | Logistic regression |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Halilaj, I.; Oberije, C.; Chatterjee, A.; van Wijk, Y.; Rad, N.M.; Galganebanduge, P.; Lavrova, E.; Primakov, S.; Widaatalla, Y.; Wind, A.; et al. Open Source Repository and Online Calculator of Prediction Models for Diagnosis and Prognosis in Oncology. Biomedicines 2022, 10, 2679. https://doi.org/10.3390/biomedicines10112679
Halilaj I, Oberije C, Chatterjee A, van Wijk Y, Rad NM, Galganebanduge P, Lavrova E, Primakov S, Widaatalla Y, Wind A, et al. Open Source Repository and Online Calculator of Prediction Models for Diagnosis and Prognosis in Oncology. Biomedicines. 2022; 10(11):2679. https://doi.org/10.3390/biomedicines10112679
Chicago/Turabian StyleHalilaj, Iva, Cary Oberije, Avishek Chatterjee, Yvonka van Wijk, Nastaran Mohammadian Rad, Prabash Galganebanduge, Elizaveta Lavrova, Sergey Primakov, Yousif Widaatalla, Anke Wind, and et al. 2022. "Open Source Repository and Online Calculator of Prediction Models for Diagnosis and Prognosis in Oncology" Biomedicines 10, no. 11: 2679. https://doi.org/10.3390/biomedicines10112679
APA StyleHalilaj, I., Oberije, C., Chatterjee, A., van Wijk, Y., Rad, N. M., Galganebanduge, P., Lavrova, E., Primakov, S., Widaatalla, Y., Wind, A., & Lambin, P. (2022). Open Source Repository and Online Calculator of Prediction Models for Diagnosis and Prognosis in Oncology. Biomedicines, 10(11), 2679. https://doi.org/10.3390/biomedicines10112679








