Nitro Capsaicin Suppressed Microglial Activation and TNF-α-Induced Brain Microvascular Endothelial Cell Damage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Capsaicin and Nitro Capsaicin Material
2.2. Cell Culture
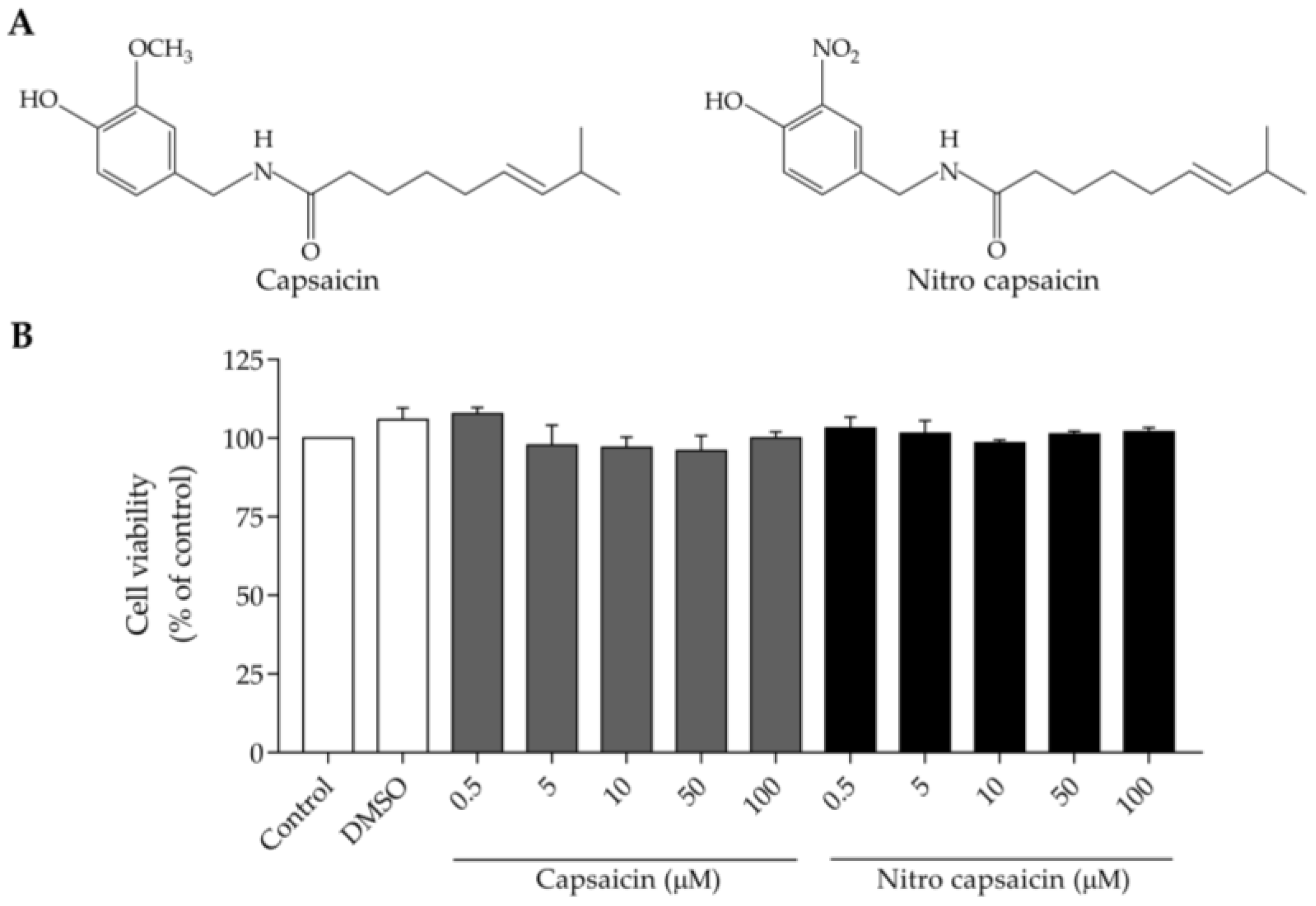
2.3. Cell Viability Assay
2.4. Nitric Oxide Assay
2.5. Reverse-Transcription Polymerase Chain Reaction
2.6. Enzyme-Linked Immunosorbent Assay
2.7. Western Blot
2.8. Immunofluorescence Staining Assay
2.9. Statistical Analysis
3. Results
3.1. Cytotoxic Potency of Nitro Capsaicin in SIMA9 Cells
3.2. Nitro Capsaicin Inhibited Nitric Oxide Production, iNOS Expression, and NF-κB Activation in SIMA9 Cells
3.3. Nitro Capsaicin Suppressed TNF-α, IL-1β, and IL-6 Production in SIMA9 Cells
3.4. Nitro Capsaicin Enhanced IL-4 and IL-10 Production in SIMA9 Cells
3.5. Nitro Capsaicin Exerted Anti-Inflammatory Effects and Suppressed NF-κB Activation in hCMEC/D3 Cells
3.6. Nitro Capsaicin Inhibited Proinflammatory Cytokine Production in hCMEC/D3 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Griciuc, A.; Patel, S.; Federico, A.N.; Choi, S.H.; Innes, B.J.; Oram, M.K.; Cereghetti, G.; McGinty, D.; Anselmo, A.; Sadreyev, R.I.; et al. TREM2 Acts Downstream of CD33 in Modulating Microglial Pathology in Alzheimer’s Disease. Neuron 2019, 103, 820–835.E7. [Google Scholar] [CrossRef] [PubMed]
- Njie, E.G.; Boelen, E.; Stassen, F.R.; Steinbusch, H.W.; Borchelt, D.R.; Streit, W.J. Ex vivo cultures of microglia from young and aged rodent brain reveal age-related changes in microglial function. Neurobiol. Aging 2012, 33, 195.e1–195.e12. [Google Scholar] [CrossRef] [Green Version]
- Kouli, A.; Camacho, M.; Allinson, K.; Williams-Gray, C.H. Neuroinflammation and protein pathology in Parkinson’s disease dementia. Acta Neuropathol. Commun. 2020, 8, 211. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Nicola, D.; Boche, D. Post-mortem analysis of neuroinflammatory changes in human Alzheimer’s disease. Alzheimers Res. Ther. 2015, 7, 42. [Google Scholar] [CrossRef] [Green Version]
- Hansen, D.V.; Hanson, J.E.; Sheng, M. Microglia in Alzheimer’s disease. J. Cell Biol. 2018, 217, 459–472. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.G.; Moon, M.; Choi, J.G.; Park, G.; Kim, A.J.; Hur, J.; Lee, K.T.; Oh, M.S. Donepezil inhibits the amyloid-beta oligomer-induced microglial activation in vitro and in vivo. Neurotoxicology 2014, 40, 23–32. [Google Scholar] [CrossRef]
- Ramírez, E.; Sánchez-Maldonado, C.; Mayoral, M.A.; Mendieta, L.; Alatriste, V.; Patricio-Martínez, A.; Limón, I.D. Neuroinflammation induced by the peptide amyloid-β (25-35) increase the presence of galectin-3 in astrocytes and microglia and impairs spatial memory. Neuropeptides 2019, 74, 11–23. [Google Scholar] [CrossRef]
- Montagne, A.; Barnes, S.R.; Sweeney, M.D.; Halliday, M.R.; Sagare, A.P.; Zhao, Z.; Toga, A.W.; Jacobs, R.E.; Liu, C.Y.; Amezcua, L.; et al. Blood-brain barrier breakdown in the aging human hippocampus. Neuron 2015, 85, 296–302. [Google Scholar] [CrossRef] [Green Version]
- Nation, D.A.; Sweeney, M.D.; Montagne, A.; Sagare, A.P.; D’Orazio, L.M.; Pachicano, M.; Sepehrband, F.; Nelson, A.R.; Buennagel, D.P.; Harrington, M.G.; et al. Blood–brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat. Med. 2019, 25, 270–276. [Google Scholar] [CrossRef]
- Brás, J.P.; Bravo, J.; Freitas, J.; Barbosa, M.A.; Santos, S.G.; Summavielle, T.; Almeida, M.I. TNF-alpha-induced microglia activation requires miR-342: Impact on NF-kB signaling and neurotoxicity. Cell Death Dis. 2020, 11, 415. [Google Scholar] [CrossRef]
- Nishioku, T.; Matsumoto, J.; Dohgu, S.; Sumi, N.; Miyao, K.; Takata, F.; Shuto, H.; Yamauchi, A.; Kataoka, Y. Tumor necrosis factor-α mediates the blood-brain barrier dysfunction induced by activated microglia in mouse brain microvascular endothelial cells. J. Pharmacol. Sci. 2010, 112, 251–254. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.M.; Shang, D.S.; Zhao, W.D.; Fang, W.G.; Chen, Y.H. Microglial TNF-α-dependent elevation of MHC class I expression on brain endothelium induced by amyloid-beta promotes T cell transendothelial migration. Neurochem. Res. 2013, 38, 2295–2304. [Google Scholar] [CrossRef] [PubMed]
- Paik, S.; Somvanshi, R.K.; Kumar, U. Somatostatin Maintains Permeability and Integrity of Blood-Brain Barrier in β-Amyloid Induced Toxicity. Mol. Neurobiol. 2019, 56, 292–306. [Google Scholar] [CrossRef] [PubMed]
- Duperray, A.; Barbe, D.; Raguenez, G.; Weksler, B.B.; Romero, I.A.; Couraud, P.O.; Perron, H.; Marche, P.N. Inflammatory response of endothelial cells to a human endogenous retrovirus associated with multiple sclerosis is mediated by TLR4. Int. Immunol. 2015, 27, 545–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernhart, E.; Kogelnik, N.; Prasch, J.; Gottschalk, B.; Goeritzer, M.; Depaoli, M.R.; Reicher, H.; Nusshold, C.; Plastira, I.; Hammer, A.; et al. 2-Chlorohexadecanoic acid induces ER stress and mitochondrial dysfunction in brain microvascular endothelial cells. Redox Biol. 2018, 15, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Sato, N.; Matsumoto, T.; Kawaguchi, S.; Seya, K.; Matsumiya, T.; Ding, J.; Aizawa, T.; Imaizumi, T. Porphyromonas gingivalis lipopolysaccharide induces interleukin-6 and c-c motif chemokine ligand 2 expression in cultured hCMEC/D3 human brain microvascular endothelial cells. Gerodontology 2022, 39, 139–147. [Google Scholar] [CrossRef]
- Stephan, D.; Sbai, O.; Wen, J.; Couraud, P.O.; Putterman, C.; Khrestchatisky, M.; Desplat-Jégo, S. TWEAK/Fn14 pathway modulates properties of a human microvascular endothelial cell model of blood brain barrier. J. Neuroinflamm. 2013, 10, 9. [Google Scholar] [CrossRef] [Green Version]
- Balleza-Tapia, H.; Crux, S.; Andrade-Talavera, Y.; Dolz-Gaiton, P.; Papadia, D.; Chen, G.; Johansson, J.; Fisahn, A. TrpV1 receptor activation rescues neuronal function and network gamma oscillations from Aβ-induced impairment in mouse hippocampus in vitro. Elife 2018, 7, e37703. [Google Scholar] [CrossRef]
- Chung, Y.C.; Baek, J.Y.; Kim, S.R.; Ko, H.W.; Bok, E.; Shin, W.H.; Won, S.Y.; Jin, B.K. Capsaicin prevents degeneration of dopamine neurons by inhibiting glial activation and oxidative stress in the MPTP model of Parkinson’s disease. Exp. Mol. Med. 2017, 49, e298. [Google Scholar] [CrossRef] [Green Version]
- Bok, E.; Chung, Y.C.; Kim, K.-S.; Baik, H.H.; Shin, W.-H.; Jin, B.K. Modulation of M1/M2 polarization by capsaicin contributes to the survival of dopaminergic neurons in the lipopolysaccharide-lesioned substantia nigra in vivo. Exp. Mol. Med. 2018, 50, 1–14. [Google Scholar] [CrossRef]
- Glas, B.; Claeson, A.S. Skin sensitivity to capsaicin, perceived stress and burn out among patients with building-related symptoms. Int. Arch. Occup. Environ. Health 2021, 94, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Nasrawi, C.W.; Pangborn, R.M. Temporal effectiveness of mouth-rinsing on capsaicin mouth-burn. Physiol. Behav. 1990, 47, 617–623. [Google Scholar] [CrossRef]
- Petruzzi, M.; Lauritano, D.; De Benedittis, M.; Baldoni, M.; Serpico, R. Systemic capsaicin for burning mouth syndrome: Short-term results of a pilot study. J. Oral Pathol. Med. 2004, 33, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Herrera-López, J.A.; Mejía-Rivas, M.A.; Vargas-Vorackova, F.; Valdovinos-Díaz, M.A. Capsaicin induction of esophageal symptoms in different phenotypes of gastroesophageal reflux disease. Rev. Gastroenterol. Mex. 2010, 75, 396–404. [Google Scholar]
- Laolob, T.; Bunyapraphatsara, N.; Waranuch, N.; Pongcharoen, S.; Punyain, W.; Chancharunee, S.; Sakchaisri, K.; Pratuangdejkul, J.; Chongruchiroj, S.; Kielar, F.; et al. Enhancement of Lipolysis in 3T3-L1 Adipocytes by Nitroarene Capsaicinoid Analogs. Nat. Prod. Commun. 2021, 16, 1934578X20987949. [Google Scholar] [CrossRef]
- Dave, K.M.; Ali, L.; Manickam, D.S. Characterization of the SIM-A9 cell line as a model of activated microglia in the context of neuropathic pain. PLoS ONE 2020, 15, e0231597. [Google Scholar] [CrossRef] [Green Version]
- Nagamoto-Combs, K.; Kulas, J.; Combs, C.K. A novel cell line from spontaneously immortalized murine microglia. J. Neurosci. Methods 2014, 233, 187–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weksler, B.; Romero, I.A.; Couraud, P.O. The hCMEC/D3 cell line as a model of the human blood brain barrier. Fluids Barriers CNS 2013, 10, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Q.; Sun, W.; Qu, M. Anti-neuro-inflammatory effects of the bioactive compound capsaicin through the NF-κB signaling pathway in LPS-stimulated BV2 microglial cells. Pharmacogn. Mag. 2018, 14, 489–494. [Google Scholar] [CrossRef]
- Bhatia, H.S.; Roelofs, N.; Muñoz, E.; Fiebich, B.L. Alleviation of Microglial Activation Induced by p38 MAPK/MK2/PGE(2) Axis by Capsaicin: Potential Involvement of other than TRPV1 Mechanism/s. Sci. Rep. 2017, 7, 116. [Google Scholar] [CrossRef]
- Asiimwe, N.; Yeo, S.G.; Kim, M.S.; Jung, J.; Jeong, N.Y. Nitric Oxide: Exploring the Contextual Link with Alzheimer’s Disease. Oxidative Med. Cell. Longev. 2016, 2016, 7205747. [Google Scholar] [CrossRef] [PubMed]
- Liberatore, G.T.; Jackson-Lewis, V.; Vukosavic, S.; Mandir, A.S.; Vila, M.; McAuliffe, W.G.; Dawson, V.L.; Dawson, T.M.; Przedborski, S. Inducible nitric oxide synthase stimulates dopaminergic neurodegeneration in the MPTP model of Parkinson disease. Nat. Med. 1999, 5, 1403–1409. [Google Scholar] [CrossRef] [PubMed]
- Yuste, J.E.; Tarragon, E.; Campuzano, C.M.; Ros-Bernal, F. Implications of glial nitric oxide in neurodegenerative diseases. Front. Cell Neurosci. 2015, 9, 322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bal-Price, A.; Brown, G.C. Inflammatory neurodegeneration mediated by nitric oxide from activated glia-inhibiting neuronal respiration, causing glutamate release and excitotoxicity. J. Neurosci. 2001, 21, 6480–6491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergamini, S.; Rota, C.; Canali, R.; Staffieri, M.; Daneri, F.; Bini, A.; Giovannini, F.; Tomasi, A.; Iannone, A. N-acetylcysteine inhibits in vivo nitric oxide production by inducible nitric oxide synthase. Nitric Oxide 2001, 5, 349–360. [Google Scholar] [CrossRef]
- Majano, P.L.; Medina, J.; Zubía, I.; Sunyer, L.; Lara-Pezzi, E.; Maldonado-Rodríguez, A.; López-Cabrera, M.; Moreno-Otero, R. N-Acetyl-cysteine modulates inducible nitric oxide synthase gene expression in human hepatocytes. J. Hepatol. 2004, 40, 632–637. [Google Scholar] [CrossRef]
- Aktan, F. iNOS-mediated nitric oxide production and its regulation. Life Sci. 2004, 75, 639–653. [Google Scholar] [CrossRef]
- Hwang, C.J.; Choi, D.Y.; Park, M.H.; Hong, J.T. NF-κB as a Key Mediator of Brain Inflammation in Alzheimer’s Disease. CNS Neurol Disord Drug Targets 2019, 18, 3–10. [Google Scholar] [CrossRef]
- Reale, M.; Iarlori, C.; Feliciani, C.; Gambi, D. Peripheral chemokine receptors, their ligands, cytokines and Alzheimer’s disease. J. Alzheimers Dis. 2008, 14, 147–159. [Google Scholar] [CrossRef]
- Mudò, G.; Frinchi, M.; Nuzzo, D.; Scaduto, P.; Plescia, F.; Massenti, M.F.; Di Carlo, M.; Cannizzaro, C.; Cassata, G.; Cicero, L.; et al. Anti-inflammatory and cognitive effects of interferon-β1a (IFNβ1a) in a rat model of Alzheimer’s disease. J. Neuroinflamm. 2019, 16, 44. [Google Scholar] [CrossRef] [Green Version]
- Yao, K.; Zu, H.B. Microglial polarization: Novel therapeutic mechanism against Alzheimer’s disease. Inflammopharmacology 2020, 28, 95–110. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.W.; Sun, X.; Shen, X.F.; Lu, Y.; Wang, J.Q.; Sun, Z.R.; Miao, C.H.; Chen, J.W. Propofol attenuates TNF-α-induced MMP-9 expression in human cerebral microvascular endothelial cells by inhibiting Ca2+/CAMK II/ERK/NF-κB signaling pathway. Acta Pharmacol. Sin. 2019, 40, 1303–1313. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Cai, J.; Liu, W.; Wu, X.; Gao, C. Cysteinyl leukotriene receptor type 1 (CysLT1R) antagonist zafirlukast protects against TNF-α-induced endothelial inflammation. Biomed. Pharmacother. 2019, 111, 452–459. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, L.; Xu, H.; Liu, S.; Zhu, F.; Yan, F.; Shen, S.; Zhu, M. TRPV1 agonism inhibits endothelial cell inflammation via activation of eNOS/NO pathway. Atherosclerosis 2017, 260, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Watanabe, K.; Yokoyama, S.; Matsumoto, C.; Hirata, M.; Tominari, T.; Inada, M.; Miyaura, C. Capsaicin, a TRPV1 Ligand, Suppresses Bone Resorption by Inhibiting the Prostaglandin E Production of Osteoblasts, and Attenuates the Inflammatory Bone Loss Induced by Lipopolysaccharide. ISRN Pharmacol. 2012, 2012, 439860. [Google Scholar] [CrossRef] [Green Version]
- Shen, Z.; Bao, X.; Wang, R. Clinical PET Imaging of Microglial Activation: Implications for Microglial Therapeutics in Alzheimer’s Disease. Front. Aging Neurosci. 2018, 10, 314. [Google Scholar] [CrossRef]
- Tang, Y.; Le, W. Differential Roles of M1 and M2 Microglia in Neurodegenerative Diseases. Mol. Neurobiol. 2016, 53, 1181–1194. [Google Scholar] [CrossRef] [PubMed]
- Qian, K.; Wang, G.; Cao, R.; Liu, T.; Qian, G.; Guan, X.; Guo, Z.; Xiao, Y.; Wang, X. Capsaicin Suppresses Cell Proliferation, Induces Cell Cycle Arrest and ROS Production in Bladder Cancer Cells through FOXO3a-Mediated Pathways. Molecules 2016, 21, 1406. [Google Scholar] [CrossRef] [Green Version]
- Mendivil, E.J.; Sandoval-Rodriguez, A.; Zuñiga-Ramos, L.M.; Santos-Garcia, A.; Armendariz-Borunda, J. Capsaicin and sulforaphane prevent experimental liver fibrosis via upregulation of peroxisome proliferator-activated receptor gamma and nuclear factor (erythroid-derived 2)-like 2. J. Funct. Foods 2019, 52, 382–388. [Google Scholar] [CrossRef]
- Choi, J.H.; Jin, S.W.; Choi, C.Y.; Kim, H.G.; Lee, G.H.; Kim, Y.A.; Chung, Y.C.; Jeong, H.G. Capsaicin Inhibits Dimethylnitrosamine-Induced Hepatic Fibrosis by Inhibiting the TGF-β1/Smad Pathway via Peroxisome Proliferator-Activated Receptor Gamma Activation. J. Agric. Food Chem. 2017, 65, 317–326. [Google Scholar] [CrossRef]
- Saria, A.; Skofitsch, G.; Lembeck, F. Distribution of capsaicin in rat tissues after systemic administration. J. Pharm. Pharmacol. 1982, 34, 273–275. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.W.; Sum, A.K. Molecular dynamics study of the properties of capsaicin in an 1-octanol/water system. J. Phys. Chem. B 2006, 110, 2351–2357. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamornwan, S.; Chokpanuwat, T.; Uppakara, K.; Laorob, T.; Wichai, U.; Ketsawatsomkron, P.; Saengsawang, W. Nitro Capsaicin Suppressed Microglial Activation and TNF-α-Induced Brain Microvascular Endothelial Cell Damage. Biomedicines 2022, 10, 2680. https://doi.org/10.3390/biomedicines10112680
Jamornwan S, Chokpanuwat T, Uppakara K, Laorob T, Wichai U, Ketsawatsomkron P, Saengsawang W. Nitro Capsaicin Suppressed Microglial Activation and TNF-α-Induced Brain Microvascular Endothelial Cell Damage. Biomedicines. 2022; 10(11):2680. https://doi.org/10.3390/biomedicines10112680
Chicago/Turabian StyleJamornwan, Sopana, Tanida Chokpanuwat, Kwanchanok Uppakara, Thanet Laorob, Uthai Wichai, Pimonrat Ketsawatsomkron, and Witchuda Saengsawang. 2022. "Nitro Capsaicin Suppressed Microglial Activation and TNF-α-Induced Brain Microvascular Endothelial Cell Damage" Biomedicines 10, no. 11: 2680. https://doi.org/10.3390/biomedicines10112680
APA StyleJamornwan, S., Chokpanuwat, T., Uppakara, K., Laorob, T., Wichai, U., Ketsawatsomkron, P., & Saengsawang, W. (2022). Nitro Capsaicin Suppressed Microglial Activation and TNF-α-Induced Brain Microvascular Endothelial Cell Damage. Biomedicines, 10(11), 2680. https://doi.org/10.3390/biomedicines10112680





