High-Frequency Transcranial Random Noise Stimulation for Auditory Hallucinations of Schizophrenia: A Case Series
Abstract
1. Introduction
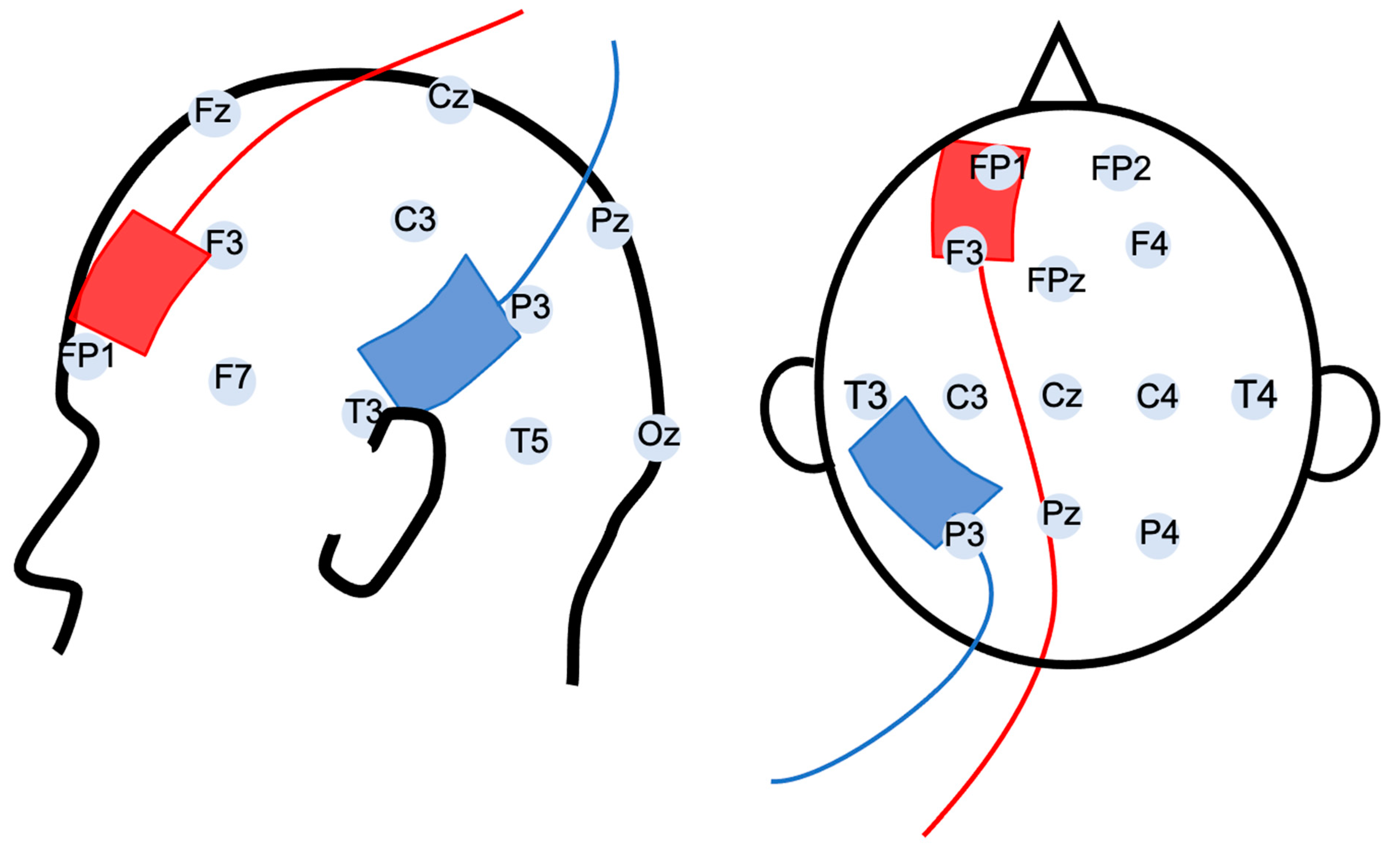
2. Materials and Methods
3. Results
3.1. Effects on Auditory Hallucinations
3.2. Effects on Other Symptoms of Schizophrenia
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Samara, M.T.; Nikolakopoulou, A.; Salanti, G.; Leucht, S. How Many Patients With Schizophrenia Do Not Respond to Antipsychotic Drugs in the Short Term? An Analysis Based on Individual Patient Data From Randomized Controlled Trials. Schizophr. Bull. 2018, 45, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Kane, J.M.; Agid, O.; Baldwin, M.L.; Howes, O.; Lindenmayer, J.-P.; Marder, S.; Olfson, M.; Potkin, S.G.; Correll, C.U. Clinical Guidance on the Identification and Management of Treatment-Resistant Schizophrenia. J. Clin. Psychiatry 2019, 80, 2783. [Google Scholar] [CrossRef] [PubMed]
- Iasevoli, F.; Giordano, S.; Balletta, R.; Latte, G.; Formato, M.V.; Prinzivalli, E.; De Berardis, D.; Tomasetti, C.; de Bartolomeis, A. Treatment resistant schizophrenia is associated with the worst community functioning among severely-ill highly-disabling psychiatric conditions and is the most relevant predictor of poorer achievements in functional milestones. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2016, 65, 34–48. [Google Scholar] [CrossRef]
- Lefaucheur, J.-P.; Wendling, F. Mechanisms of action of tDCS: A brief and practical overview. Neurophysiol. Clin. Neurophysiol. 2019, 49, 269–275. [Google Scholar] [CrossRef]
- Brunelin, J.; Mondino, M.; Gassab, L.; Haesebaert, F.; Gaha, L.; Suaud-Chagny, M.-F.; Saoud, M.; Mechri, A.; Poulet, E. Examining Transcranial Direct-Current Stimulation (tDCS) as a Treatment for Hallucinations in Schizophrenia. Am. J. Psychiatry 2012, 169, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Jardri, R.; Pouchet, A.; Pins, D.; Thomas, P. Cortical Activations During Auditory Verbal Hallucinations in Schizophrenia: A Coordinate-Based Meta-Analysis. Am. J. Psychiatry 2011, 168, 73–81. [Google Scholar] [CrossRef]
- Ćurčić-Blake, B.; Ford, J.M.; Hubl, D.; Orlov, N.; Sommer, I.; Waters, F.; Allen, P.; Jardri, R.; Woodruff, P.W.; David, O.; et al. Interaction of language, auditory and memory brain networks in auditory verbal hallucinations. Prog. Neurobiol. 2016, 148, 1–20. [Google Scholar] [CrossRef]
- Mondino, M.; Sauvanaud, F.; Brunelin, J. A Review of the Effects of Transcranial Direct Current Stimulation for the Treatment of Hallucinations in Patients With Schizophrenia. J. ECT 2018, 34, 164–171. [Google Scholar] [CrossRef]
- Valiengo, L.; Goerigk, S.; Gordon, P.C.; Padberg, F.; Serpa, M.H.; Koebe, S.; Dos Santos, L.A.; Lovera, R.A.M.; De Carvalho, J.B.; Van De Bilt, M.; et al. Efficacy and Safety of Transcranial Direct Current Stimulation for Treating Negative Symptoms in Schizophrenia. JAMA Psychiatry 2020, 77, 121–129. [Google Scholar] [CrossRef]
- Koops, S.; Blom, J.D.; Bouachmir, O.; Slot, M.I.; Neggers, B.; Sommer, I.E. Treating auditory hallucinations with transcranial direct current stimulation in a double-blind, randomized trial. Schizophr. Res. 2018, 201, 329–336. [Google Scholar] [CrossRef]
- Lefaucheur, J.-P.; Antal, A.; Ayache, S.S.; Benninger, D.H.; Brunelin, J.; Cogiamanian, F.; Cotelli, M.; De Ridder, D.; Ferrucci, R.; Langguth, B.; et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin. Neurophysiol. 2016, 128, 56–92. [Google Scholar] [CrossRef]
- Fregni, F.; El-Hagrassy, M.M.; Pacheco-Barrios, K.; Carvalho, S.; Leite, J.; Simis, M.; Brunelin, J.; Nakamura-Palacios, E.M.; Marangolo, P.; Venkatasubramanian, G.; et al. Evidence-Based Guidelines and Secondary Meta-Analysis for the Use of Transcranial Direct Current Stimulation in Neurological and Psychiatric Disorders. Int. J. Neuropsychopharmacol. 2020, 24, 256–313. [Google Scholar] [CrossRef] [PubMed]
- Terney, D.; Chaieb, L.; Moliadze, V.; Antal, A.; Paulus, W. Increasing Human Brain Excitability by Transcranial High-Frequency Random Noise Stimulation. J. Neurosci. 2008, 28, 14147–14155. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.-A.; Taylor, J.; Loo, C. Comparison of the Effects of Transcranial Random Noise Stimulation and Transcranial Direct Current Stimulation on Motor Cortical Excitability. J. ECT 2015, 31, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Moliadze, V.; Fritzsche, G.; Antal, A. Comparing the Efficacy of Excitatory Transcranial Stimulation Methods Measuring Motor Evoked Potentials. Neural Plast. 2014, 2014, 1–6. [Google Scholar] [CrossRef]
- Inukai, Y.; Saito, K.; Sasaki, R.; Tsuiki, S.; Miyaguchi, S.; Kojima, S.; Masaki, M.; Otsuru, N.; Onishi, H. Comparison of Three Non-Invasive Transcranial Electrical Stimulation Methods for Increasing Cortical Excitability. Front. Hum. Neurosci. 2016, 10, 668. [Google Scholar] [CrossRef]
- Murphy, O.; Hoy, K.; Wong, D.; Bailey, N.; Fitzgerald, P.; Segrave, R. Transcranial random noise stimulation is more effective than transcranial direct current stimulation for enhancing working memory in healthy individuals: Behavioural and electrophysiological evidence. Brain Stimul. 2020, 13, 1370–1380. [Google Scholar] [CrossRef]
- van der Groen, O.; Potok, W.; Wenderoth, N.; Edwards, G.; Mattingley, J.B.; Edwards, D. Using noise for the better: The effects of transcranial random noise stimulation on the brain and behavior. Neurosci. Biobehav. Rev. 2022, 138. [Google Scholar] [CrossRef]
- Palm, U.; Hasan, A.; Keeser, D.; Falkai, P.; Padberg, F. Transcranial random noise stimulation for the treatment of negative symptoms in schizophrenia. Schizophr. Res. 2013, 146, 372–373. [Google Scholar] [CrossRef]
- Haesebaert, F.; Mondino, M.; Saoud, M.; Poulet, E.; Brunelin, J. Efficacy and safety of fronto-temporal transcranial random noise stimulation (tRNS) in drug-free patients with schizophrenia: A case study. Schizophr. Res. 2014, 159, 251–252. [Google Scholar] [CrossRef]
- Chang, C.-C.; Lin, Y.-Y.; Tzeng, N.-S.; Kao, Y.-C.; Chang, H.-A. Adjunct high-frequency transcranial random noise stimulation over the lateral prefrontal cortex improves negative symptoms of schizophrenia: A randomized, double-blind, sham-controlled pilot study. J. Psychiatr. Res. 2021, 132, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Dondé, C.; Dondé, C.; Haesebaert, F.; Haesebaert, F.; Poulet, E.; Poulet, E.; Mondino, M.; Mondino, M.; Brunelin, J.; Brunelin, J. Validation of the French Version of the Auditory Hallucination Rating Scale in a Sample of Hallucinating Patients with Schizophrenia: Validation de la version française de l’échelle d’évaluation des hallucinations auditives dans un échantillon de patients souffrant de schizophrénie et ayant des hallucinations. Can. J. Psychiatry 2019, 65, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, R.E.; Hawkins, K.A.; Gueorguieva, R.; Boutros, N.N.; Rachid, F.; Carroll, K.; Krystal, J.H. Transcranial Magnetic Stimulation of Left Temporoparietal Cortex and Medication-Resistant Auditory Hallucinations. Arch. Gen. Psychiatry 2003, 60, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Bose, A.; Shivakumar, V.; Agarwal, S.M.; Kalmady, S.V.; Shenoy, S.; Sreeraj, V.S.; Narayanaswamy, J.C.; Venkatasubramanian, G. Efficacy of fronto-temporal transcranial direct current stimulation for refractory auditory verbal hallucinations in schizophrenia: A randomized, double-blind, sham-controlled study. Schizophr. Res. 2018, 195, 475–480. [Google Scholar] [CrossRef]
- Wagner, E.; Honer, W.G.; Sommer, I.E.; Koops, S.; Blumberger, D.M.; Daskalakis, Z.J.; Lange, J.J.D.-D.; Bais, L.; Knegtering, H.; Aleman, A.; et al. Repetitive transcranial magnetic stimulation (rTMS) for schizophrenia patients treated with clozapine. World J. Biol. Psychiatry 2020, 22, 14–26. [Google Scholar] [CrossRef]
- Mondino, M.; Fonteneau, C.; Simon, L.; Dondé, C.; Haesebaert, F.; Poulet, E.; Brunelin, J. Advancing clinical response characterization to frontotemporal transcranial direct current stimulation with electric field distribution in patients with schizophrenia and auditory hallucinations: A pilot study. Eur. Arch. Psychiatry Clin. Neurosci. 2021, 271, 85–92. [Google Scholar] [CrossRef]
- Marquardt, L.; Kusztrits, I.; Craven, A.R.; Hugdahl, K.; Specht, K.; Hirnstein, M. A multimodal study of the effects of tDCS on dorsolateral prefrontal and temporo-parietal areas during dichotic listening. Eur. J. Neurosci. 2020, 53, 449–459. [Google Scholar] [CrossRef]
- Datta, A.; Bansal, V.; Diaz, J.; Patel, J.; Reato, D.; Bikson, M. Gyri-precise head model of transcranial direct current stimulation: Improved spatial focality using a ring electrode versus conventional rectangular pad. Brain Stimul. 2009, 2, 201–207.e1. [Google Scholar] [CrossRef]
- Dmochowski, J.P.; Datta, A.; Bikson, M.; Su, Y.; Parra, L.C. Optimized multi-electrode stimulation increases focality and intensity at target. J. Neural Eng. 2011, 8, 046011. [Google Scholar] [CrossRef]
- Kuo, H.-I.; Bikson, M.; Datta, A.; Minhas, P.; Paulus, W.; Kuo, M.-F.; Nitsche, M.A. Comparing Cortical Plasticity Induced by Conventional and High-Definition 4 × 1 Ring tDCS: A Neurophysiological Study. Brain Stimul. 2013, 6, 644–648. [Google Scholar] [CrossRef]
- Huang, Y.; Thomas, C.; Datta, A.; Parra, L.C. Optimized tDCS for Targeting Multiple Brain Regions: An Integrated Implementation. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; pp. 3545–3548. [Google Scholar] [CrossRef]
- Bose, A.; Shivakumar, V.; Chhabra, H.; Parlikar, R.; Sreeraj, V.S.; Dinakaran, D.; Narayanaswamy, J.C.; Venkatasubramanian, G. Feasibility and Clinical Utility of High-definition Transcranial Direct Current Stimulation in the Treatment of Persistent Hallucinations in Schizophrenia. East Asian Arch. Psychiatry 2017, 27, 162–164. [Google Scholar] [PubMed]
- Sreeraj, V.S.; Dinakaran, D.; Parlikar, R.; Chhabra, H.; Selvaraj, S.; Shivakumar, V.; Bose, A.; Narayanaswamy, J.C.; Venkatasubramanian, G. High-definition transcranial direct current simulation (HD-tDCS) for persistent auditory hallucinations in schizophrenia. Asian J. Psychiatry 2018, 37, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Sheffield, J.G.; Ramerpresad, S.; Brem, A.-K.; Mansfield, K.; Orhan, U.; Dillard, M.; McKanna, J.; Plessow, F.; Thompson, T.; Santarnecchi, E.; et al. Blinding efficacy and adverse events following repeated transcranial alternating current, direct current, and random noise stimulation. Cortex 2022, 154, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Brunelin, J.; Mondino, M.; Haesebaert, J.; Attal, J.; Benoit, M.; Chupin, M.; Dollfus, S.; El-Hage, W.; Galvao, F.; Jardri, R.; et al. Examining transcranial random noise stimulation as an add-on treatment for persistent symptoms in schizophrenia (STIM’Zo): A study protocol for a multicentre, double-blind, randomized sham-controlled clinical trial. Trials 2021, 22, 964. [Google Scholar] [CrossRef] [PubMed]

| Patient | Sex | Age | AHRS | PANSS | Antipsychotic Medication |
|---|---|---|---|---|---|
| 1 | F | 67 | 26 | 62 | olanzapine LP 300 mg/15d + amisulpride 800 mg/d |
| 2 | M | 44 | 26 | 43 | clozapine 500 mg/d |
| 3 | F | 26 | 38 | 91 | drug free |
| 4 | F | 38 | 28 | 70 | clozapine 600 mg/d + levomepromazine 100 mg/d |
| 5 | M | 27 | 32 | 83 | clozapine 500 mg/d + chlorpromazine 200 mg/d + risperidone 6 mg/d |
| 6 | F | 38 | 22 | 66 | clozapine 300 mg/d + cyamemazine 150 mg/d |
| 7 | M | 42 | 31 | NA | clozapine 400 mg/d + aripiprazole 15 mg/d |
| 8 | F | 22 | 22 | NA | aripiprazole 25 mg/d |
| 9 | M | 59 | 24 | 68 | olanzapine 30 mg/d + clozapine 25 mg/d |
| 10 | F | 36 | 25 | 83 | clozapine 600 mg/d |
| n | Pre-hf-tRNS | Post-hf-tRNS | p | |
|---|---|---|---|---|
| PANSS total score | 6 | 69.17 (16.8) | 56.33 (13.4) | 0.140 |
| Positive symptoms | 7 | 16.86 (4.10) | 14.57 (3.51) | 0.020 |
| Negative symptoms | 7 | 19.00 (4.97) | 15.57 (4.89) | 0.142 |
| General psychopathology | 6 | 34.33 (10.05) | 27.67 (6.41) | 0.156 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mondino, M.; Janin, D.; Galvao, F.; Brunelin, J. High-Frequency Transcranial Random Noise Stimulation for Auditory Hallucinations of Schizophrenia: A Case Series. Biomedicines 2022, 10, 2698. https://doi.org/10.3390/biomedicines10112698
Mondino M, Janin D, Galvao F, Brunelin J. High-Frequency Transcranial Random Noise Stimulation for Auditory Hallucinations of Schizophrenia: A Case Series. Biomedicines. 2022; 10(11):2698. https://doi.org/10.3390/biomedicines10112698
Chicago/Turabian StyleMondino, Marine, Delphine Janin, Filipe Galvao, and Jérôme Brunelin. 2022. "High-Frequency Transcranial Random Noise Stimulation for Auditory Hallucinations of Schizophrenia: A Case Series" Biomedicines 10, no. 11: 2698. https://doi.org/10.3390/biomedicines10112698
APA StyleMondino, M., Janin, D., Galvao, F., & Brunelin, J. (2022). High-Frequency Transcranial Random Noise Stimulation for Auditory Hallucinations of Schizophrenia: A Case Series. Biomedicines, 10(11), 2698. https://doi.org/10.3390/biomedicines10112698






